 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A novel virtual screening approach is implemented herein, which is a further improvement of our previously published “target-bound pharmacophore modeling approach”. The generated pharmacophore library is based only on highly contributing amino acid residues, instead of arbitrary pharmacophores, which are most commonly used in the conventional approaches in literature. Highly contributing amino acid residues were distinguished based on free binding energy contributions obtained from calculation from molecular dynamic (MD) simulations. To the best of our knowledge; this is the first attempt in the literature using such an approach; previous approaches have relied on the docking score to generate energy-based pharmacophore models. However, docking scores are reportedly unreliable. Thus, we present a model for a per-residue energy decomposition, constructed from MD simulation ensembles generating a more trustworthy pharmacophore model, which can be applied in drug discovery workflow. This work is aimed at introducing a more rational approach to the field of drug design, rather than comparing the validity of this approach against those previously reported. We recommend additional computational and experimental work to further validate this approach. This approach was used to screen for potential reverse transcriptase inhibitors using the pharmacophoric features of compound GSK952. The complex was subjected to docking, thereafter, MD simulation confirmed the stability of the system. Experimentally determined inhibitors with known HIV-reverse transcriptase inhibitory activity were used to validate the protocol. Two potential hits (ZINC46849657 and ZINC54359621) showed a significant potential with regard to free binding energy. Reported results obtained from this work confirm that this new approach is favorable in the future of the drug design industry.
Introduction
HIV infection is the leading cause of death across the globe.Citation1 There are two strains of HIV, namely, HIV-1 and HIV-2. HIV-1 is the most infectious and prevalent globally.Citation2 Worldwide statistics by the American foundation for AIDS Research reported that sub-Saharan Africa is the most affected region, with approximately 70% of adults and 91% of children HIV positive.Citation3
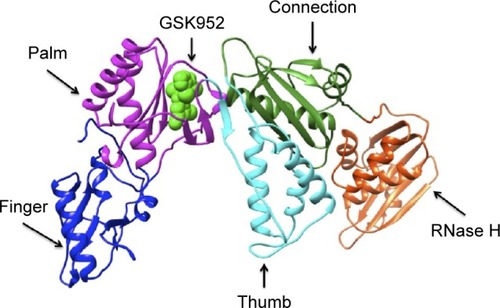
HIV-1 reverse transcriptase (RT) is currently an essential target for US Food and Drug Administration (FDA) approved HIV-1 therapyCitation4 and a prominent target of many approved anti-HIV drugs that are key components of highly active antiretroviral therapies.Citation5 The HIV-1 RT enzyme catalyzes the conversion of viral RNA into cDNA, which enters the host nucleus and is incorporated into host chromosomal DNA of the host cell by the IN enzyme.Citation6 It is the sole viral enzyme required for the catalytic formation of cDNA generated from viral RNA, hence playing a central role in HIV replication,Citation7 making it a prime target for HIV-1 therapy. HIV-1 RT is a heterodimer consisting of two subunits; a p66 subunit (DNA polymerization site and RNase H active site), which is responsible for the replication of the single-stranded RNA genome found in virions into the double-stranded DNA; and a p51 subunit, which is responsible for the proper folding of p66 rather than enzymatic activities subunit ().Citation8
Figure 1 Ribbon representation of HIV-1 RT-GSK952 complex.
Abbreviations: RT, reverse transcriptase; PDB, Protein Data Base.
