Abstract
Background
To investigate hypoglycemic activity and elucidate the active composition of the fruit blueberry (Vaccinium corymbosum).
Methods
Methanol extracts of blueberry (MEB) were separated using a D101 macroporous resin column to yield quinic acid derivative (Fr.1)- and flavonoid (Fr.2)-rich fractions. The effects of the blueberry extracts on mRNA expression of GLUT-2 (glucose transporter type 2) and PPARγ (peroxisome proliferator-activated receptor-γ), as well as on the activities of PPRE (peroxisome proliferator response element) and NF-κB were analyzed in LO2 normal liver cells. Real-time PCR was used to detect the expression of GLUT-2, PPARγ, TNF-α, IL-1β, and IL-6 mRNA. The PPRE and NF-κB activities were detected by a luciferase reporter assay. Western blotting was used to detect the levels of PPARγ, GLUT-2, and p65. The active compositions were isolated using various chromatography columns, and were analyzed by NMR.
Results
mRNA and protein expression of GLUT-2 and PPARγ were significantly increased upon treatment with 400 μg/mL extracts of blueberry (P<0.05). The PPRE activity was also significantly increased in a dose-dependent manner upon administration of MEB (P<0.05). Furthermore, the NF-κB activity induced by lipopolysaccharides was inhibited by MEB (P<0.05). No fraction separated from MEB exhibited PPRE activation or NF-κB inhibition activity. Blueberry extract may execute its hypoglycemic activity by stimulating expression of GLUT-2 and PPARγ, and by inhibiting the inflammatory pathway. Together, quinic acid derivatives and flavonoids may result in a synergistic effect. Fourteen phenolic acids, including eight flavonoids, four quinic acid derivatives, and two other phenolic acids, were isolated and identified, and caffeoylquinic acid derivatives and quercetin glycosides were found to be the major constituents of blueberry.
Conclusion
Blueberry may have hypoglycemic activity that functions through synergistic effects with caffeoylquinic acid derivatives and quercetin glycosides.
Introduction
Diabetes is a type of endocrine and metabolic disease caused by an absolute deficiency (type I) or relative deficiency (type II, insulin resistance) of insulin secretion, which is characterized by hyperglycemia, as well as complications, such as chronic impairment of the eyes, kidney, heart, blood vessels, and nervous system.Citation1 Currently, diabetes is one of the most harmful diseases to human health, and the number of diabetes patients in China has reached more than 150 million.Citation2 Patients with type II diabetes account for more than 90% of the total number of diabetes patients, and the incidence of diabetes is associated with many factors, including changes in dietary patterns, unhealthy lifestyles, reduction of physical activity, increase in mental stress, environmental pollution, smoking, lack of public awareness and self-awareness, and population aging.Citation3 The main target drugs for the treatment of type II diabetes include insulin secretagogues (sulfonylureas, glibenclamides), insulin sensitizers (thiazolidinediones, biguanides), amylin analogs (Planklin), α-glucosidase and amylase inhibitors (acarbose), DPP-VI inhibitors, glucagon-like peptide-1 (GLP-1), sodium-glucose cotransporter-2 (SGLT-2) inhibitors, protein tyrosine phosphatase 1B (PTP-1B) inhibitors, and peroxisome proliferator-activated receptor-γ (PPARγ) agonists, among others.Citation4–Citation9 Glucose transporter 2 (GLUT-2) is a membrane protein that mediates glucose transport. Hepatic cell expression of GLUT-2 not only plays an important role in glucose metabolism, but also has a close association with diabetes or its associated complications. In the diabetic rat model, glucose-impaired insulin secretion has been proven to be associated with the reduction or inhibition of GLUT-2 expression by islet b cells, which plays an important role in the development of diabetes.Citation10 Recent studies have shown that inhibition of CB1R can downregulate GLUT-2 expression and reduce glucose reabsorption, which may support the rationale for clinical testing of peripherally restricted CB1R antagonists or the development of novel renal-specific GLUT-2 inhibitors against diabetic nephropathy.Citation11 PPARγ is a nuclear receptor that is mainly expressed in the large intestine, adipose tissue, and liver. PPARγ binds to PPRE (peroxisome proliferator response element), which has a variety of biological effects and plays an important role in adipocyte differentiation, glucose and lipid metabolism, insulin resistance, and the inflammatory response. PPARγ is also the effective target of thiazolidinedione (TZD) hypoglycemic drugs for the treatment of type II diabetes. PPARγ expression is down-regulated in liver tissues in type II diabetic db/db mice, and amelioration of hyperlipidemia and hyperglycemia are related to the up-regulation of PPARγ expression.Citation12 The transcription factor nuclear factor (NF-κB) mediates inflammation and stress signals. Numerous studies have indicated that there is a close relationship between NF-κB signaling and diabetes. Inhibition of the NF-κB cell signaling pathway improves insulin sensitivity in the liver.Citation13 Suppression of the NF-κB signaling pathway prevents diabetic liver injury in type II diabetic rat models.Citation14
Blueberry belongs to the genus Vaccinium of Ericaceae. Vaccinium are perennial shrub fruit trees that originated in North America. There are more than 400 species of Vaccinium all over the world. Blueberries are mainly grown in the US, and are also known as American blueberries. Wild blueberries in China are mostly grown on Changbai Mountain, the Da Hinggan Mountains, and in the forest area of the Xiao Hinggan Mountains, most of which are in the Da Hinggan Mountain region. Previous research has shown that blueberries contain abundant nutritional elements, such as anthocyanins, organic acids, phenolic acids, superoxide dismutase (SOD), pectin, polysaccharides, and pterostilbene, among others.Citation15–Citation18 Blueberry is one of the five healthy fruits recommended by the Food and Agriculture Organization of the United Nations (FAO), because dietary consumption of blueberries has been demonstrated to be very beneficial to human health and provides effective protection against diseases, including lowering blood pressure, inhibiting tumorigenesis, and potentially preventing neurodegenerative disease.Citation19–Citation21 These effects play an important role in the treatment of diseases such as diabetes, liver disease, cancer, cardiovascular disease, and anemia, among others.Citation22,Citation23 A previous report showed that blueberry extract has good hypoglycemic activity. The study demonstrated that anthocyanins from blueberry have the potency to alleviate symptoms of hyperglycemia using a diabetic mice model;Citation24 however, its effective mechanism is not clear. To investigate the underlying mechanism of blueberry extract in decreasing the blood glucose level, the intent of the present research was to investigate the effect of blueberry extract on GLUT-2 and PPARγ mRNA expression, as well as on PPRE and NF-κB activity in liver cells, and to identify the chemical composition of the main active components by means of separation using various chromatography columns to clarify the hypoglycemic mechanism of blueberry.
