Abstract
Background
MetaGeM is a wide gender-medicine project comprising post hoc and meta-analyses by gender of clinical outcomes, therapeutic approaches, and safety data from previously conducted observational studies to explore possible gender differences in real-life clinical settings. We report the results of the safety meta-analysis of seven MetaGeM studies, evaluating gender differences in adverse event (AE) incidence and severity.
Methods
Data were collected between February 2002 and July 2013. Male and female patients were compared for the main safety variables, using Student’s t-test, χ2 test, or Fisher’s exact test as appropriate. As supportive analysis, a logistic regression model was estimated to evaluate associations between gender and outcome.
Results
In total, 4,870 patients (46% females, 54% males) were included in the analysis; age was higher for females (mean ± standard deviation 61.2±18.3 years) than males (56.3±16.6 years). Overall, 264 AEs were reported (59.1% in males). There were no significant gender differences in the percentage of patients with at least one AE: 3.0% for females versus 3.9% for males, χ2 test P>0.05. According to the logistic regression model results, no association between gender and AEs occurrence seems to exist. A statistically significant gender difference in the percentage of drug-related AEs emerged (37.6% in females vs 20.8% in males, χ2 P=0.0039). Slightly significantly more AEs in females were addressed with treatment compared with males (78.1% vs 66.7%, χ2 P=0.0485). Total serious AEs (SAEs) were 47 (72% in males). The frequency of patients with ≥1 SAE was 0.6% in females versus 1.2% in males (χ2 test P=0.0246).
Conclusion
This safety analysis on a large sample of almost 5,000 patients with different diseases and treated with a wide range of different drugs provides a useful overview on possible gender differences in drug tolerability, which may be helpful in more accurately designing future clinical trials from a gender-specific perspective.
Introduction
Clinical data suggest that male and female patients exhibit differences regarding the pharmacology and toxicity of medicationsCitation1 and differ in their response to drug treatment, not only as a result of physiological differences, such as body weight, surface area, and extracellular and intracellular water, but also in terms of differences in pharmacokinetics (PK) and pharmacodynamics (PD).Citation2–Citation4 It is known that sex hormones influence drug absorption, distribution, metabolism, PD, and adverse events (AEs).Citation5 In 2001, on the basis of its Adverse Events Reporting System, the US Food and Drug Administration stated that females experience more and more serious AEs (SAEs) than males.Citation6 It has been hypothesized that this may be due to overdosing, different PK and PD, the fact that females are more likely to report AEs than males, or that females take more medications than males. It has been shown that females use more medicines than males do and have a higher rate of chronic diseases, but they also generally pay more attention to their health and have more consciousness and care about themselves.Citation6
Despite the increasing evidence of physiological and pathological differences between genders, beyond those related to reproduction,Citation7–Citation11 females still represent a small percentage (22%) of participants in Phase I trials which are essential to verify drug dosage, AEs, and safety.Citation12 Medical research has been conducted for decades with a large prevalence of male participants in clinical studies, yet the findings of these studies have often been applied to both genders.Citation12 Only with the new millennium has this changed with the European Union promoting females’s participation in research projects and the World Health Organization including gender medicine in the Equity Act in order to achieve gender-appropriate care.Citation13 However, to date, the analyses provided by the pharmaceutical industry to the regulatory authorities often do not classify safety and efficacy data by gender.
In 2013, the Italian Drug Agency (Agenzia Italiana del Farmaco) invited pharmaceutical companies to process data divided by gender during the submission of regulatory documentation, so as to highlight possible differences.Citation14 In the same year, Novartis Italy put in place a wide gender-medicine project, called MetaGeM, which included analysis by gender of the data from nine previously conducted observational studies. These studies were performed between 2002 and 2013, and covered a range of different clinical areas, including immune-mediated disorders (psoriasis and psoriatic arthritis), transplantation medicine (liver and kidney transplants), infectious diseases (hepatitis B), and the central nervous system (Parkinson’s disease and Alzheimer’s disease [AD]). The aim of the MetaGeM project was to analyze and describe clinical outcomes, therapeutic approaches, and safety data by gender, using post hoc analyses and meta-analyses, in order to explore possible gender differences. The methodology of the MetaGeM project has been described in detail elsewhere.Citation15 The present paper reports the results of the overall safety analysis of the MetaGeM studies, aimed at evaluating possible gender differences in the incidence and severity of AEs and the potential association between gender and safety.