HIV-1 RT does not possess any proofreading activity. Thus, DNA synthesis prone to errors can be carried out by HIV-1 RT, resulting in a higher mutation rate and the production of multiple HIV variants.Citation2 Etravirine and rilpivirine are the most recent non-nucleoside reverse transcriptase inhibitors (NNRTIs) to which mutated RT viruses are resistant.Citation9 To date, NNRTIs’ resistance still poses a challenge with regard to NNRTI therapy. Thus, there is a clear need for the discovery of new drugs with greater resistance profiles capable of inhibiting HIV-I RT mutated viruses. Numerous studies have made significant attempts in the discovery of new potent NNRTIs using pharmacophore model approaches.Citation10–Citation12
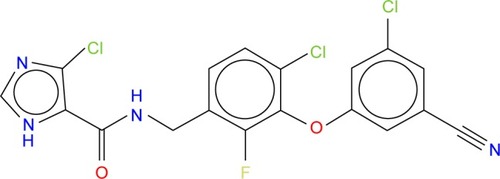
The potential anti-HIV drug needs to be effective against resistant strains. The present study looked at GSK952, a newly discovered NNRTI. The crystal structure (Protein Data Base [PDB] 2YNI)Citation8 of the catalytic domain of HIV-1 RT enzyme bound to GSK952 is available. GSK952 shows high potency against the mutated Y188L strain as well as the mutated Y188C and K103N strains. Previous work on this compound confirmed it has a good inhibitory activity against HIV-1 RT, including the mutated viral Y188L HIV-1 RT strains.Citation8 It exhibited a high antiviral profile when compared to a group of compounds sharing a common imidazole-amide biaryl ether scaffold.Citation8 It possesses a linear arrangement of hydrogen bond acceptors (C=O) and donors (NH), which are required for binding to HIV-1 RT and to form hydrogen bonds with residues in the active site, including K101, K103, and P236.Citation13 GSK952 forms hydrogen bonds with NH and C=O groups (), which are positioned along the protein backbone rather than the side chains. This could explain the inhibitory activity against the K103N mutation.Citation14
In this mutation, the side chain of K103 is tilted away from the inhibitor due to the mutation of the wild type to Asn103.Citation8 GSK952 () has revealed an extraordinary antiviral activity against a wide range of NNRTI-resistant virusesCitation8 and forms favorable pharmacokinetic profiles across multiple species. We believe it has great potential and deserves further exploration. Thus, it is an ideal compound for the current study.
Figure 2 The 2D structure of ligand GSK952 used to generate the pharmacophore model.
Computational tools and capability escalation have led to virtual screening (VS) becoming a routine method in pharmaceutical drug discovery.Citation15 Numerous computational screening tools are accessible for mining of inhibitors with properties of interest.Citation16 VS is one of the most trusted and convenient tools in drug design. Literature confirms its reliability in the discovery of novel HIV-1 RT inhibitors through VS.Citation17 VS is either ligand based or structure based.Citation18 Structure-based VS uses the three-dimensional (3D) structure of the receptor to search for potential ligands.Citation19 Ligand-based VS (LBVS) explores features or properties of known bioactive ligands and searches for compounds with similarities.Citation20 In LBVS, also known as pharmacophore-based VS, favorable features of a known active site are used to build a pharmacophore model. Among other models, pharmacophore searches are best at discovering a range of chemical structures with feasible features, hence the principal method for the initial selection of compounds.Citation21 Ligand-based pharmacophore approaches generate libraries based on a set of known ligands illustrative of crucial interactions between the ligands and a particular target;Citation22 whereas structure-based pharmacophore models are based on the knowledge of the 3D structure of the target.Citation23 Numerous studies have combined LBVS and structure-based VS with the aim of improving the VS process.Citation24,Citation25 A number of studies have attempted to improve VS and pharmacophore models.Citation26–Citation30 A previous study proposed a target-bound generated pharmacophore model to further improve pharmacophore-based VS. It has been confirmed that target-bound pharmacophore-based VS is a more rational approach.Citation19 Yet to this date, general standards for VS with regard to method evaluation are insufficient. The aim of this work is to identify more potential NNRTIs by exploiting the structural features of GSK952 using a pharmacophore model.
We propose implementing an approach that aims to improve and refine the current pharmacophore approach. This approach is centered on the type of interactions witnessed at a molecular level, which includes hydrogen bonding, charge, and hydrophobic interactions (HPIs).Citation31 This approach will search for compounds that interact with the highly contributing residues based on the free binding energy (FBE).
In this study, we performed molecular dynamic (MD) simulations, pharmacophore-based VS and per-residue energy decomposition (PRED) analysis for residues with the greatest FBE contributions. VS depends primarily on docking calculations, although results based exclusively on docking calculations are rather questionable.Citation32 To test the validity of our proposed approach, we applied the same docking procedure to a set of experimentally determined inhibitors with known HIV-1 RT inhibition activity. In our approach, we intend to unshackle the limitations of previous approaches, such as using noncrucial residues, which retrieves a large number of hits with assumed activity. In an attempt to enhance the accuracy of the pharmacophore model, we only selected highly contributing amino acid residues (HCAAR). A target-bound ensemble was employed in the current study, in the hope of implementing a better approach. A pharmacophore model was created using HCAAR in the protein’s active site, whereas conventional methods use pharmacophore maps created without considering the energy contributions of interacting residues. Only potential pharmacophore moieties were considered; thus, the library of potential compounds generated is more concise and direct. In our approach, the energy-based pharmacophore map is generated using the FBE calculated from MD, which is a more reliable approach, compared to the conventional approach using the docking score. We believe this approach will be more effective than the conventional approach due to these key factors highlighted earlier. The refinement of the method proposed in this study could be implemented as a potential tool for medicinal chemists in the search for more potent HIV-1 RT inhibitors.
