Abstract
Background
Amiodarone is a frequently used antiarrhythmic drug in patients with end-stage heart failure. Given its long half-life, pre-transplant use of amiodarone has been controversially discussed, with divergent results regarding morbidity and mortality after heart transplantation (HTX).
Aim
The aim of this study was to investigate the effects of long-term use of amiodarone before HTX on early post-transplant atrial fibrillation (AF) and mortality after HTX.
Methods
Five hundred and thirty patients (age ≥18 years) receiving HTX between June 1989 and December 2012 were included in this retrospective single-center study. Patients with long-term use of amiodarone before HTX (≥1 year) were compared to those without long-term use (none or <1 year of amiodarone). Primary outcomes were early post-transplant AF and mortality after HTX. The Kaplan–Meier estimator using log-rank tests was applied for freedom from early post-transplant AF and survival.
Results
Of the 530 patients, 74 (14.0%) received long-term amiodarone therapy, with a mean duration of 32.3±26.3 months. Mean daily dose was 223.0±75.0 mg. Indications included AF, Wolff–Parkinson–White syndrome, ventricular tachycardia, and ventricular fibrillation. Patients with long-term use of amiodarone before HTX had significantly lower rates of early post-transplant AF (P=0.0105). Further, Kaplan–Meier analysis of freedom from early post-transplant AF showed significantly lower rates of AF in this group (P=0.0123). There was no statistically significant difference between patients with and without long-term use of amiodarone prior to HTX in 1-year (P=0.8596), 2-year (P=0.8620), 5-year (P=0.2737), or overall follow-up mortality after HTX (P=0.1049). Moreover, Kaplan–Meier survival analysis showed no statistically significant difference in overall survival (P=0.1786).
Conclusion
Long-term use of amiodarone in patients before HTX significantly reduces early post-transplant AF and is not associated with increased mortality after HTX.
Introduction
Life-threatening arrhythmias are the most common cause for sudden cardiac death and pose a great danger for patients with end-stage heart failure.Citation1 Antiarrhythmic treatment with amiodarone for atrial or ventricular arrhythmias is effective and is commonly used in clinical practice.Citation2,Citation3 Amiodarone has been demonstrated to maintain normal sinus rhythm in patients with atrial fibrillation (AF) and to decrease the rate of recurrence of ventricular tachycardia.Citation2,Citation3 Especially in patients on the waiting list for heart transplantation (HTX), it remains a preferred antiarrhythmic medication as it can be used in patients with end-stage heart failure and severely reduced left ventricular ejection fraction for the management of persistent arrhythmias.Citation4,Citation5
Since it is a highly lipophilic drug, amiodarone is extensively distributed into the body’s tissues with a reported half-life ranging from 60 days in patients receiving short-term therapy up to 120 days in patients with long-term use of amiodarone.Citation6–Citation8 This raises the question whether the use of amiodarone before HTX may be associated with increased morbidity and mortality after HTX as the newly transplanted heart is exposed to this drug. On the other hand, the long half-life of amiodarone could be a potential safeguard against arrhythmias in the early post-transplant period.
Hence, the aim of this large retrospective single-center study was to investigate the effects of long-term use of amiodarone before HTX on early post-transplant AF and mortality after HTX.
Methods
Patients
This study was reviewed and approved by the ethics committee of the University of Heidelberg, Heidelberg, Germany, and was conducted in accordance with the ethical standards of the 2013 Declaration of Helsinki. All adult patients (≥18 years) receiving HTX at the Heidelberg Heart Center, Heidelberg, Germany, between June 1989 and December 2012 were included in this study. Patients with repeated cardiac transplantation were excluded. Data were retrieved from the Heidelberg Registry for Heart Transplantation. Written informed consent was obtained for the Heidelberg HTX register. Patients were divided into two groups based on their treatment with amiodarone before HTX: patients with at least 1 year of pre-transplant use of amiodarone immediately before HTX were included in the “long-term use of amiodarone” group, whereas patients with no or less than 1 year of pre-transplant use of amiodarone prior to HTX were included in the “no long-term use of amiodarone” group. Moreover, duration (in months) and daily dose (in milligrams) of pre-transplant long-term use of amiodarone were reviewed and evaluated. Primary outcomes were early post-transplant AF (AF≤30 days after HTX) lasting 30 seconds or longer and mortality after HTX.