Materials and methods
General experimental procedures
1H and 13C nuclear magnetic resonance (NMR) data were recorded on a Varian 500 MHz instrument (Varian Inc., Palo Alto, USA) with TMS as the internal standard. Electrospray ionization mass spectral (ESI-MS) data were acquired on a Q-Star Elite mass spectrometer (Applied Biosystems MDS, Waltham, MA, USA). The UV spectra were measured on a SHIMADZU UV-2450 UV-visible spectrophotometer (Shimadzu Corporation, Kyoto, Japan). High-performance liquid chromatography (HPLC) was performed on a Hitachi Elite LaChrom system (Elite Lachrom Hitachi, Japan) consisting of a L2130 pump, L-2200 autosampler, and L-2455 diode array detector, all of which were operated by EZChrom Elite software (Scientific Software, Agilent Technologies, Santa Clara, USA). All solvents were of either analytical or HPLC grade and were purchased from Wilkem Scientific (Thermo Fisher Scientific, Shanghai, China).
Cell culture
Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco (Grand Island, NY, USA). Human non-tumor hepatic LO2 cells were purchased from the Chinese Academy of Sciences (Shanghai, China). Cells were maintained in DMEM supplemented with 10% FBS and incubated in a humidified incubator at 37°C in 5% CO2.
Extraction and isolation
The fruits of the blueberry species (Vaccinum corymbosum) were collected locally from Xinqizhou blueberry farms (Nanchang, Jiangxi, China) in June 2015. Fresh fruits (10.0 kg, fresh weight) were extracted exhaustively with MeOH (3×20 L) at room temperature to yield MeOH extracts. A part of the extracts (50 g) was dissolved in distilled deionized water (250 mL), filtered, and then loaded onto a D101 macroporous resin column (5×60 cm).Citation25 A 5-fold column volume of water was first used to wash the polysaccharide and protein materials. Next, a 5-fold column volume of 30% ethanol was used to wash the quinic acid derivatives (Fr.1), and 70% ethanol was further used to wash the flavonoid (Fr.2) constituents.
Fr.1 (15 g) was chromatographed on a C18 MPLC column (3.5×40 cm) and eluted with a gradient system of MeOH/H2O (1:9 to 8:2, v/v) to afford five sub-fractions (A1–A5), which were combined based on analytical HPLC analyses. Fraction A2 was separated using a Sephadex LH-20 column (3.5×120 cm), eluted with MeOH, further separated by semi-preparative HPLC, and eluted with a gradient system of MeOH/H2O to yield compounds 9–12. Fraction A4 was separated by semi-preparative HPLC and eluted with a gradient system of MeOH/H2O to yield compounds 13 and 14.
Fr.2 (11 g) was separated using a Sephadex LH-20 column (3.5×120 cm) and eluted with MeOH to afford three sub-fractions (B1–B3). Fraction B2 was separated by a C18 MPLC column (3.5×40 cm) and eluted with a gradient system of MeOH/H2O (2:8 to 8:2, v/v) to afford five sub-fractions (C1–C5), which were combined based on analytical HPLC analyses. Sub-fraction C2 was separated by semi-preparative HPLC and eluted with MeOH/H2O to yield compound 6. Sub-fraction C3 was separated by semi-preparative HPLC and eluted with a gradient system of MeOH/H2O to yield compounds 1–4. Sub-fraction C4 was separated by semi-preparative HPLC and eluted with a gradient system of MeOH/H2O to yield compounds 5, 7, and 8.
Luciferase reporter assays
For detecting PPRE activity, LO2 cells were co-transfected with p-PPRE-luc and pSV40-β-galactosidase.Citation26 After 6 h, the cells were subjected to different fractions of blueberry extracts for 24 h and then harvested to measure the luciferase activity. For detection of the NF-κB activity, LO2 cells were co-transfected with p-NF-κB-luc and pSV40-β-galactosidase. After 6 h, the cells were subjected to lipopolysaccharides or different fractions of blueberry extracts for 24 h and then harvested for measurement of the luciferase activity.
Quantitative real-time RT-PCR analyses
Total RNA was isolated from hepatocytes using TRIzol reagent according to the manufacturer’s instructions (Invitrogen Life Technology, Carlsbad, CA, USA). Total RNA was reverse-transcribed to cDNA using ReverTraAce (TOYOBO, Tokyo, Japan), as instructed. Quantitative real-time PCR was performed by standard methods using species-specific primer pairs (). The 18S rRNA expression levels were amplified and used for the calibration of real-time RT-PCR.