Computational methodology
The computational tools implemented in this study are represented in the workflow in . A diagrammatic representation of the pharmacophore model used in this study is shown in . The residues with the highest energy contributions are numbered and ranked from 1 to 6, with 1 being the highest FBE contributing residue (). The highest ranked contributors were used to select the corresponding pharmacophore moieties on the ligand to generate a pharmacophore model used to screen a database for potential hit compounds.
Figure 3 A schematic representation of the VS workflow used in the current study.
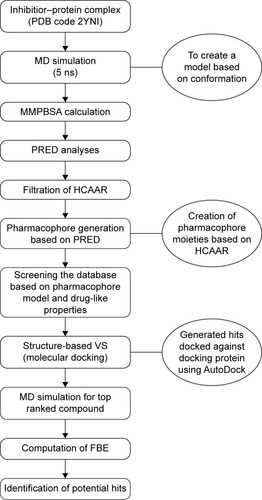
Figure 4 A diagrammatic representation of the pharmacophore model.
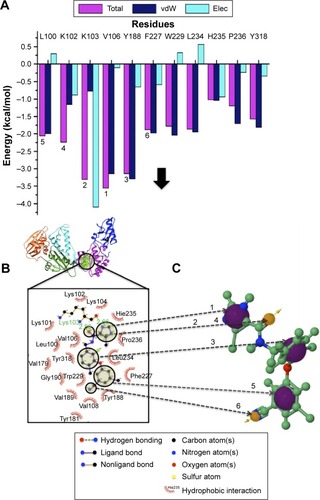
Abbreviations: PRED, per-residue energy decomposition; FBE, free binding energy; vdW, van der Waals; Elec, electrostatic; 2D, two dimensional.
Ligand and receptor preparation
The crystal structure of HIV-1 RT-GSK952 complex was obtained from PDB (2YNI).Citation8 The steepest decent method and MMFF94S force field in Avogadro softwareCitation33 were used to minimize conformation of HIV-1 RT-GSK952 complex. The crystal structure was opened on UCSF chimeraCitation34 to delete chain B and solvents (H2O, MG [magnesium atoms] and TAR [tartaric acid]). GSK952 was selected and deleted. GSK952 was prepared by adding hydrogen. The ligand was prepared using Antechamber and tLeap of Amber14Citation35 to optimize both HIV-1 RT (enzyme) and GSK952 (ligand), respectively, to ensure all parameters are present for MD simulations. Topology files generated were submitted for MD simulation.
MD simulations
Solvation of MD simulationsCitation36 was performed on HIV-1 RT-GSK952 using the graphics processing units version of the PMEMD (Particle Mesh Ewald Molecular Dynamics) engine integrated with Amber14.Citation35 Leap module in Amber 14Citation35 was used to add hydrogen atoms to the proteins. The standard Amber force field was employed to treat the receptor for bioorganic systems (ff99sb).Citation37 A box with equilibrated TIP3PCitation38 water molecules that were arranged around the receptor at a distance of 10 Å around the enzyme and Cl− ions was added to neutralize the systems. Cubic periodic boundary conditions were employed, and particle-mesh Ewald methodCitation39 applied in Amber12 was used to treat the long-range electrostatic interactions with nonbonding at 2fs integration step. The initial minimization of the system was carried out using the steepest descent method for 1,000 steps. A canonical ensemble (amount of substance volume temperature [NVT]) MD was carried out for 50 ps, during which the system was progressively heated up from 0 to 300 K by means of a Langevin thermostat.Citation40 The system was then equilibrated at 300 K under 1 atm pressure while preserving the force constants on the restrained solute. Throughout all MD simulations, the SHAKE algorithmCitation41 was employed on atoms covalently bonded to a hydrogen atom. A production run was achieved, with no restraints, for 5 ns in an isothermal isobaric (amount of substance, pressure, and temperature [NPT]). ensemble employing a Berendsen barostat.Citation42 Trajectory exploration including the root-mean-square deviation (RMSD), root-mean-square fluctuation (RMSF), and radius of gyration (Rg) were carried out using PTRAJ (process trajectory) and CPPTRAJ modules applied in AMBER14.Citation35
The trajectory was saved every 1 ps and examined every 1 ps using the PTRAJ module applied in Amber14.Citation35 The structure was visualized using graphical user interface of the UCSF chimera package.Citation34 Data were plotted using the GUI of Microcal Origin data analysis software Version 6 (www.originlab.com).
