Abstract:
Ethionamide (ETA) and prothionamide (PRO) are interchangeably used in tuberculosis (TB) chemotherapy regimens. Subtle discrepancies between biochemical and genetic information on the modes of sensitivity and resistance of isoniazid (INH) and ETA warrants further studies. We report a new mutation – EthAW21R – in Mycobacterium bovis Bacillus Calmette-Guérin that corresponds with co-resistance to both PRO and ETA, which to the best of our knowledge has not been reported before. Our findings suggest that mutation EthAW21R could be used as a marker site for testing PRO and ETA cross-resistance.
The thioamide, ethionamide (ETA), and its propyl-analog prothionamide (PRO) are interchangeably used in tuberculosis (TB) chemotherapy regimens to treat multidrug-resistant TB (MDR-TB),Citation1–Citation3 drug-susceptible TB meningitis (TBM), and miliary TB in some settings, due to their good cerebrospinal fluid (CSF) penetration ability.Citation4
PRO is associated with better tolerance compared with ETA in the treatment of MDR-TB, and both are structurally similar to isoniazid (INH).Citation2,Citation5,Citation6 The only notable distinction in their mechanism(s) of action is the lack of cross-resistance to INH.Citation7,Citation8
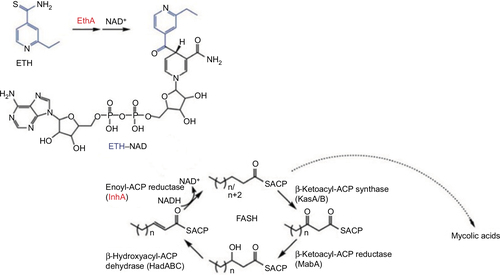
Both ETA and PRO are prodrugs whose enzymatic activation by Mycobacterium tuberculosis’ EthA inhibits InhA, which subsequently inhibits the M. tuberculosis’ mycolic acid synthesis ().Citation2,Citation9 Mutations in the ethA gene often underlie ETA and PRO monoresistance.Citation2 Hanoulle et alCitation10 postulated that both are further transformed by EthA enzyme to a metabolite that accumulates intracellularly and acts as the final toxic compound. As illustrated in ,Citation11 activated ETA and PRO form adducts with nicotinamide adenine dinucleotide (NAD), which is the inhibitor of the InhA enzyme in M. tuberculosis.Citation1,Citation12,Citation13 Thee et alCitation2 suggested that the correlation between mutations conferring ETA resistance and the MIC warrants further studies because of the subtle discrepancies between biochemical and genetic information on the modes of sensitivity and resistance in the cases of INH and ETA.
Figure 1 The mechanism of action for ETA.
Abbreviations: ETA, ethionamide; NAD, nicotinamide adenine dinucleotide.
Here, we report a new mutation – EthAW21R – in Mycobacterium bovis Bacillus Calmette-Guérin (BCG) that corresponds with co-resistance to PRO and ETA, which to the best of our knowledge has not been reported before.
We screened wild-type M. bovis BCG Tice on high PRO concentrations and obtained one drug-resistant colony at 30 µg/mL PRO-containing 7H11 plate. To confirm the phenotypic resistance of the single colony, we similarly re-tested it on 30 and 40 µg/mL PRO-containing 7H11 plate. We sequenced the six reported genes (ethR, ethA, inhA katG, ndh, and ahpC; ; BGI, Shenzhen, China) associated with ETA and PRO resistance and found a single-nucleotide mutation in ethA gene leading to W21R mutation while the other five genes had no mutation(s).
Table 1 PCR and sequencing primers used to delineate target-based spontaneous genotypic resistance mechanisms of M. bovis BCG Tice
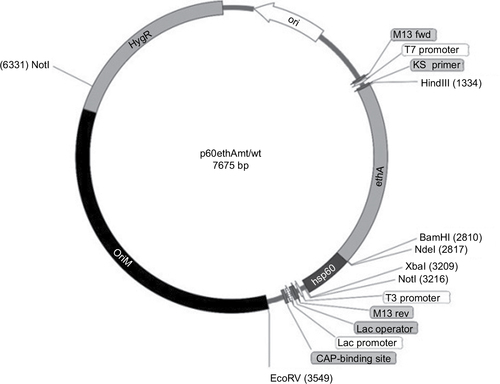
We then overexpressed this mutated 1.4kb ethAW21R and the M. bovis ethAwt genes by cloning them at the NdeI and HindIII sites of extrachromosomal p60LuxN plasmid bearing the M. tuberculosis hsp60 promoter ().Citation14 Recombinant plasmids p60ethAW21Rand p60ethAwt constructs were verified by enzyme digestion and sequencing (BGI). Wild-type M. bovis BCG Tice and M. tuberculosis H37Rv strains were transformed with the plasmids p60ethAW21Rand p60ethAwt through electroporation as described previously with some modifications.Citation15 Positive selection was confirmed by PCR amplification of the hygromycin resistance marker gene (hyg) in p60ethAW21Randp60ethAwt using primers hyg-r and hyg-f ().
Figure 2 E. coli–mycobacteria shuttle plasmids p60ethAMt/Wt.
Abbreviation: E. coli, Escherichia coli.
