Abstract
Nitric oxide (NO) is an important regulator of vascular tone, and is also an antithrombotic, anti-inflammatory, antiproliferative, and antiatherogenic factor. Endothelial function is altered in patients with coronary artery disease, stroke, and peripheral artery disease, and endothelial dysfunction correlates with the risk factor profile for a patient. Hypertension and type 2 diabetes are risk factors for vascular disease, and are both pathologies characterized by loss of NO activity. Indeed, endothelial dysfunction is usually present in diabetic and/or hypertensive patients. Tetrahydrobiopterin is an essential cofactor for the NO synthase enzyme, and insufficiency of this cofactor leads to uncoupling of the enzyme, release of superoxide, endothelial dysfunction, progression of hypertension, and finally, proatherogenic effects. Tetrahydrobiopterin is also an important mediator of NO synthase regulation in type 2 diabetes and hypertension, and may be a rational therapeutic target to restore endothelial function and prevent vascular disease in these patients. The aim of this paper is to review the rationale for therapeutic strategies directed to biopterins as a target for vascular disease in type 2 diabetic hypertensive patients.
Introduction
The endothelium maintains the integrity of the vascular system via interaction between nitric oxide (NO) and vasoconstrictive factors.Citation1 Endothelial dysfunction develops when the bioavailability of NO decreases, triggering a vasoconstrictive, proliferative, proinflammatory, and procoagulant condition that facilitates vascular damage.Citation1,Citation2
Both type 2 diabetes and hypertension increase oxidative stress and lead to endothelial dysfunction.Citation1 Endothelial dysfunction plays a key role in the pathophysiology of atherogenesis and diabetes-associated vascular disease, and explains, at least in part, the enhanced progression of cardiovascular disease in type 2 diabetes.Citation3
Despite being a radical, oxygen is sparingly reactive because its two unpaired electrons are situated in different molecular orbits. However, in endothelial cells, oxygen undergoes univalent reduction to form superoxide by means of enzymes such as nicotinamide adenine dinucleotide phosphate (NADH/NADPH) oxidase.Citation4 Vascular NADH/NADPH oxidase is active during normal metabolism,Citation5 and sustained activation of this enzyme occurs in response to several stimuli, including angiotensin II, thrombin, platelet-derived growth factor, endothelin-1, tumor necrosis factor-alpha (TNFα), hypercholesterolemia, and hyperglycemia.Citation6 Also, some vascular flow conditions may determine regulation of NADPH oxidase, whereby laminar shear stress downregulates NADPH oxidase activity, whereas oscillatory shear stress induces a sustained increase in oxidase activity.Citation4
Tetrahydrobiopterin
It was once believed that the only function of tetrahydrobiopterin (BH4) was as a cofactor for the activity of phenylalanine, tyrosine, and tryptophan hydroxylases during neurotransmitter synthesis. However, 20 years ago, when nitric oxide synthase (NOS) was characterized, BH4 was soon identified as one of its essential cofactors.Citation7 Since this observation, BH4 has been implicated as a significant determinant of NO bioavailability and concomitant conduit/resistance vessel functions.
Each monomer of endothelial NOS (eNOS) has one BH4 binding site in the oxygenase domain and because the enzyme acts functionally as a dimer, two molecules of BH4 are incorporated into each eNOS complex.Citation8 In the active site, BH4 stabilizes the ferrous-dioxygen complex, and the cofactor also donates electrons to the oxygenase domain, and this is the initiating step of L-arginine oxidation.Citation9,Citation10 If BH4 is reduced, electron transfer from eNOS becomes uncoupled from L-arginine oxidation, the ferrous-dioxygen complex dissociates, and the enzyme produces superoxide instead of NO.