Molecular docking
AutoDock Vina was used for docking calculation.Citation43 Geister partial charges were allocated during docking. AutoDock Graphical user interface provided by MGL tools were used to outline the AutoDock atom types.Citation44 The docked conformations were obtained using the Lamarckian genetic algorithm.Citation45 The magnitude of the grid box was x =14 Å, y =14 Å, z =18 Å, enclosing the anticipated active site residues including the highest contributing Leu100, Lys102, Lys103, Val106, Try188, and Phe227 residues. The classification of the compounds was in accordance with their docking score (DS) in a descending order.
FBE calculations
The FBE of the docked complexes was calculated to support the docking calculations and to predict the binding efficiencies of the HIV-1 RT against the targets. The FBE predictions are performed using molecular mechanics/Poisson–Boltzmann surface area (MMPBSA) that incorporates EquationEquation 1(1) Citation46 and molecular mechanics/generalized-Boltzmann surface area (MMGBSA) method that incorporates EquationEquation 2
(2) .Citation47
Results and discussion
PRED pharmacophore model
The pharmacophore model exploits both the structural features of the proteins as well as the chemical features of ligands. To generate a PRED-based pharmacophore model, PRED decomposition was computed from MMPBSA calculations after 5 ns MD simulations of the (2YNI-GSK952) complex. Residues Leu100, Lys102, Lys103, Val106, Try188, and Phe227 were found to be the highest contributing residues that interact with the ligands (). The pharmacophoric features of the ligands’ HPI, hydrogen acceptor, and hydrogen bond interactions were found to interact with Leu100, Lys102, Val106, Try188, Lys103, Phe227, and Lys103, respectively. These ligand features were set as a query to generate a PRED-based pharmacophore model in ZINCpharmer.Citation48 Furthermore, the PRED-based pharmacophore model () was used to screen the ZINC databaseCitation49 for compounds with similar features to obtain the novel hits. Additionally, a further selection criterion was implemented when screening ZINCpharmer database. Seven hundred and eighty-eight hits were obtained from the ZINC database.
Molecular docking
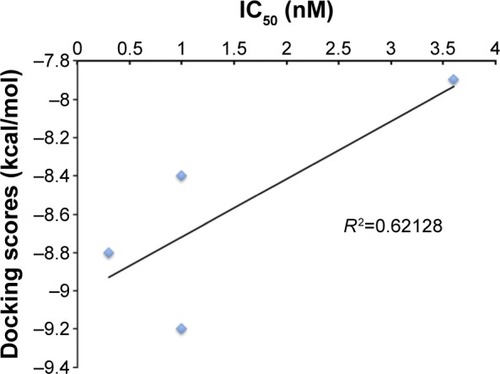
All 788 hits were docked into the crystal structure (2YNI) to assess their chemical and physical feasibility. Thus, only ones with the correct pose and physical properties were selected for further consideration. This provided valuable insights into the nature of the binding site and the key ligand–protein interactions that are responsible for the molecular recognition and served as a validation step in the proposed workflow. A set of four compounds with experimentally determined activity (half maximal inhibitory concentration [IC50] values) was selected to further validate our findings. These four compounds were docked into the crystal structure of 2YNI as described earlier in the Molecular docking section. Calculated DS were correlated against the inhibitors’ experimentally determined IC50 values (). DS correlated (R2=0.62128) () with the IC50 values. The comparison by means of correlation serves as an additional validation step and adds robustness and validity to the docking protocol used in the current study. After the validation, molecular docking was carried out for all 788 hits.
Table 1 Validation of molecular docking approach
Figure 5 Validation of molecular docking: docking score vs half maximal inhibitory concentration (IC50).
The top ten compounds with the highest DS were selected from the library of 788 hits. The DS for the top ten compounds ranged from −11.5 to −12.4 kcal/mol (). It should be noted that there is not much difference in the binding energy of the top ten compounds with a rough range of 0.9 kcal/mol. Hit compounds were found to be more stable due to conservation of vital pharmacophoric properties when generating the pharmacophore model.