Follow-up
All available records pertaining to heart rhythm in the early post-transplant period (≤30 days after HTX) were assessed. During the initial hospital stay, patients were monitored using continuous telemetry. Additionally, 12-lead electrocardiography (ECG) was performed on a regular basis and in case of any noted arrhythmia on telemetry. Also, 24-hour Holter recording was performed to detect arrhythmic disorders.
After the initial hospital stay, patients were seen on a monthly basis during the first 6 months after HTX, then bimonthly until the end of the first year, and thereafter three times per year (or if clinically indicated).Citation9,Citation10 Routine follow-up included medical history taking, physical examination, 12-lead ECG, and routine laboratory analysis (including immunosuppressive drug monitoring). Further, according to center standard, echocardiography and endomyocardial biopsy were performed.Citation11,Citation12
Post-transplant medication
Post-transplant medication including immunosuppressive drug therapy was applied according to center standard. Immunosuppressive drug target levels were routinely monitored by laboratory analysis. The initial immunosuppressive regimen of cyclosporine A (CsA) and azathioprine (AZA) was subsequently replaced by CsA and mycophenolate mofetil (MMF) from 2001 onward, and by tacrolimus (TAC) and MMF from 2006 onward. Simultaneously, steroids (prednisolone) were also administered.Citation9,Citation10
Statistical analysis
Statistical analysis was performed with SAS (Version 9.3, SAS Institute, Cary, NC, USA). Data were expressed as mean ± standard deviation (SD) or as absolute number (n) with percentage (%). Student’s t-test was used for continuous variables and chi-squared test for categorical variables. The Kaplan–Meier estimator using log-rank tests was applied for freedom from early post-transplant AF and for survival after HTX.
Analysis of maximum-likelihood estimation was applied to evaluate a possible association between time of pre-transplant use of amiodarone and survival of patients after HTX. Time of pre-transplant long-term use of amiodarone (in months) was used as the analysis variable and survival of patients after HTX (in months) as the dependent variable. A P-value of <0.05 was considered to be statistically significant.
Demographic data included recipient age, sex, body mass index (BMI), coronary artery disease (CAD), arterial hypertension, dyslipidemia, diabetes mellitus, and renal insufficiency. Donor data included age, sex, and BMI. Principal diagnoses for HTX were categorized as nonischemic cardiomyopathy (CMP), ischemic CMP, valvular heart disease, and cardiac amyloidosis. Further, transplant sex mismatch and the perioperative data (type of anastomosis [biatrial, bicaval, or total orthotopic HTX] and ischemic time) were analyzed.
Results
Patient characteristics
Five hundred and thirty patients (age ≥18 years) receiving HTX at the Heidelberg Heart Center between June 1989 and December 2012 were included in this study. Among these, 74 patients received long-term therapy with amiodarone, whereas 456 patients did not receive long-term therapy with amiodarone.
In patients receiving long-term therapy with amiodarone, mean recipient age at HTX was 53.1±8.1 years, and the mean follow-up period after HTX was 7.2±6.5 years, with 59 patients being male (79.7%). Mean donor age was 40.4±13.2 years, with 30 donors being male (40.5%).
In the other group, mean recipient age at HTX was 51.5±10.8 years, and mean follow-up period after HTX was 6.8±6.1 years, with 353 patients being male (77.4%). Mean donor age was 39.5±13.3 years, with 207 donors being male (45.4%).
With regard to BMI, no statistically significant differences between the recipient (P=0.0854) and donor groups (P=0.8131) were detected. Comorbidities at the time of HTX, including CAD (P=0.6108), arterial hypertension (P=0.6527), dyslipidemia (P=0.1390), and diabetes mellitus (P=0.8765), were not statistically significantly different except for renal insufficiency. Patients with long-term use of amiodarone had a significantly higher rate of renal insufficiency (52 of 74 [70.3% of subgroup] vs 253 of 456 [55.5% of subgroup], P=0.0170).
