Abstract
Background
The purpose of this work was to describe the efficacy and safety of a telmisartan 80 mg + hydrochlorothiazide 25 mg (T80/H25) single-pill combination therapy in patients with moderate-severe hypertension (mean seated trough cuff systolic blood pressure [BP] ≥ 160 mmHg and diastolic BP ≥ 100 mmHg) in specific patient subpopulations.
Methods
This was a planned analysis of a double-blind, multicenter, parallel-group trial that demonstrated the superiority of a single-pill combination of T80/H25 versus T80 monotherapy in terms of systolic BP change from baseline to week 7. Subpopulations included older (aged ≥ 65 years) versus younger, gender, race, hypertension severity, and prior antihypertensive therapy. Endpoints were change from baseline in mean seated trough cuff systolic and diastolic BP, proportion of patients achieving their BP goal (systolic/diastolic BP < 140/90 mmHg), and proportion of patients attaining systolic BP reductions of >30 mmHg and >40 mmHg.
Results
Across all subgroups, the T80/H25 single-pill combination provided consistently greater systolic and diastolic BP reductions than T80 and more patients had systolic BP reductions of >30 mmHg. In the T80 and T80/H25 groups, BP control was achieved in 34.1% and 48.8% of men, 35.5% and 62.7% of women, 34.5% and 56.6% of Asians, 22.6% and 38.6% of blacks, 36.7% and 57.8% of whites, 36.9% and 57.5% of patients < 65 years, 29.3% and 49.3% ≥65 years, 44.2% and 66.2% of those with grade 2 hypertension, 20.4% and 39.4% of those with grade 3 hypertension, 38.9% and 53.2% of previously untreated patients, 38.1% and 62.5% of patients previously treated with one antihypertensive, and 29.7% and 48.9% of patients previously treated with two or more antihypertensive agents respectively. Treatment was generally well tolerated across the patient subgroups.
Conclusion
The T80/H25 single-pill combination provides consistent BP reductions and higher goal attainment rates versus T80 across a range of hypertensive patient subgroups, which are likely to have a positive impact on patients’ cardiovascular risk.
Introduction
Antihypertensive monotherapy is often ineffective in achieving adequate blood pressure (BP) control. Elevated BP often has a multifactorial cause, which may not be addressed through single-agent therapy, and the initial BP-lowering effects of monotherapy may be opposed by reflex counter-regulatory mechanisms.Citation1,Citation2 While clinicians often begin treatment of patients with mild (grade 1) hypertension by prescribing a single antihypertensive agent, data suggest that at least 75% of patients with hypertension will require combination therapy in order to reach BP goals recommended by the guidelines.Citation1,Citation3 This contributes to the advice within current clinical guidelines that, for many patients, more than one antihypertensive agent is usually required in order to achieve the goal of systolic/diastolic BP < 140/90 mmHg.Citation4,Citation5
Scientific rationale exists for using antihypertensive agents having complementary mechanisms of action in combination.Citation1,Citation2 Coadministration of appropriate antihypertensive agents can both improve levels of BP control and reduce cardiovascular events,Citation6 and combination therapy may offer improved tolerability and safety compared with high-dose monotherapy.Citation1 Furthermore, single-pill combination dosing is simplified and can improve long-term adherence with treatment.Citation1,Citation7
The use of an angiotensin II receptor blocker plus a thiazide diuretic is a combination therapy for the management of hypertension that is endorsed in current international guidelines.Citation4,Citation5 Several large-scale randomized trials in patients with stages 1 and 2 hypertension have demonstrated the antihypertensive efficacy of once-daily telmisartan-hydrochlorothiazide combinations, with evidence of greater 24-hour BP control, achieved with favorable tolerability.Citation8 A recently reported international, double-blind, double-dummy study showed that, compared with telmisartan 80 mg (T80) alone, initial treatment with a single-pill combination of T80 mg/hydrochlorothiazide (H25) mg (T80/H25) provided significant reductions in BP after 2 weeks of treatment and higher BP goal attainment in patients with moderate-to-severe hypertension.Citation9
Despite the widespread use of combination treatment with an angiotensin II receptor blocker and hydrochlorothiazide, there are few prospective assessments of how the efficacy and tolerability of these combinations vary among different demographic groups. Prior evidence indicates that hypertension severity at baseline and duration of hypertension can affect BP goal attainment,Citation9–Citation11 and that ethnic and age-related variations in the response to antihypertensive treatment also exist.Citation12,Citation13 Our recently reported studyCitation9 comprised a large and diverse patient population and provides an opportunity for subanalysis of the response to a single-pill combination of T80/H25 within these different patient subpopulations. This prospectively defined analysis describes the efficacy of a single-pill combination of T80/H25 according to specific patient subpopulations, based on age, gender, race, prior antihypertensive treatment history, and stages and severities of baseline hypertension, with additional post hoc analyses of safety and tolerability in these subpopulations.
Patients and methods
Study design
This was an international, multicenter, 7-week, Phase IV, randomized, double-blind, double-dummy, active-controlled, parallel-group, forced-titration study in patients with grade 2 or 3 hypertension (ClinicalTrials.gov registration: NCT00926289). The design has been described in detail elsewhere.Citation9 In brief, after an open-label, placebo run-in treatment period of 1–14 days, patients were randomized to double-blind treatment with T40/H12.5 single-pill combination therapy or T40 monotherapy for one week, before being uptitrated to the target dose of T80/H25 single-pill combination therapy or T80 alone, respectively, for the remaining 6 weeks of the study. The trial was conducted under guidelines set forth in the Declaration of Helsinki (1996) and the International Conference on Harmonisation Tripartite Harmonised Guideline for Good Clinical Practice, and was approved by the health authority of each country and by the institutional review board or independent ethics committee of each center. Study participants provided their written informed consent.