Table 2 Representation of the top ten compounds displaying 3D shapes, HBD, HBA, xlogP, MW, and calculated DS and RB
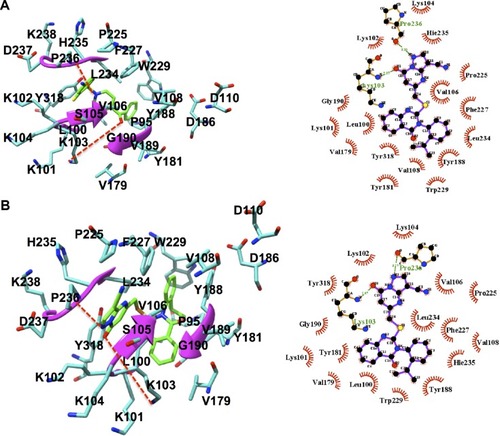
It is of high importance to consider residues that are directly accountable for the efficacy of HIV-1 RT when evaluating whether the molecules bind strongly with HIV-1 RT. This will ensure effective inhibition. Lig-plot analysis is best suited for displaying the two-dimensional interaction between the ligand and the residue contributing to the ligand binding. Lig-plot shows the interacting residues with Leu100, Lys102, Lys103, Val106, Tyr188, and Phe227 with the highest FBE contributing to the ligand binding. Leu100, Lys102, Val106, Phe227, Pro229, and Y188 have HPIs whereas Lys103 displays a hydrogen interaction with the inhibitor (). The PRED calculations presented in this study are valuable tools that can be employed to highlight the most significant residues involved in the binding of the inhibitor.
As a result, this will serve as a guidance in the design of drug candidates that can strongly bind with such residues. The anti-HIV activity of the top ten hits is not evidenced, as the FDA-approved anti-HIV drugs were omitted throughout the entire screening process to guarantee the design of compound libraries grounded on novel structural scaffolds. Such findings verify the refined pharmacophore approach adopted in this work, rendering the suggested concept secure and dependable enough for the mining of novel drug candidates against the enzyme of interest. We believe the conveyed methodology in this current work can be applied to more biological drug targets with determined protein structures and binders that are recognized.
MD simulations and MMPBSA calculations
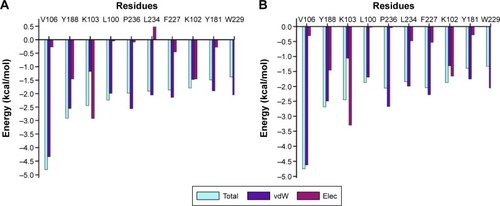
As previously mentioned, docking alone cannot provide reliable results. Hence, it is of high importance to correlate docking results with MD simulations. MD simulation (5 ns) was performed on the top ten screened hits from the molecular docking to confirm the change in mobility resulting from the binding of each hit to HIV-1 RT. Common MD-type force fields were employed to evaluate and rescore the docked complexes, this will lead to more accurate estimates of the binding affinities. All the free energy components are representative of average values over the 5 ns MD simulations calculated using the MMPBSA approach shown in . Among the top ten, ZINC54359621 and ZINC46849657 resulted in the highest FBE. The total calculated FBE (ΔGbind) of GSK952 against HIV-1 RT protein is −58.8 kcal/mol compared to −58.5 kcal/mol and −59.0 kcal/mol of ZINC46849657 and ZINC54359621, respectively. These findings are more reliable than the energy contributions obtained from the docking calculations. It was also observed that the ΔEvdW of ZINC54359621 and ZINC46849657 was higher (; ), which is advantageous and a contributing factor toward the high binding affinity.Citation50 Electrostatic forces contribute to inhibitor molecules gaining binding energy.Citation51 ZINC54359621 and ZINC46849657 electrostatic interactions are similar to that of GSK952, which explains their higher binding affinity and stability.
Table 3 A comparison of GSK952’s binding affinity with that of the top two hits ZINC54359621 and ZINC46849657
Figure 6 The per residue graphs showing FBE contribution for both ZINC54359621 and ZINC46849657.
Abbreviations: FBE, free binding energy; vdW, van der Waals; Elec, electrostatic.
PRED of the top two hits showed consistency compared to that of GSK952. The residues that contributed to the binding of GSK952 to HIV-1 RT protein also contributed to the binding of the top two hits ().
It is noted that ZINC54359621 has a hydrogen bond between the oxygen atom of Pro236 and the NH group of the aromatic ring, NH group of Lys103 and the oxygen atom of the aromatic ring. ZINC46849657 has a hydrogen bond between the oxygen atom of Pro236 and the NH group of the aromatic ring, the NH group of residue Lys103 and the oxygen atom of the aromatic ring (). These are one of the key interactions required for the binding to HIV-1 RT. This indicates the compounds’ suitability as an RT inhibitor.
Figure 7 Binding mode of compounds.
Abbreviation: RT, reverse transcriptase.