Considering the principal diagnoses for HTX, significantly more patients with nonischemic CMP were observed in the long-term use of amiodarone group (48 of 74 [64.9%] vs 236 of 456 [51.7%], P=0.0359), whereas significantly more patients with valvular heart disease (29 of 456 [6.4%] vs 0 of 74 [0.0%], P=0.0257) and cardiac amyloidosis (36 of 456 [7.9%] vs 0 of 74 [0.0%], P=0.0123) were observed in the opposite group. No statistically significant difference was observed for ischemic CMP as the principal diagnosis for HTX (26 of 74 [35.1%] vs 155 of 456 [34.0%], P=0.8474). Furthermore, there was no statistically significant difference between the two groups with regard to the transplant sex mismatch (37 of 74 [50.0% of subgroup] vs 192 of 456 [42.1% of subgroup], P=0.2035).
Perioperative data including anastomosis type, with further subgroups of biatrial HTX (P=0.6786), bicaval HTX (P=0.1242), and total orthotopic HTX (P=0.3751), as well as ischemic time (P=0.5763) showed no statistically significant difference between the groups. Patient characteristics are presented in .
Table 1 Patient characteristics
Duration, daily dose, and indications for long-term use of amiodarone
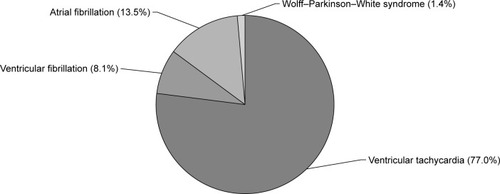
The mean duration of long-term therapy of amiodarone before HTX was 32.3±26.3 months, ranging from 12 to 168 months. The mean daily amiodarone dose was 223.0±75.0 mg, ranging from 100 to 400 mg per day. Indications for long-term therapy with amiodarone were AF in ten patients (13.5% of subgroup), Wolff–Parkinson–White syndrome in one patient (1.4% of subgroup), ventricular tachycardia in 57 patients (77.0% of subgroup), and ventricular fibrillation in six patients (8.1% of subgroup). Indications for pre-transplant use of amiodarone are displayed in .
Figure 1 Indications for pre-transplant use of amiodarone.
Initial post-transplant medication
Comparison of the initial immunosuppressive drug therapy showed no statistically significant differences between patients with or without long-term use of amiodarone before HTX with regard to the use of CsA (P=0.2426) or TAC (P=0.2426). Neither the administration of AZA (P=0.7485) nor the use of MMF (P=0.7485) showed a statistically significant difference. Moreover, there were no statistically significant differences regarding the use of acetylsalicylic acid (P=0.5496), angiotensin-converting-enzyme inhibitor/sartan (P=0.2562), or statin (P=0.7005). In addition, neither the administration of beta-blocker (P=0.1561) nor the use of calcium channel blocker (P=0.3792) showed a statistically significant difference. Medication used within 30 days after HTX are given in .
Table 2 Initial post-transplant medication
Early post-transplant AF
Sixty-one of the 530 HTX patients (11.5%) developed AF in the early post-transplant period. The chi-squared test was applied to determine whether there was a significant difference in the occurrence of early post-transplant AF (AF ≤30 days after HTX) between patients with and without long-term use of amiodarone before HTX by comparing the corresponding expected and observed frequencies.
In patients with long-term amiodarone use prior to HTX, the expected frequencies predicted 8.5 patients with AF after HTX (8.5 of 74 [11.5% of all patients with long-term amiodarone use], 8.5 of 61 [14.0% of patients with AF], and 8.5 of 530 [1.6% of all patients]). In the other group, the expected frequencies predicted 52.5 patients with AF after HTX (52.5 of 456 [11.5% of all patients without long-term amiodarone use], 52.5 of 61 [86.0% of patients with AF], and 52.5 of 530 [9.9% of all patients]).
In contrast to these results, the observed frequencies differed significantly (P=0.0105), showing that patients with long-term use of amiodarone prior to HTX had lower rates of early post-transplant AF, with only two patients with AF after HTX (2 of 74 [2.7% of all patients with long-term use of amiodarone], 2 of 61 [3.3% of patients with AF], and 2 of 530 [0.4% of all patients]).