Patients
Eligible patients included those with grade 2 or 3 hypertension (defined as mean seated trough cuff systolic BP ≥ 160 mmHg and diastolic BP ≥ 100 mmHg) who met the inclusion criteria as described in the primary study publication. They were recruited at 102 participating centers in eight countries (Bulgaria, China, France, Georgia, Romania, Russia, South Korea, and the US).Citation9
Patients were evaluated at baseline for demographic characteristics that allowed for the analysis of data according to specific patient subpopulations: older (≥65 years) versus younger, men versus women, race (black, white, Asian), baseline hypertension severity (moderate [grade 2] defined as 160–179/100–109 mmHg versus severe [grade 3] defined as ≥180–199/110–119 mmHg), and prior antihypertensive treatment history (no prior antihypertensive therapy, one prior antihypertensive therapy, two or more prior antihypertensive therapies).
Efficacy and safety evaluations
At each scheduled study visit, seated trough cuff BP was measured approximately 24 (20–30) hours after last study drug intake, using a standard manual cuff sphygmomanometer or other validated device. Three consecutive measurements, taken approximately 2 minutes apart, were recorded and the average calculated.
Blood pressure measurements were performed at screening, at the start of the open-label placebo run-in treatment period, at the end of the run-in treatment period prior to randomization (ie, at baseline), and after one, 3, 5, and 7 weeks of double-blind treatment.
Efficacy endpoints were assessed at weeks 3, 5, and 7 and included the primary endpoint measure of change from baseline to the final visit (week 7) in mean seated trough cuff systolic BP. Other secondary endpoints included change from baseline to final visit in mean seated trough cuff diastolic BP, the proportion of patients achieving their systolic BP goal (defined as a mean seated trough cuff systolic BP < 140 mmHg), the proportion of patients achieving diastolic BP goal (defined as a mean seated trough cuff diastolic BP < 90 mmHg), the proportion of patients achieving their overall BP goal (defined as a mean seated trough cuff systolic/diastolic BP < 140/90 mmHg), the proportion of patients with a mean seated trough cuff systolic BP reduction of >30 mmHg, and the proportion of patients with mean seated trough cuff systolic BP reduction of >40 mmHg, the proportion of patients with systolic BP response (systolic BP < 140 mmHg or a reduction of ≥15 mmHg), as well as the proportion of patients with a diastolic BP response (diastolic BP < 90 mmHg or a reduction of ≥10 mmHg), all determined at week 7.
All adverse events that occurred throughout the study period were recorded and classified according to the Medical Dictionary for Regulatory Activities version 13.0, with intensity and causal relationship to study drug determined by the investigator. Treatment discontinuations and serious adverse events were also recorded.Citation9 Laboratory tests were conducted at screening, randomization, and at the final study visit. To ensure standardization in laboratory parameters, all blood samples were analyzed by a central laboratory.
Statistical analysis
The efficacy analyses were performed on the full analysis set, which was defined as randomized patients who took at least one dose of double-blind trial medication, and for whom a baseline measurement and at least one postdose trough efficacy measurement during the high-dose double-blind treatment period were available (with the proviso that the measurements were taken on the same arm).Citation9 Safety analysis was performed on all randomized patients who received at least one dose of the allocated treatment.
These analyses were not powered per se to examine the antihypertensive efficacy of the single-pill combination T80/H25 versus T80 in the five different patient subpopulations and to test for treatment-by-subgroup interactions, nor were statistical models utilized to determine independence of these subgroups. For example, the white patients could be either <65 years or older, either male or female, with either grade 2 or 3 hypertension at randomization, and with different treatment histories. Finally, these analyses did not employ multiplicity adjustments.
A restricted maximum likelihood-based repeated-measures approach, using baseline and all available longitudinal observations at each postbaseline visit during the high-dose treatment phase was used for analysis of the primary endpoint as well as the change from baseline in diastolic BP. The model included the fixed, categorical effects of treatment, country, week, and treatment-by-week interaction, subgroup, and treatment-by-subgroup interaction, with the continuous covariates of baseline mean seated trough cuff systolic BP or diastolic BP and baseline-by-week interaction. An unstructured covariance structure was used to model within-patient errors. The difference in treatments (T80/H25 versus T80) with a 95% confidence interval (CI) was calculated for each subgroup.
Blood pressure goal rates, BP responses, and great systolic BP reductions (>30 or >40 mmHg) were evaluated using logistic regression, with fixed effects for treatment, country, subgroup, treatment-by-subgroup interaction, and the respective baseline value (diastolic BP or systolic BP) as a covariate. For these dichotomous endpoints, last trough observation carried forward was used to account for missing data. Odds ratios (ORs) with 95% CIs were calculated for the effect of T80/H25 versus T80 monotherapy in the different patient subpopulations. For both the described analyses, P values were calculated for the interaction of subgroup and treatment.