HIV-1 RT is highly flexible in nature,Citation52,Citation53 and therefore the stability of the system during MD simulation was analyzed by computing 1) RMSD () and 2) RMSF (). The average values of RMSD for ZINC54359621 and ZINC46849657 are 3.12 Å and 2.58 Å, respectively. Literature on HIV-1 RT with RMSD with a similar behavior as RMSD () validates the system’s stabilityCitation54,Citation55 due to the high flexibility of HIV-1 RT. RMSF shows good stability of all residues interacting with the ligands from residues 100 to 236. The average values of RMSF for ZINC54359621 and ZINC4684965 are 1.87 Å and 1.53 Å, respectively. Further, the compactness of the system was analyzed by computing Rg of the systems. The average values of Rg for ZINC54359621 and ZINC46849657 are 51.2 Å and 51.2 Å, respectively. Results show that the target protein folded correctly and was able to retain a stable compact structure ().
Conclusion
VS was carried out to identify the potential inhibitors against mutated HIV-1 RT based on 1) PRED-based pharmacophore model, 2) molecular docking and, 3) MMPBSA approaches. The study identified two novel hits ZINC54359621 and ZINC46849657. These compounds may be representatives of a new series of NNRTIs possessing high resistance profiles against mutated HIV viruses. They have the potential to inhibit the transcription of the viral RNA by binding to the active site of the RT’s p66 subunit, thereby decreasing the replication rate of the virus. The pharmacophore approach was further refined, where the candidate molecules for structure-based VS were chosen based on their orientation and chemical features in relation to the 3D structure. The PRED was also calculated in order to obtain more insights into the crucial protein residues involved in ligand binding. This will potentially aid in the design of potent inhibitors that bind to these HCAAR. The results presented in this study present a map to the innovation and design of potent drug candidates against different biological targets. Results have shown that choosing highly contributing FBE candidates from a library of compounds generated from a PRED target-bound pharmacophore map is a more reliable and accurate approach.
Supplementry materials
Figure S1 Two key binding interactions exist between GSK952 and the backbone NH and C=O groups of Lys103 of HIV-1 RT.
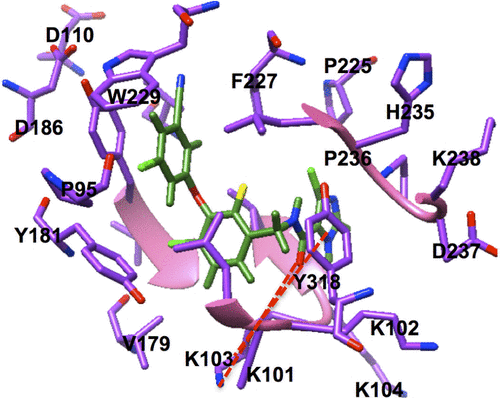
Figure S2 PRED-based pharmacophore model.
Figure S3 MD simulation results of ZINC54359621 and ZINC46849657.
Abbreviations: MD, molecular dynamic; RMSD, root-mean-square deviation; RMSF, root-mean-square fluctuation; Rg, radius of gyration.
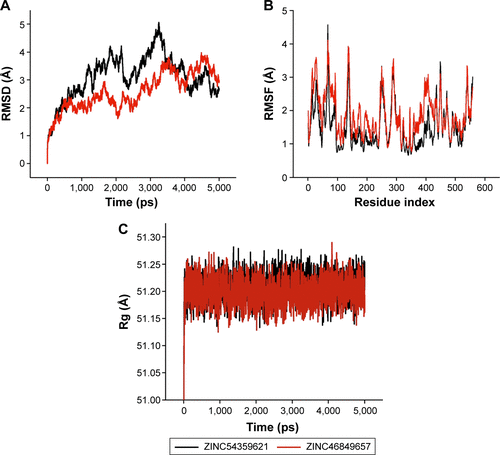
Table S1 PRED of highly interacting residues
Acknowledgments
Ethics committee approval was not required by our institute for this study because it did not involve human nor animal participants. The authors would like to acknowledge the School of Health Science, University of KwaZulu-Natal for financial support. Computational resources granted by the Center for High Performance Computing (http://www.chpc.ac.za) are highly appreciated.
Disclosure
The authors report no conflicts of interest in this work.
