Abstract
5-Methoxyindole-2-carboxylic acid (MICA) is a potent hypoglycemic agent that inhibits gluconeogenesis in the liver. It is also a well-known inhibitor of mitochondrial dihydrolipoamide dehydrogenase. MICA was extensively studied in the 1960s and 1970s and was once tested for its antidiabetic effect in diabetic Chinese hamsters, whereby MICA was shown to exhibit pronounced glucose-lowering ability while also leading to increased rate of death of the diabetic animals. Since then, MICA’s potential ability in lowering blood glucose in diabetes has never been revisited. In my opinion, MICA should be comprehensively reexplored for its antidiabetic properties in a variety of rodent diabetes models. For a given animal model, its dose-dependent effect and the effects of different routes of administrations as well as its synergistic effects with other glucose-lowering drugs should also be investigated. More studies in the future on this chemical may provide novel insights into its role as an antidiabetic agent.
Introduction
Diabetes mellitus, as an insidious disease, has become epidemic and is now a leading health issue globally. This disease is caused by dysregulation of glucose metabolism resulting in persistently high levels of blood glucose that imparts glucotoxicity.Citation1,Citation2 In adult-onset diabetes, also known as type 2 diabetes, development and progression of the disease is initiated by insulin resistance followed by β-cell dysfunction and insulin deficiency.Citation1,Citation3 As type 2 diabetes is a chronic disease that is aging-related, blood glucose control in many patients often requires the use of multipharmacological agents. Hence, there is always a need to develop novel pharmacological agents or chemicals that can be used to help fighting diabetes.
5-methoxyindole-2-carboxylic acid as a hypoglycemic agent
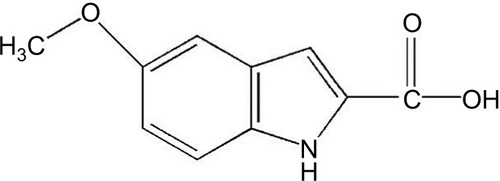
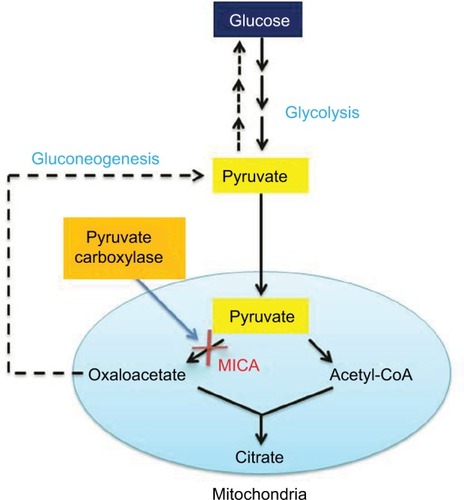
During our studies of mitochondrial dysfunction in stroke injury, we stumbled upon a chemical called 5-methoxyindole-2-carboxylic acid (MICA) (), which exhibits a potential role in neuroprotection against stroke injury when applied as either a preconditioning or postconditioning agent.Citation4,Citation5 MICA is often regarded as a biomarker for malignant melanoma,Citation6 but intriguingly, a quick literature search indicates that MICA is actually also a hypoglycemic agent.Citation7,Citation8 In fact, this chemical, having an indole nucleus core structure that is known to possess antidiabetic properties,Citation9,Citation10 was studied extensively in 1960s and 1970s in terms of its glucose-lowering ability. It was found that MICA could inhibit liver gluconeogenesis by blocking carboxylation of pyruvate,Citation11–Citation14 a key reaction in the gluconeogenic pathway (). The mechanism of this inhibition remains unknown as MICA was found to have no inhibitory effect on purified pyruvate carboxylase when incubated with the enzyme in vitro.Citation14
Figure 2 Diagram showing MICA block of pyruvate conversion to oxaloacetate in the gluconeogenesis pathway.