Results
Patient baseline characteristics
The baseline characteristics of the entire cohort of 888 patients randomized and treated in the study have been described previously elsewhere.Citation9 Baseline BP characteristics of different patient subpopulations according to treatment group are shown in . Within the 858 patients in the full analysis set, 515 had grade 2 hypertension and 339 had grade 3 hypertension and most patients had received at least one prior antihypertensive therapy. No marked imbalances were found between the two treatment groups across the different patient subpopulations. Grade 3 hypertension was recorded in approximately 40% of all patients, but grade 3 hypertension was noted at a higher rate in the relatively small black subpopulation, where 86% had BP ≥ 180/110 mmHg.
Table 1 Baseline BP characteristics in the different patient subpopulations (based on full analysis set)
Efficacy
Across the entire study population, at week 7, single-pill T80/H25 combination therapy significantly reduced the adjusted mean ± standard error seated trough cuff systolic/diastolic BP from baseline (−37.0 ± 0.62/−18.6 ± 0.38 mmHg) as compared with T80 monotherapy (−28.5 ± 0.88/−15.4 ± 0.55 mmHg [adjusted mean difference −8.5/−3.2 mmHg; 95% CI −10.6, −6.4/−4.5, −1.9; P < 0.0001]) and allowed more patients to achieve the BP target of systolic/diastolic BP < 140/90 mmHg (55.5% versus 34.7%; OR, 2.39; 95% CI 1.76, 3.26; P < 0.0001).Citation9
The adjusted mean reductions in systolic/diastolic BP from baseline according to treatment group and patient sub-populations are given in , with treatment differences (including 95% CI) for the T80/H25 single-pill combination versus T80, with regard to changes in systolic BP and diastolic BP from baseline depicted in . The ORs and 95% CIs for BP goal rates, and for the proportion of patients achieving systolic BP reductions >30 or >40 mmHg, for treatment with the T80/H25 single-pill combination versus T80 in the different patient subpopulations, are shown in and , respectively.
Figure 1 Treatment difference (95% confidence interval) of single-pill combination T80/H25 versus T80 on changes in mean seated trough cuff (A) systolic blood pressure and (B) diastolic blood pressure from baseline to week 7 in the different patient populations full analysis set.
Abbreviations: H25, hydrochlorothiazide 25 mg; T80, telmisartan 80 mg.
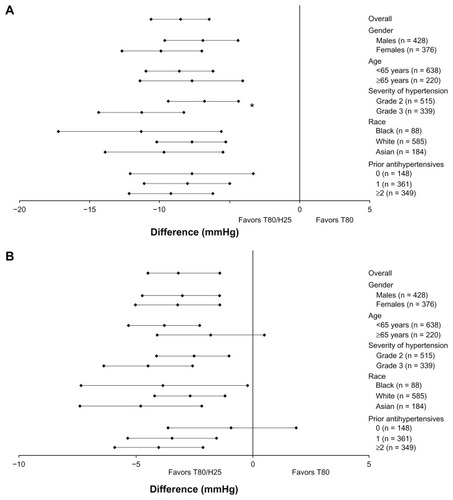
Figure 2 Odds ratios (95% confidence interval) of the single-pill combination of T80/H25 versus T80 in blood pressure goal rates. (A) Systolic blood pressure (<140 mmHg), (B) diastolic blood pressure (<90 mmHg), and (C) systolic/diastolic blood pressure control (<140/90 mmHg) at week 7 in the different patient subpopulations.
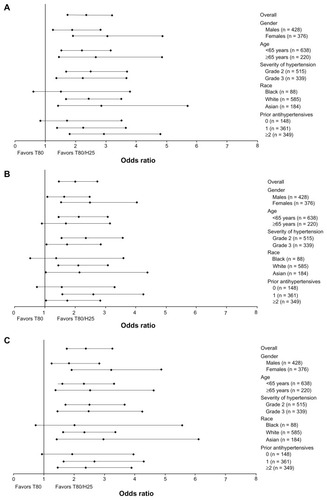
Figure 3 Odds ratios (95% confidence interval) of a single-pill combination of T80/H25 versus T80 on proportion of patients with seated trough cuff systolic blood pressure reduction (A) >30 mmHg and (B) >40 mmHg at week 7 in the different patient subpopulations.
Abbreviations: H25, hydrochlorothiazide 25 mg; T80, telmisartan 80 mg.
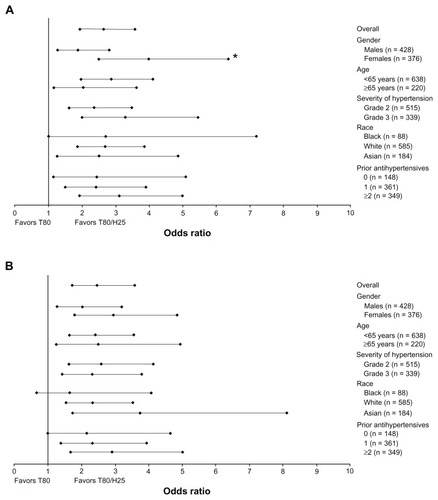
Table 2 Systolic and diastolic BP reductions (mmHg) from baseline to week 7 in overall patient population and different patient subpopulations in the full analysis set
Responses to treatment were, in general, similar in the different populations. Descriptions of results according to different subpopulations follow (except where specified, there were no statistically significant interactions [defined as an interaction P value < 0.05] between subgroup and treatment outcomes). Any reported statistically significant interactions should be regarded as quantitative rather than qualitative interactions.