When large amounts of reactive oxygen species (ROS) are present in the endothelial cell, electron transfer within the active site of eNOS becomes “uncoupled” from L-arginine oxidation. This process is known as eNOS uncoupling and under those conditions, electron flow through the enzyme results in reduction of molecular oxygen at the prosthetic heme site rather than formation of NO, and molecular oxygen is reduced to form superoxide, leading to endothelial dysfunction.Citation11
Several studies have shown that when BH4 is oxidized to dihydrobiopterin (BH2), the bioavailability of BH4 for eNOS is reduced. This is seen when BH4 reacts with super-oxide or with peroxynitrite, which leads to eNOS uncoupling and finally, to endothelial dysfunction.Citation11 In addition, BH2 (which has no cofactor activity) may compete with BH4 for the oxygenase domain in eNOS, leading to decreased eNOS activity.Citation8
Tetrahydrobiopterin synthesis
Biosynthesis of BH4 can occur by one of three pathways, ie, from guanosine triphosphate cyclohydrolase I (GTP-CHI) via a de novo synthetic pathway, from sepiapterin via the salvage pathway, and via recycling pathways.Citation12
Via the de novo pathway, BH4 synthesis is initiated by the action of GTP-CHI, which represents the rate-controlling enzyme and initiates GTP degradation to 7,8 dihydroneopterin triphosphate, which is converted to 6-pyruvoyl-tetrahydropterin by the 6-6-pyruvoyl synthase enzyme. Finally, the 6-pyruvoyl-tetrahydropterin is reduced to BH4 by NADPH-dependent sepiapterin reductase ().
Figure 1 Tetrahydrobioterin synthesis.
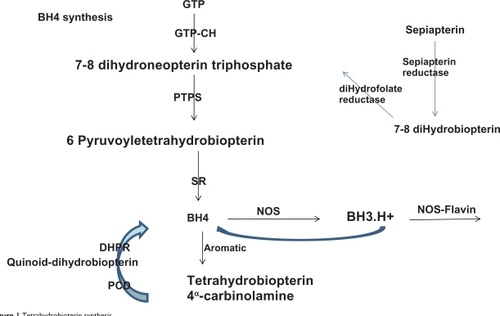
Table 1 Factors that increase guanosine triphosphate cyclohydrolase I activity
Via the salvage pathway, sepiapterin is metabolized to 7,8-BH2 and finally, to BH4 by two NADPH-dependent enzymes, ie, sepiapterin reductase and dehydrofolate reductase (). However, sepiapterin is not a physiologic metabolite in humans or animals.Citation9
In the recycling pathway, BH4 is generated differently, depending on the enzymes involved. When aromatic aminohydrolases are present, BH4 is regenerated from BH4α-carbinolamine in a two-step process, whereby pterin-4α carbinolamine dehydratase produces the quinonoid BH2 intermediate, which is subsequently reduced by 6,7-dihydropteridine reductase. When NOS is involved, BH4 is transformed to a BH3.H+ radical, which is subsequently reduced in the next catalytic cycle by electron transfer from eNOS flavinsCitation9 ().
Regulation of tetrahydrobiopterin
As mentioned in the previous section, the GTP-CH enzyme is critical for BH4 synthesis, and several studies have demonstrated that vascular agonists may increase BH4 production through GTP-CH mRNA induction. Incubation of endothelial cells with phenylalanine increases mRNA levels for GTP-CH by 2.2-fold after 6 hours,Citation13 and interestingly, similar concentrations of phenylalanine increase BH4 levels in bovine coronary endothelial cells by 16%.Citation14 Insulin increases mRNA for GTP-CH, which enhances endothelial BH4 through the activation of the de novo pathway.Citation15 Addition of 17β-estradiol to culture medium elevated both mRNA levels for CGT-CH and intracellular BH4 levels in brain microvascular endothelial cells.Citation16 Inflammatory cytokines, such as interleukin (IL)-1β, interferon-γ or TNFα, increase BH4 synthesis, probably through the effect of increasing mRNA for GTP-CH.Citation17 Finally, statins and cyclosporine can increase BH4 synthesis and GTP-CH mRNA in cultures of endothelial cells.Citation14,Citation18
On the other hand, several studies have demonstrated that some vascular agonists may decrease BH4 production through GTP-CH mRNA downregulation. Johns et al found that dexamethasone decreases the expression of GTP-CH mRNA and BH4 levels in endothelial cells.Citation19 Similarly, inflammatory cytokines, such as IL-4, IL-10, and transforming growth factor-beta, decrease BH4 synthesis, probably through GTP-CH mRNA downregulation.Citation20