Methods
Seven of the nine observational studies of the MetaGeM project were included in this safety analysis on 2,612 (53.6%) males and 2,258 (46.4%) females: they are listed and briefly described in . With regard to the two excluded studies, the GENDER-ATTENTION (“The female in her real dimension: effect of gender and hormonal status on adverse events’ incidence in psoriatic patients treated with cyclosporine”) study (Colombo et al, unpublished data, 2013a; Colombo et al, unpublished data, 2013b; Colombo et al, unpublished data, 2014a; Colombo et al, unpublished data, 2014b; Colombo et al, unpublished data, 2016) was not included because a gender-specific safety assessment was the primary objective of the study itself, while the Studio Osservazionale Italiano per la valutazione dell’insUfficienza Renale in pazienti con trapianto di Fegato (SURF) (“Italian Observational Study for the evaluation of renal insufficiency in liver transplant patients”) study (Donato et al, unpublished data, 2013) was also not considered because it did not include safety data collection.
Table 1 MetaGeM observational studies and patients included in the analysis
An AE was defined as any unfavorable or unintended sign, symptom, or disease that occurred from the time the signed informed consent was obtained until the end of the patient observation period. An AE was defined as a SAE if it resulted in death, was judged life-threatening, required inpatient hospitalization or prolongation of existing hospitalization, resulted in persistent or significant disability/incapacity, or caused a congenital anomaly/birth defect in the child of the observed patient. In cases where such information was available, the possible relationship with therapy, as evaluated by the investigator, was reported.
Data were collected between February 2002 (first patient first visit; PSYCHAE studyCitation16,Citation17) and July 2013 (last patient last visit; ICEBERG study [Rizzetto et al, unpublished data, 2011; Bandiera et al, unpublished data, 2012]); first patient first visit and last patient last visit for each study are shown in . Monitoring visits were conducted to verify whether enrollment was performed consecutively and according to inclusion/exclusion criteria. Moreover, before statistical analysis, a data cleaning process was run to check the collected data for completeness and accuracy.
Patients were considered evaluable for this analysis if they were evaluable for the individual study in which they participated and if gender was recorded in the case report form (CRF), as detailed elsewhere.Citation16–Citation23
Evaluable patients were described according to sociodemographic (such as age) and clinical (such as ongoing specific therapies) features. Moreover, male and female patients were compared with regard to the main safety variables: AE occurrence and characteristics (description, intensity, possible correlation with a drug, evolution, and therapeutic intervention, if any), and SAE occurrence and description. Comparisons were performed by Student’s t-test, χ2 test, or Fisher’s exact test as appropriate. For the post hoc analyses, all P-values presented are exploratory. Patients with missing data in selected parameters were not excluded from analysis, but were simply not evaluated for these parameters.
Data collection was performed for all studies through the CRF; however, not all variables of interest were present in all studies or, if present, not all had the same answer categories. For these reasons, AE CRF fields that were common to all studies were identified through a preliminary analysis, and a new variable for each outcome of interest (eg, AE description, AE intensity) was created considering the value in each study. Fields that were missing in some studies were analyzed only where present.
Different answer modalities were present in the original CRFs for AE intensity, possible correlation of AE with a drug, and AE evolution, and so recoding was performed. Briefly, the AE intensity was classified as mild, moderate, high, or not determined, as originally reported in most studies. In the EVOLUTION study, grade 1 intensity (based on Common Terminology Criteria for Adverse Events v3.0) was considered as mild, grade 2 as moderate, and grades 3–5 as high, while in the ICEBERG study (Rizzetto et al, unpublished data, 2011; Bandiera et al, unpublished data, 2012), AEs defined as severe were considered to be of high intensity. For the CETRA study, the intensity of AEs could not be analyzed because this variable was not collected on the CRF. Possible correlation with a drug was defined as present (yes), absent (no), or not determined; if changes in therapy had been introduced, these were evaluated in terms of dosage escalation or reduction, therapy discontinuation, or “other”. Concerning their evolution, AEs were considered as resolved (including resolved without sequelae), unresolved, toward resolution, resolved without sequelae, or resolution unknown. SAEs were reported as death, hospitalization, disability, persistent or significant inability, life-threatening events, or not determined.