Gender
In general, men and women did not have significant differences with regard to BP reductions (, and ), with a single-pill combination of T80/H25 providing more effective systolic/diastolic BP reductions than T80 (adjusted mean treatment difference: in men, −6.9/−3.0 mmHg; 95% CI −9.6, −4.3/−4.7, −1.4; in women, −9.9/−3.2 mmHg; 95% CI −12.7, −7.0/−5.0, −1.4). Among men, BP control was achieved by 34.1% and 48.8% in the T80 and T80/H25 single-pill combination groups, respectively. Among women, BP control was achieved in 35.5% and 62.7% in the T80 and T80/H25 single-pill combination groups, respectively. Altogether, 60.9% of men and 75.4% of women achieved a systolic BP reduction > 30 mmHg with the T80/H25 single-pill combination (P = 0.0171 for interaction, ). In the T80/H25 single-pill combination group, a systolic BP response was seen in 90.2% of men and 93.8% of women, and a diastolic BP response was seen in 81.5% of men and 87.3% of women.
Age
Younger and older study participants did not show significant differences with regard to BP reductions (, and ). In both age groups, the T80/H25 single-pill combination provided more effective systolic/diastolic BP reductions than T80 (adjusted mean treatment difference: in patients < 65 years, −8.6/−3.8 mmHg; 95% CI −11.0, −6.2/−5.3, −2.3; in patients ≥ 65 years, −7.7/−1.8 mmHg; 95% CI −11.4, −4.1/−4.1, 0.5). Among patients < 65 years, BP control was achieved by 36.9% and 57.5% in the T80 and T80/H25 single-pill combination groups, respectively. Among patients ≥ 65 years, BP control was achieved by 29.3% and 49.3% in the T80 and T80/H25 single-pill combination groups, respectively. Altogether, 70.1% of those aged < 65 years and 60.9% of those aged ≥ 65 years achieved a systolic BP reduction > 30 mmHg with the T80/H25 single-pill combination (). In the T80/H25 single-pill combination group, a systolic BP response was seen in 92.6% of those aged < 65 years and in 89.9% of those aged ≥ 65 years, and a diastolic BP response was seen in 84.1% of those aged < 65 years and in 84.8% of those aged ≥ 65 years.
Hypertension severity
In patients with grade 2 and 3 hypertension, the T80/H25 single-pill combination provided more effective systolic/diastolic BP reductions than T80 monotherapy (adjusted mean treatment difference: in patients with grade 2 hypertension −6.8/−2.5 mmHg; 95% CI −9.4, −4.3/−4.1, −1.0; in patients with grade 3 hypertension −11.3/−4.5 mmHg; 95% CI −14.4, −8.3/−6.4, −2.6). The reductions in systolic BP in the T80/H25 single-pill combination group over those seen in the T80 group were significantly greater for patients with grade 3 hypertension than in those with grade 2 hypertension (P < 0.05 for interaction, , and ). Blood pressure control was achieved in 44.2% and 66.2% of those with grade 2 hypertension, and in 20.4% and 39.4% of those with grade 3 hypertension, in the T80 and the T80/H25 single-pill combination groups, respectively. Altogether, 63% of those with grade 2 hypertension and 76.5% of those with grade 3 hypertension achieved a systolic BP reduction > 30 mmHg with T80/H25 (). In the T80/H25 group, a systolic BP response was seen in 90.7% of those with grade 2 hypertension and in 94.2% of those with grade 3 hypertension (P < 0.05 for interaction); a diastolic BP response was seen in 87.5% of those with grade 2 hypertension and in 80.1% of those with grade 3 hypertension.
Racial group
No statistically significant differences in BP reduction were found between black, Asian, and white patients (, and ). In all racial subpopulations, the T80/H25 single-pill combination provided more effective systolic/diastolic BP reductions than T80 (adjusted mean treatment difference: in black patients, −11.3/−3.8 mmHg; 95% CI −17.1, −5.6/−7.3, −0.2; in white patients, −7.7/−2.7 mmHg; 95% CI −10.2, −5.3/−4.2, −1.2; in Asian patients, −9.7/−4.8 mmHg; 95% CI −13.9, −5.5/−7.4, −2.2). Blood pressure control was achieved in 22.6% and 38.6% of black, 36.7% and 57.8% of white, and 34.5% and 56.6% of Asian patients treated with T80 and T80/H25, respectively. Overall, 78.9% of black, 65.5% of white, and 69.8% of Asian patients achieved a systolic BP reduction > 30 mmHg with T80/H25 (). Treatment with T80/H25 showed a systolic BP response rate of 91.2% of black, 91.7% of white, and 93.0% of Asian patients, and a diastolic BP response was seen in 61.4% of black, 87.6% of white, and 84.5% of Asian patients.
Prior antihypertensive use
Differences in prior use of antihypertensives at baseline did not significantly affect treatment differences in BP reductions during this study (, and ). In all prior treatment groups, the T80/H25 single-pill combination provided greater systolic BP/diastolic BP reductions than T80 (adjusted mean treatment difference: in previously untreated patients, −7.7/−0.9 mmHg; 95% CI −12.2, −3.3/−3.6, 1.9; in patients previously treated with one antihypertensive, −8.0/−3.4 mmHg; 95% CI −11.1, −5.0/−5.3, −1.5; in patients previously treated with at least two antihypertensive agents −9.2/−4.0 mmHg; 95% CI −12.2, −6.2/−5.9, −2.1). Blood pressure control was achieved in 38.9% and 53.2% of previously untreated patients, in 38.1% and 62.5% of patients previously treated with one antihypertensive, and in 29.7% and 48.9% of patients previously treated with at least two antihypertensive agents in the T80 and T80/H25 single-pill combination groups, respectively. In the T80/H25 single-pill combination group, 72.3% of previously untreated patients, 67.3% of patients previously treated with one antihypertensive, and 66.7% of patients previously treated with two or more antihypertensive agents achieved a systolic BP reduction > 30 mmHg (). In the T80/H25 single-pill combination group, a systolic BP response was seen in 91.5% of previously untreated patients, in 92.3% of patients previously treated with one antihypertensive, and in 91.8% of patients previously treated with at least two antihypertensive agents. A diastolic BP response was seen in 87.2% of previously untreated patients, 83.9% of patients previously treated with one antihypertensive, and 83.5% of patients previously treated with two or more antihypertensive agents.