In addition to regulation of BH4 synthesis through these already described mRNA mechanisms, there are two other conditions where changes in BH4 bioavailability have been demonstrated. It has recently been reported that increasing concentrations of L-arginine raised intracellular levels of BH4 production in cultured bovine coronary endothelial cells. This effect is specific for the L-isomer and is not affected by the use of arginase inhibitors.Citation14 This in vitro observation was further supported by in vivo studies in which dietary supplementation of L-arginine increased BH4 availability in coronary endothelial cells from control and diabetic rats.Citation21 In light of the reducing properties of BH4, the possibility that oxidative stress may affect BH4 availability arises. Indeed, the use of peroxynitrite in intact arteries demonstrated that oxidative stress is associated with BH4 reduction.Citation22 Moreover, the hypothesis that BH4 acts as a reducing agent is further supported by the demonstration that vitamin C stabilizes BH4 in cultured endothelial human cells, through a mechanism not related to the interaction of vitamin C and superoxide anions.Citation23
Interaction between tetrahydrobiopterin and nitric oxide synthase
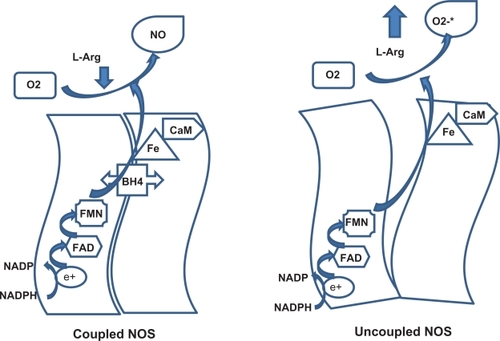
NO is synthesized from L-arginine by NOS. Molecular cloning has identified three distinct NOS isoforms,Citation24 two of which are expressed constitutively in neurons and vascular endothelial cells, and are activated by increased intracellular calcium levels. The expression of the third isoform is induced in a calcium-independent fashion by several agonists. All NOS isoforms catalyze the reaction of 1.5 mol NADPH + 1 mol L-arginine to 1 mol citrulline + 1 mol NO + 1.5 NADP+. This reaction is fully dependent on BH4. NOS contains a prosthetic heme group catalyzing the reductive activation of molecular oxygen which is required for L-arginine oxidation, as well as tightly bound flavins shuttling NADPH-derived electrons to the heme ().Citation25 In this reaction, heme Fe3+ is reduced by flavin mononucleotide to heme Fe2+, which binds molecular oxygen to form an unstable dioxygen complex (heme-Fe-O-O) that, in the presence of BH4, is converted to heme-Fe3+-O-OH, then converted to heme-Fe5+=O, to participate in the release of NO (). BH4 in this reaction participates in the following events: BH4 binding to NOS stabilizes loose dimer conformation;Citation26 BH4 binding to NOS enhances L-arginine binding to heme and inhibits superoxide release from the heme oxygenase domain of the enzyme.Citation27
NO synthesis by eNOS can be affected by different stimuli, ie, signaling molecules (integrated into caveolae), phosphorylation (by Akt) at serine 177 residues in response to shear stress or hormones (such as estrogens or insulin) activating the enzyme, whereas phosphorylation at threonine 459 (by protein kinase C) decreases the activation of eNOS. Dephosphorylation of threonine 459 in response to bradykinin activates eNOS.Citation8
Deficient BH4 levels in several in vitro and in vivo animal models have correlated with low NO production in vascular tissue.Citation28 Recent studies suggest that disruption of the zinc-thiolate complex at the dimer interface, close to the BH4 binding site, leads to loss in BH4 from the binding site, enzymatic uncoupling, and destabilization of eNOS dimers.Citation29
The data suggest that eNOS uncoupling and increased nitrosylation of eNOS, decreased expression of GTP-CHI and sepiapterin reductase, and subsequent reduced BH4 bioavailability, may be important contributors to endothelial dysfunction and its consequences.
