Abstract
Background
Waterborne cryptosporidiosis is the second cause of diarrhea in young children and immunocompromised hosts after rotavirus. Except for nitazoxanide (NTZ), there is no accredited cryptosporidiosis treatment to date. Therefore, there is an urgent need to find an effective and safe treatment for cryptosporidiosis. This study aimed to investigate the possible anti-protozoal effects of Syzygium aromaticum (clove) oil, Anethum graveolens (dill) seeds oil, Lactobacillus acidophilus LB, and zinc against Cryptosporidium parvum in comparison to NTZ.
Methods
Besides the negative control, mice from six experimental groups (T1–T6) were infected with Cryptosporidium parvum oocysts. On the seventh day post-infection (PID), mice from five groups were treated for 8 consecutive days with NTZ, clove oil, dill seed oil, Lactobacillus acidophilus LB, and zinc commercial forms (T2–T5). Oocysts shedding rate, differences of mice body weight, serum IL10, and TNF-α, cryptosporidial antigen, and cd3 at the intestinal mucosa were evaluated at the end of the experiment.
Results
The mean of the C. parvum oocysts’ shedding rate was significantly lower in all treated groups than in the non-treated group. The oocysts reduction rate was the highest in zinc-treated mice (98.3%), Lactobacillus acidophilus LB and dill-treated groups (95.77%), and the NTZ-treated group (91.55%). Clove oil was the least effective, with a 74.65% reduction rate. Excluding the clove oil-treated group, immunohistochemical analysis revealed the clearance of the Cryptosporidium antigen in the intestinal tissue in all treated groups.
Conclusion
The study has provided a rational basis for using these safe, cheap, and commercially available alternatives in treating cryptosporidiosis combined with NTZ.
Plain Language Summary
Cryptosporidiosis is one of the most worldwide distributed neglected tropical diseases and is difficult to be treated in immunocompromised hosts. The current study tried to find out effective, safe, available, cheap, and natural therapeutic alternatives. Clove oil, dill seed oil, Lactobacillus acidophilus LB probiotic, and zinc commercial forms were tested in cryptosporidiosis infected animals. All tested alternatives greatly reduced the Cryptosporidium oocysts shedding rate. The oocysts reduction rate was the highest in zinc-treated mice, while it was the lowest in the clove oil-treated group. Dill, Lactobacillus spp., zinc, and NTZ cause 100% clearance cryptosporidial infection from intestinal mucosal tissue. The study provides a rational foundation to use the four alternatives in treating cryptosporidiosis together with NTZ.
Introduction
Cryptosporidium, an apicomplexan parasite, is a major human parasite in the last and early part of the current century. Water is an important environmental transmission route for Cryptosporidium spp.Citation1 Treating infected waters and patients costs developed countries a large amount of money annually. Infected water consumption was responsible for most of the cryptosporidiosis outbreaks recorded worldwide.Citation2 There is little information on Cryptosporidium in Egyptian drinking water. However, available data show variable prevalence rates of between 3% and 100%.Citation3–7 The results may vary depending on different diagnostic techniques, sample sizes, and geographic locations.
Watery diarrhea and malabsorption are the most common clinical forms of the parasite. The parasite targets epithelial cells in the small intestines of humans and animals. The severity of the clinical manifestation increases in young children and immunocompromised individuals, leading to long-lasting diarrhea, considerable deformation of the intestinal epithelium, and malnutrition.Citation8 In contrast, a good immune system in immunocompetent hosts ends up with self-limiting signs and symptoms.