Safety and tolerability
Adverse events
describes the total adverse events, treatment-related adverse events, and adverse events leading to discontinuation, for all treatment arms and including the low-dose treatment period. The proportion of patients experiencing treatment-related adverse events in the T80/H25 single-pill combination and T80 monotherapy groups was low and similar across most subpopulations. The proportion of patients with adverse events leading to treatment discontinuation was low and similar across the different patient subpopulations (). Only one patient, in the T40 group prior to uptitration, experienced a serious adverse event during the treatment period. There were no deaths during the course of the study.
Table 3 Summary of adverse events in the different patient subpopulations, based on treated patients
Among male patients receiving T80/H25, the most frequent adverse events were pollakiuria (n = 5; 1.6%), and cough, dizziness, and nasopharyngitis (each n = 4; 1.3%). The most frequent adverse events for female patients receiving T80/H25 were dizziness (n = 7; 2.5%) and nasopharyngitis (n = 4; 1.4%). Among the subpopulation of younger (<65 years) patients receiving T80/H25, the most frequent adverse events were dizziness (n = 10; 2.2%), nasopharyngitis (n = 6; 1.3%), and pollakiuria (n = 5; 1.1%). Among patients with grade 2 hypertension receiving T80/H25, dizziness and nasopharyngitis were each reported in five patients (1.4%). Among those patients with grade 3 hypertension receiving T80/H25, dizziness was reported in five patients (2.2%), and vertigo and pollakiuria were the next most frequently reported (each n = 4; 1.7%).
The percentage of patients with treatment-related adverse events was numerically higher in black patients (12.9% and 9.8% for T80 and the T80/H25 single-pill combination) when compared with white and Asian patients (1.0% and 3.1%, and 3.6% and 6.8% for T80 and the T80/H25 single-pill combination, respectively); also, any adverse events were more frequent in this small subpopulation. The adverse events observed with the highest frequency among the subpopulation of black patients receiving T80/H25 were pollakiuria and upper respiratory tract infection (n = 4, 6.6% and n = 3, 4.9%). Other adverse events reported in black patients receiving T80/H25 with a frequency of 1.6% (n = 1) included sinusitis, vulvovaginal mycotic infection, dizziness, asthma, cough, oropharyngeal pain, constipation, arthralgia, myalgia, musculoskeletal stiffness, elevated blood alkaline phosphatase, arthropod bite, and upper limb fracture. Among Asian patients receiving T80/H25, dizziness was reported in seven patients (5.3%), and nasopharyngitis, chest discomfort, and blood uric acid increase were the next most frequently reported (each n = 3; 2.3%). Among the subpopulation of white patients receiving T80/H25, nasopharyngitis was the most frequently reported adverse event (n = 5; 1.3%).
The most frequent adverse events in those without any prior antihypertensive treatment were dizziness and chest discomfort (each n = 3; 3.1%). Other adverse events reported in this subpopulation with a frequency of n = 2; 2.1% were upper respiratory tract infection, vertigo, nausea, and chest pain. In patients treated with T80/H25 who had a history of prior use of one antihypertensive agent, the most frequent adverse events were dizziness (n = 4; 1.6%), nasopharyngitis, and pollakiuria (each n = 3; 1.2%). In patients treated with T80/H25 who had a history of prior use of at least two anti-hypertensive agents, the most frequent adverse events were dizziness and nasopharyngitis (each n = 4; 1.7%).
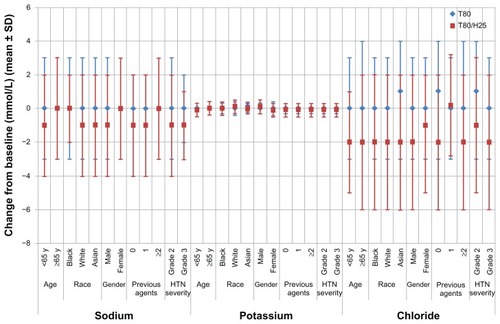
Electrolytes
Mean changes from baseline for sodium, potassium, and chloride were negligible in the overall population, in both treatment groups. Decreases in electrolytes from “high” or “normal” at baseline to “low” were observed at low frequencies in the T80/H25 group (sodium 0.7%, potassium 2.1%, chloride 0.4%). There were no such decreases in the T80 group. Overall, two patients had possibly clinically significant alterations related to electrolyte levels, both in the T80/H25 group. The criteria for clinically significant abnormalities based on normalized potassium values were defined as serum levels below 3.0 mmol/L or above 5.8 mmol/L. One patient had an increase in serum potassium from 4.5 mmol/L at baseline to 6.6 mmol/L at week 5. This was resolved post study, with serum potassium restored to 4.1 mmol/L by week 8. One patient had a decrease in serum potassium from 3.3 mmol/L at baseline to 2.8 mmol/L at week 6. This was resolved by week 7, with serum levels restored to 3.1 mmol/L.
