Abstract
This study aimed to evaluate the efficacy and safety of topical treatment with natural herbal medicines on recurrent aphthous stomatitis (RAS). Nine electronic databases were searched to identify the randomized controlled trials and clinical controlled trials that reported the potential effect of natural herbal medicines on RAS published in Chinese or English. Ulcer size and duration, and remission of pain were assessed as main outcome measures. The methodological quality of the studies was evaluated using the Cochrane Handbook for Systemic Review of Interventions and Rev Man software. Thirteen trials with a total of 1,515 patients were included in the present analysis, which showed that topical treatment with natural herbal medicines seemed to benefit RAS patients by reducing ulcer size, shortening ulcer duration, and relieving pain without severe side effects. In conclusion, there is some evidence of the efficacy of topically applied natural herbal medicines with regards to improved RAS outcome measures and fewer side effects. However, given the limitations of this study, the evidence remains insufficient. Well-designed and high-quality randomized controlled trials are required for further exploration.
Introduction
Recurrent aphthous stomatitis (RAS), or recurrent aphthous ulcer (RAU), is one of the most common oral diseases, with an estimated prevalence of 25%.Citation1 Many factors may be involved in its progression, such as genetic predisposition, immunological abnormalities, microbial infection, psychological stress, and hormonal state.Citation2 Since the etiology and pathogenesis of RAS remains unclear, there is currently no consensus regarding a definitive curative therapy. The commonly accepted treatment strategy is to lessen the pain and duration of lesions.Citation3 Topical corticosteroids, antibiotics, and analgesics are highly recommended for patients with RAS.Citation4 However, longer treatment and frequent exposure to these medications may cause fungal infection and drug resistance, which may further lead to more severe adverse effects or even life-threatening complications.Citation5
Natural herbal medicines as an alternative therapy for RAS have been widely used in many countries for decades.Citation6–Citation8 Clinical studies on the use of such remedies have reported favorable benefits to patients by reducing the discomfort and duration of ulcers.Citation8–Citation11 However, no evidence-based reviews regarding the efficacy and safety of the topical application of these medicines on RAS have been available in the literature to date. Therefore, we conducted this analysis to evaluate the efficacy and safety of topical treatment with natural herbal medicines on RAS.
Materials and methods
Database and search strategy
This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines.Citation12 All clinical trials that reported the effect of topical treatment with natural herbal medicines on RAS were selected in the Chinese National Knowledge Infrastructure, the Chinese Biomedical Literature Database, the Chinese Scientific Journal Database (VIP), WANFANG data (WANFANG), PubMed, Excerpta Medica dataBASE (EMBASE), Science Citation Index (SCI), Current Controlled Trials (CCT), and the Cochrane Central Register of Controlled Trials in the Cochrane Library (up to April 2015). Publication language was confined to Chinese and English. The following terms were searched individually or combined: “Chinese patent medicine”, “Chinese patent drugs”, “traditional Chinese medicine”, “Chinese herbology”, “Chinese medicine”, “Chinese material medica”, “Chinese herbs”, “Chinese herbal medicine”, “natural herbal medicine”, “Chin Tradit Pat Med”, “herbal medicine”, “recurrent aphthous ulcer”, “recurrent aphthous stomatitis”, “recurrent oral ulcer”, and “recurrent oral ulceration”. Manual searching was used as a complementation.
Inclusion and exclusion criteria
Inclusion criteria
Randomized controlled trials (RCTs) and clinical controlled trials (CCTs) that evaluated the efficacy and safety of topical treatment with natural herbal medicines on RAS were collected. In accordance with “The diagnosis and management of recurrent aphthous stomatitis: a consensus approach”,Citation13 patients meeting the diagnostic criteria for RAS were included, with no restrictions on age, sex, or race. Patients in the experimental group received natural herbal medicines locally, without combined topical Western medications or systemic administration. Patients in the control group had to receive placebo treatment, chlorhexidine rinse. The outcome measures included the assessment of ulcer size, lesion duration, and remission of pain.
Exclusion criteria
Studies which there was duplication of study subjects were excluded, as were case reports or clinical observations without control groups, reviews, workshop summaries, translated papers or abstracts, animal studies, research reports without relevant or adequate information on participants, and interventions.
Data extraction and quality assessment
Two independent reviewers (CL Li and HL Huang) identified studies using the search strategy. If the eligibility of a study was not unanimous, a third reviewer party (H Hua and WC Wang) was consulted. Data were extracted from the included studies as follows: journal citation, author, year of publication, title, sample size, mediations, methodological details, therapeutic duration, and clinical outcomes.
