Abstract
Characterized by its acute onset, critical condition, poor prognosis, and high mortality rate, severe acute pancreatitis (SAP) can cause multiple organ failure at its early stage, particularly acute lung injury (ALI). The pathogenesis of ALI is diffuse alveolar damage, including an increase in pulmonary microvascular permeability, a decrease in compliance, and invasion of many inflammatory cells. Corticosteroids are the main treatment method for ALI; however, the associated high toxicity and side effects induce pain in patients. Recent studies show that the effective components in many traditional Chinese medicines can effectively inhibit inflammation with few side effects, which can decrease the complications caused by steroid consumption. Based on these observations, the main objective of the current study is to investigate the effect of alpinetin, which is a flavonoid extracted from Alpinia katsumadai Hayata, on treating lung injury induced by SAP and to explore the mechanism underlying the alpinetin-mediated decrease in the extent of ALI. In this study, we have shown through in vitro experiments that a therapeutic dose of alpinetin can promote human pulmonary microvascular endothelial cell proliferation. We have also shown via in vitro and in vivo experiments that alpinetin upregulates aquaporin-1 and, thereby, inhibits tumor necrosis factor-α expression as well as reduces the degree of lung injury. Overall, our study shows that alpinetin alleviates SAP-induced ALI. The likely molecular mechanism includes upregulated aquaporin expression, which inhibits tumor necrosis factor-α and, thus, alleviates SAP-induced ALI.
Introduction
Severe acute pancreatitis (SAP) is characterized by a critical condition and high mortality rate; its pathogenesis remains debatable. Scholars believe that the key step includes pancreatic enzymes that induce autodigestion of the pancreas via a series of activation processes, which produces pancreatic cellular and interstitial edema, adiponecrosis, and hemorrhage and subsequently activates inflammatory cascade reactions throughout the entire body. In severe cases, SAP can result in systemic inflammatory response syndrome, which causes multiple organ dysfunction.Citation1,Citation2 Among such cases, the most common syndrome is acute lung injury (ALI),Citation3 which features the characteristics of rapid disease progression and a high mortality rate. ALI remains a clinically treated disease. One of the main challenges of treating ALI is that the pathogenesis remains unknown. Therefore, it is imperative to explore the origin and development of SAP-induced lung injuries. Many studies show that the pathogenesis of pancreatitis-associated lung injury is related to many factors, such as an excess release of cytokine and inflammatory mediators,Citation4,Citation5 apoptosis,Citation6 and an overaccumulation of neutrophils and macrophages.Citation7 However, currently no mechanism can fully explain all of the problems related to pancreatitis-associated lung injury. Scholars generally recognize that the excess release of cytokines and inflammatory mediators, which affects human pulmonary microvascular endothelial cell (HPMVEC) permeability and causes ALI with the pulmonary edema as the main pathological feature, plays a key role in the pathogenesis of pancreatitis-associated lung injury. In particular, tumor necrosis factor (TNF)-α is one of the important inflammatory mediators.Citation8,Citation9
The protein aquaporin (AQP) was discovered by Peter Agre through isolating and purifying polypeptides in red cell membranes, and it is a specific water channel protein in cells.Citation10,Citation11 Currently, 13 subtypes (AQP0–12) have been discovered in mammals. The main AQPs distributed in lung tissues are AQP-1, AQP-3, AQP-4, and AQP-5, among which the AQP-1 content is relatively high in HPMVECs.Citation12 AQP-1 is involved in the pathogenesis of many lung injuries.Citation13,Citation14 The role of AQP, particularly AQP-1, which is widely distributed in HPMVECs, in SAP-induced lung injury pathogenesis has recently been studied. Studies show that AQP-1 expression in SAP-induced lung injury is negatively correlated with the extent of pulmonary edema.Citation15,Citation16
Alpinetin is the main component of seeds from the Zingiberaceae plant, Alpinia katsumadai Hayata. The molecular structure of alpinetin is 7-hydroxy-5-methoxyflavanone. A. katsumadai Hayata is an aromatic drug used to resolve dampness; it dries dampness, strengthens the spleen, warms the stomach, and prevents vomiting. A. katsumadai Hayata has been used to treat internal retention of cold and wetness, epigastric pain, belching and hiccups, and poor appetite in traditional Chinese medicine. In recent years, studies on alpinetin, which is extracted from A. katsumadai Hayata, show that it exhibits antibacterial and anti-inflammatory effects.Citation17,Citation18 However, few studies have reported on SAP treatment and, particularly, SAP-induced ALI. However, such treatments may have broad clinical implications.