Consequently, patients without long-term use of amiodarone prior to HTX had higher rates of early post-transplant AF, including 59 patients with AF after HTX (59 of 456 [12.9% of all patients without long-term use of amiodarone], 59 of 61 [96.7% of patients with AF], and 59 of 530 [11.1% of all patients]). The influence of long-term use of amiodarone on post-transplant AF is shown in .
Table 3 Influence of long-term use of amiodarone on early post-transplant atrial fibrillation
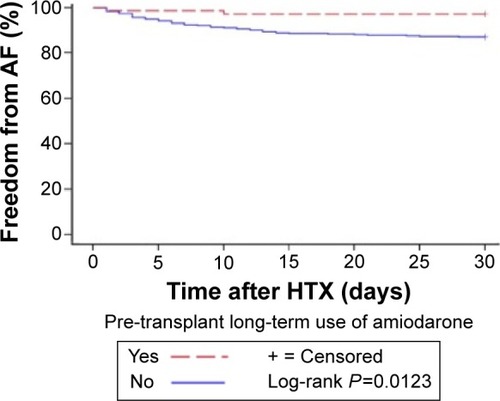
Further, Kaplan–Meier analysis of freedom from early post-transplant AF showed significantly lower rates of AF in patients with long-term use of amiodarone before HTX (2 of 61 [3.3% of subgroup] vs 59 of 61 [96.7% of subgroup], P=0.0123). The Kaplan–Meier estimator (freedom from early post-transplant AF) is given in .
Post-transplant mortality
Of the 530 HTX patients, 254 (47.9%) died during the overall follow-up. There was no statistically significant difference between the patients with and without long-term use of amiodarone prior to HTX in 1-year follow-up mortality (amiodarone: 16 of 71 patients [22.5% of subgroup] vs no amiodarone: 97 of 449 patients [21.6% of subgroup], P=0.8596), 2-year follow-up mortality (amiodarone: 17 of 69 patients [24.6% of subgroup] vs no amiodarone: 114 of 445 patients [25.6% of subgroup], P=0.8620), 5-year follow-up mortality (amiodarone: 18 of 60 patients [30.0% of subgroup] vs no amiodarone: 144 of 386 patients [37.3% of subgroup], P=0.2737), or overall follow-up mortality (amiodarone: 29 of 74 patients [39.2% of subgroup] vs no amiodarone: 225 of 456 patients [49.3% of subgroup], P=0.1049). The influence of long-term use of amiodarone on post-transplant mortality is shown in .
Table 4 Influence of long-term use of amiodarone on mortality after HTX
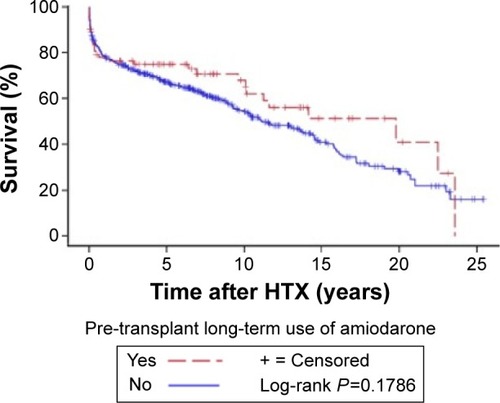
In addition, the Kaplan–Meier survival analysis showed no statistically significant difference in the overall survival between patients with and without long-term use of amiodarone prior to HTX (P=0.1786). The Kaplan–Meier estimator (overall follow-up survival) is given in . Moreover, analysis of maximum-likelihood estimation showed no evidence in this study for an association between time of pre-transplant long-term use of amiodarone (ranging from 12 to 168 months) and survival of patients after HTX (hazard ratio [HR] =1.000; 95% HR confidence interval [CI]: 0.983–1.017; P=0.9765).
Discussion
Background
The use of amiodarone in patients with end-stage heart failure and arrhythmias prior to HTX has been controversially discussed.Citation4,Citation5,Citation13–Citation18 Due to its lipophilic character, large volume of distribution, reduced clearance rate, and long half-life, the redistribution of amiodarone from the recipient’s tissues to the transplanted donor’s heart has been reported.Citation6–Citation8,Citation19 This exposure of the newly transplanted heart to amiodarone may have an influence on post-transplant arrhythmias and mortality after HTX. Therefore, the purpose of this study was to investigate the effects of long-term use of amiodarone before HTX on early post-transplant AF and mortality after HTX.