NO produced by endothelial cells protects blood vessels from thrombosis, and has antiatherosclerotic activity. Enhanced NO bioactivity reduces atherosclerosis progression through multiple mechanisms (). All major risk factors for atherosclerosis are associated with impairment of NO activity.Citation15
Table 2 Antiatherosclerotic effects of nitric oxide
Superoxide generation has been implicated in a variety of experimental and clinical vascular disease states, including diabetes, cigarette smoking, hypertension, chronic nitrate tolerance, and overt atherosclerosis, not only because of the increased production of ROS but also because of the reduced formation of the protective molecule NO.Citation31
Tetrahydrobiopterin in disease
In the absence of enough BH4, instead of oxidizing L-arginine, eNOS reduces molecular oxygen to superoxide, leading to endothelial dysfunction.Citation32 When NO reacts with superoxide, it loses its vasodilatory, antiatherogenic, antithrombotic, anti-inflammatory, and antiproliferative effects.Citation33
BH4 prevents peroxynitrite-induced nitration of proteins, and the prevention of nitration of tyrosine residues in cells stimulated by proinflammatory cytokines avoids the affectation of proteins involved in energy production, fatty acid metabolism, apoptosis, and oxidative stress induced by peroxynitrite.Citation34
Experimental evidence reveals that altered glucose metabolism results in low BH4 bioavailability, and also that BH4 levels are reduced in diabetic rats. Interestingly, dietary supplementation with BH4 in insulin-resistant rats prevents impaired endothelial-dependent vasodilatation.Citation12
The main cause of impaired endothelium-dependent relaxation in insulin-resistant rats is abnormal metabolism of BH4. The mechanism seems to be an imbalance between NO and superoxide anion.Citation32 NO regulates vascular tone and blood pressure, and a reduction in NO bioavailability increases vascular tone and raises blood pressure,Citation4 so BH4 may be involucrate in the maintenance of blood pressure and a decrease in its bioavailability may play a role in the pathways that lead to hypertension.Citation12
Therapeutic considerations
BH4 as a therapeutic agent
Several in vivo studies in animals and in humans have shown the beneficial effect of BH4 on endothelial dysfunction and its vasoprotective properties. Therefore, enhancing BH4 synthesis or bioavailability in endothelial cells may be a good strategy for the prevention and treatment of cardiovascular disease, especially in high-risk patients, such as hypertensive and diabetic subjects.Citation34
When BH4 is administrated in healthy volunteers, the bioavailability of NO is increased. Several clinical trials have evaluated the use of BH4 for the management of endothelial dysfunction, and it has been shown that this cofactor increases acetylcholine-induced vasodilatation in coronary arteries in patients with coronary heart disease.Citation35
The administration of BH4 improves forearm circulation in smokers, and diabetic or hypercholesterolemic patients, then, actions that lead to improve BH4 availability may be effective in restoring NO-mediated endothelial function and limiting vascular disease progression in several conditions, such as atherosclerosis, diabetes, and hypertension.Citation36 Indeed, infusion of BH4 improves the endothelial-dependent vasodilation response to acetylcholine but not the endothelium-independent vasodilation response to nitroprusside. In patients with type 2 diabetes, the cofactor has no effect in control subjects.Citation32
In healthy subjects, impairment of endothelial function induced by an oral glucose challenge was reversed by the active cofactor BH4, but not by an inactive stereoisomer.Citation37 In patients with type 2 diabetes, infusion of BH4 corrects endothelial dysfunction via an NO-dependent pathway.Citation12
The administration of BH4 to spontaneously hypertensive rats is associated with improvement of endothelial function and prevention of hypertension and ventricular hypertrophy.Citation12
Porkert et al found that oral administration of BH4 400 mg had a significant antihypertensive effect in poorly controlled hypertensive patients.Citation38 They also found an improvement of endothelial function in this study, and the beneficial effect of BH4 was maintained during the eight-week study duration without tachyphylaxis. However, only patients receiving the BH4 dose of 400 mg or higher responded to the biopterin, whereas patients at a daily dose of 200 mg did not achieve a significant reduction in blood pressure.