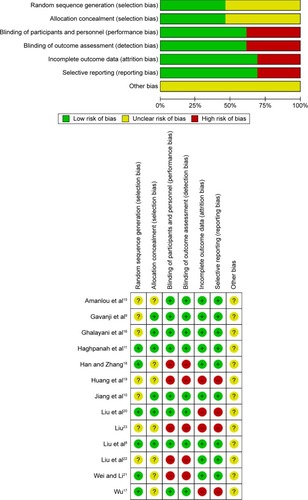
The quality of the enrolled publications was assessed according to the Cochrane Handbook for Systemic Review of Interventions, Version 5.1.0, and Rev Man 5.3 software.Citation14 The assessment criteria were random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias.
Data analysis
Due to the poor homogeneity of the included publications, only descriptive analysis was conducted in the present study.
Results
Description of the assessed publications
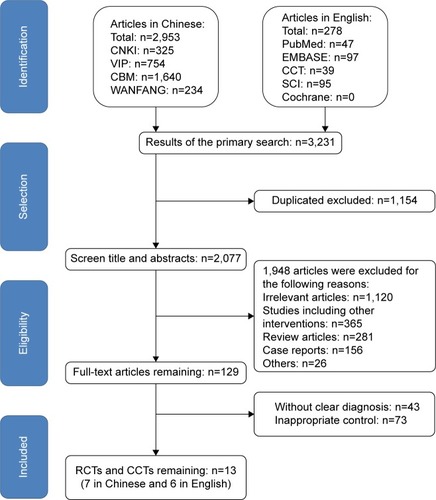
A total of 3,231 abstracts (2,953 in Chinese and 278 in English) were selected from nine databases; 1,154 studies were excluded for duplication. According to the exclusion criteria, screening of the titles and abstracts resulted in the removal of 1,948 articles. Full-text review of the remaining 129 publications indicated that 13 studies met the inclusion criteria and they were enrolled into the final analysis.Citation8–Citation11,Citation15–Citation23 The flowchart displayed in shows the detailed selection process.
Figure 1 Study selection process.
To share more information with regard to this topic, part of excluded articles were provided as Supplementary material.
Characteristics of the studies
The characteristics of the 13 studies are summarized in . A total of 1,515 RAS patients were studied in this review. The sample size ranged from 15 to 150 patients in each study. Twelve different types of herbal medicine, in the form of gargles, membranes, powders, tablets, toothpastes, and gelatin preparations, were used, among which eight were traditional Chinese medicines and four were Iranian herbal medicines. The experimental period in these trials ranged from 3 to 10 days, with an average time of 5.92±2.21 days.
Table 1 Characteristics of the enrolled studies
Risk of bias and quality assessment of the studies
Among the included studies, 12 were RCTs and one was self-controlled. The quality of over 50% of enrolled studies was rated as poor, according to the Cochrane quality assessment criteria ( and ).
Table 2 Quality assessment of included studies
Effects of the interventions
Due to the heterogeneity of the enrolled trials, only descriptive analysis was conducted in this study.
Main outcome measure
The main outcome measures assessed in this study were ulcer size, lesion duration, and remission of pain.
Size of ulcer
Four out of 13 trials reported a change in ulcer size, which was measured by different methods. Haghpanah et alCitation11 evaluated the average diameter of all ulcers; however, Jiang et alCitation10 and LiuCitation23 evaluated the maximum diameter of the ulcer, while Liu et alCitation8 determined the size of the ulcer by the maximum diameter and its vertical diameter. Nevertheless, all the studies reported a statistically significant reduction of the ulcer size in patients receiving herbal medicine therapy compared with the controls, with the exception of the study by Haghpanah et al,Citation11 who reported that a significant difference between the herbal medicine group and the control group was only observed in the first day of the intervention, with no statistically significant differences between the groups for the remaining time points.
Duration of ulcer
Four trials reported data on the duration of the ulcer. Ghalayani et alCitation16 reported a significant difference in the mean healing time between 8.6±0.99 days of placebo treatment and 5.3±0.81 days of treatment with Punica granatum extract (P<0.001). In the study by Liu et al,Citation22 it was found that Tian-zhu aerosol oral rinse remarkably reduced the duration of the ulcer compared with chlorhexidine rinse (P<0.05); however, no detailed information was described. In the study by Amanlou et al,Citation15 the average time for complete healing of the lesions in patients receiving Satureja khuzestanica extract or its essential oil was 5.90±1.24 days and 6.85±1.3 days, respectively, which were both significantly less than those receiving placebo treatment (10.40±1.66 days, P=0.0001). Moreover, there was no significant difference between the experimental groups. In the study by Wei and Li,Citation21 the healing time of the lesions in patients receiving Fufangjiaolianzhiji (2.79±0.30 days) was significantly shorter than when patients received a chlorhexidine rinse (6.02±1.45 days, P<0.05).