Patient characteristics
Analysis of recipient data between patients with and with-out pre-transplant long-term amiodarone therapy showed no statistically significant differences with regard to age, male sex, BMI, CAD, arterial hypertension, dyslipidemia, or diabetes mellitus. Donor age, sex, BMI, and transplant sex mismatch did not vary significantly between the groups. Furthermore, type of anastomosis (biatrial, bicaval, or total orthotopic HTX) and ischemic time were not statistically different.
Patients with long-term use of amiodarone had a significantly higher rate of renal insufficiency, although amiodarone is cleared predominantly via hepatic metabolism and <1% is excreted via urine.Citation6,Citation8 However, rhabdomyolysis – which can lead to kidney failure and death – may occur in patients treated simultaneously with amiodarone and simvastatin, especially in doses greater than 20 mg per day.Citation20 Additionally, renal dysfunction has been considered a risk factor for death after HTX.Citation21
With regard to principal diagnoses for HTX, groups differed slightly as more patients with nonischemic CMP were found in the long-term use of amiodarone group, while more patients with valvular heart disease and cardiac amyloidosis were found in the opposite group. However, no difference between groups was found in patients with ischemic CMP as the principal diagnosis for HTX.
Therefore, the comparison of patients with and without long-term amiodarone use indicated no major differences in demographic data, providing two similar groups principally varying only in terms of prior amiodarone therapy.
Duration, daily dose, and indications for long-term use of amiodarone
Former studies have reported a wide spectrum of duration of pre-transplant amiodarone therapy ranging from <1 week to a minimum duration of 3 months.Citation5,Citation16 A longer duration of pre-transplant amiodarone therapy has been associated with an elevated post-transplant mortality.Citation5 Accordingly, we focused on long-term-use of amiodarone before HTX with a minimum use of 12 months to minimize bias regarding duration of intake and achievement of steady state due to the long half-life of amiodarone ranging up to 120 days.Citation8
Increased mortality with amiodarone use may not only be associated with the duration of intake but also with the amount of daily dose. In this study, the mean daily dose was 223.0±75.0 mg, which is comparable to Macdonald et alCitation13 reporting a mean daily dose of 247.0±31.0 mg without an elevated post-transplant mortality in patients with previous amiodarone therapy. In contrast, Chin et alCitation5 described a mean daily dose of 327.0±130.0 mg with an increased post-transplant mortality in patients with pre-transplant use of amiodarone for more than 4 weeks.
Amiodarone has been used for the treatment of supraventricular and ventricular arrhythmias.Citation2,Citation3 Indications for pre-transplant long-term therapy with amiodarone in this study were AF (13.5%), Wolff–Parkinson–White syndrome (1.4%), ventricular tachycardia (77.0%), and ventricular fibrillation (8.1%).
Initial post-transplant medication
Atrial tachyarrhythmias after HTX have been associated with acute rejection episodes.Citation22 As rejection episodes are influenced by immunosuppressive drug therapy,Citation12 we compared the initial immunosuppressive regimen within 30 days after HTX of patients with and without long-term use of amiodarone prior to HTX. We found no statistically significant differences (CsA, TAC, AZA, or MMF, all P-values were not significant).
Antiarrhythmic drugs other than amiodarone (beta-blockers or calcium channel blockers) can influence the occurrence or frequency of AF.Citation3 To reveal the effects of concomitantly administered antiarrhythmic drugs, we compared the initial post-transplant medication within 30 days after HTX of patients with and without long-term use of amiodarone before HTX. The use of beta-blocker, ivabradine, or calcium channel blocker did not differ significantly between the groups (all P-values were not significant), minimizing the potential bias with regard to antiarrhythmic drugs.
Early post-transplant AF
Pre-transplant amiodarone therapy has been associated with post-transplant sinus bradycardia as a result of its negative chronotropic effects through calcium channel inhibition and beta-receptor blockade, requiring temporary or permanent pacing support in some patients.Citation4,Citation23,Citation24 In contrast, however, its potential as a safeguard against tachyarrhythmias in the early post-transplant period – due to the long half-life of pre-transplant use of amiodarone – has been poorly studied.