References
- FriedrichBMDziubaNLiGEndsleyMAMurrayJLFergusonMRHost factors mediating HIV-1 replicationVirus Res2011161210111421871504
- JonckheereHDe ClercqEAnnéJFidelity analysis of HIV-1 reverse transcriptase mutants with an altered amino-acid sequence at residues Leu74, Glu89, Tyr115, Tyr183 and Met184Eur J Biochem200026792658266510785387
- amfAR. [webpage on the Internet]Statistics: Worldwide: The Foundation for AIDS Research: HIV/AIDS Research Available from http://www.amfar.org/worldwide-aids-stats/Accessed March 8, 2016
- MoonsamySDashRCSolimanMEIntegrated computational tools for identification of CCR5 antagonists as potential HIV-1 entry inhibitors: homology modeling, virtual screening, molecular dynamics simulations and 3D QSAR analysisMolecules20141945243526524762964
- SinghKMarchandBKirbyKAMichailidisESarafianosSGStructural aspects of drug resistance and inhibition of HIV-1 reverse transcriptaseViruses20102260663820376302
- AbadMJBedoyaLMBermejoPTangYi-WeiHIV-screening strategies for the discovery of novel HIV-inhibitorsRecent Translational Research in HIV/AIDS200722457468
- Jacobo-MolinaAArnoldEHIV reverse transcriptase structure-function relationshipsBiochemistry19913026635163561711368
- ChongPSebaharPYoungmanMRational design of potent non-nucleoside inhibitors of HIV-1 reverse transcriptaseJ Med Chem20125523106011060923137340
- UsachIMelisVPerisJENon-nucleoside reverse transcriptase inhibitors : a review on pharmacokinetics, pharmacodynamics, safety and tolerabilityJ Int AIDS Soc20131611424008177
- BarrecaMLRaoADe LucaLDiscovery of novel benzimidazolones as potent non-nucleoside reverse transcriptase inhibitors active against wild-type and mutant HIV-1 strainsBioorg Med Chem Lett20071771956196017276064
- DistintoSEspositoFKirchmairJIdentification of HIV-1 reverse transcriptase dual inhibitors by a combined shape-, 2D-fingerprint- and pharmacophore-based virtual screening approachEur J Med Chem20125021622922361685
- KellerPABirchCLeachSPTyssenDGriffithRNovel pharmacophore-based methods reveal gossypol as a reverse transcriptase inhibitorJ Mol Graph Model200321536537312543135
- PentaAGangulySMurugesanSDesign and synthesis of tetrahydrophthalimide derivatives as inhibitors of HIV-1 reverse transcriptaseOrg Med Chem Lett201331823968361
- RenJMiltonJWeaverKLShortSAStuartDIStammersDKStructural basis for the resilience of efavirenz (DMP-266) to drug resistance mutations in HIV-1 reverse transcriptaseStructure20008101089109411080630
- ZavodszkyMIRohatgiAVan VoorstJRYanHKuhnLAScoring ligand similarity in structure-based virtual screeningJ Mol Recognit200922428029219235177
- SolimanMEA hybrid structure/pharmacophore-based virtual screening approach to design potential leads: A computer-aided design of South African HIV-1 subtype C protease inhibitorsDrug Dev Res2013745283295
- GuWImpact of virtual screening on HIV reverse transcriptase inhibitor discoveryJacobs Journal of AIDS/HIV20141415
- VyasVJainAJainAGuptaAVirtual screening: A fast tool for drug designSci Pharm200876333360
- SkeltonAAMaharajYRSolimanMETarget-bound generated pharmacophore model to improve the pharmacophore-based virtual screening: Identification of G-protein human CCR2 receptors inhibitors as anti-inflammatory drugsCellular and Molecular Bioengineering2014714557
- LavecchiaADi GiovanniCVirtual screening strategies in drug discovery: a critical reviewCurr Med Chem201320232839286023651302
- YoungDCComputational Drug Design: A Guide for Computational and Medicinal ChemistsWiley-Interscience2009
- YangSYPharmacophore modeling and applications in drug discovery: challenges and recent advancesDrug Discov Today20101511–1244445020362693
- MallipeddiPLKumarGWhiteSWWebbTRRecent advances in computer-aided drug design as applied to anti-influenza drug discoveryCurr top Med Chem201414161875188925262796
- WillettPEnhancing the effectiveness of ligand-based virtual screening using data fusionQSAR Comb Sci2006251211431152
- DrwalMNGriffithRCombination of ligand- and structure-based methods in virtual screeningDrug Discov Today Technol2013103e395e40124050136
- KhannaMWangFJoITargeting multiple conformations leads to small molecule inhibitors of the uPAR·uPA protein-protein interaction that block cancer cell invasionACS Chem Biol20116111232124321875078
- AmaroREBaronRMcCammonJAAn improved relaxed complex scheme for receptor flexibility in computer-aided drug designJ Comput Aided Mol Des200822969370518196463