As supportive analysis, a logistic regression model was estimated to evaluate the association between gender (male, female) and outcome (“the patient had at least one AE during the study”). The model provided estimates of the odds ratios of experiencing an AE in female versus male patients. All analyses were performed with SAS v9.2 and Enterprise Guide v4.3. (SAS v9.2: Copyright © 2009 by SAS Institute Inc., Cary, NC, USA; Enterprise Guide v4.3 Copyright © 2006–2010 by SAS Institute Inc., Cary, NC, USA).
Results
In total, 4,870 patients, 46% females and 54% males, were included in the analysis, as detailed in . Age by gender and study is reported in : overall, mean age was higher in females (61.2±18.3 years) than in males (56.3±16.6 years). Disease-specific therapies ongoing at enrollment in the studies are summarized in .
Table 2 Demographic characteristics of patients
Table 3 Specific medications taken at inclusion in the study
Overall, 264 AEs were reported, 59.1% in males; summarizes the type of AEs, according to the Medical Dictionary for Regulatory Activities (MedDRA) system organ class.
Table 4 AEs according to system organ class (MedDRA)
There was no significant gender difference in the percentage of patients with at least one AE (ie, patients with 1, 2, or ≥3 AEs): 3.0% for females versus 3.9% for males, χ2 test P≥0.05. Details about the occurrence and intensity of AEs by gender and study are reported in . The results of the logistic regression model also showed no association between gender and AE occurrence: odds ratio =0.764 (females vs males), 95% confidence interval: 0.559–1.044, but a statistically significant gender difference in the percentage of drug-related AEs emerged (37.6% in females vs 20.8% in males, χ2 test P=0.0039). AE evolution is depicted in . Overall, more AEs were addressed with some kind of treatment in females compared with males (78.1% vs 66.7%, χ2 P=0.0485).
Table 5 Occurrence of AEs during the study
Figure 1 Evolution of AEs by gender.
Abbreviation: AEs, adverse events.
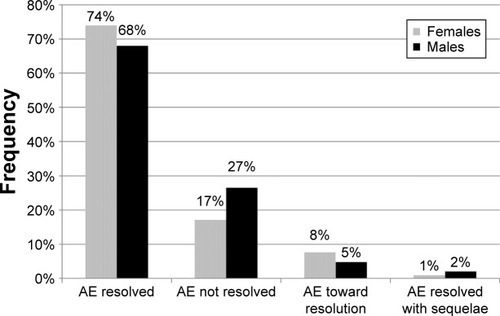
Total SAEs were 47 (72% in males). The frequency of patients with ≥1 SAE was 0.6% in females versus 1.2% in males (χ2 test P=0.0246). Number and type of SAEs are detailed in .