AF in the early post-transplant period after HTX has a reported incidence of 7.9%–18.2%.Citation25,Citation26 In this study, 61 of 530 patients (11.5% of total) developed AF in the early post-transplant period. Patients with AF are at an increased risk of morbidity and mortality as a result of thromboembolic complications such as stroke.Citation27 Moreover, several studies have shown an increased mortality in patients with AF after HTX compared to patients with sinus rhythm.Citation25,Citation26,Citation28 In order to investigate the influence of long-term use of amiodarone on post-transplant AF between patients with and without long-term amiodarone use prior to HTX, the expected and observed frequencies of AF of both groups were compared. Here, patients with long-term use of amiodarone before HTX had significantly lower rates of early post-transplant AF (P=0.0105). Additionally, Kaplan–Meier analysis of freedom from early post-transplant AF showed significantly lower rates of AF in patients with long-term use of amiodarone before HTX compared to those without (P=0.0123).
Post-transplant mortality
With regard to post-transplant mortality, the scientific community is divided, as some studies reported increased post-transplant mortality in patients with amiodarone use before HTX,Citation15,Citation16 whereas other studies showed no difference.Citation13,Citation14,Citation17 Macdonald et alCitation13 compared post-transplant mortality between 19 patients with pre-transplant amiodarone therapy (mean duration of therapy: 9.0±2.2 months; mean daily dose: 247.0±31.0 mg) and 31 patients as control. They found no statistically significant differences of prior amiodarone therapy on early allograft inotropic function or on clinical outcomes such as length of hospital stay or mortality within the first month after HTX (mortality was 5.3% and 9.7% in patients with and without amiodarone use, respectively).Citation13
Similar results regarding morbidity and mortality were reported by Chelimsky-Fallick et al.Citation14 They retrospectively matched 29 amiodarone patients (mean duration of therapy: 11.0±22.0 months; mean daily dose: 360.0±230.0 mg) with 29 control patents. The 30-day mortality did not differ significantly as two patients in the amiodarone group (6.9%) and one patient in the control group (3.4%) died.Citation14 In contrast, Konertz et alCitation15 reported a higher rate of complications after HTX in patients with pre-transplant use of amiodarone, which included mortality, difficult weaning from extracorporeal circulation, elevated inotropic doses, and prolongation of respiratory support. Two of six patients (33.3%) with pre-transplant amiodarone therapy died compared to three of 26 patients (11.5%) without prior use of amiodarone. The duration of amiodarone therapy or mean daily dose were not reported.Citation15
The three aforementioned studies were of rather small sample size and covered only a short follow-up period after HTX (mean follow-up <1 year). Further, they did not focus on differences in duration of pre-transplant amiodarone therapy. This approach was pursued by Chin et al,Citation5 who split 106 patients into groups based on their pre-transplant use of amiodarone: <1 week (one patient, mean dose of 300 mg), 1–4 weeks (five patients, mean dose of 928±492 mg), and >4 weeks (32 patients, mean dose of 327±130 mg). Five patients (no mean dose of amiodarone provided) received amiodarone for an unspecific period, and 63 patients had no prior amiodarone therapy. There was a threefold increase of in-hospital mortality in patients treated with amiodarone for more than 4 weeks (ten of 32 [31.3%]) in comparison to patients without amiodarone (six of 63 [9.5%]), indicating that a longer pre-transplant use of amiodarone may be associated with increased mortality.Citation5 Likewise, in the aforementioned studies, sample size was small and follow-up covered only a short period after HTX, although focusing on duration of pre-transplant use of amiodarone.