In the present study, we investigated the role of alpinetin in treating SAP-induced ALI in both in vivo and in vitro cell culture. In addition, the detailed mechanism by which alpinetin regulates AQP-1 and TNF-α expression to alleviate ALI was examined to provide guidance for clinical treatment.
Materials and methods
Animals
We used adult male Sprague Dawley rats with weights between 200 and 250 g (provided by Laboratory Animal Centre of Guilin Medical University, Guangxi, People’s Republic of China) and AQP-1 knockout male rats with weights between 200 and 250 g (Cyagen Biosciences, Santa Clara, CA, USA). All rats were raised in specific-pathogen-free conditions. All experimental procedures were approved by the Animal Care and Use Committee of Guilin Medical University.
Antibody and reagents
We used ursodeoxycholic acid sodium salt and alpinetin (Mengry Bio-Technology Co., Ltd., Shanghai, People’s Republic of China); an AQP-1 primer (Invitrogen, Carlsbad, CA, USA); AQP-1 antibody and TNF-α (Santa Cruz Bio-technology Inc., Dallas, TX, USA); lipopolysaccharide (LPS; Sigma-Aldrich Co., St Louis, MO, USA); 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT assay; Sigma-Aldrich); and the Apoptosis Detection Kit (BD Biosciences, San Jose, CA, USA).
Cell culture
We used HPMVECs (American Type Culture Collection, Manassas, VA, USA); Dulbecco’s Modified Eagle’s Medium (DMEM), high glucose, and fetal bovine serum (FBS; Gibco, Gaithersburg, MD, USA); and 0.25% pancreatin (Keygentec, Nanjing, People’s Republic of China).
Animal model and grouping
The rats were divided into a sham group, model group, AQP-1 knockout pancreatitis-associated lung injury group (AQP-1−/−group), dexamethasone group, and alpinetin group (40, 80, 160, and 320 μg/mL); each group included ten rats. We prepared the pancreatitis-associated lung injury models as follows. The rats fasted for 12 hours prior to surgery with free access to water. Chloral hydrate (20 g/L) was injected intraperitoneally for anesthesia. The rats’ hair was shaved at the abdomen, and the surgical skins were sterilized. Under aseptic conditions, a 1 mL small syringe needle was inserted into the sidewall from the duodenum papilla in the abdomen from the anterior medial incision; the needle entered the pancreatic duct from the opening of the pancreatic duct duodenum papilla. Simultaneously, the junction for the bile duct and liver was clamped using a small bulldog clamp, and 15 g/L ursodeoxycholic acid sodium salt (1 mL/kg) was injected in a retrograde manner for 30 seconds. After the model was constructed, 2 mg/kg dexamethasone was injected from the femoral vein for the dexamethasone group. Different doses of alpinetin (40, 80, 160, and 320 μg/mL) were intragastrically administered in the alpinetin group after the model was constructed. The rats in each group were sacrificed at 6, 12, and 24 hours to collect the bilateral lungs and blood from the inferior vena cava. The serum was obtained by centrifuging the venous blood and stored in a −80°C refrigerator.
Cell culture and grouping
HPMVECs were cultured in a DMEM high glucose growth medium with 10% FBS using American Type Culture Collection guidelines. Simultaneously, 100 U/mL of penicillin and 100 U/mL of streptomycin were added to the growth medium. The cell culture dish was placed into a 5% CO2 and 95% air incubator at 37°C. The experiments were performed after the cells reached a logarithmic growth phase. The groups include the normal control group (control), LPS group (1.0 mg/mL), alpinetin group, and LPS + alpinetin group. Different concentrations of alpinetin were used (0.01, 0.1, 1.0, and 10.0 μg/mL) in each group; the application time was 24 hours.