Remission of pain
Remission of pain was described in all the trials included in this literature review and it was described as the main outcome index in six studies. In addition, a visual analog scale (VAS) was applied in five out of the six studies to record the level of pain. In these studies, the visual analog scale score was significantly decreased in the herbal medicine groups than in the control groups.Citation8–Citation11,Citation16 In addition, two trials reported the average period of pain elimination, which was significantly shorter in patients receiving herbal medicine than placebo treatment.Citation15,Citation16
Side effects
Five trials did not report side effects,Citation9,Citation16,Citation18,Citation21,Citation22 while five trials reported that there were no side effectsCitation10,Citation11,Citation17,Citation19,Citation23 and three trials reported slight side effects.Citation8,Citation15,Citation20 In the study by Liu et al,Citation20 one patient receiving herbal medicine and one patient in the control group reported side effects, but no detailed information was provided. In the study by Amanlou et al,Citation15 two participants reported a slight burning sensation after receiving S. khuzestanica essential oil (prepared in an ethanol/water mixture: 50% v/v) on the first application. Liu et alCitation8 noted that two patients in the placebo group complained of slight lingual numbness, which spontaneously remedied.
Discussion
RAS is one of the most common oral disorders and its etiology is not well understood. Its management is mainly directed toward symptomatic, supportive treatment.Citation3 Therefore, corticosteroids and analgesics serve as the first choice for RAS patients.Citation4 However, longer treatment times and frequent exposure to such drugs may induce severe complications, such as secondary fungal infections and drug resistance.Citation5 There has been a long history of the use of natural herbal medicines for various disorders, including RAS, worldwide and such remedies have been studied both in clinic trials and experimental studies.Citation8,Citation9,Citation24,Citation25 In this study, we focused on the efficacy and safety of the topical application of natural herbal medicines for the treatment of RAS.
A total of 1,515 subjects in 13 clinical trials were analyzed in the present analysis. Compared with controls, topical natural herbal medicines greatly improved the patients’ symptoms by reducing ulcer size, shortening ulcer duration, and relieving pain without severe complications. However, only a weak conclusion can be drawn due to several limitations. First, the homogeneity of the studies was quite poor, with variables such as the various types of treatment, dosage, formula, application method, sample size, and experiment duration. Second, the quality of the studies was not sufficient because of poor study design and high risks in the performance, detection, attrition, and reporting bias. Therefore, a meta-analysis could not be conducted based on the current data. Consequently, this analysis unveiled the need for well-designed multicenter RCTs, which are of paramount importance for further exploration. Furthermore, precise criteria and standard methodologies should be established to ensure high-quality data.
The rationale of study designs can be a guarantee of strong clinical trials. The herbal medicine–oriental model or disease–oriental model may greatly affect the inclusion and exclusion criteria of future meta-analyses.
Detailed information of the herbal medicine used in future studies, including the formula, dosage, therapy duration, application protocols, and control intervention, should be provided. If possible, the dosage and application protocol should be homogenized for different forms of the same medicine.
In the studies included in this review, the therapy duration ranged from 3 to 10 days. However, we found that the natural course of an untreated ulcer was 9.5±1.3 daysCitation15,Citation16 and the duration of herbal medicine treatment was 5.21±1.73 days.Citation15,Citation16,Citation21,Citation22 It is therefore suggested that the experimental period should exceed 7 days to avoid missing valuable data. Moreover, follow-up is highly recommended.
In addition to the above, the index and measurement of outcomes should be described clearly and consistently in future studies. According to the current data, ulcer size, lesion duration, and level of pain are commonly considered as the main outcome indicators. However, researchers often failed to assess them in a standard way, especially with regards to the ulcer size. Actually, manifestation of RAS may be a single ulcer or several round or elliptic recurrent ulcers in the oral mucosa.Citation4 Therefore, measurement of the maximum diameter may not be suitable to determine the ulcer size accurately. Theoretically, evaluation of the ulcer area may be better in describing the lesion size; however, the precise calculation may restrict its application in clinic. We suggest that measurement of the maximum diameter and its vertical diameter should be made, as described in the study by Liu et al,Citation8 as it is a precise and convenient way to determine the size of lesions. Finally, appropriate statistical methods also represent an important part of study protocols and can allow sample size calculation and data analysis.
In summary, the current data show favorable benefits of the topical treatment of RAS with natural herbal medicines and only three of the included studies reported slight and/or transient side effects during the clinical trial period. Thus, there is some evidence to suggest that topical herbal medicine therapy is an effective and safe alternative option to current Western medicine-based treatments for RAS.