Settergren et al showed that the administration of L-arginine and BH4 improves endothelial function and reduces endothelial dysfunction induced by ischemia-reperfusion in type 2 diabetic patients with coronary artery disease.Citation39 However, both agents were given in combination, so it was not possible to evaluate if administration of each agent alone would demonstrate the same results or if the combination is necessary. It is important to say that L-arginine as monotherapy was not effective, and perhaps harmful, in patients with intermittent claudication and peripheral artery disease.Citation40
BH4 has shown efficacy in the treatment of erectile dysfunction. Sommer et al found that BH4 administration led to a significant increase in duration of penile rigidity, making BH4 suitable as a treatment for erectile dysfunction.Citation41
Oral administration of BH4 slows the progression of atherosclerosis and reduces the expression of NADPH oxidase and inflammatory factors in hypercholesterolemic apolipoprotein E-knockout mice.Citation42
Other pharmacologic interventions to restore BH4 bioavailability and eNOS function
Nitrates
Nitrates (isosorbide, nitroglycerin) have been used as NO donors because they release NO.Citation43 Unfortunately, chronic administration of nitroglycerin has been associated with vascular production of superoxide and poor clinical benefits.Citation12,Citation44 A long-acting nitrate, pentaerythritol tetranitrate, may confer a better long-term cardiovascular prognosis.Citation44
L-arginine
Several clinical studies have shown vasodilatation and enhanced NO production after administration of L-arginine. Drexler et al (cited by Tenenbaum et alCitation45) showed that L-arginine enhanced the blood flow response to acetylcholine in patients with coronary artery disease but not in controls. Since then, there have been many studies with L-arginine in healthy human subjects and in patients with various cardiovascular conditions.Citation45
In contrast, there have been several relatively small clinical studies with experimental endpoints that failed to show beneficial effects of L-arginine on vascular function. The evidence suggests that there may be subgroups of patients whose vascular function is improved by L-arginine supplementation, and other subgroups of patients who do not respond to this intervention. Because patients on “optimized medical treatment” and those with advanced coronary stenoses showed less effect, and L-arginine was more effective when early changes in vascular function were chosen as endpoints. It is possible that this amino acid may have a role as a nutraceutical agent (daily doses below 2 to 3 g/day appear to be without beneficial effect) in the modification of functional impairment and in the prevention of vascular disease, but not as a therapy to reverse manifest atherosclerosis.Citation45,Citation46 In animal models, long-term administration of L-arginine inhibits the formation of atherosclerotic plaques.Citation44
Folic acid
Because folic acid participates in the BH4 recycling pathway, several studies have been performed to investigate its use for the treatment of cardiovascular disease. Some results had shown that this agent may restore endothelial function in diabetic patients, as well as in hypercholesterolemic subjects, and that it reduces atherosclerotic plaques in mice.Citation44,Citation46 Unfortunately, those results have not been confirmed by recent clinical trials.Citation47
Ascorbate
Ascorbate increases BH4 in endothelial cells via prevention of oxidation of this biopterin, without an effect on its synthesis, and may explain the effect of ascorbate on blood pressure in hypertensive patients.Citation33
Sepiapterin
Because sepiapterin is a precursor in the alternative BH4 synthesis pathway, it has been proposed as a therapeutic approach for endothelial dysfunction.Citation12 Indeed, acute studies have shown that administration of sepiapterin restores endothelial function.Citation44 However, because sepiapterin competes with BH4 for the oxygenase domain in eNOS, higher doses of this precursor may increase superoxide production and decrease NO bioavailability.Citation12,Citation44 There are no studies that have evaluated the long-term effects of sepiapterin in vascular disease. This agent did not increase BH4 levels, but decreased BH2 concentrations,Citation44 and a recent study shows that the BH4/BH2 ratio may be even more important than absolute BH4 levels for eNOS functioning.Citation49