Cell viability assays
At the logarithmic growth phase, HPMVECs from the LPS group were collected, digested with 0.25% pancreatin-ethylene-diaminetetraacetic acid, and washed with phosphate-buffered saline. Next, the DMEM high glucose growth medium containing 10% FBS was used to suspend single cells, which were then inoculated in a 96-well plate (1×104 cells/well) and incubated in a CO2 incubator overnight. The supernatant was assimilated and discarded. Thereafter, different concentrations of alpinetin (0.01, 0.1, 1.0, and 10.0 μg/mL) were added. Blank well plates (only growth medium was added) were prepared as a control. Each concentration group consisted of five repeating wells, which were incubated for 24, 48, and 72 hours. At 4 hours prior to the measurements, the supernatant was discarded. MTT assay (20 μL) (0.5 mg/mL) was added to each well, and the cells were incubated for 4 hours at 37°C in the incubator. After adding dimethyl sulfoxide at 100 μL, a microplate reader was used to measure the optical density values at 490 nm for each well.
Pathologic lung tissue morphology
The right lung tissue was fixed with formaldehyde embedded in paraffin and sectioned into 5 μm thick slices. The slices were then deparaffinized using xylene and washed with different grades of ethanol and water and incubated in xylene (I) for 5 minutes and then in xylene (II) for 5 minutes (with I and II representing the different containers). Thereafter, the samples were sequentially incubated in 100% ethanol for 2 minutes, 95% ethanol for 1 minute, 80% ethanol for 1 minute, and 75% ethanol for 1 minute; finally, the samples were rinsed with distilled water for 2 minutes. The slices were stained with hematoxylin for 5 minutes and washed with tap water. The slices were differentiated using hydrochloric ethanol for 30 seconds, incubated in tap water for 15 minutes or warm water (~50°C) for 5 minutes, and placed in eosin solution for 2 minutes. The slices were then dehydrated, developed, sealed, and examined under a microscope.
Measuring lung water (lung wet-to-dry weight ratio)
The wet weight of the left lung from the sacrificed rats in each group was obtained, and the lung was dried in a 60°C oven for 24 hours to remove the water. Next, the dry weight was obtained to calculate the wet-to-dry weight ratio (W/D).
Reverse transcription polymerase chain reaction measurement
The total RNA was extracted from the right lung and from the corresponding cells. Reverse transcription was performed in accordance with the manufacturer’s instructions and based on the AQP-1 primers, including the upstream primer sequence 5′-GAAGCTCTTCTGGAGGGCTG-3′ and the downstream sequence 5′-GGCTTCATCTCCACCCTGGAG-3′. β-Actin was used as an internal reference to measure the expression level of AQP-1 mRNA with the upstream primer sequence 5′-GATATCGCTGCGCTCGTCGTC-3′ and the downstream sequence 5′-CATGAGGTAGTCTGTCAGGTC-3′. The amplification conditions were as follows: initial denaturation for 5 minutes at 94°C, denaturation for 30 seconds at 98°C, annealing for 30 seconds at 55°C, extension for 1 minute at 72°C, recycling 30 times, extension for 5 minutes at 72°C, and, finally, a decrease to 4°C to terminate the reaction. The polymerase chain reaction reactants were recorded and analyzed using agarose gel electrophoresis and a gel documentation system.
Western blot
The right lung cells were collected to extract the total protein. The bicinchoninic acid assay was used to determine the protein concentration. The protein was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis, and then semidry transferred to polyvinylidene difluoride film and treated with skim milk for 1 hour. The film was washed three times using mixture of Tris-buffered saline and Tween 20, incubated in the first antibody (AQP-1 antibody dilution factor: 1:1,000, TNF-α antibody dilution factor: 1:1,000) at 4°C overnight, washed with mixture of Tris-buffered saline and Tween 20, and incubated with a horseradish oxidase-labeled second antibody at 37°C for 1 hour. The results were filmed and developed.