Histological changes caused by intestinal cryptosporidiosis are non-specific, but they include dampening of villi, hyperplasia of intestinal crypt cells, and inflammatory cells infiltration into the luminal propria. All previous changes are associated with the intensity of C. parvum infection. However, the pathophysiological mechanisms underlying intestinal C. parvum infection are not well understood. Studies discussing parasite pathogenesis are limited, and all are sourced from experimental animal studies.Citation9
To date, there is no accredited efficient treatment for cryptosporidiosis. Infected hosts eradicate the parasite through innate and acquired immunity.Citation10 Therefore, the hypothesis that cryptosporidiosis might be controlled by the administration of natural commercially available products came to the surface recently. Modulation of innate and acquired immunity at mucosal and systemic levels is the supposed target mechanism by which they overcome cryptosporidial infection.Citation11,Citation12
In recent decades, medicinal plants have gained greater recognition due to the awareness that these plants have lesser side effects and improved efficacy than their synthetic counterparts.Citation13 Several studies documented variable degrees of antibacterial, antiviral, anticarcinogenic, antifungal, and antiparasitic activities of different herbs, including cinnamon, oregano, clove, thyme, dill, garlic, black seed, and mint.Citation14,Citation15 However, Syzygium aromaticum, popular as clove and Anethum graveolens known as dill, gained much attention among other spices due to its strong antimicrobial, including antiparasitic and antioxidant properties.Citation16,Citation17 Both herbs are cultivated widely worldwide. They have a very long history of use, going back to Ancient Egypt. Syzygium aromaticum and Anethum graveolens seeds essential oil and extracts exerted anti-inflammatory activity against various microorganisms, including Entamoeba histolytica and Helicobacter pylori.Citation13,Citation18
Nitazoxanide (NTZ) is used to treat cryptosporidiosis. It has a broad-spectrum parasiticidal activity approved by the US FDA for treating diarrhea in both children and adults.Citation19
Several probiotics, mainly Lactobacillus strains, have been investigated for cryptosporidiosis treatment and prevention. According to Travers et al, the possible mechanisms of action of Lactobacillus include modulating the intestinal environment by competing with pathogens (nutrients, adhesion site, etc), improving barrier function, and modulating peristalsis and mucus secretion, which favor beneficial microflora. In addition, probiotics can also modulate host immunity by stimulating CDs’ differentiation, stimulating Th1/Th2 response; cytokines induction (IL10, IL12, IFNγ); stimulation of IgA producing cells and IgA secretion.Citation20
Although zinc supplementation proved beneficial in managing diarrhea, its effects on intestinal cryptosporidial infection remain unknown. Therefore, whether the benefits of zinc supplementation in diarrhea are similar to cryptosporidial infection remains unclear. The expected possible effects of zinc in Cryptosporidium spp. infection arises from the famous role of zinc in priming the immune system and tissue repair.Citation21 Epithelial cell and tissue integrity are maintained partially by trace nutrients as zinc promotes cell growth and suppressing apoptosis; thus, it may protect against Cryptosporidium infection.Citation22,Citation23
The study aimed to investigate the effects of four naturally commercially available products, including Syzygium aromaticum, Anethum graveolens seeds oil, Lactobacillus acidophilus LB, and zinc to treat cryptosporidiosis in C. parvum challenged mice compared to NTZ-treated mice.
Materials and Methods
Ethics Concerns
The study protocol was reviewed and approved by the Ethical Committee of the Faculty of Medicine, Assiut University (approval no. 17300385), following the internationally accepted principles for laboratory animal use and care, under the International Guiding Principles for Biomedical Research Involving Animals developed by the Council for International Organizations of Medical Sciences (CIOMS).Citation24
The Experimental Animal Models
Forty-two pathogen-free Swiss albino male mice, 8 weeks old with 32 grams mean weight, were used in the study.
The Parasite
Cryptosporidium parvum oocysts were isolated from positive water samples collected from the local community drinking water supply according to the previous recommendation of Hassan et al using a specially designed filter system.Citation25 Discontinuous sucrose gradient flotation was used to isolate and purify the parasite.Citation26 Water samples were subjected to molecular characterization using nested polymerase chain technique (nPCR).Citation25 Briefly, DNA extraction was performed by FavorPrep DNA Isolation Mini Kit (Favorgen Biotech Co., Taiwan) following the manufacturer’s instructions then amplified, using two sets of primers as shown in . Cryptosporidium oocyst wall protein (COWP) fragments digestion was determined by electrophoresis in 3.2% agarose gels comprising ethidium bromide, thereafter, fragments visualization was done under UV light to explore the Cryptosporidium genotype.
Table 1 Primers Sets Used for nPCR Targeting COWP Gene
The Treatment Forms
Nitaxanide Preparation
Mice received Cryptonaz (Copad Pharma, Egypt) commercially available as 100 mg/5 mL in a 60-mL syrup bottle.
Syzygium aromaticum Oil
Clove plant oil in a 30-mL commercial container was available as Zongle USDA Certified Organic Clove Essential Oil (Zongle Therapeutics, Georgia, USA).
Anethum graveolens Oil
The commercial form of pure dill seed oil was supplied in a 30-mL container by Hemani Award (Dubai, United Arab Emirates).
Lactobacillus acidophilus LB
A commercial form of Lactobacillus acidophilus LB from human origin was provided as Lactéol Fort 340-mg sachets each containing 10 billion L. acidophilus LB (Axcan Pharma, Egypt).
Zinc Supplements
It was supplied as zinc sulfate preparation in the commercial form as zinc Origin 10 mg/5 mL (Origin International Pharma, Egypt).