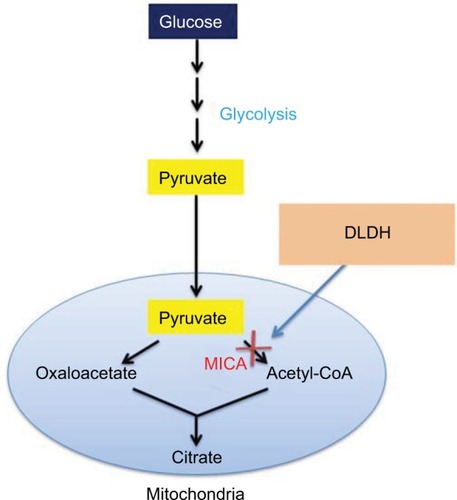
MICA is also able to inhibit aerobic glucose degradation. The mechanism by which this works is that MICA can inhibit mitochondrial dihydrolipoamide dehydrogenase (DLDH),Citation14–Citation16 a component in the pyruvate dehydrogenase complexCitation17,Citation18 (). This inhibition is reversible and can be reversed by lipoic acid,Citation14 a cofactor for DLDH. It should be noted that MICA inhibition of the conversion of pyruvate to acetyl-CoA occurs not only just in the liver but also in many other tissues such as adipose, testis, brain, and kidney.Citation5,Citation12,Citation19,Citation20 The establishment that MICA inhibits DLDH is based on the evidence that the cofactor lipoic acid instead of other cofactors such as thiamine and thiamine pyrophosphate can reverse MICA inhibition of DLDH.Citation14
Figure 3 Diagram showing MICA block of pyruvate oxidation to acetyl-CoA in glucose combustion pathway.
Abbreviations: DLDH, dihydrolipoamide dehydrogenase; MICA, 5-methoxyindole-2-carboxylic acid.
While the concept that MICA can exert its hypoglycemic effect via inhibition of the gluconeogenic pathway is straightforward, whether MICA can decrease blood glucose via inhibition of DLDH remains to be evaluated. Conceptually, when MICA blocks oxidation of pyruvate to acetyl-CoA, pyruvate would accumulate and more glucose would be consumed for ATP production by the glycolytic pathway in order to meet the body’s energy needs. This would be similar to one of the mechanisms by which metformin increases glucose consumption by the glycolytic pathway via inhibiting mitochondrial complex I, leading to lowering of blood glucose.Citation21 Therefore, MICA may also be able to lower blood glucose by inhibiting DLDH in diabetic subjects.
MICA’s metabolic effects and mortality rate in one diabetic animal model
The study of MICA’s hypoglycemic effect in the 1970s perhaps culminated by a short report that diabetic Chinese hamsters, when treated by MICA (100 mg/kg body weight, via gavage) for approximately a week, showed a depressed blood glucose levels but also died at a greater rate than did control animals.Citation22 The conclusion of this study was that while MICA could lower blood glucose in diabetic Chinese hamsters, it might not be a good antidiabetic agent because it increased the death rate of the treated diabetic animals. Since then, the property of MICA as a hypoglycemic agent has never been evaluated again, and most studies involving the use of MICA have been focused on its function as a DLDH inhibitor.Citation20,Citation23,Citation24 It should be pointed out that in this short and limited study that tested the hypoglycemic effect of MICA on diabetic Chinese hamsters, only one dosage and one means of MICA administration (via gavage) were tested.Citation22 It should also be noted that this single study was very limited in scope and probably also dampened any further interest in studying MICA as a therapeutic drug for diabetes. In my opinion, such a single study is not enough to draw a conclusion that MICA is not a useful tool for managing diabetes. In particular, we and others have demonstrated that different administration routes could be important to minimize MICA’s toxic effects and get beneficial results.Citation4,Citation5,Citation7,Citation8
Perspectives
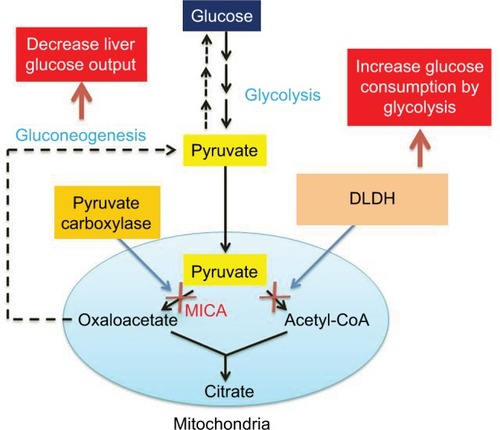
Based on the above discussions that MICA is a potent inhibitor of the gluconeogenic pathway in the liver and is also a robust inhibitor of mitochondrial DLDH (as summarized in ), I think MICA should be further tested in many other rodent models of diabetes for its potential ability in lowering blood glucose. For a given diabetes model, the hypoglycemic effect of MICA and its toxic effect should be tested with different dosages in combination with different routes of administration. It may also be tested at different dosages with well-known antidiabetic drugs such as metformin and acarbose for its potential synergistic effect. In this regard, its tissue distribution and whether or how it is metabolized in the body should also be investigated.