Statistical analysis
All data are shown as the mean ± standard deviation. All analyses were evaluated using the SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). Analysis of variance was used to analyze the mean value for each group. P<0.05 was considered a significant difference.
Results
The effect of SAP on lung injury
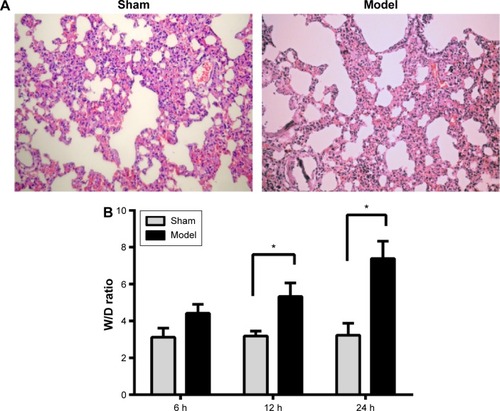
The rats were divided into a sham group and model group and were sacrificed at 6, 12, and 24 hours. Immunohistochemical staining was used to observe pathomorphological changes in the lung tissues. The lung W/D was used to measure the effect of SAP on the lung tissue. Using immunohistochemical staining, we observed injury to the lungs in the model group compared with the sham group. The lung structure in the sham group was clear, no edema fluid and no blood congestion were observed in the interstitial veins, and no invasion neutrophils were observed inside an alveolar cavity. However, we found that the model group of rats with pulmonary interstitial inflammatory cell infiltration was observed to have congestion and hemorrhage, and a little pale red edema fluid was seen in some of the alveolar cavity (). The W/D in the model group was significantly greater compared with that of the sham group (P<0.05), as shown in .
Figure 1 The effect of SAP on lung tissue.
Abbreviations: HE, hematoxylin and eosin; SAP, severe acute pancreatitis; W/D, wet-to-dry weight ratio.
Pancreatitis-associated lung injury can decrease AQP-1 expression
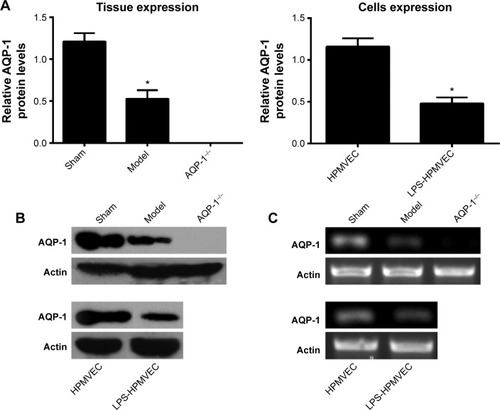
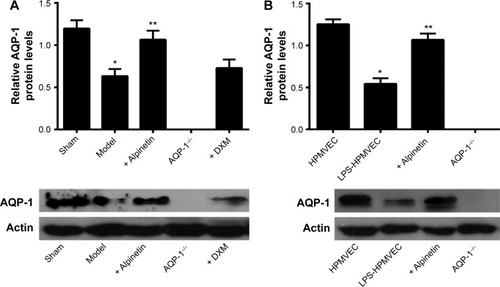
We used reverse transcription polymerase chain reaction and Western blot to measure lung AQP-1 expression in the sham group, model group, and AQP-1−/− group as well as AQP-1 expression in the HPMVEC and LPS-HPMVEC groups. The level of AQP-1 expression was significantly lower in the model group compared with the sham group, but the AQP-1 was almost unexpressed in the AQP-1−/− group (). In the cell experiments, the level of AQP-1 expression was significantly lower in the LPS-HPMVECs compared with the HPMVEC group. These results led us to conclude that AQP-1 expression decreases in pancreatitis-associated lung injury.
Figure 2 The level of AQP-1 expression in the pancreatitis-associated lung injuries measured in the animal models and at the cellular level.