Conclusion
There is weak evidence with regards to the efficacy of the topical application of natural herbal medicines for the treatment of RAS patients’ conditions, with few side effects reported. However, given the limitations of the trials included in this assessment and the methodologies employed in the current data, a definitive conclusion cannot be drawn. Well-designed and high-quality RCTs are required for further exploration.
Acknowledgments
This work was supported by the National Key Clinic Program (2013).
Disclosure
The authors report no conflicts of interest in this work.
References
- ScullyCPorterSOral mucosal disease: recurrent aphthous stomatitisBr J Oral Maxillofac Surg200846319820617850936
- GreenberyMGlickMBurket’s Oral Medicine Diagnosis and Treatment11th edHamiltonBC Decker20086365
- PorterSRHegartyAKaliakatsouFHodgsonTAScullyCRecurrent aphthous stomatitisClin Dermatol200018556957811134852
- FemianoFLanzaABuonaiutoCGuidelines for diagnosis and management of aphthous stomatitisPediatr Infect Dis J200726872873217848886
- EisenDCarrozzoMBagan SebastianJVThongprasomKNumberVOral lichen planus: clinical features and managementOral Dis200511633834916269024
- McBrideDRManagement of aphthous ulcersAm Fam Physician200062114915416010905785
- RattanJSchneiderMArberNGorskyMDayanDSucralfate suspension as a treatment of recurrent aphthous stomatitisJ Intern Med199423633413438077892
- LiuXGuanXChenRHuaHLiuYYanZRepurposing of yunnan baiyao as an alternative therapy for minor recurrent aphthous stomatitisEvid Based Complement Alternat Med2012201228462023258985
- GavanjiSLarkiBBakhtariAThe effect of extract of Punica granatum var. pleniflora for treatment of minor recurrent aphthous stomatitisIntegr Med Res2014328390
- JiangXWZhangYZhuYLEffects of berberine gelatin on recurrent aphthous stomatitis: a randomized, placebo-controlled, double-blind trial in a Chinese cohortOral Surg Oral Med Oral Pathol Oral Radiol2013115221221723246229
- HaghpanahPMoghadamniaAAZarghamiAMotallebnejadMMuco-bioadhesive containing ginger officinale extract in the management of recurrent aphthous stomatitis: a randomized clinical studyCaspian J Intern Med2015613826221489
- MoherDLiberatiATetzlaffJAltmanDGGroupPPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementInt J Surg20108533634120171303
- ScullyCGorskyMLozada-NurFThe diagnosis and management of recurrent aphthous stomatitis: a consensus approachJ Am Dent Assoc2003134220020712636124
- HigginsJPTGreenSCochrane Handbook for Systemic Reviews of Interventions, Version 5.1.0.2011The Cochrane Collaboration Available from: http://handbook.cochrane.org/Accessed January 5, 2015
- AmanlouMBabaeeNSaheb-JameeMSalehniaAFarsamHTohidast AkradZEfficacy of Satureja khuzistanica extract and its essential oil preparations in the management of recurrent aphthous stomatitisDaru2007154231235
- GhalayaniPZolfagharyBFarhadARTavangarASoleymaniBThe efficacy of Punica granatum extract in the management of recurrent aphthous stomatitisJ Res Pharm Pract201322889224991610
- WuGYThe effect of treating ROU with the membrane of nourishing yin and promoting granulationJ Clin Stomatol2000163172173
- HanFZhangZJEffect of qingmei ulcer membrance on recurrent oral ulcer in 88 casesJ Zhengzhou University: Med Sci2003385810811
- HuangHFXuXMengZYChenLEffect of the membrance of nourishing yin and promoting granulation on recurrent aphthous stomatitisJin Ling Yi Yuan Xue Bao199692173174
- LiuYPZhangLChenSJClinical studies of the effect of KouChuangNing tablets on recurrent aphthous stomatitis with syndrome of accumulated heat in heart and spleenJ TCM Univ Human20062633637
- WeiQWLiWDEffect of Fufangjiaolianzhiji on recurrent oral ulcer in 63 casesShan Xi Zhong Yi2003238714725
- LiuYXLiuXLiHYuFZhengXYHuangZYObservation on clinical therapeutic efficacy of tian-zhu aerosol oral rinse treatment of 83 cases with oral ulcenJ Chengdu Univ TCM20133648082
- LiuGLEffect of kangfuxinye on recurrent oral ulcer: a clinic observationChin J Mis Diagn201010286826
- ZhengLWHuaHCheungLKTraditional Chinese medicine and oral diseases: today and tomorrowOral Dis201117171220646230
- LiCLHeJLiZGZhengLWHuaHEffects of total glucosides of paeony for delaying onset of Sjogren’s syndrome: an animal studyJ Craniomaxillofac Surg201341761061523333492