Table 1 Primers used for quantitative real-time PCR
Western blotting
LO2 cells were washed in ice cold saline, collected, and lysed with a lysis buffer contained protease inhibitors cocktail (Roche Diagnostics, Neuilly SurSeine, France). The total protein was extracted by NE-PER (Pierce Biotechnology, Rockford, IL, USA), and the concentration of protein was quantified by bichinconinic acid (BCA) protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the protocols of manufacturer. Samples (40 μg proteins per lane) were boiled for 100°C for 5 min and loaded on 8%–12% SDS–polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins of each lane representing one mouse were transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, MA, USA) and blocked in 5% nonfat milk solution for 1 h at room temperature with gentle shaking. Membranes were incubated with primary antibodies for GAPDH, GLUT-2, PPARγ, and NF-κB p65 (Abcam, MA, USA), diluted in 5% nonfat milk/TBST at 4°C overnight. Subsequently, each membrane was washed with TBST three times following incubation with horseradish peroxidase-conjugated secondary antibodies (rabbits and mouse) for 1 h at room temperature. Finally, specific bands of the membrane were performed using chemiluminescence (ECL) reagents (Millipore) and detected by Fluorescent Image Analyzer (FUJIFILM Corp, Tokyo, Japan).
Statistical analyses
All statistical analyses were performed with GraphPad Prism software. Values are expressed as the mean±SEM. Pairwise comparisons were performed with Student’s t-test (two-tailed), and multiple-group comparisons were performed with one-way ANOVA with Bonferroni’s post-hoc test. A P-value<0.05 was considered to be significant.
Results
Effects of blueberry extracts on PPRE activity
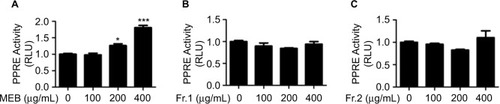
Quinic acid derivatives and flavonoid glycosides were two major classes of the chemical constituents of blueberry. Methanol extracts of blueberry (MEB) were separated using a D101 macroporous resin column. A 5-fold column volume of water was used to wash the polysaccharide and protein materials. Next, a 5-fold column volume of 30% ethanol was used to wash the quinic acid derivatives (Fr.1), and 70% ethanol was further used to wash the flavonoids (Fr.2). The UV spectra of Fr.1 and Fr.2 were typical of quinic acid derivatives and flavonol, respectively. PPRE activity in liver cells has been linked to beneficial hypoglycemic effects. To determine the potential hypoglycemic activities of blueberry extracts, the effects of blueberry extracts on PPRE activity were determined by luciferase reporter assays. Three extracts (MEB, Fr.1, and Fr.2) from blueberry were used to treat human non-tumor hepatic LO2 cells. As shown in , MEB treatment (200 and 400 μg/mL) stimulated PPRE activity by 1.3- and 1.8-fold compared with the control. As indicated, there was no significant difference in the PPRE activity upon administration of extracts Fr.1 and Fr.2 of blueberry (). These results indicate that MEB may affect the hypoglycemic activity of blueberry through the synergistic effects of quinic acid derivatives and flavonols.
Figure 1 Effects of blueberry extract on PPRE activity. (A) Effects of MEB on PPRE activity. (B) Effects of extract Fr.1 of blueberry on PPRE activity. (C) Effects of extract Fr.2 of blueberry on PPRE activity. LO 2 cells were co-transfected with p-PPRE-luc and pSV40-β-galactosidase. After 6 h, the cells were subjected to different extracts of blueberry for 24 h and then harvested for measurement of the luciferase activity. All of the results are presented as the means±SD of three independent experiments (n=3). *p<0.05; ***p<0.001.
Effects of blueberry extracts on NF-κB activity
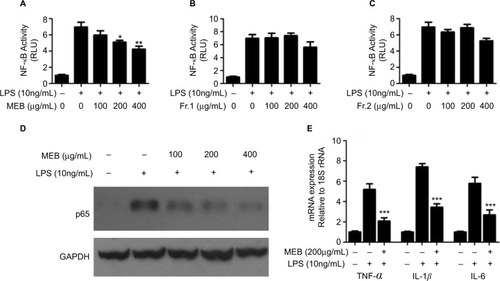
Taking into ccount the importance of the NF-κB signaling pathway in diabetic liver injury and insulin resistance,Citation27 we evaluated the effects of blueberry extracts on NF-κB activity using luciferase reporter assays. In consistent with the results of previous reports, LPS (10 ng/mL) activated the reporter gene by 6.9-fold compared with the control.Citation28 MEB (100, 200, and 400 μg/mL) markedly repressed the NF-κB-driven luciferase gene by 15%, 27%, and 40% (), respectively, compared with the levels in LPS-stimulated cells. However, extracts Fr.1 and Fr.2 of blueberry had no inhibitory effect on LPS-increased NF-κB activity (). To further explore the influence of MEB on the major inflammatory signaling pathway of NF-κB, p65 was then examined by Western blotting (). LPS increased protein levels of p65 compared to the control cell, while MEB inverted these variations (). Moreover, to test whether MEB suppressed downstream inflammatory cytokines of the pathway in mRNA, RT-qPCR was utilized to detect the expression of TNF-α, IL-1β, and IL-6 following LPS treatment (). Interestingly, MEB did produce a marked change with a significant decrease in these inflammation mediators when compared to LPS treated cells. These results confirmed that MEB are effective components of blueberry with regards to its hypoglycemic activity.