We then evaluated the MICs of PRO and ETA against the recombinant and parental strains (control) using the classical agar plate method.Citation16 We show that after overexpressing the mutated ethAW21R in wild-type BCG and M. tuberculosis H37Rv, both PRO and ETA MIC rose by 256- and 128-fold, respectively (). Additionally, no observable differences were noted in the MICs of the overexpressed ethAwt recombinants and the parent strains (MIC =0.25 and 0.5 µg/mL; ). Our findings suggest that the mutation ethAW21R could be used as a marker site for testing PRO and ETA cross-resistance.
Table 2 MICs of PRO and ETA for wild-type and recombinant strains
Acknowledgments
This work was supported by the National Mega project of China for Innovative Drugs (2018ZX09721001-003-003) and for Main Infectious Diseases (2017ZX10302301-003-002), the National Natural Science Foundation of China (81572037), the Chinese Academy of Sciences Grants (154144KYSB20150045, KFZD-SW-207, and YJKYYQ20170036) and Guangzhou Municipal Industry and Research Collaborative Innovation Program (201508020248 and 201604020019). It was also partially supported by the Public Research and Capacity Building Project of Guangdong Province (2017A020212004), the Guangzhou Municipal Clinical Medical Centre Program (155700012) and the Key Project Grant (SKLRD2016ZJ003) from the State Key Laboratory of Respiratory Disease, Guangzhou Institute of Respiratory Diseases, First Affiliated Hospital of Guangzhou Medical University. TZ received support from Science and Technology Innovation Leader of Guangdong Province (2016TX03R095). JM is a recipient of the University of Chinese Academy of Sciences PhD Fellowship Program while GM and GC are recipients of the CAS-TWAS President’s PhD Fellowship Program for International Students. The work was undertaken at the following institutes, which are mandated to undertake tuberculosis studies in Southern China: 1) State Key Laboratory of Respiratory Disease, Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences, Guangzhou, China, and 2) State Key Laboratory of Respiratory Disease, Department of Clinical Laboratory, Guangzhou Chest Hospital, Guangzhou, China. The facilities are compliant with biosafety level 2+ and 3 requirements for handling infectious materials.
Disclosure
The authors report no conflicts of interest in this work.
References
- Wang F, Langley R, Gulten G, et al. Mechanism of thioamide drug action against tuberculosis and leprosy. J Exp Med. 2007;204(1):73–78.
- Thee S, Garcia-Prats A, Donald P, Hesseling A, Schaaf H. A review of the use of ethionamide and prothionamide in childhood tuberculosis. Tuberculosis. 2016;97:126–136.
- Di Perri G, Bonora S. Which agents should we use for the treatment of multidrug-resistant Mycobacterium tuberculosis? J Antimicrob Chemother. 2004;54(3):593–602.
- Donald PR, Seifart HI. Cerebrospinal fluid concentrations of ethionamide in children with tuberculous meningitis. J Pediatr. 1989;115(3):483–486.
- Scardigli A, Caminero JA, Sotgiu G, Centis R, Ambrosio L, Migliori GB. Efficacy and tolerability of ethionamide versus prothionamide: a systematic review. Eur Respir J. 2016;48(3):946.
- Jenner P, Ellard G, Gruer P, Aber V. A comparison of the blood levels and urinary excretion of ethionamide and prothionamide in man. J Antimicrob Chemother. 1984;13(3):267–277.
- Winder FG. Mode of action of the antimycobacterial agents and associated aspects of the molecular biology of the mycobacteria. Biol Mycobact. 1982;1:353–438.
- Fattorini L, Iona E, Ricci ML, et al. Activity of 16 antimicrobial agents against drug-resistant strains of Mycobacterium tuberculosis. Microb Drug Resist. 1999;5(4):265–270.
- DeBarber AE, Mdluli K, Bosman M, Bekker L-G, Barry CE. Ethionamide activation and sensitivity in multidrug-resistant Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2000;97(17):9677–9682.
- Hanoulle X, Wieruszeski J-M, Rousselot-Pailley P, et al. Selective intracellular accumulation of the major metabolite issued from the activation of the prodrug ethionamide in mycobacteria. J Antimicrob Chemother. 2006;58(4):768–772.
- Vilchèze C, Jacobs WR Jr. Resistance to isoniazid and ethionamide in Mycobacterium tuberculosis: genes, mutations, and causalities. Microbiol Spectr. 2014;2(4):MGM2-0014-2013.
- Banerjee A, Dubnau E, Quemard A, et al. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science. 1994;263(5144):227–229.
- Vilchèze C, Weisbrod TR, Chen B, et al. Altered NADH/NAD+ ratio mediates coresistance to isoniazid and ethionamide in mycobacteria. Antimicrob Agents Chemother. 2005;49(2):708–720.
- Liu T, Wang B, Guo J, et al. Role of folP1 and folP2 genes in the action of sulfamethoxazole and trimethoprim against mycobacteria. J Microbiol Biotechnol. 2015;25(9):1559–1567.
- Yang F, Njire MM, Liu J, et al. Engineering more stable, selectable marker-free autoluminescent mycobacteria by one step. PLoS One. 2015;10(3):e0119341.
- Zhang T, Bishai WR, Grosset JH, Nuermberger EL. Rapid assessment of antibacterial activity against Mycobacterium ulcerans by using recombinant luminescent strains. Antimicrob Agents Chemother. 2010;54(7):2806–2813.