Table 6 Serious adverse events (SAEs) occurred during study
Discussion
Gender-specific medicine is the study of how diseases differ between males and females in terms of prevention, clinical manifestations, therapeutic approach, outcomes and tolerability, prognosis, and psychological and social impact. There has been increasing evidence in recent years of physiological and pathological differences between the genders. Focusing on the diseases examined in the studies included in our analysis, many gender differences have been reported for patients with psoriasis and psoriatic arthritis, in terms of epidemiology,Citation24 pattern and burden of disease,Citation25 search for care,Citation26 and choice of therapy;Citation27,Citation28 for example, peripheral joint involvement, pain, and functional impairment are more frequent for female than for male patients with psoriasis.Citation29–Citation32 Moreover, the PSYCHAE studyCitation16 pointed out that the psychological status of females was also worse than that of males, independently of the severity of psoriasis. There is also an extensive literature on gender differences in AD, as recently reviewed by Li and Singh.Citation33 Clinical studies have shown differences between males and females in specific cognitive ability domains and risk of AD at later age. Several major biological hypotheses have been postulated, such as differences in age-related sex hormone reduction (estrogens, progesterone, testosterone), impact from risks of other diseases (diabetes, depression), and age-related decline in brain volume. In Parkinson’s disease as well, gender-related differences have been recognized, although these are still poorly understood. Prevalence and incidence of Parkinson’s disease are significantly higher in males than in females.Citation34,Citation35 From a clinical point of view, females with Parkinson’s disease have shown worse capacity in activities of daily living and more severity of levodopa-induced dyskinesia in several studies,Citation36–Citation38 while male gender was shown to predict worse rigidity score and higher risk for sleep behavior disorder, dementia, and death.Citation37,Citation39–Citation41 A 2014 cross-sectional surveyCitation42 found that males reported a greater disease burden and greater daily levodopa equivalent doses than females. The greater burden of disease score in males was significantly associated with gender even after controlling for age and disease duration. Concerning transplants, gender-based disparities have been observed in post-liver transplantation outcomes, together with a continuous decline in the number of liver transplantations in females.Citation43
The effect of gender on drug tolerability has begun to raise interest only in recent years, mainly resulting in the observation that females experience more frequent and more SAEs than males, possibly due to different PK and PD, higher rates of AE reporting, or greater use of medications in females compared to males.Citation6,Citation44–Citation46 Clinical research sponsored by Novartis Italy in the past decade included several large observational studies in important medical areas, including psoriasis.Citation16,Citation17,Citation22,Citation47 Central nervous system disorders, such as Parkinson’s disease and AD,Citation18–Citation21 and transplantation,Citation23 but none of those studies, except one (the aforementioned GENDER-ATTENTION study [Colombo et al, unpublished data, 2013a; Colombo et al, unpublished data, 2013b; Colombo et al, unpublished data, 2014a; Colombo et al, unpublished data, 2014b; Colombo et al, unpublished data, 2016]), adopted a gender-specific approach in data analysis. Based on the increasing interest in gender medicine and prompted by the large quantity of clinical data available through these large national studies, it was decided to reanalyze these data from a gender perspective (the MetaGeM project). This paper focuses on the overall safety data from seven MetaGeM studies.
The overall MetaGeM sample (N=4,870) consisted of 2,612 (53.6%) males, with females being predominant only in the two AD studies, EVOLUTION and AXEPT (59.7% and 63.6%, respectively, of those enrolled are female). This prevalence of female patients in the AD studies probably accounts for the overall higher mean age in females compared with males.
There were no significant gender differences in the frequency of patients with at least one AE (3.0% for females vs 3.9% for males, χ2 test P>0.05), with 59.1% of global AEs occurring in males. Considering only those reported as drug-related AEs, the incidence was significantly higher in females, and this is consistent with several reports revealing that females are more prone to adverse drug reactions (ADRs) than males, as confirmed by the evidence that eight of ten drugs that have been dropped out from the US market were responsible for more ADRs in females than in males. Indeed, risk factors for ADRs, such as polytherapy, aging (females become generally older than males), and depression, are in general more frequent in females than in males.Citation44–Citation46,Citation48,Citation49 Other factors hypothesized to explain why females usually report more ADRs are the fact that females have a higher prevalence of pain (headache, migraine, musculoskeletal pain) and pay greater attention to their health status. Moreover, physiological aspects, such as menstrual cycles, pregnancy, and menopause, are likely to have a relevant impact on the PK and PD of drugs.