Figure 4 Scheme combined from showing the potential mechanisms by which MICA lowers blood glucose.
Abbreviations: DLDH, dihydrolipoamide dehydrogenase; MICA, 5-methoxyindole-2-carboxylic acid.
Additionally, as MICA targets nearly all tissues in the body and has a variety of effects on protein expression and cellular pathways in numerous experimental settings or disease models,Citation4,Citation5,Citation12,Citation15,Citation20,Citation25–Citation27 its potential effect on pancreatic β-cell function and insulin secretion in the context of diabetes should also be investigated. Moreover, a comprehensive evaluation of MICA on glucose metabolism, in particular, gluconeogenesis in cultured hepatocytes should also be performed.
Finally, it should be noted that in archaebacterium Haloferax volcanii that lacks lipoic acid in its less complex pyruvate dehydrogenase complex,Citation28 MICA is a potent inhibitor of the bacterial potassium ion transport and respiration,Citation29 suggesting that MICA has other biological targets that could bear the toxic brunt of MICA while yielding overall hypoglycemic effects. In fact, in animal models, MICA has been shown to be able to target d-amino acid oxidase, though the inhibitory efficacy is very low.Citation30 Therefore, it would not be surprising if MICA is found to target other proteins, which may also be implicated for its hypoglycemic function.
Conclusion
MICA, as a potential hypoglycemic agent, should be comprehensively reevaluated using a variety of diabetic animal models. In particular, its effect on mitochondrial bioenergetics, its pharmacokinetics and pharmacodynamics, its adverse effects, and its mechanisms of action on lowering blood glucose should all be studied. Moreover, its synergistic effect, if any, with other antidiabetic drugs, should also be investigated. Additionally, derivatives of MICA exhibiting decreased adverse effects may also be explored. MICA may well be a promising agent for fighting diabetes until proven otherwise.
Acknowledgments
This publication was supported in part by the National Institute of Neurological Disorders and Stroke (Grant number: R01NS079792) and by UNTHSC intramural grants RI10015 and RI10039.
Disclosure
The author reports no conflicts of interest in this work.
References
- Barnett AH Type 2 Diabetes 2nd ed Oxford, UK Oxford University Press 2012
- Yan LJ Pathogenesis of chronic hyperglycemia: from reductive stress to oxidative stress J Diabetes Res 2014 2014 137919 25019091
- Luo X Wu J Jing S Yan LJ Hyperglycemic stress and carbon stress in diabetic glucotoxicity Aging Dis 2016 7 1 90 110 26816666
- Wu J Jin Z Yang X Yan LJ Post-ischemic administration of 5-methoxyindole-2-carboxylic acid at the onset of reperfusion affords neuroprotection against stroke injury by preserving mitochondrial function and attenuating oxidative stress Biochem Biophys Res Commun 2018 497 1 444 450 29448100
- Wu J Li R Li W Administration of 5-methoxyindole-2-carboxylic acid that potentially targets mitochondrial dihydrolipoamide dehydrogenase confers cerebral preconditioning against ischemic stroke injury Free Radic Biol Med 2017 113 244 254 29017857
- Karnell R von Schoultz E Hansson LO Nilsson B Arstrand K Kagedal B S100B protein, 5-S-cysteinyldopa and 6-hydroxy-5-methoxyindole-2-carboxylic acid as biochemical markers for survival prognosis in patients with malignant melanoma Melanoma Res 1997 7 5 393 399 9429222
- Hanson RL Ray PD Walter P Lardy HA Mode of action of hypoglycemic agents. I. Inhibition of gluconeogenesis by quinaldic acid and 5-methoxyindole-2-carboxylic acid J Biol Chem 1969 244 16 4351 4359 5806581
- Reed J Lardy HA Mode of action of hypoglycemic agents. 3. Studies on 5-methoxy indole-2-carboxylic acid and quinaldic acid J Biol Chem 1970 245 20 5297 5303 5469167
- Li YY Wu HS Tang L The potential insulin sensitizing and glucose lowering effects of a novel indole derivative in vitro and in vivo Pharmacol Res 2007 56 4 335 343 17889553