Figure 2 Effects of blueberry extracts on NF-κB activity. (A) Effects of MEB on NF-κB activity. (B) Effects of extract Fr.1 of blueberry on NF-κB activity. (C) Effects of extract Fr.2 of blueberry on NF-κB activity. LO2 cells were co-transfected with p-NF-κB-luc and pSV40-β-galactosidase. After 6 h, the cells were subjected to LSP (10 ng/mL) and/or different extracts of blueberry for 24 h, and then harvested for measurement of the luciferase activity. (D) Effects of MEB on p65 expression. (E) Effects of MEB on mRNA expression of TNF-a, IL-1β, and IL-6. The LO2 cells were subjected to LPS (10 ng/mL) and/or different extracts of blueberry for 24 h and then harvested for Western blotting and quantitative real-time RT-PCR. All results are presented as the means±SD of three independent experiments (n=3). *p<0.05; **p<0.01; ***p<0.001.
Effects of MEB on the expression of GLUT-2 and PPARγ mRNA and protein
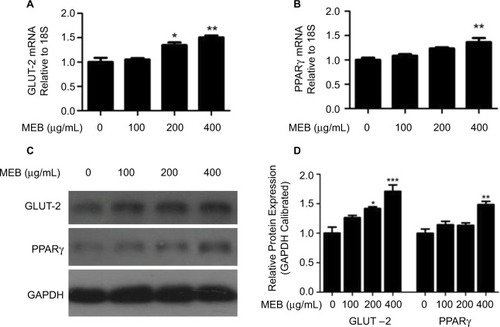
To further detect the hypoglycemic activity of MEB, mRNA expression of GLUT-2 and PPARγ were measured in LO2 cells under treatment with MEB. We found that MEB induced GLUT-2 and PPARγ mRNA expression. Blueberry extract at a dose of 400 μg/mL enhanced GLUT-2 and PPARγ mRNA expression by 1.5- and 1.4-fold (), respectively, compared with the control. The effects of MEB on the mRNA expression of GLUT-2 and PPARγ were confirmed by the Western blotting results in .
Figure 3 Effects of MEB on the mRNA expression of GLUT-2 (A), PPARγ (B), and protein expression of GLUT-2, PPARg (C and D). LO2 cells were maintained in DMEM supplemented with 10% FBS and incubated in a humidified incubator at 37°C in 5% CO2 for 24 h. Then, the cells were further incubated with MEB (100, 200, and 400 μg/mL) for another 24 h. Cells were then collected for quantitative real-time RT-PCR and Western blotting. All of the results are presented as the means±SD of three independent experiments (n=3). *p<0.05; **p<0.01; ***p<0.001.
Chemical compositions analysis of blueberry extracts
To elucidate the chemical compositions of the blueberry extracts, MEB were first fractionated using a D101 macroporous resin column to yield the Fr.1 and Fr.2 fractions. The two fractions were further purified using various chromatographic columns, including a Sephadex LH-20, octadecylsilane, and semi-preparative HPLC, to yield pure compounds. The isolated compounds were characterized by 1H-unclear magnetic resonance (1H-NMR), 13C-NMR, and ESI-MS. Fourteen phenolic acids, including eight flavonoids, four quinic acid derivatives, and two other phenolic acids, were isolated, and their structures were identified as isoquercetin (1),Citation29 hyperin (2),Citation29 guajavarin (3),Citation29,Citation30 quercitrin (4),Citation29 astragalin (5),Citation31 isomyricitrin (6),Citation32 helichrysoside (7),Citation33 tiliroside (8),Citation33,Citation34 5-O-caffeoylquinic acid (9),Citation31,Citation35 3-O-caffeoylquinic acid (10),Citation35 5-O-caffeoylquinic acid methyl ester (11),Citation33 3-O-caffeoylquinic acid methyl ester (12),Citation35 methyl cinnamate (13),Citation33 and methyl caffeate (14).Citation33
Compounds 1–4 were obtained as yellowish amorphous powders. The UV spectrum showed a λmax at approximately 255 nm (band II) and 355 nm (band I). The 1H-NMR spectrum () showed two meta proton peaks at approximately δ 6.10 (1H, d, J =2.0 Hz) and 6.30 (1H, d, J =2.0 Hz) ppm, consistent with the H-6 and H-8 on A-ring of flavonoid, and an ABX system at approximately 7.25–7.74 (1H, d, J=2.2 Hz, H-2′), 7.20–7.48 (1H, dd, J=2.2 Hz, 8.4 Hz, H-6′), and 6.78 (1H, d, J=8.4 Hz, H-5′), corresponding to the catechol protons on the B-ring. One anomeric proton signal was found at [1: δ 5.15(1H, d, J=7.6), 2: δ 5.07(1H, d, J=7.6), 3: δ 5.07(1H, d, J=6.6), 4: δ 5.27(1H, d, J=1.1)]. These signals showed that compounds 1–4 contained the same aglycone of quercetin. Additionally, one methyl signal at δ 0.87(3H, d, J=6.1) of compound 4 was found, which can be easily identified as quercitrin (4). Through careful comparison of the 13C-NMR data of the sugar moiety carbon signals of compounds 1−3 at [1: 102.8(C-1″), 74.3(C-2″), 76.7(C-3″), 69.8(C-4″), 77.0(C-5″), 61.1(C-6″). 2: 103.9(C-1″), 71.7(C-2″), 73.7(C-3″), 68.6(C-4″), 75.7(C-5″), 60.5(C-6″). 3: 102.3(C-1″), 71.4(C-2″), 72.7(C-3″), 67.7(C-4″), 65.6(C-5″)], the compounds were identified as isoquercetin (1), hyperin (2), and guajavarin (3), respectively. Compounds 5 and 6 showed the same sugar moiety proton signals as those of compound 1 in the 1H-NMR and 13C-NMR data, although the B-rings were different. An AA’BB’ system was found at 7.97 (2H, d, J=8.8 Hz, H-2′, 6′) and 6.84 (2H, d, J=7.8 Hz, H-3′, 5′) of compound 5 and at 7.30 (2H, s, H-2′, 6′) of compound 6; therefore, compounds 5 and 6 were identified as astragalin (5) and isomyricitrin (6).