- OkimotoNFutatsugiNFujiHHigh-performance drug discovery: computational screening by combining docking and molecular dynamics simulationsPLoS Comput Biol2009510e100052819816553
- WangLGuQZhengXDiscovery of new selective human aldose reductase inhibitors through virtual screening multiple binding pocket conformationsJ Chem Inf Model20135392409242223901876
- RastelliGEmerging topics in structure-based virtual screeningPharm Res20133051458146323468050
- IslamMAPillayTSExploration of the structural requirements of HIV-protease inhibitors using pharmacophore, virtual screening and molecular docking approaches for lead identificationJ Mol Graph Model201556203025541527
- ShoichetBKMcGovernSLWeiBIrwinJJLead discovery using molecular dockingCurr Opin Chem Biol20026443944612133718
- HanwellMDCurtisDELonieDCVandermeerschTZurekEHutchisonGRAvogadro: an advanced semantic chemical editor, visualization, and analysis platformJ Cheminform20124111722236646
- PettersenEFGoddardTDHuangCCUCSF Chimera – a visualization system for exploratory research and analysisJ Comput Chem200425131605161215264254
- CaseDABerrymanJTBetzRMAMBER 2015University of CaliforniaSan Francisco Available from ambermd.org/doc12/Amber15.pdf
- GötzAWWilliamsonMJXuDPooleDLe GrandSWalkerRCRoutine microsecond molecular dynamics simulations with AMBER on GPUs. 1. Generalized BornJ Chem Theory Comput2012851542155522582031
- DuanYWuCChowdhurySA point-charge force field for molecular mechanics simulations of proteins based on condensed-phase quantum mechanical calculationsJ Comput Chem200324161999201214531054
- Antony DhivyanJEAnoopMNVirtual screening and lead optimization to identify novel inhibitors for HDAC-820129158 Available from http://arxiv.org/ftp/arxiv/papers/1209/1209.2793.pdf
- HarveyMJDe FabritiisGAn implementation of the Smooth Particle Mesh Ewald Method on GPU HardwareJ Chem Theory Comput2009592371237726616618
- WuXBrooksBRSelf-guided Langevin dynamics simulation methodChem Phys Lett2003381512518
- KräutlerVVan GunsterenWFHünenbergerPHA fast SHAKE algorithm to solve distance constraint equations for small molecules in molecular dynamics simulationsJ Comput Chem2001225501508
- BerendsenHJCPostmaJPMvan GunsterenWFDiNolaAHaakJRMolecular dynamics with coupling to an external bathJ Chem Phys19848136843690
- TrottOOlsonAJAutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreadingJ Comput Chem201031245546119499576
- mgltools.scripps.edu [homepage on the Internet]MGLTools Website – Welcome – MGLTools Available from: http://mgltools.scripps.edu/Accessed February 14, 2016
- MorrisGMGoodsellDSHallidayRSAutomated docking using a Lamarckian genetic algorithm and an empirical binding free energy functionJ Comput Chem1998191416391662
- HouTWangJLiYWangWAssessing the performance of the molecular mechanics/Poisson Boltzmann surface area and molecular mechanics/generalized Born surface area methods. II. The accuracy of ranking poses generated from dockingJ Comput Chem201132586687720949517
- LynePDLambMLSaehJCAccurate prediction of the relative potencies of members of a series of kinase inhibitors using molecular docking and MM-GBSA scoringJ Med Chem200649164805480816884290
- KoesDRCamachoCJZINCPharmer: pharmacophore search of the ZINC databaseNucleic Acids Res201240Web Server issue409414
- IrwinJJShoichetBKZINC – a free database of commercially available compounds for virtual screeningJ Chem Inf Model200545117718215667143
- KawasakiYFreireEFinding a better path to drug selectivityDrug Discov Today20111698599021839183
- Kroeger SmithMBRouzerCATaneyhillLAMolecular modeling studies of HIV-1 reverse transcriptase nonnucleoside inhibitors: total energy of complexation as a predictor of drug placement and activityProtein Sci1995410220322228535257
- RodgersDWGamblinSJHarrisBAThe structure of unliganded reverse transcriptase from the human immunodeficiency virus type 1Proc Natl Acad Sci U S A1995924122212267532306
- DasKMartinezSEBandwarRPArnoldEStructures of HIV-1 RT-RNA/DNA ternary complexes with dATP and nevirapine reveal conformational flexibility of RNA/DNA: insights into requirements for RNase H cleavageNucleic Acids Res201442128125813724880687
- MadridMJacobo-MolinaADingJArnoldEMajor subdomain rearrangement in HIV-1 reverse transcriptase simulated by molecular dynamicsProteins199935333233710328268
- TemizNABaharIInhibitor binding alters the directions of domain motions in HIV-1 reverse transcriptaseProteins2002491617012211016