Unexpectedly, we found that 2% of SAEs occurred in males, and the frequency of patients reporting at least one SAE was significantly higher in males, despite the older mean age of females compared with males. These results are in contrast with the large quantity of published data showing that females experience more AEs and that these are generally also more serious.Citation6,Citation30–Citation32 However, it has to be considered that the overall patient population was fairly unbalanced by the CETRA study on renal transplantation (inclusion criteria: age ≥18 years, renal allograft functioning for at least 6 months, and serum creatinine level <2.5 mg/dL), which included 64% male patients and accounted for 77% of total SAEs (36 of 47). This may be reasonably explained by the fact that the CETRA patients were transplanted patients, frequently hospitalized for transplant-related events. For these reasons, in order to have a more homogeneous population, we repeated the analysis excluding CETRA patients. This analysis showed no significant difference between the genders in terms of SAEs (0.2% of females vs 0.4% of males had at least one SAE; χ2 test, P>0.05).
We had no adequate information about the degree of disease severity in the patients considered for this analysis and therefore were unable to analyze for possible correlations between severity of disease and incidence of AE; however, we hypothesized that the type and number of therapies administered could mirror the severity of the disease, at least for the DEEP study (Parkinson’s disease) and the PSYCHAE and SYNERGY studies (psoriasis ± psoriatic arthritis). Following specific analyses, we found no correlations between the incidence of AEs and the number of drugs administered to patients (1–2 and ≥3) in the DEEP study, or the type of therapy (topical or systemic) in the other two studies in psoriasis. This seems to confirm that the observed gender differences were not biased by the severity of the disease.
Limitations
Our study has some limitations. As a post hoc analysis, it was not originally designed to assess gender differences in patients’ safety profile, and statistical analysis was mainly descriptive with only explorative P-values. Moreover, owing to the inclusion of several studies, large differences in patient population, study design, and ongoing treatments have to be taken into account. For this reason, a common approach for data cleaning, recoding, and statistical analysis was followed. Finally, one single study, CETRA, which studied the quite complex and serious clinical condition of renal transplantation, accounted for the majority of AEs (183/264) and SAEs (36/47), and this may have strongly affected the results of our analysis.
Conclusion
This safety analysis on a large sample of almost 5000 patients affected with different diseases and treated with a wide range of different drugs provides a useful overview, within the considered disease areas, of possible gender differences in drug tolerability, which may be helpful in more accurately designing future clinical trials from a gender-specific perspective.
Acknowledgments
The study was supported by an unrestricted educational grant from Novartis Farma S.p.A., Origgio (VA), Italy. The authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE), and were fully responsible for all aspects of manuscript development. We are grateful to MediNeos (Modena, Italy) for data collection and statistical analysis and to Renata Perego for help in writing the manuscript.
Disclosure
DC is a part-time employee of Novartis Farma Italy and received grants from Allergan and Aventis. EZ and MN are employees of Novartis Farma Italy. SR and AO are employees of MediNeos srl. GB was an employee of Novartis Farma Italy during study execution and manuscript submission, but now is an employee of UCB Pharma Italy. The authors report no other conflicts of interest in this work.
References
- BaggioGCorsiniAFloreaniAGianniniSZagonelVGender medicine: a task for the third millenniumClin Chem Lab Med201351471372723515103
- KorenGNordengHMacLeodSGender differences in drug bioequivalence: time to rethink practicesClin Pharmacol Ther201393326026223299647
- UenoKSatoHSex-related differences in pharmacokinetics and pharmacodynamics of anti-hypertensive drugsHypertens Res201235324525022089536
- SoldinOPMattisonDRSex differences in pharmacokinetics and pharmacodynamicsClin Pharmacokinet200948314315719385708
- SpoletiniIVitaleCMalorniWRosanoGMSex differences in drug effects: interaction with sex hormones in adult lifeHandb Exp Pharmacol20122149110523027447
- MillerMAGender-based differences in the toxicity of pharmaceuticals – the Food and Drug Administration’s perspectiveInt J Toxicol200120314915211488556
- LegatoMJPrinciples of Gender-Specific Medicine1st edSan DiegoElsevier, Academic Press20041396
- ChenMLLeeSCNgMJSchuirmannDJLeskoLJWilliamsRLPharmacokinetic analysis of bioequivalence trials: implications for sex-related issues in clinical pharmacology and biopharmaceuticsClin Pharmacol Ther20006851051711103754
- GandhiMAweekaFGreenblattRMBlaschkeTFSex differences in pharmacokinetics and pharmacodynamicsAnn Rev Pharmacol Toxicol20044449952314744256
- SchwartzJBThe influence of sex on pharmacokineticsClin Pharmacokinet20034210712112537512
- AndersonGDSex and racial differences in pharmacological response: where is the evidence? Pharmacogenetics, pharmacokinetics, and pharmacodynamicsJ Womens Health (Larchmt)200514192915692274
- CasseseMZuberVClinical trials and gender medicineAnn Ist Super Sanita201147110010321430348
- Agenda for research on women’s health for the 21st centuryA report of the Task Force on the NIH Women’s Health Research Agenda for the 21st CenturyNational Institute of Health Publication 99-4389Bethesda, MDU.S. Department of Health and Human Services, Public Health Service, National Institutes of Health1999
- AIFA Farmaci e Genere – Avviso alle Aziende Farmaceutiche 30/01/2013 [The Italian Medicines Agency (AIFA) Medicines and Gender – Communication to Pharmaceutical Companies 30th January 2013].