Table 2 1H-NMR (500 MHz, CD3OD) characteristics of flavonol glycosides 1–6 isolated from blueberry extracts
Compound 7 showed the same aglycone as seen in compounds 1–4. The UV spectrum showed λmax values at 259 nm (band II) and 314 nm (band I), typical of the UV spectrum of the coumaroyl substituent quercetin glycoside.Citation36 1H-NMR data confirmed this speculation, showing signals of quercetin glycoside signals [δ 7.60 (1H, d, J=2.0 Hz, H-2′), 7.55 (1H, dd, J=8.4, 2.0 Hz, H-6′), 6.81 (1H, d, J=8.4 Hz, H-5′), 6.28 (1H, d, J=2.0 Hz, H-8), 6.11 (1H, d, J=2.0 Hz, H-6), 5.28 (1H, d, J=7.6 Hz, H-1″), 4.33 (1H, d, J=11.5 Hz, H-6″a), 4.21 (1H, dd, J=11.5, 6.7 Hz, H-6″b), 3.55–3.40 (3H, H-2″, 3″, 5″), and 3.32 (1H, t, J=9.6 Hz, H-4″)]. One additional coumaroyl signal was detected at δ 7.40 (1H, d, J=15.8 Hz, H-7‴), 7.30 (2H, d, J=8.2 Hz, H-2‴, 6‴), 6.78 (2H, d, J=8.2 Hz, H-3‴, 5‴), and 6.07 (1H, d, J=15.8 Hz, H-8‴). The downfield shift of H-6 (δ 4.33 and 4.21) indicated that coumaroyl was linked to C-6 of the sugar. Compound 7 was identified as helichrysoside (7), which was further confirmed by HR-ESI-MS, m/z 611.1097 [M+H]+ (calcd. for C30H27O14, 611.1401). Compound 8 showed the same aglycone as that of 5, and the UV spectrum showed λmax values at 266 nm (band II) and 312 nm (band I), which is the typical UV spectrum of the coumaroyl substituent kaempferol glycoside.Citation36 Thus, compound 8 was identified as tiliroside (8). 1H-NMR (500 MHz, CD3OD) δ 7.98 (2H, dd, J=8.8, 2.0 Hz, H-2′,6′), 6.82 (2H, d, J=8.8, 2.0 Hz, H-3′,5′), 7.40 (1H, d, J=15.9 Hz, H-7‴), 7.31 (2H, d, J=8.2 Hz, H-2‴, 6‴), 6.80 (2H, d, J=8.2 Hz, H-3‴, 5‴), 6.30 (1H, d, J=2.0 Hz, H-8), 6.12 (1H, d, J=2.0 Hz, H-6), 6.00 (1H, d, J=15.9 Hz, H-8‴), 5.24 (1H, d, J=7.6 Hz, H-1″), 4.30 (1H, brd, J=11.5 Hz, H-6″a), 4.19 (1H, dd, J=11.5, 6.7 Hz, H-6″b), 3.40–3.48 (3H, H-2″, 3″, 5″), 3.31 (1H, m, H-4″).
Compounds 9−12 showed similar 1H-NMR spectra () and UV spectra, with λmax values at 327, 298 (shoulder), and 242 nm, suggesting that compounds 9−12 were caffeoyl-substituted quinic acid derivatives.Citation35 The substituted position of caffeoyl was used to identify the oxygenated methine protons of the quinic acid core, as detailed in the discussion previously.Citation35 Compounds 9 and 10 were identified as 5-O-caffeoylquinic acid (9) and 3-O-caffeoylquinic acid (10). There was one additional OCH3 signal found in the spectra of compounds 11 and 12, for which the other proton signals were very similar to those of compounds 9 and 10. Compounds 11 and 12 were identified as 5-O-caffeoylquinic acid methyl ester (11) and 3-O-caffeoylquinic acid methyl ester (12). m/z 201.0480 [M+Na]+ (calcd. for C10H10NaO3, 201.0528). 1H-NMR (500 MHz, CD3OD): δ 7.60 (1H, d, J=16.1 Hz, H-7), 7.44 (2H, d, J=8.3 Hz, H-2, 6), 6.80 (2H, d, J=8.3 Hz, H-3, 5), 6.30 (1H, d, J=16.1 Hz, H-8), 3.75 (3H, s, OCH3). Compound 13 was identified as methyl cinnamate.
Table 3 1H-NMR (500 MHz, CD3OD) characteristics of compounds 9–12 from blueberry extracts
The UV spectrum of compound 14 showed λmax at 325, 297, 240 nm, (+) HR-ESIMS, m/z 217.0453 [M+Na]+ (calcd for C10H10NaO4, 217.0477). 1H-NMR (500 MHz, CD3OD): δ 7.57 (1H, d, J=15.9 Hz, H-7), 7.04 (1H, d, J=1.8 Hz, H-2), 6.94 (1H, dd, J=8.2, 1.8 Hz, H-6), 6.79 (1H, d, J=8.2 Hz, H-5), 6.28 (1H, J=15.9 Hz, H-8), 3.79 (3H, s, OCH3). Compound 14 was identified as methyl caffeate.