Considering former studies with short-term follow-ups, Blomberg et alCitation16 conducted a study with 20 patients receiving amiodarone for a minimum of 3 months before HTX, and 65 HTX-patients without prior amiodarone therapy served as control. They provided a 5-year survival analysis. Here, eleven of 20 patients in the amiodarone group (55.0%) and 15 of 65 in the other group (23.1%) died.Citation16 Interestingly, one-third of deaths in the amiodarone group occurred between 30 and 60 days after HTX, leaving room for speculations that a longer follow-up in the former studies might have had an impact on mortality assessment.Citation13,Citation14
A more recent large retrospective study with a total of 396 patients by Sanchez-Lazaro et alCitation17 observed no significant differences in acute graft failure or early mortality. They examined 25 patients with a minimum use of amiodarone for at least 30 days before transplantation and 371 patients with-out pre-transplant use of amiodarone. Three of 25 patients (12.0%) with pre-transplant amiodarone therapy died versus 39 of 371 patients (10.5%) without prior use of amiodarone. The mean daily dose of amiodarone was not reported.Citation17
The latest contribution to this issue was presented by Yerebakan et alCitation18 at the 34th Annual Meeting of the Inter-national Society for Heart and Lung Transplantation in San Diego, California, USA, in April 2014. The authors used a propensity score to match 86 patients with pre-transplant use of amiodarone to 86 controls. There was a significantly increased in-hospital mortality after HTX in patients receiving prior treatment with amiodarone (7.0% vs 0.0%, P=0.01). However, long-term survival after HTX was similar between groups (P=0.72). As the final data set has not been published yet, preliminary results should be interpreted cautiously.Citation18
Study limitations
Our findings were derived from a single-center study with 530 patients including 74 patients with long-term therapy of amiodarone before HTX. Therefore, sufficient power to compare results with multi-center studies was provided. Moreover, in contrast to multicenter studies, patients received a comparable center-specific induction regimen and follow-up, thus minimizing potential confounders. Second, the retrospective nature of this analysis carries the limitations of such a study design.Citation9,Citation10 Third, a possible era effect cannot be ruled out, given the long follow-up period.Citation11,Citation12
Conclusion
The use of amiodarone in patients with end-stage heart failure and arrhythmias before HTX has been controversially discussed.Citation4,Citation5,Citation13–Citation18 This large retrospective single-center study with 530 patients aimed to investigate the effects of long-term use of amiodarone before HTX on early post-transplant AF and mortality after HTX.
Notably, patients with long-term use of amiodarone before HTX had significantly lower rates of early post-transplant AF (P=0.0105). In addition, Kaplan–Meier analysis of freedom from early post-transplant AF showed significantly lower rates of AF in this group (P=0.0123). Moreover, we found no evidence of increased post-transplant mortality in patients with pre-transplant long-term use of amiodarone with regard to 1-year (P=0.8596), 2-year (P=0.8620), 5-year (P=0.2737), or overall follow-up mortality after HTX (P=0.1049). Further, Kaplan–Meier survival analysis showed no statistically significant difference in the overall survival (P=0.1786).
Therefore, based on our results and the efficacy of amiodarone in the treatment of atrial and ventricular arrhythmias in patients with end-stage heart failure, we consider amiodarone an important antiarrhythmic drug in patients with severe heart failure awaiting HTX. Nevertheless, each indication for amiodarone has to be carefully taken into consideration for balancing its benefits and disadvantages. In addition, further large prospective randomized controlled studies are required to assess the effects of pre-transplant use of amiodarone on post-transplant outcomes.
Acknowledgments
The authors thank Gerda Baumann, Viola Deneke, and Berthold Klein for their assistance and advice.
Disclosure
The authors report no conflicts of interest in this work.