The mean changes from baseline in serum sodium, potassium, and chloride at week 7 by patient subpopulation are displayed in . T80/H25 treatment was associated with small mean reductions in sodium levels (−1 mmol/L) in the majority of the patient subpopulations treated with T80/H25. No mean changes in sodium were observed within subpopulations of older, black, or female patients, or those who had received prior treatment with at least two antihypertensive agents. At week 7, mean potassium levels were unchanged in older, black, and Asian patients taking T80/H25. All other patient subpopulations receiving T80/H25 had small mean reductions in serum potassium of −0.1 mmol/L at week 7. Treatment with T80/H25 was generally associated with a small decline in chloride levels of between −1 and −2 mmol/L.
Substrates
For most substrates analyzed, changes between baseline and end of treatment were small and comparable between the patient subpopulations. Overall, there were only two possibly clinically significant decreases for the substrates analyzed (glucose in two patients in the T80/H25 group). There were no clinically significant increases for high-density lipoprotein, low-density lipoprotein (LDL), or urea. In patients receiving T80/H25, mean creatinine levels were unchanged at week 7, with the exception of the subpopulation of patients who had a history of prior treatment with at least 2 agents, in whom a mean increase of 0.1 mg/dL was observed. There were no mean changes in creatinine at week 7 in the T80 treatment group.
Within the T80/H25 treatment arm, mean blood glucose was increased by 1–7 mg/dL in most patient subpopulations. Females receiving T80/H25 had no change from baseline in mean blood glucose, and older patients ≥ 65 years receiving T80/H25 exhibited some reduction in mean blood glucose at week 7 (−1.0 mg/dL). Within the T80 treatment group, mean blood glucose was increased by 1–6 mg/dL in most patient subpopulations. Male patients and those <65 years receiving T80 showed no change from baseline in mean blood glucose. Reductions in mean blood glucose between −1 and −4 mg/dL were observed with T80 treatment in black patients, patients with no history of previous antihypertensive use, and those with grade 2 hypertension.
The effect of T80/H25 treatment on LDL was consistent across most patient subpopulations, although there appeared to be a differential effect of race and history of previous antihypertensive treatment; in white and black patients, there was a mean increase from baseline in LDL of 1.0% and 9.6%, respectively, whereas Asian patients had a mean decrease of −3.4%. In patients who had received previous treatment with one or two or more agents, there was a mean increase from baseline in LDL of 0.5% and 1.6%, respectively, whereas patients who had not received previous treatment with other antihypertensive agents had a mean decrease in LDL of −1.1%. In the T80 treatment group, a mean increase from baseline in LDL between 0.6% and 3.1% was observed in female, black, and Asian patients, those with history of any prior antihypertensive treatment, younger patients and those with grade 3 hypertension. Mean LDL was unchanged at week 7 in white patients receiving T80. Male patients, those with no prior antihypertensive treatment, patients with grade 2 hypertension, and older patients experienced mean decreases in LDL of −0.5 to −1.6%.
Triglycerides increased in the T80/H25 group by 15 mg/dL but decreased in the T80 group by 9 mg/dL, and these effects were broadly consistent across the patient subpopulations, with the exception of Asian patients, in whom a higher mean increase of 31 mg/dL (25.4%) was observed for T80/H25 treatment and in patients who had received at least two previous antihypertensive treatments, in whom a mean increase of 20 mg/dL (15.9%) was observed for T80/H25 treatment. Older patients ≥ 65 years receiving T80/H25 had the smallest mean increase in triglycerides of 6 mg/dL (5.2%). Uric acid increased by 1 mg/dL in the T80/H25 group but remained constant in the T80 group, and these effects were broadly consistent across the patient subpopulations.
Discussion
The prospectively defined efficacy analysis of this randomized, international, multicenter study showed the superiority of a single-pill combination of T80/H25 over T80 monotherapy in patients with grade 2 or 3 hypertension. This analysis was performed to establish whether there was any difference in response to treatment in patients according to their gender, race, age, severity of hypertension at baseline, and the number of antihypertensive agents they had received before study entry. In these analyses, the single-pill combination of T80/H25 consistently provided additional BP lowering, irrespective of these different patient subpopulations.
High rates of BP response to the single-pill combination of T80/H25 were achieved in both men and women. While women were more likely than men to achieve large systolic BP reductions (ie, reduction in systolic BP > 30 mmHg) in response to the T80/H25 single-pill combination, overall, both men and women achieved excellent additional BP reductions with the combination therapy (60.9% of men and 75.4% of women achieved systolic BP reductions > 30 mmHg).
Comparable efficacy of treatment was observed regardless of patient race, with substantial responses to the T80/H25 single-pill combination noted in white, black, and Asian subpopulations. Older patients, those with severe hypertension, and patients who had previously been treated with two or more antihypertensive agents responded to treatment as well as younger patients, those with less severe hypertension, and those with less exposure to prior antihypertensive therapy. Moreover, treatment with the T80/H25 single-pill combination was effective across all baseline hypertension severities and effected greater systolic BP reductions among patients with grade 3 hypertension relative to systolic BP reductions in patients with grade 2 hypertension, as expected.