Discussion
Most experimental and clinical studies have strongly suggested that blueberry is a source of bioactive compounds for the treatment of obesity and type II diabetes.Citation37 However, the mechanisms have not been illuminated in detail. A D101 macroporous resin column was used to separate MEB, and the effect of the resulting fractions on PPRE and NF-κB activity were detected. Inflammation contributes to the pathogenesis of type II diabetes, and anti-inflammation strategies for the treatment of this disease simultaneously lower blood glucose levels and potentially reduce the severity and prevalence of the associated complications.Citation38 Previous research has shown that blueberry polyphenol extract effectively inhibits the LPS-induced inflammatory response and decreases the activity of NF-κB,Citation39 lowering proinflammatory mediators, including nitric oxide (NO), tumor necrosis factor-alpha (TNF-α), and interleukin-1 beta (IL-1β), and down-regulating NO synthase (iNOS) and cyclooxygenase 2 (COX2). Inconsistent with the results of previous reporting, MEB markedly repressed the NF-κB-driven luciferase gene. MEB were separated into two groups, quinic acid derivatives (Fr.1) and flavonoid glycosides (Fr.2). Interestingly, neither of the separated MEB fractions demonstrated inhibitory effects on NF-κB activity. This phenomenon supported the notion that quinic acid derivatives and flavonoids result in a synergistic effect in the repression of NF-κB activity. Hyperglycemia induces inflammatory responses, producing free radicals that can lead to type II diabetes.Citation40 Polyphenols exert their protective effects against diabetes mellitus by suppressing inflammatory responses.Citation41,Citation42 It is supposed that the hypoglycemic effects of MEB are mediated, at least in part, by the inhibition of NF-κB activity. Activation of PPARs in the treatment of type II diabetes mellitus (T2DM) has proven effective in improving insulin sensitivity, hyperglycemia, and lipid metabolism.Citation43
PPARs are important in controlling the expression of genes involved in the regulation of glucose, lipid, and cholesterol metabolism; cell growth; and cell differentiation by binding to specific PPREs at the enhancer sites of target genes.Citation44 Activation of PPARs contributed to anti-inflammatory effects in several cell types, including liver cells.Citation12 The anti-inflammatory properties of PPARs are often associated with the repression of transcriptional pathways involved in inflammatory responses, such as modulation of NF-κB signaling. Currently, MEB have been shown to stimulate PPRE activity and induce up-regulation of PPARγ mRNA expression. This suggested that the repression of NF-κB activity by MEB is closely related to the simultaneous PPRE activity. This result is further supported by the observation that quinic acid derivatives and flavonoids have no effect on PPRE activity. However, the existing result could not rule out the contribution of PPARα and PPARβ to PPRE activity. The potential mechanism of PPRE activity mediation by MEB requires further study.
Recently, GLUT-2 has drawn attention as a molecule that might be involved in the pathogenesis of diabetes mellitus. Improvement of insulin signaling in HepG2 is involved in enhancing the GLUT-2 expression.Citation45 GLUT-2 is a glucose transporter that is mainly present in the plasma membrane of pancreatic β-cells. GLUT-2 has been shown to have blood glucose regulation functions through the control of insulin secretion,Citation46 and it was reported that GLUT-2 mRNA and protein expression were reduced by hyperinsulinemia and increased by hyperglycemia in the liver of diabetic rats.Citation47 Rutin, a flavonoid isolated from Toona sinensis Roem, has the ability to enhance insulin-dependent receptor kinase (IRK) activity and glucose transporter 4 (GLUT4) translocation in differentiated myotubes.Citation48 Chlorogenic acid (CGA), a common dietary polyphenol with numerous biologically activities, reversed the downregulation of GLUT-2 induced by a HFD (high-fat diet).Citation49 Consistently, the present study demonstrated that MEB stimulated GLUT-2 mRNA expression in liver cells. Polyphenol-rich Chrysanthemum morifolium extract (CME) also showed the ability to reverse the decline of PPARα/γ and GLUT-2 induced by alloxan. Chemical constituents analysis showed that chlorogenic acid, dicaffeoylquinic acid, and apigenin were the major polyphenols of CME, and those polyphenols might exert a synergic hypoglycemic effect via PPARα/γ-mediated mechanisms.Citation50Pongamia glabra (PBME) and Ficus glomerata (FBME) produced a synergistic hypoglycemic effect with combined therapy at low doses. The primary constituents in the two plants were flavonoids, furanoflavonoids, sterols, saponins, glycosides, glaunol, tannins, and other polyphenol compounds.Citation51 Through inhibition of oxidative stress, polyphenols protect against the effects of chronic diseases mediated by inflammatory responses.Citation52
Taken together, blueberry extract may exert hypoglycemic properties through the synergistic effects of caffeoylquinic acid derivatives and quercetin glycosides, and the hypoglycemic effect is involved in an increase of GLUT-2 and PPARγ expression and inhibition of the relevant inflammatory pathways. Although further efforts are needed to define the hypoglycemic mechanisms of blueberry extract in diabetic animal model, a diet rich in blueberry extract may be a potential chemopreventive tool useful for the management of diabetes.