Abbreviations: AQP-1, aquaporin-1; HPMVEC, human pulmonary microvascular endothelial cell; LPS, lipopolysaccharide; RT-PCR, reverse transcription polymerase chain reaction.
Alpinetin can reduce SAP-induced ALI
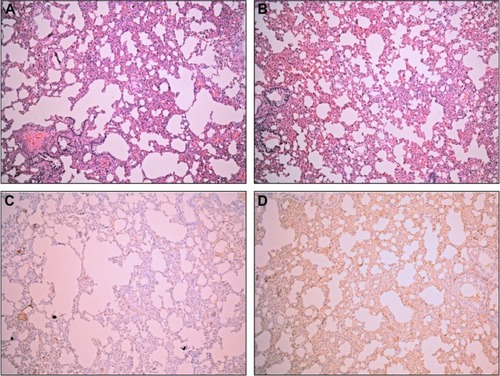
To confirm whether alpinetin can reduce SAP-induced ALI, we divided the rats into a sham group, model group, alpinetin group (40, 80, 160, and 320 μg/mL), dexamethasone group, and AQP-1−/− group, and raised the rats under the same conditions. The rats were sacrificed at 6, 12, and 24 hours. We observed the pathomorphological changes and changes in the lung tissue W/D. Compared with the model group, the lung conditions significantly improved in the alpinetin group through adding 160 μg/mL alpinetin, which showed no significant difference compared with lung tissue in the dexamethasone group. In contrast, compared with the alpinetin group, the lung injury did not improve in the AQP-1−/− group (). As demonstrated by the change in the lung W/D data, the W/D for the alpinetin group was more decreased than in the model group (P<0.05), but did not differ significantly in the sham group and dexamethasone group; however, the W/D was significantly higher in the AQP-1−/− group than the sham group, as shown in . Therefore, alpinetin alleviates SAP-induced ALI, which might be related to AQP-1 expression.
Table 1 The change in the lung W/D (x ± s)
Figure 3 The effect of alpinetin on lung tissue in pancreatitis-associated lung injury.
Abbreviations: AQP-1, aquaporin-1; HE, hematoxylin and eosin.
Alpinetin can promote LPS-HPMVEC proliferation
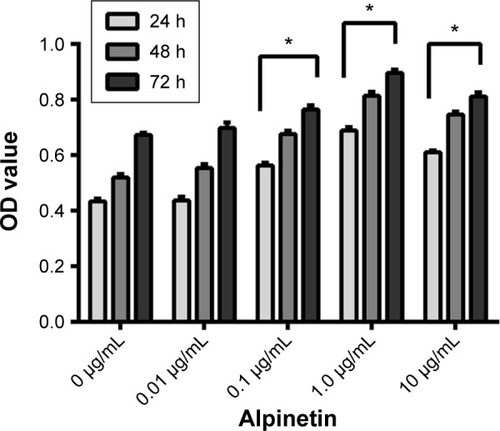
Different alpinetin doses (0.01, 0.1, 1.0, and 10.0 μg/mL) were applied to the LPS-HPMVECs for 24, 48, and 72 hours; the MTT assay was used to measure cell viability. In the range of 0, 0.01, 0.1, and 1.0 μg/mL, alpinetin promoted HPMVEC proliferation in a time- and dose-dependent manner (). With an increase in alpinetin concentration from 0.01 to 10.0 μg/mL, the A490 value in the HPMVECs gradually increased and was most pronounced at 1.0 μg/mL.
Figure 4 Alpinetin can promote the HPMVEC proliferation.
Abbreviations: HPMVEC, human pulmonary microvascular endothelial cell; LPS, lipopolysaccharide; MTT assay, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoli-umbromide; OD, optical density.