Achieving BP reductions in all subpopulations of patients with moderate to severe hypertension is an important treatment goal that affects long-term risk for cardiovascular morbidity and mortality. Despite this, few systematic, prospective analyses are reported regarding the consistency of treatment response among different demographic groups. The INCLUSIVE (IrbesartaN/HCTZ bLood pressUre reductionS in dIVErse patient populations) trial found that irbesartan 300 mg/H25 mg was similarly effective in age, gender, and racial subgroups,Citation14–Citation16 although unlike the current analysis, this was an open-label study and used a 10-week titration phase. A pooled analysis of two double-blind trials of initial therapy with valsartan 160 mg/H12.5 likewise found consistent effects across subgroups, although this study did not assess the effects of combination with high-dose hydrochlorothiazide. Citation17 A pooled analysis of two large, double-blind studies comparing T80/H25 with valsartan 160 mg/H25 found consistent superiority for the T80/H25 combination, regardless of age, gender, or race.Citation18
The most commonly reported adverse events in patients who were receiving treatment with the T80/H25 combination included nasopharyngitis and dizziness. The actual frequency of nasopharyngitis among patients receiving T80/H25 was generally low (1.4%), and was similar, regardless of gender, age, or severity of hypertension. The rate of dizziness was also low in the T80/H25 treatment group (1.9%), and dizziness was more frequently observed in females compared with males, in younger patients compared with older patients, in Asians compared with black or white patients, and also more frequently in those with no history of previous antihypertensive treatment.
The use of thiazide diuretics has been associated with adverse electrolytic changes, including hyponatremia, hypokalemia, and hyperuricemia, although this effect may be offset with concomitant use of renin-angiotensin system inhibitors.Citation19,Citation20 This study only reports the short-term effects of T80/H25 treatment on electrolyte balance up to 7 weeks. At the end of this short trial, mean serum potassium levels were unchanged in older, black, and Asian patients receiving T80/H25. The remainder of the patient subpopulations receiving T80/H25 had small mean reductions in serum potassium of −0.1 mmol/L at week 7. The changes observed with T80/H25 treatment during this short trial are smaller than those observed in studies of thiazide diuretics alone, and appear negligible in light of studies that indicate potassium reductions of a much larger magnitude (about 1 mmol/L) are clinically relevant.Citation21,Citation22 Inhibitors of the renin-angiotensin system, such as angiotensin II receptor blockers, are associated with small serum potassium elevations, generally <0.3 mmol/L, and this may help to offset the effects of thiazide diuretics when used in combination.Citation19
Hyponatremia would typically be expected to present within 2 weeks of initiating treatment with a thiazide diuretic.Citation23 During this short study, T80/H25 treatment was associated with small mean reductions in serum sodium levels (−1 mmol/L) in several of the observed patient subpopulations. Females, older patients, and those with a history of two or more antihypertensive drugs, ie, subpopulations that might be typically predisposed to thiazide-induced hyponatremia, did not experience a change in mean serum sodium with T80/H25 treatment during this study.
Mean uric acid levels were increased by 1 mg/dL in patients receiving T80/H25, but remained constant in the T80 monotherapy group, effects that were found to be broadly consistent across the different patient subpopulations. The magnitude of the observed changes are in accordance with long-term changes in uric acid levels during the SHEP (Systolic Hypertension in the Elderly Program) study involving diuretic antihypertensive treatment with chlorthalidone monotherapy 25–50 mg.Citation24 The increases in triglycerides that were observed in most patient subpopulations receiving T80/H25 (mean 15 mg/dL) are smaller than those observed during the SHEP study (mean 21 mg/dL).Citation24 Asian patients had a mean increase in triglycerides of 31 mg/dL during the present study. Previous studies have indicated that increases in triglyceride and uric acid levels are associated with diuretic use, but may not be independent cardiovascular risk factors.Citation24,Citation25
Observational studies have reported an increase in the incidence of new-onset diabetes mellitus with thiazide diuretics compared with renin-angiotensin system blockers or calcium channel blockers, especially in hypertensive patients with risk factors of prediabetes or metabolic syndrome. Where an increase in blood glucose did occur following treatment with T80/H25 during this study, this increase was modest. The results of the metabolic analyses reported here are in accordance with other studies, which have indicated that the positive metabolic effects that result from adding an angiotensin II receptor blocker, such as telmisartan, may offset the negative metabolic effects of thiazide diuretic therapy.Citation26,Citation27
This planned analysis suggests that the T80/H25 single-pill combination provides consistent BP reductions in patients with moderate-to-severe hypertension across each of the different subpopulations studied. Furthermore, there were no discernible differences in treatment safety or tolerability between men and women, older and younger patients, racial subgroup, according to hypertension severity grade, or between previously untreated patients and those previously managed with one, two, or more antihypertensive agents.
Conclusion
In this planned analysis of a randomized, international, multicenter study in patients with grade 2 or 3 hypertension, response rates to treatment with a T80/H25 single-pill combination were, in general, similar across the different subpopulations of men and women, older and younger patients, those with grade 2 versus grade 3 hypertension, different races (black, Asian, white), and history of prior antihypertensive use. Treatment with the T80/H25 single-pill combination resulted in large BP reductions across all studied patient subpopulations. Single-pill combination treatment with T80/H25 was well tolerated in all the patient subgroups studied, with no marked difference in treatment-related adverse events compared with monotherapy. The percentage of patients with treatment-related adverse events was numerically higher in black patients compared with white and Asian patients in both treatment groups. The proportion of patients with adverse events leading to discontinuation was low and similar across the different patient subgroups. In conclusion, treatment with T80/H25 appears to provide large BP reductions and high goal attainment rates in patients regardless of gender, age, race, severity of hypertension, and prior antihypertensive treatment, with favorable safety and tolerability, which is likely to result in an overall positive impact on cardiovascular risk.