Author contributions
Weifeng Huang and Chunpeng Wan conceived and designed the experiments; Liangliang Yao, Xiao He, Lei Wang, Mingxi Li, and Youxin Yang performed the experiments; Weifeng Huang and Chunpeng Wan analyzed the data; Chunpeng Wan contributed reagents/materials/analysis tools; Weifeng Huang and Chunpeng Wan wrote the paper. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The project was supported by the National Natural Science Foundation of China (31500286) and the Natural Science Foundation of Jiangxi Province (20161BAB214167). We thank Nature Research Editing Service for its linguistic assistance during the preparation of this manuscript.
Disclosure
The authors declare no conflicts of interest in this work.
References
- American Diabetes Association Diagnosis and classification of diabetes mellitus Diabetes Care 2014 37 S1 S81 S90 24357215
- Ma RC Lin X Jia W Causes of type 2 diabetes in China Lancet Diabetes Endocrinol 2014 2 12 980 991 25218727
- Chan JC Zhang Y Ning G Diabetes in China: a societal solution for a personal challenge Lancet Diabetes Endocrinol 2014 2 12 969 979 25218728
- Deacon CF Mannucci E Ahrén B Glycaemic efficacy of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors as add-on therapy to metformin in subjects with type 2 diabetes—a review and meta analysis Diabetes Obes Metab 2012 14 8 762 767 22471248
- Nair S Wilding JP Sodium glucose cotransporter 2 inhibitors as a new treatment for diabetes mellitus J Clin Endocrinol Metab 2010 95 1 34 42 19892839
- Bhandari MR Jong-Anurakkun N Hong G Kawabata J α-Glucosidase and α-amylase inhibitory activities of Nepalese medicinal herb Pakhanbhed (Bergenia ciliata, Haw.) Food Chem 2008 106 1 247 252
- Green BD Flatt PR Bailey CJ Dipeptidyl peptidase IV (DPP IV) inhibitors: a newly emerging drug class for the treatment of type 2 diabetes Diab Vasc Dis Res 2006 3 3 159 165 17160910
- An T Hong D Hu L Li J Protein tyrosine phosphatases 1B inhibitors from traditional Chinese medicine ACS Publications 2006 143 156
- Huang TH Peng G Kota BP Anti-diabetic action of Punica granatum flower extract: Activation of PPARγ and identification of an active component Toxicol Appl Pharmacol 2005 207 2 160 169 16102567
- Abe N Watanabe T Ozawa S Pancreatic endocrine function and glucose transporter (GLUT)–2 expression in rat acute pancreatitis Pancreas 2002 25 2 149 153 12142737
- Jourdan T Szanda G Rosenberg AZ Overactive cannabinoid 1 receptor in podocytes drives type 2 diabetic nephropathy Proc Natl Acad Sci USA 2014 111 50 E5420 E5428 25422468
- Zhong J Gong W Lu L Irbesartan ameliorates hyperlipidemia and liver steatosis in type 2 diabetic db/db mice via stimulating PPARγ, AMPK/Akt/mTOR signaling and autophagy Int Immunopharmacol 2017 42 176 184 27919004
- Ke B Zhao Z Ye X Inactivation of NF-κB p65 (RelA) in liver improves insulin sensitivity and inhibits cAMP/PKA pathway Diabetes 2015 64 10 3355 3362 26038580
- Han L Li C Sun B Protective effects of celastrol on diabetic liver injury via TLR4/MyD88/NF-κB signaling pathway in type 2 diabetic rats J Diabetes Res 2016 2016 2641248 27057550
- Su X Zhang J Wang H Phenolic acid profiling, antioxidant, and anti-inflammatory activities, and miRNA regulation in the polyphenols of 16 blueberry samples from China Molecules 2017 22 2 pii: E312
- Khalid S Barfoot KL May G Lamport DJ Reynolds SA Williams CM Effects of acute blueberry flavonoids on mood in children and young adults Nutrients 2017 9 2 pii: E158
- Diaconeasa Z Leopold L Rugină D Ayvaz H Socaciu C Antiproliferative and antioxidant properties of anthocyanin rich extracts from blueberry and blackcurrant juice Int J Mol Sci 2015 16 2 2352 2365 25622252
- Castrejón ADR Eichholz I Rohn S Kroh LW Huyskens-Keil S Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosum L.) during fruit maturation and ripening Food Chem 2008 109 3 564 572
- Szajdek A Borowska EJ Bioactive compounds and health-promoting properties of berry fruits: a review Plant Foods Hum Nutr 2008 63 4 147 156 18931913
- Neto CC Cranberry and blueberry: evidence for protective effects against cancer and vascular diseases Mol Nutr Food Res 2007 51 6 652 664 17533651
- Rodriguez-Mateos A Ishisaka A Mawatari K Vidal-Diez A Spencer JP Terao J Blueberry intervention improves vascular reactivity and lowers blood pressure in high-fat-, high-cholesterol-fed rats Br J Nutr 2013 109 10 1746 1754 23046999
- Stull AJ Blueberries’ impact on insulin resistance and glucose intolerance Antioxidants 2016 5 4 pii: E44
- Bingül İ Başaran-Küçükgergin C Tekkeşin MS Olgaç V Doğru-Abbasoğlu S Uysal M Effect of blueberry pretreatment on diethyl-nitrosamine-induced oxidative stress and liver injury in rats Environ Toxicol Pharmacol 2013 36 2 529 538 23811110
- Grace MH Ribnicky DM Kuhn P Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, Vaccinium angustifolium Aiton Phytomedicine 2009 16 5 406 415 19303751