- Sharma V Kumar P Pathak D Biological importance of the indole nucleus in recent years: a comprehensive review J Heterocycl Chem 2010 47 491 502
- Daligcon BC Oyama J Hannak K Increased gluconeogenesis in rats exposed to hyper-G stress Life Sci 1985 37 3 235 241 4010477
- Garcia-Salguero L Aranda F Peragon J Corpas FJ Lupianez JA Metabolic adaptation of renal carbohydrate metabolism. IV. The use of site-specific liver gluconeogenesis inhibitors to ascertain the role of renal gluconeogenesis Arch Int Physiol Biochim Biophys 1991 99 3 237 242 1717058
- Bauman N Pease BS Effects of 5-methoxyindole-2-carboxylic acid on carbohydrate metabolism Biochem Pharmacol 1969 18 5 1093 1101 5789776
- Bauman N Hill CJ Inhibition of gluconeogenesis and α-keto oxidation by 5-methoxyindole-2-carboxylic acid Biochemistry 1968 7 4 1322 1327 4878041
- Haramaki N Han D Handelman GJ Tritschler HJ Packer L Cytosolic and mitochondrial systems for NADH- and NADPH-dependent reduction of α-lipoic acid Free Radic Biol Med 1997 22 3 535 542 8981046
- Miller JA Runkle SA Tjalkens RB Philbert MA 1,3-Dinitrobenzene induced metabolic impairment through selective inactivation of the pyruvate dehydrogenase complex Toxicol Sci 2011 122 2 502 511 21551353
- Williams CHJr Lipoamide dehydrogenase, glutathione reductase, thioredoxin reductase, and mercuric ion reductase-a family of flavoenzyme transhydrogenases Muller F Chemistry and Biochemistry of Flavoenzymes III Boca Raton, FL, USA CRC Press 1992 121 212
- Yan LJ Yang SH Shu H Prokai L Forster MJ Histochemical staining and quantification of dihydrolipoamide dehydrogenase diaphorase activity using blue native PAGE Electrophoresis 2007 28 7 1036 1045 17315258
- Gorin E Zendowski S Effect of methoxyindole 2-carboxylic acid and 4-pentenoic acid on adipose tissue metabolism Biochim Biophys Acta 1975 388 2 268 278 1138899
- Siva AB Panneerdoss S Sailasree P Singh DK Kameshwari DB Shivaji S Inhibiting sperm pyruvate dehydrogenase complex and its E3 subunit, dihydrolipoamide dehydrogenase affects fertilization in Syrian hamsters PLoS One 2014 9 5 e97916 24852961
- Andrzejewski S Gravel SP Pollak M St-Pierre J Metformin directly acts on mitochondria to alter cellular bioenergetics Cancer Metab 2014 2 12 25184038
- Schillinger E Loge O Metabolic effects and mortality rate in diabetic Chinese hamsters after long-term treatment with 5-methoxyindole-2-carboxylic acid (MICA) Arzneimittelforschung 1976 26 4 554 556 989012
- Kumar V Kota V Shivaji S Hamster sperm capacitation: role of pyruvate dehydrogenase A and dihydrolipoamide dehydrogenase Biol Reprod 2008 79 2 190 199 18401010
- Yan LJ Thangthaeng N Sumien N Forster MJ Serum dihydrolipoamide dehydrogenase is a labile enzyme J Biochem Pharmacol Res 2013 1 1 30 42 23646291
- Richarme G el Yaagoubi A Kohiyama M The MglA component of the binding protein-dependent galactose transport system of Salmonella typhimurium is a galactose-stimulated ATPase J Biol Chem 1993 268 13 9473 9477 8387496
- Sun Z Park SY Hwang E Thymus vulgaris alleviates UVB irradiation induced skin damage via inhibition of MAPK/AP-1 and activation of Nrf2-ARE antioxidant system J Cell Mol Med 2017 21 2 336 348 27641753
- Aomine M Effects of 5-methoxyindole-2-carboxylic acid on growth, glucose uptake and glycogen content in Tetrahymena pyriformis GL Int J Biochem 1977 8 1 27 32
- Kerscher L Oesterhelt D Pyruvate: ferredoxin oxidoreductase – new findings on an ancient enzyme Trends Biochem Sci 1982 7 10 371 374
- Meury J 5-Methoxyindole-2-carboxylic acid is a potent inhibitor of respiration and potassium ion transport in the archaebacterium Haloferax volcanni FEMS Micobiol Lett 1993 108 3 271 274
- Heefner DL Currie MG Rossi RF Zepp CM inventors Sepracor Inc., assignee D-amino acid oxidase inhibitors for learning and memory United States patent US 20030162825A1 2003