Disclosure
Medical writing assistance, supported f inancially by Boehringer Ingelheim, was provided by Danielle Russell, PhD, and Winnie McFadzean, PhD, of PAREXEL during the preparation of this article. The authors were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development, and have approved the final version. Dr Bays has received research grant support from Boehringer-Ingelheim, as well as numerous other pharmaceutical companies. Dr Voelker and Ms Mattheus are employees of Boehringer Ingelheim Pharma GmbH & Co. KG. The other authors report no conflicts of interest in this work.
References
- GradmanAHBasileJNCarterBLBakrisGLCombination therapy in hypertensionJ Clin Hypertens (Greenwich)201113314615421366845
- SeverPSMesserliFHHypertension management 2011: optimal combination therapyEur Heart J201132202499250621697169
- FranklinSSNeutelJMInitial combination therapy for rapid and effective control of moderate and severe hypertensionJ Hum Hypertens200923141118615100
- ChobanianAVBakrisGLBlackHRSeventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressureHypertension20034261206125214656957
- ManciaGDeBGDominiczakA2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC)J Hypertens20072561105118717563527
- CorraoGNicotraFParodiACardiovascular protection by initial and subsequent combination of antihypertensive drugs in daily life practiceHypertension201158456657221825231
- BangaloreSLeyLImproving treatment adherence to antihypertensive therapy: the role of single-pill combinationsExpert Opin Pharmacother201213334535522220825
- PloskerGLWhiteWBTelmisartan/hydrochlorothiazide: a review of its use as fixed-dose combinations in essential hypertensionDrugs200868131877189918729541
- ZhuDLBaysHGaoPMattheusMVoelkerBRuilopeLMEfficacy and tolerability of initial therapy with single-pill combination telmisartan/hydrochlorothiazide 80/25 mg in patients with grade 2 or 3 hypertension: a multinational, randomized, double-blind, active-controlled trialClin Ther20123471613162422717420
- ChapmanABSchwartzGLBoerwinkleETurnerSTPredictors of antihypertensive response to a standard dose of hydrochlorothiazide for essential hypertensionKidney Int20026131047105511849460
- NeutelJMLong-term blood pressure control: what can we do?Postgrad Med20111231889321293088
- JohnsonJAEthnic differences in cardiovascular drug response: potential contribution of pharmacogeneticsCirculation2008118131383139318809808
- GuptaAKRacial differences in response to antihypertensive therapy: does one size fits all?Int J Prev Med20101421721921566775
- OfiliEOFerdinandKCSaundersEIrbesartan/HCTZ fixed combinations in patients of different racial/ethnic groups with uncontrolled systolic blood pressure on monotherapyJ Natl Med Assoc200698461862616623075
- CushmanWCNeutelJMSaundersEEfficacy and safety of fixed combinations of irbesartan/hydrochlorothiazide in older vs younger patients with hypertension uncontrolled with monotherapyAm J Geriatr Cardiol2008171273618174757
- OfiliEOCableGNeutelJMSaundersEEfficacy and safety of fixed combinations of irbesartan/hydrochlorothiazide in hypertensive women: the inclusive trialJ Womens Health (Larchmt)200817693193818681815
- NashDTCrikelairNZappeDAchieving BP goals with valsartan and HCTZ alone and in combination: pooled analysis of two randomized, double-blind, placebo-controlled studiesCurr Med Res Opin20082492617262618687165
- WhiteWBDavidaiGSchumacherHImpact of angiotensin receptor blockade in combination with hydrochlorothiazide 25 mg in 2121 patients with stage 1–2 hypertensionJ Hum Hypertens2009231281782519357698
- WeirMRRolfeMPotassium homeostasis and renin-angiotensin-aldosterone system inhibitorsClin J Am Soc Nephrol20105353154820150448
- KohKKQuonMJHanSHDistinct vascular and metabolic effects of different classes of anti-hypertensive drugsInt J Cardiol20101401738119059660
- SrivastavaTNYoungDBImpairment of cardiac function by moderate potassium depletionJ Card Fail1995131952009420651
- FranseLVPahorMDiBMSomesGWCushmanWCApplegateWBHypokalemia associated with diuretic use and cardiovascular events in the Systolic Hypertension in the Elderly ProgramHypertension20003551025103010818057
- HwangKSKimGHThiazide-induced hyponatremiaElectrolyte Blood Press201081515721468197
- SavagePJPresselSLCurbJDInfluence of long-term, low-dose, diuretic-based, antihypertensive therapy on glucose, lipid, uric acid, and potassium levels in older men and women with isolated systolic hypertension: The Systolic Hypertension in the Elderly Program. SHEP Cooperative Research GroupArch Intern Med199815877417519554680
- BrandFNMcGeeDLKannelWBStokesJIIICastelliWPHyperuricemia as a risk factor of coronary heart disease: the Framingham StudyAm J Epidemiol1985121111183964986
- SowersJRRaijLJialalIAngiotensin receptor blocker/diuretic combination preserves insulin responses in obese hypertensivesJ Hypertens20102881761176920498618
- KalraSKalraBAgrawalNCombination therapy in hypertension: An updateDiabetol Metabol Syndr2010214454