- Shi D Xu M Ren M Immunomodulatory effect of flavonoids of blueberry (vaccinium corymbosum L.) leaves via the NF-κB signal pathway in LPS-stimulated RAW 264.7 cells J Immunol Res 2017 2017 5476903 29445755
- Smale ST Luciferase assay Cold Spring Harb Protoc 2010 2010 5 pdb.prot5421
- Baker RG Hayden MS Ghosh S NF-κB, inflammation, and metabolic disease Cell Metab 2011 13 1 11 22 21195345
- Chassin C Hornef MW Bens M Hormonal control of the renal immune response and antibacterial host defense by arginine vasopressin J Exp Med 2007 204 12 2837 2852 17967904
- Lu Y Foo LY Identification and quantification of major polyphenols in apple pomace Food Chem 1997 59 2 187 194
- Fraisse D Heitz A Carnat A Carnat AP Lamaison JL Quercetin 3-arabinopyranoside, a major flavonoid compound from Alchemilla xanthochlora Fitoterapia 2000 71 4 463 464 10925029
- Tan C Wang Q Luo C Chen S Li Q Li P Yeast α-glucosidase inhibitory phenolic compounds isolated from Gynura medica leaf Int J Mol Sci 2013 14 2 2551 2558 23358246
- Scharbert S Holzmann N Hofmann T Identification of the astringent taste compounds in black tea infusions by combining instrumental analysis and human bioresponse J Agric Food Chem 2004 52 11 3498 3508 15161222
- Wan C Yuan T Cirello AL Seeram NP Antioxidant and α-glucosidase inhibitory phenolics isolated from highbush blueberry flowers Food Chem 2012 135 3 1929 1937 22953942
- Zhang Y Seeram NP Lee R Feng L Heber D Isolation and identification of strawberry phenolics with antioxidant and human cancer cell antiproliferative properties J Agric Food Chem 2008 56 3 670 675 18211028
- Wan C Li S Liu L Chen C Fan S Caffeoylquinic acids from the aerial parts of chrysanthemum coronarium L Plants 2017 6 1 pii. E10
- Wang XM Wan CP Zhou SR Qiu Y Two new flavonol glycosides from Sarcopyramis bodinieri var. delicate Molecules 2008 13 6 1399 1405 18596665
- Shi M Loftus H McAinch AJ Su XQ Blueberry as a source of bioactive compounds for the treatment of obesity, type 2 diabetes and chronic inflammation J Funct Foods 2017 30 16 29
- Donath MY Shoelson SE Type 2 diabetes as an inflammatory disease Nat Rev Immunol 2011 11 2 98 107 21233852
- Lau FC Joseph JA McDonald JE Kalt W Attenuation of iNOS and COX2 by blueberry polyphenols is mediated through the suppression of NF-kB activation J Funct Foods 2009 1 3 274 283
- Pickup JC Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes Diabetes Care 2004 27 3 813 823 14988310
- Tzeng TF Liu WY Liou SS Hong TY Liu IM Antioxidant-rich extract from plantaginis semen ameliorates diabetic retinal injury in a streptozotocin-induced diabetic rat model Nutrients 2016 8 9 pii. E572
- Liu HW Wei CC Chang SJ Low-molecular-weight polyphenols protect kidney damage through suppressing NF-κB and modulating mitochondrial biogenesis in diabetic db/db mice Food Funct 2016 7 4 1941 1949 26960417
- Davidson MA Mattison DR Azoulay L Krewski D Thiazolidinedione drugs in the treatment of type 2 diabetes mellitus: past, present and future Crit Rev Toxicol 2018 48 1 52 108 28816105
- Berger J Moller DE The mechanisms of action of PPARs Annu Rev Med 2002 53 409 435 11818483
- Cordero-Herrera I Martín MA Bravo L Goya L Ramos S Cocoa flavonoids improve insulin signalling and modulate glucose production via AKT and AMPK in HepG2 cells Mol Nutr Food Res 2013 57 6 974 985 23456781
- Guillam MT Hümmler E Schaerer E Early diabetes and abnormal postnatal pancreatic islet development in mice lacking Glut-2 Nat Genet 1997 17 3 327 330 9354799
- Burcelin R Eddouks M Kande J Assan R Girard J Evidence that GLUT-2 mRNA and protein concentrations are decreased by hyperinsulinaemia and increased by hyperglycaemia in liver of diabetic rats Biochem J 1992 288 2 675 679 1463468
- Hsu CY Shih HY Chia YC Rutin potentiates insulin receptor kinase to enhance insulin-dependent glucose transporter 4 translocation Mol Nutr Food Res 2014 58 6 1168 1176 24668568
- Peng BJ Zhu Q Zhong YL Xu SH Wang Z Chlorogenic acid maintains glucose homeostasis through modulating the expression of SGLT-1, GLUT-2, and PLG in different intestinal segments of Sprague-Dawley rats fed a high-fat diet Biomed Environ Sci 2015 28 12 894 903 26777909
- Shang X Zhu ZY Wang F Liu JC Liu JY Xie ML Hypoglycemic effect of Chrysanthemum morifolium extract on alloxan-induced diabetic mice is associated with peroxisome proliferator-activated receptor α/γ-mediated hepatic glycogen synthesis J Appl Biomed 2017 15 1 81 86
- Heroor S Beknal A Mahurkar N Synergistic activity of bark extracts of Pongamia glabra and Ficus glomerata in alloxan-induced diabetic rats World J Pharm Pharm Sci 2013 2 6 6640 6652
- Mitjavila MT Moreno JJ The effects of polyphenols on oxidative stress and the arachidonic acid cascade. Implications for the prevention/treatment of high prevalence diseases Biochem Pharmacol 2012 84 9 1113 1122 22858365