Alpinetin can alleviate SAP-induced ALI by upregulating AQP-1 expression
We used reverse transcription polymerase chain reaction and Western blot to measure lung AQP-1 expression in the sham group, model group, alpinetin group (160 μg/mL), dexamethasone group, and AQP-1−/− group as well as AQP-1 expression in the HPMVEC, LPS-HPMVEC, and LPS-HPMVEC cells treated with alpinetin groups. In the animal experiments, the level of AQP-1 expression was significantly lower in the model group compared with the sham group; in contrast, the level of AQP-1 expression increased after the model group was treated with therapeutic doses of alpinetin. However, this effect was not observed in the dexamethasone and AQP-1−/− groups (). In the cell experiments, the level of AQP-1 expression was significantly greater in the LPS-HPMVECs treated with alpinetin compared with the HPMVEC group without treatment. These results indicate that alpinetin can mitigate pancreatitis-associated lung injury by restoring the level of AQP-1 expressed.
Figure 5 The level of AQP-1 expressed in pancreatitis-associated lung injury was measured in the animal models and at the cellular level.
Abbreviations: AQP-1, aquaporin-1; DXM, dextromethorphan; HPMVEC, human pulmonary microvascular endothelial cell; LPS, lipopolysaccharide.
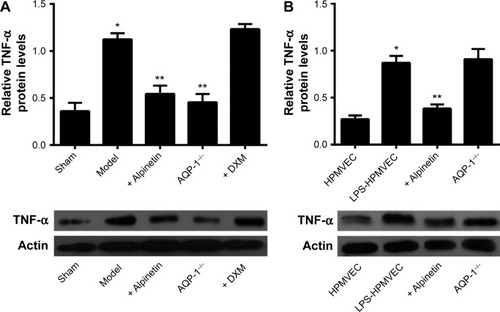
Alpinetin can reduce SAP-induced ALI by decreasing the TNF-α expression
We extracted the total protein from the lung tissue in the sham group, model group, alpinetin group (160 μg/mL), dexamethasone group, and AQP-1−/− group as well as from the HPMVECs, LPS-HPMVECs, and alpinetin-treated LPS-HPMVECs. Western blot was then used to measure the TNF-α factor expression levels. In the animal experiments, the level of TNF-α expressed was significantly greater than in the model group compared with the sham group; in contrast, the level of TNF-α expressed was lower in the model group treated with therapeutic doses of alpinetin and dexamethasone. However, this effect was not observed in the AQP-1−/− group (). In the cell experiments, the level of TNF-α expressed was significantly lower in the alpinetin-treated LPS-HPMVECs compared with the HPMVECs without treatment (). These results indicate that alpinetin can mitigate pancreatitis-associated lung injury through decreasing the level of TNF-α expressed and may be related to the level of AQP-1 expressed.
Figure 6 The level of TNF-α expressed in pancreatitis-associated lung injury was measured in the animal models and at the cellular level.
Abbreviations: AQP-1, aquaporin-1; DXM, dextromethorphan; HPMVEC, human pulmonary microvascular endothelial cell; LPS, lipopolysaccharide; TNF, tumor necrosis factor.
Discussion
SAP is characterized by its acute onset, critical condition, poor prognosis, and high mortality rate; it can cause multiple organ failure in early stages, particularly ALI,Citation19 which can further develop into acute respiratory distress syndrome and result in patient death. The pathogenesis of SAP-induced ALI is complicated and can be caused by many factors. Scholars generally recognize that the excess release of cytokine and inflammatory mediators, which induces changes in HPMVEC permeability and produces ALI with pulmonary edema as the main pathological feature, plays a key role. Among the inflammatory factors involved in this process, TNF-α is a crucial factor that appears in the early stage of SAP and can be used as an early indicator to determine the extent of SAP and prognosis.Citation8 TNF-α can induce a series of cascade reactions in the cell nucleus and at the subcellular level, which induces the secretion of other inflammatory mediators and cytokines, induces inflammation, and exacerbates pancreatitis-associated lung injury. We also found that TNF-α exhibited high levels of expression in lungs with pancreatitis-associated lung injury and was positively correlated with the extent of lung injury.Citation20 We also found that TNF-α expression is greater in pancreatitis-associated lung injury compared with normal lung tissue. Inhibiting TNF-α expression might reduce the extent of pancreatitis-associated lung injury.