Statins
Statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) are used in the management of dyslipidemia. These drugs have cholesterol-independent effects, may upregulate NO expression, and there is evidence that statins modulate atherogenesis, plaque rupture, and thrombosis.Citation50
Hattori et al found that statins elevate GTP-CH mRNA. Because GTP-CH is the rate-limiting step in the synthesis of BH4, statins elevate levels of this biopterin in vascular endothelial cells, and they also enhance eNOS expression and increase the ratio of BH4/BH2.Citation51
Because statins enhance BH4 synthesis, increase NO production, and prevent relative shortages of BH4, they also inhibit vascular NADPH oxidase and reduce oxidative stress,Citation44 thereby normalizing endothelial function. In humans, statins not only reduce cardiovascular morbidity and mortality, but also reduce progression of coronary atherosclerosis in patients with coronary heart disease.Citation52
Antagonism of the renin-angiotensin-aldosterone system
Angiotensin II activates NADPH oxidase via AT1 receptor activation in vascular cells. However, NADPH oxidase is probably not the only source of ROS stimulated by angiotensin II. As we have reviewed, one consequence of increased superoxide production in response to angiotensin II is inactivation of NO and resulting endothelial dysfunction, which is one of the earliest steps in the atherosclerotic process.Citation52
Several studies have shown that inhibition of AT1 receptor activation by AT1 receptor antagonists or angiotensin-converting enzyme (ACE) inhibitors normalizes oxidative stress and improves endothelial dysfunction, and ACE inhibitors are known to retard the progression of atherosclerosis and heart failure.Citation53 Both AT1 receptor antagonists and ACE inhibitors provide an equivalent beneficial effect on risk of death and cardiovascular events.Citation54
Like angiotensin II, aldosterone may also promote endothelial dysfunction and vascular disease. Imanishi et al found that eplerenone, an aldosterone antagonist, alone or combined with enalapril, reduces NADPH activity, and elevates both BH4 levels and NO bioavailability in endothelial cells of hyperlipidemic rabbits.Citation55
Aliskiren, a direct renin inhibitor, as monotherapy or in combination with an AT1 receptor antagonist, also decreases NADPH oxidase activity, increases BH4 levels, and enhances NO bioavailability. Aliskiren also has an antiatherosclerotic effect.Citation56
Conclusion
BH4 availability is a critical determinant of eNOS regulation in atherosclerosis and is a rational therapeutic target to restore NO-mediated endothelial function and reduce disease progression in high-risk patients.
Strategies aimed at increasing BH4 biosynthesis or levels, reducing BH4 oxidation, or enhancing BH4 regeneration seem to be useful and safe therapeutic options for the prevention of macrovascular complications in patients at high risk for cardiovascular complications, in particular patients with type 2 diabetes and/or hypertension.
Disclosure
AFRG is a research fellow, and received a grant from Consejo Nacional de Ciencia y Tecnologia (CONACYT) Mexico in support of this research.
References
- GhanemFAMovahedAInflammation in high blood pressure: A clinical perspectiveJ Am Soc Hypertens2007111311920409841
- Rubio-GuerraAFVargas-RoblesHVargas-AyalaGRodríguez-LopezLEscalante-AcostaBAThe effect of trandolapril and its fixed-dose combination with verapamil on circulating adhesion molecules levels in hypertensive patients with type 2 diabetesClin Exp Hypertens20083068268818855271
- RubioAFVargasHMacedaALozanoJJEscalanteBACorrelation between the levels of circulating adhesion molecules and atherosclerosis in type-2 diabetic normotensive patientsCell Adh Migr2009336937219717975
- MadamanchiNRVendrovARungeMSOxidative stress and vascular diseaseArterioscler Thromb Vasc Biol200525293815539615