References
- SaravananPDavidsonNCRisk assessment for sudden cardiac death in dialysis patientsCirc Arrhythm Electrophysiol20103555355920959609
- PedersenCTKayGNKalmanJEHRA/HRS/APHRS expert consensus on ventricular arrhythmiasHeart Rhythm20141110166196
- JanuaryCTWannLSAlpertJS2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm SocietyJ Am Coll Cardiol2014642117624998121
- JenningsDLMartinezBMontalvoSLanfearDEImpact of pre-implant amiodarone exposure on outcomes in cardiac transplant recipientsHeart Fail Rev201520557357825925244
- ChinCFeindelCChengDDuration of preoperative amiodarone treatment may be associated with postoperative hospital mortality in patients undergoing heart transplantationJ Cardiothorac Vasc Anesth199913556256610527225
- LatiniRTognoniGKatesREClinical pharmacokinetics of amiodaroneClin Pharmacokinet1984921361566370540
- ZipesDPPrystowskyENHegerJJAmiodarone: electrophysiologic actions, pharmacokinetics and clinical effectsJ Am Coll Cardiol198434105910716368644
- SomaniPBasic and clinical pharmacology of amiodarone: relationship of antiarrhythmic effects, dose and drug concentrations to intracellular inclusion bodiesJ Clin Pharmacol19892954054122544634
- DoeschAOMüllerSKonstandinMMalignancies after heart transplantation: incidence, risk factors, and effects of calcineurin inhibitor withdrawalTransplant Proc20104293694369921094840
- RiviniusRHelmschrottMRuhparwarAAnalysis of malignancies in patients after heart transplantation with subsequent immunosuppressive therapyDrug Des Devel Ther2014993102
- HelmschrottMBeckendorfJAkyolCSuperior rejection profile during the first 24 months after heart transplantation under tacrolimus as baseline immunosuppressive regimenDrug Des Devel Ther2014813071314
- HelmschrottMRiviniusRRuhparwarAAdvantageous effects of immunosuppression with tacrolimus in comparison with cyclosporine A regarding renal function in patients after heart transplantationDrug Des Devel Ther2015912171224
- MacdonaldPHackworthyRKeoghASivathasanCChangVSprattPThe effect of chronic amiodarone therapy before transplantation on early cardiac allograft functionJ Heart Lung Transplant1991107437491958681
- Chelimsky-FallickCMiddlekauffHRStevensonWGAmiodarone therapy does not compromise subsequent heart transplantationJ Am Coll Cardiol1992207155615611452930
- KonertzWWeyandMDeiwickMScheldHHIs pretransplant anti-arrhythmic drug therapy a risk factor?Transplant Proc1992246267726781465898
- BlombergPJFeingoldADDenofrioDComparison of survival and other complications after heart transplantation in patients taking amiodarone before surgery versus those not taking amiodaroneAm J Cardiol200493337938114759399
- Sanchez-LazaroIJAlmenarLMartinez-DolzLDoes amiodarone influence early mortality in heart transplantation?Transplant Proc20063882537253817097993
- YerebakanHNakaYSorabellaRAmiodarone treatment prior to heart transplantation is associated with acute graft dysfunction and early mortality: a propensity-matched comparisonJ Heart Lung Transplant2014334S105
- GiardinaEGSchneiderMBarrMLMyocardial amiodarone and desethylamiodarone concentrations in patients undergoing cardiac transplantationJ Am Coll Cardiol19901649439472212375
- KarimiSHoughABeckeyCParraDResults of a safety initiative for patients on concomitant amiodarone and simvastatin therapy in a Veterans Affairs medical centerJ Manag Care Pharm201016747248120726676
- BourgeRCNaftelDCCostanzo-NordinMRPretransplantation risk factors for death after heart transplantation: a multiinstitutional study. The Transplant Cardiologists Research Database GroupJ Heart Lung Transplant19931245495628369318
- ScottCDDarkJHMcCombJMArrhythmias after cardiac transplantationAm J Cardiol19927011106110631384302
- BertoletBDEagleDAContiJBMillsRMBelardinelliLBradycardia after heart transplantation: reversal with theophyllineJ Am Coll Cardiol19962823963998800116
- DiBiaseATseTMSchnittgerIWexlerLStinsonEBValantineHAFrequency and mechanism of bradycardia in cardiac transplant recipients and need for pacemakersAm J Cardiol19916716138513892042569
- DasariTWPavlovic-SurjancevBPatelNIncidence, risk factors, and clinical outcomes of atrial fibrillation and atrial flutter after heart transplantationAm J Cardiol2010106573774120723655
- PavriBBO’NunainSSNewellJBRuskinJNWilliamGPrevalence and prognostic significance of atrial arrhythmias after orthotopic cardiac transplantationJ Am Coll Cardiol1995257167316807759722
- HohnloserSHPajitnevDPogueJIncidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: an ACTIVE W SubstudyJ Am Coll Cardiol200750222156216118036454
- AhmariSABunchTJChandraAPrevalence, pathophysiology, and clinical significance of post-heart transplant atrial fibrillation and atrial flutterJ Heart Lung Transplant2006251536016399531