Recent studies show that a decrease in AQP in lung tissue also exacerbates the extent of pancreatitis-associated lung injury. AQP proteins are membrane proteins that are closely related to the rapid transport of waterCitation10,Citation11 and play an important role in maintaining water equilibrium in the body, particularly during glycerol metabolism. Four types of AQPs (1, 3, 4, and 5) are distributed throughout the lung tissue, mainly in the membranes of lung capillary endothelial cells, alveolar type I epithelial cells, and airway epithelial cells, and they are involved in the occurrence of many pulmonary edema-related diseases. Among the AQPs, the AQP-1 content was relatively high in the HPMVECs. The molecular biological mechanism of AQP in the transmembrane transport of water and other molecules is the basis for its involvement in regulating water in the lung. Studies show that, compared with normal cellular genotypes, those lacking the AQP-1 gene or AQP-5 gene were associated with a tenfold decrease in the ability to sense a permeating concentration of water transport and bypass the blood–air barrier. Further, a knockout of both genes produced a 30-fold decrease in water permeability,Citation21,Citation22 which suggests that AQP-1 plays an important role in regulating pulmonary edema. We also show low levels of AQP-1 expression in SAP-induced ALI both at the protein and gene levels. This result further confirms that AQP-1 can maintain water equilibrium in the body through the cell membranes, which reduces pulmonary edema and thus mitigates the extent of lung injury. Inflammatory factors play an important role in the pathogenesis of SAP-induced ALI. In particular, the inflammatory factor TNF-α, which appears at the early stage of SAP, exacerbates damage to lung cells. Studies suggest that AQP-1 can decrease the extent of injury in lung cells by downregulating TNF-α; however, further investigation is necessary for a detailed mechanism.
In recent years, compared with nonspecific, immunity-inhibiting steroids, several traditional Chinese medicines have attracted the attention of anti-inflammation and antioxidation clinics due to fewer side effects and lower toxicities. Alpinetin is a flavonoid extracted from A. katsumadai Hayata. It exhibits antibacterial,Citation17,Citation18 antioxidant, anticancer, antithrombotic, antiemetic, and analgesic effects; it also lowers blood pressure, blood lipids, and blood sugar. In addition, alpinetin has an inhibitory effect on LPS-induced inflammationCitation23 and can inhibit the excretion of the inflammatory mediators TNF-α, IL-6, and IL-1β. Many studies show that extracts from traditional Chinese medicines have an inhibitory effect on lung injury-induced inflammation, including through recruiting neutrophils and excreting inflammatory mediators,Citation6,Citation8,Citation16,Citation20 but the specific mechanism is not described in detail. In this study, we also found that alpinetin can inhibit inflammation caused by SAP-induced ALI and simultaneously alleviate the extent of SAP-induced ALI; however, the detailed mechanisms remain unknown. Further, AQP-1 was correlated with ALI to a certain extent; AQP-1 expression decreased in the injured lung tissues but increased in the alpinetin model group; and TNF-α expression as well as pulmonary edema decreased. These results suggest that alpinetin can increase water permeability in the cell membrane by upregulating AQP-1 and reducing pulmonary edema by decreasing the expression of inflammatory mediators.
SAP is a common disease with a complicated pathogenesis and many complications, but ALI is only one of the main reasons for mortality. We treated SAP using alpinetin, which is an extract from traditional Chinese medicine. An exploration of the underlying mechanisms may provide guidance for clinical treatment. Alpinetin has a broad application potential.
Acknowledgments
This research was supported in part by the Pharmaceutical Technology Special Project of the Health Department in Guangxi (GZPT13-45), the Construction Project of Key Laboratory of Molecular Medicine in Liver Damage and Repair, Guangxi (SYS2013009), and Self-financed Projects of the Guangxi Department of Health (Z2013464).
Disclosure
The authors report no conflicts of interest in this work.