- GriendlingKKSorescuDUshio-FukaiMNAD(P)H oxidase: Role in cardiovascular biology and diseaseCirc Res20008649450110720409
- KimJMontagnamiMKonKQuonMJReciprocal relations between insulin resistance and endothelial dysfunctionCirculation20061131888190416618833
- KwonNSNathanCFStuerhrDJReduced biopterin as a cofactor in the generation of nitrogen oxides by murine macrophagesJ Biol Chem198926420496205012584226
- AlpNJChannonKMRegulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular diseaseArterioscler Thromb Vasc Biol20052441342014656731
- GrossSJonesSHattoriCLRamanCSTetrahydrobiopterin: An essential cofactor of nitric oxide synthase with an elusive roleIgnarroLJNitric Oxide Biology and PathobiologyNew York, NYAcademic Press2000
- HurshmanARKrebsCEdmondsonDHuynhBHMarlettaMAFormation of a pterin radical in the reaction of the heme domain of inducible nitric oxide synthase with oxygenBiochemistry199938156891569610625434
- BauersachsJWidderJDTetrahydrobiopterin, endothelial nitric oxide synthase, and mitochondrial function in the heartHypertension20095390790819398653
- SchmidtTSAlpNJMechanisms for the role of tetrahydrobiopterin in endothelial function and vascular diseaseClin Sci2007113476317555404
- GesierichANiroomandFTiefenbavherCPRole of human GTP cyclohydrolase I and its regulatory protein in tetrahydrobiopterin metabolismBasic Res Cardiol200298697512607127
- WuGKellyKAHatakeyamaKMeiningerCJRegulation of endothelial tetrahydrobiopterin synthesis by l-arginineBlauNThonyBPterins, Folates and Neurotransmitters in Molecular MedicineHeilbronnSPS Verlagsgesellschaft mbH2004
- IshiiMShimizuSNagaiTShiotaKKiruchiYYamamotoTStimulation of tetrahydrobiopterin synthesis by insulin: Possible involvement of phosphatidylinositol 3-kinaseInt J Biochem Cell Biol200133657311167133
- ShiotaKIshiiMYamamotoTShimizuSKiuchiYStimulation of tetrahydrobiopterin synthesis by 17β-estradiol in brain microvascular endothelial cellsPteridines200011129132
- Rosenkrantz-WeissPSessaWCMilsteinSKaufmanSWatsonCAPoberJSRegulation of nitric oxide synthesis by proinflammatory cytokines in human umbilical vein endothelial cellsJ Clin Invest199493223622437514193
- IshiiMShimizuSShiotaKYamamotoSKiuchiYYamamotoTStimulation of tetrahydrobiopterin synthesis by cyclosporin A during lipopolysaccharide treatment in vascular endothelial cellsPteridines2002138993
- JohnsDGDorranceAMTramontiniNLWebbRCGlucocorticoids inhibit tetrahydrobiopterin-dependent endothelial functionExp Biol Med20012262731
- SchoedonGScheemannMBlauNEdgellCJSChafnerAModulation of human endothelial cell tetrahydrobiopterin synthesis by activating and deactivating cytokines: New perspectives on endothelium-derived relaxing factorBiochem Biophys Res Comm1993196134313488250889
- KohliRMeiningerCJHaynesTEYanWSelfJTWuGDietary L-arginine supplementation enhances endothelial nitric oxide synthesis in streptozotocin-induced diabetic ratsJ Nutr200413460060914988454
- LaursenJBSomersMKurzSEndothelial regulation of vasomotion in ApoE-deficient mice. Implications for interactions between peroxynitrite and tetrahydrobiopterinCirculation20011031282128811238274
- BakerHMilstienSKatusikZSEffect of vitamin C on the availability of tetrahydrobiopterin in human endothelial cellsJ Cardiovasc Pharmacol20013733333811243424
- KnowlesRGMoncadaSMammalian nitric oxide synthaseBiochem J19942982492547510950
- CosentinoFLuscherTFTetrahydrobiopterin and endothelial nitric synthase activityCardiovasc Res19994327427810536654
- WhitsettJMartasekPZhaoHEndothelial cell superoxide anion radical generation is not dependent on endothelial nitric oxide synthaseserine 1179 phosphorylation and endothelial nitric oxide dimer/monomer distributionFree Rad Biol Med2006402056205816716906
- Vazquez-VivarJTetrahydrobiopterin, superoxide and vascular dysfunctionFree Rad Biol Med2009471108111919628033
- CrabtreeMJSmithCILamGGoligorskyMSGrossSSRatio of 5,6,7,8-tetrahydrobiopterin to 7,8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in NO vs superoxide production by eNOSAm J Physiol Heart Circ Physiol2008294H1530H154018192221
- ZouMHShiCCohenRAOxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitriteJ Clin Invest200210981782611901190