- ColomboDBelliaGVassellattiDZagniESgarbiSRizzoliSA gender-medicine post hoc analysis (MetaGeM) project to test sex differences in previous observational studies in different diseases: methodologyOpen Access J Clin Trials20146111116
- ColomboDCaputoAFinziAfor the PSYCHAE Study Group. Evolution of and risk factors for psychological distress in patients with psoriasis: the PSYCHAE studyInt J Immunopathol Pharmacol201023129730620378016
- ColomboDChimentiSGrossiPon behalf of SYNERGY Study GroupPrevalence of past and reactivated viral infections and efficacy of cyclosporine A as monotherapy or in combination in patients with psoriatic arthritis. The SYNERGY Study: a longitudinal observational studyBiomed Res Int2014ID941767
- AbbruzzeseGAntoniniABaronePLinguistic, psychometric validation and diagnostic ability assessment of an Italian version of a 19-item wearing-off questionnaire for wearing-off detection in Parkinson’s diseaseNeurol Sci20123361319132722307444
- StocchiFAntoniniABaronePDEEP study group. Early DEtection of wEaring off in Parkinson disease: the DEEP studyParkinsonism Relat Disord201420220421124275586
- SpallettaGCaltagironeCPadovaniAEVOLUTION study Working Group. Cognitive and affective changes in mild to moderate Alzheimer’s disease patients undergoing switch of cholinesterase inhibitors: a 6-month observational studyPLoS One201492e8921624586603
- BernabeiRRossiniPMDi CioccioLCompliance and caregiver satisfaction in Alzheimer’s Disease: results from the AXEPT StudyDement Geriatr Cogn Dis Extra20122141843223139687
- FinziAColomboDCaputoAfor the PSYCHAE Study Group. Psychological distress and coping strategies in patients with psoriasis: the PSYCHAE StudyJ Eur Acad Dermatol Venereol2007211161116917894699
- PonticelliCColomboDNovaraMBasiliscoGCETRA Study GroupGastrointestinal symptoms impair quality of life in Italian renal transplant recipients but are under-recognized by physiciansTranspl Int201023111126113420525020
- IcenMCrowsonCSMcEvoyMTDannFJGabrielSEMaradit KremersHTrends in incidence of adult-onset psoriasis over three decades: a population-based studyJ Am Acad Dermatol20096039440119231638
- EderLThavaneswaranAChandranVGladmanDDGender difference in disease expression, radiographic damage and disability among patients with psoriatic arthritisAnn Rheum Dis201372457858222589379
- BhutaniTWongJWBeboBFJrArmstrongAWAccess to health care in patients with psoriasis and psoriatic arthritis: data from National Psoriasis Foundation Survey PanelsJAMA Dermatol2013149671772123783152
- HotardRSFeldmanSRFleischerABJrSex-specific differences in the treatment of severe psoriasisJ Am Acad Dermatol200042462062310727307
- ZachariaeHZachariaeRBlomqvistKTreatment of psoriasis in the Nordic countries: a questionnaire survey from 5739 members of the psoriasis associations data from the Nordic Quality of Life StudyActa Derm Venereol200181211612111501648
- LeeWReveilleJDDavis JrJCLearchTJWardMMWeismanMHAre there gender differences in severity of ankylosing spondylitis? Results from the PSOAS cohortAnn Rheum Dis20076663363817127685
- QueiroRTejónPCotoPClinical differences between men and women with psoriatic arthritis: relevance of the analysis of genes and polymorphisms in the Major Histocompatibility Complex region and of the age at onset of psoriasisClin Dev Immunol2013201348269123690822