References
- HalonenKIPettiläVLeppäniemiAKKemppainenEAPuolakkainenPAHaapiainenRKMultiple organ dysfunction associated with severe acute pancreatitisCrit Care Med20023061274127912072681
- YuanZMeyerholzDKTwaitECKempurajDWilliardDESamuelISystemic inflammation with multiorgan dysfunction is the cause of death in murine ligation-induced acute pancreatitisJ Gastrointest Surg201115101670167821800226
- PastorCMMatthayMAFrossardJLPancreatitis-associated acute lung injury: new insightsChest200312462341235114665518
- HuaiJPSunXCChenMJMelatonin attenuates acute pancreatitis-associated lung injury in rats by modulating interleukin 22World J Gastroenterol201218365122512823049224
- AkbarshahiHRosendahlAHWestergren-ThorssonGAnderssonRAcute lung injury in acute pancreatitis – awaiting the big leapRespir Med201210691199121022749752
- WengTIWuHYChenBLLiuSHHonokiol attenuates the severity of acute pancreatitis and associated lung injury via acceleration of acinar cell apoptosisShock201237547848422258232
- KylänpääLRakonczayZJrO’ReillyDAThe clinical course of acute pancreatitis and the inflammatory mediators that drive itInt J Inflam2012201236068523304633
- ZhangYLiangDDongLAnti-inflammatory effects of novel curcumin analogs in experimental acute lung injuryRespir Res20151614325889862
- MalkaDVasseurSBödekerHTumor necrosis factor alpha triggers antiapoptotic mechanisms in rat pancreatic cells through pancreatitis-associated protein I activationGastroenterology2000119381682810982776
- DenkerBMSmithBLKuhajdaFPAgrePIdentification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubulesJ Biol Chem19882633015634156423049610
- SuXSongYJiangJBaiCThe role of aquaporin-1 (AQP1) expression in a murine model of lipopolysaccharide-induced acute lung injuryRespir Physiol Neurobiol2004142111115351300
- BaiCFukudaNSongYMaTMatthayMAVerkmanASLung fluid transport in aquaporin-1 and aquaporin-4 knockout miceJ Clin Invest1999103455556110021464
- TowneJEHarrodKSKraneCMMenonAGDecreased expression of aquaporin (AQP)1 and AQP5 in mouse lung after acute viral infectionAm J Respir Cell Mol Biol2000221344410615063
- SuXSongYJiangJBaiCThe role of aquaporin-1 (AQP1) expression in a murine model of lipopolysaccharide-induced acute lung injuryRespir Physiol Neurobiol2004142111115351300
- SongYJiangJBaiCThe role of aquaporin-1 (AQP1) expression in a murine model of lipopolysaccharide-induced acute lung injuryRespir Physiol Neurobiol2004142111115351300
- GaoZXuJSunDTraditional Chinese medicine, Qing Ying Tang, ameliorates the severity of acute lung injury induced by severe acute pancreatitis in rats via the upregulation of aquaporin-1Exp Ther Med2014861819182425371738
- MaTLiuZFunctions of aquaporin 1 and α-epithelial Na+ channel in rat acute lung injury induced by acute ischemic kidney injuryInt Urol Nephrol20134541187119623255025
- SalaARecioMCSchinellaGRAssessment of the anti-inflammatory activity and free radical scavenger activity of tilirosideEur J Pharmacol20034611536112568916
- UzelASorkunKOnçağOCogŭluDGençayOSalihBChemical compositions and antimicrobial activities of four different Anatolian propolis samplesMicrobiol Res2005160218919515881836
- FavarinDCde OliveiraJRde OliveiraCJRogerio AdePPotential effects of medicinal plants and secondary metabolites on acute lung injuryBiomed Res Int2013201357647924224172
- ZhangZQSongYLChenZHShenYBaiCXDeletion of aquaporin 5 aggravates acute lung injury induced by Pseudomonas aeruginosaJ Trauma20117151305131121502879
- LvPLiHYJiSSLiWFanLJThalidomide alleviates acute pancreatitis-associated lung injury via down-regulation of NF-κB induced TNF-αPathol Res Pract2014210955856424939146
- HuoMChenNChiGTraditional medicine alpinetin inhibits the inflammatory response in Raw 264.7 cells and mouse modelsInt Immunopharmacol201212124124822178196