- YangYHuangAKaleyGSunDeNOS uncoupling and endothelial dysfunction in aged vesselsAm J Physiol Heart Circ Physiol2009297H1829H183619767531
- StuehrDPouSRosenGMOxygen reduction by nitric-oxide synthasesJ Biol Chem2001276145331453611279231
- HeitzerTKrohnKAlbersSMeinertzTTetrahydrobiopterin improves endothelial vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitusDiabetologia2000431435143811126415
- LandmesserUDikalovSPriceSROxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertensionJ Clin Invest20031111201120912697739
- KatusicZSd’UscioLNathKAVascular protection by tetrahydrobiopterin: Progress and therapeutic prospectsTrends Pharmacol Sci200930485419042039
- ArtuncFEssigMArtuncNEffects of tetrahydrobiopterin on nitric oxide bioavailability and renal hemodynamics in healthy volunteersJ Nephrol20082185086019034869
- AlpNJMcAteerMAKhooJChoudhuryRPChannonKMIncreased endothelial tetrahydrobiopterin synthesis by targeted transgenic GTP-cyclohydrolase I overexpression reduces endothelial dysfunction and atherosclerosis in ApoE-knockout miceArterioscler Thromb Vasc Biol20042444545014707037
- IhlemannNRask-MadsenCHTetrahydrobiopterin restores endothelial dysfunction induced by an oral glucose challenge in healthy subjectsAm J Physiol2003285H875H882
- PorkertMSherSReddyUTetrahydrobiopterin: A novel anti-hypertensive therapyJ Hum Hypertens20082240140718322548
- SettergrenMBöhmFMalmströmREChannonKMPernowJL-arginine and tetrahydrobiopterin protects against ischemia/reperfusion-induced endothelial dysfunction in patients with type 2 diabetes mellitus and coronary artery diseaseAtherosclerosis2009204737818849028
- WilsonAMHaradaRNairNBalasubramanianNCookeJPL-arginine supplementation in peripheral arterial disease: No benefit and possible harmCirculation200711618819517592080
- SommerFKlotzTSteineritzDBlochWEvaluation of tetrahydrobiopterin as a potential therapeutic agent to treat erectile dysfunctionAsian J Androl2006815916716491266
- HattoriYHattoriSWangXSatohHNakanishiNKasaiKOral administration of tetrahydrobiopterin slows the progression of atherosclerosis in apolipoprotein E-knockout miceArterioscler Thromb Vasc Biol20072786587017272747
- RubioAFVargasGLozanoJJNarvaezJLRodriguezLComparison between isosorbide dinitrate aerosol and nifedipine in the management of hypertensive emergenciesJ Hum Hypertens19991347347610449212
- LiHFörstermannUPrevention of atherosclerosis by interference with the vascular nitric oxide systemCurr Pharm Des2009153133314519754387
- TenenbaumAFismanEZMotroML-arginine: Rediscovery in progressCardiology1998901531599892762
- BogerRHThe pharmacodynamics of L-arginineJ Nutr2007137S1650S1655
- StroesESGvan FaassenEEYoMFolic acid reverts dysfunction of endothelial nitric oxide synthaseCirc Res2000861129113410850963
- The Heart Outcomes Prevention Evaluation (HOPE) 2 InvestigatorsHomocysteine lowering with folic acid and B vitamins in vascular diseaseN Engl J Med20063541567157716531613
- AlpNJMussaSKhooJTetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetes by targeted transgenic GTP–cyclohydrolase I overexpressionJ Clin Invest200311272573512952921
- LaufsULa FataVPlutzkyJLiaoJKUpregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitorsCirculation199897112911359537338
- HattoriYNakanishiNAkimotoKYoshidaMKasaiKHMG-CoA reductase inhibitor increases GTP cyclohydrolase I mRNA and tetra-hydrobiopterin in vascular endothelial cellsArterioscler Thromb Vasc Biol20032317618212588756
- NissenSETuzcuEMSchoenhagenPEffect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: A randomized controlled trialJAMA20042911071108014996776
- NickeningGHarrisonDGThe AT1-type angiotensin receptor in oxidative stress and atherogenesis. Part 1Circulation200210539339611804998
- DavidBMatcharDBMcCroryDCSystematic review: Comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treating essential hypertensionAnn Intern Med2008148162917984484
- ImanishiTIkejimaHTsujiokaHAddition of eplerenone to an angiotensin-converting enzyme inhibitor effectively improves nitric oxide bioavailabilityHypertension20085173474118227404
- ImanishiTTsujiokaHIkejimaHRenin inhibitor aliskiren improves impaired nitric oxide bioavailability and protects against atherosclerotic changesHypertension20085256357218645051