- ColomboDChimentiSGrossiPAPrevalence of acute and chronic viral seropositivity and characteristics of disease in patients with psoriatic arthritis treated with cyclosporine: a post hoc analysis from a sex point of view on the observational study of infectious events in psoriasis complicated by active psoriatic arthritisClin Cosmet Investig Dermatol2015917
- ColomboDBelliaGIl paziente psoriasico e l’attenzione alle differenze di genere: lo studio Gender Attention [The patient affected by psoriasis and the attention to gender differences: the GENDER-ATTENTION study]G Ital Dermatol Venereol2013Suppl 121 Italian
- LiRSinghMSex differences in cognitive impairment and Alzheimer’s diseaseFront Neuroendocrinol201435338540324434111
- KieburtzKWunderleKBParkinson’s disease: evidence for environmental risk factorsMov Disord201328181323097348
- KowalSLDallTMChakrabartiRStormMVJainAThe current and projected economic burden of Parkinson’s disease in the United StatesMov Disord20132831131823436720
- HaaxmaCABloemBRBormGFGender differences in Parkinson’s diseaseJ Neurol Neurosurg Psych2007788819824
- BabaYPutzkeJDWhaleyWRWszolekZKUittiRJGender and the Parkinson’s disease phenotypeJ Neurol2005252101201120516151602
- HarizGMLindbergMHarizMBergenheimATGender differences in disability and health-related quality of life in patients with Parkinson’s disease treated with stereotactic surgeryActa Neurol Scand20031081283712807390
- FernandezHHLapaneKLPredictors of mortality among nursing home residents with a diagnosis of Parkinson’s diseaseMed Sci Monit20028CR241CR24611951064
- GalvinJEPollackJMorrisJCClinical phenotype of Parkinson disease dementiaNeurology20066791605161117101891
- ScaglioneCVignatelliVPlazziGREM sleep behaviour disorder in Parkinson’s disease: a questionnaire-based studyNeurol Sci200525631632115729494
- LubomskyMRushworthRLLeeWBertramKLWilliamsDRSex differences in Parkinson’s diseaseJ Clin Neurosci20142191503150624767694
- OloruntobaOOMoylanCAGender-based disparities in access to and outcomes of liver transplantationWorld J Hepatol20157346046725848470
- PirmohamedMJamesSMeakinSAdverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patientsBMJ2004329151915231615
- PatelHBellDMolokhiaMTrends in hospital admissions for adverse drug reactions in England: analysis of national hospital episode statistics 1998–2005BMC Clin Pharmacol20077917894876
- SikdarKCAlaghehbandanRMacDonaldDAdverse drug events in adult patients leading to emergency department visitsAnn Pharmacother20104464164920233911
- ColomboDGraziottinABelliaGVenaGABanfiGCassanoNInfluence of sex on incidence of side effects in plaque psoriasis patients treated with cyclosporine. Preliminary results from the Italian observational GENDER ATTENTION studyJ Am Acad Dermatol201470Suppl 1AB173
- FranconiFCampesiIPharmacogenomics, pharmacokinetics and pharmacodynamics: interaction with biological differences between men and womenBr J Pharmacol2014171358059423981051
- FattingerKRoosMVergeresPEpidemiology of drug exposure and adverse drug reactions in two Swiss department of internal medicineBr J Clin Pharmacol20004915816710671911