Design of the Experiment
Seven main different experimental conditions were evaluated. Each contained six mice.
Group C: Negative control group: None infected non-treated group.
Group T: Includes six sub-groups. Each group contained six mice. All mice in these groups were infected orally with C. parvum oocysts on the first PID. Following the recommendation of Sayed et al, the infectious dose was adjusted to contain approximately 600 oocysts:Citation7
T1 group contained six mice; each received the C. parvum infectious dose on the first PID. No treatments were given.
T2 group: Infected and treated with NTZ in a dose of 200 mg/kg/day orally on the seventh PID and for 8 consecutive days twice daily.Citation27
T3 group: Infected and treated with clove oil, 33 mg/kg was given on the seventh PID and for 8 consecutive days, once daily.Citation28
T4 group: Infected and treated with dill seed oil at a dose of 20 μL twice a day on the seventh PID and for 8 consecutive days, once daily.Citation29
T5 group: Infected on the first PID and treated with 5×108 Lactobacillus acidophilus LB dissolved in 200 µL distilled water on the seventh PID and for 8 consecutive days, once daily.Citation8
T6 group: Infected and on the seventh PID mice were treated with 5 mg of zinc in 500 µL syrup for eight consecutive days, till the end of the experiment, once daily. The zinc dose was adjusted according to the annual report of the US Environmental Protection Agency, which demonstrates the safe daily intake of zinc.Citation30 illustrates a summary of treatment type and dose given to the mice of T subgroups.
Table 2 Treatment Type and Dose Were Given to the Mice of T Subgroups
Evaluation of Experimental Conditions
Infection monitoring: After oocysts inoculation, all mice were observed daily for general health status, mortalities, fecal pellets color, and consistency. The bodyweight of mice in all groups was measured in grams and documented daily throughout the 14 days of the experiment. Pooled fecal samples from each group were collected daily from the second day and onward.Citation7 Fecal smears were performed daily along the experiment period using Modified Ziehl–Neelsen stain.Citation31 The efficacy of different treatments used and NTZ was assessed by daily measuring oocysts shedding count in feces until the experimental end by haemocytometer.Citation26 The number of oocysts was calculated as (Total no. of oocysts counted × dilution factor)/(tested stool volume in gm).Citation7
Treatments’ efficacy: The efficacy of each product was done by calculating the oocysts discharge reduction rateCitation32 as follows:
IL10 and TNF-α serum level among groups: concentrations of TNF-α and IL10 were determined in the serum samples of the animals at the end of the experiment. An enzyme-linked immunosorbent assay (ELISA) technique was performed according to the manufacturer protocol guideline, using antibody pairs from Bio Legend, Inc. (San Diego, USA).
Detection of whole Cryptosporidium oocysts antigen and CD3 by immunohistochemistry: Terminal ileum were collected from sacrificed animals, tissue sections (4 mm thick) were placed in 10 mmol/l citrate buffer solution at pH of 6.0 and 80 °C for 30 min and washed in water then application of 3.0% H2O2 in methanol for 20 min to block endogenous peroxidase activity. All slides were incubated with normal goat serum at room temperature for 20 min to avoid non-specific staining. Mouse monoclonal anti-Cryptosporidium antibody (1:50 Novus Biologicals, USA, Catalog No: NB100 65674UV) and rabbit polyclonal anti- CD3 (1:100) were then added for 1 h at room temperature. The previous steps were repeated with negative control. Next, the biotinylated secondary antibody, streptavidin horseradish peroxidase-conjugated tertiary antibody, and diaminobenzidine were applied according to the manufacturer’s instructions. Sections were then counterstained with Mayer’s Haematoxylin.
Statistical Analysis
SPSS program version 16 was used to analyze data using the chi-square test to compare qualitative variables, while the independent test was used to compare quantitative variables. Qualitative data were presented in frequencies and proportions, while the mean and standard deviation were used to express quantitative data. Statistically, significance was considered when P-value less than 0.05 and 95% confidence intervals did not overlap.
Results
Rate of Infection
The isolated C. parvum oocysts were significantly able to infect mice in all tested groups. The number of +ve samples stained with Modified Ziehl–Neelsen showed a significant decrease at the end of the experiment in all treated groups than the non-treated group except for clove oil-treated one .
Table 3 Rate of Infection Throughout the Experiment
Oocyst Shedding Count
In positive control (infected untreated group), the mean of C. parvum oocysts shedding was significantly higher than all treated groups. At the same time, maximum shedding of C. parvum oocysts in that group was observed on the 14th PID, with a mean of 4970 oocysts/gm stool (P = 0.011). In all treated groups, maximum oocyst shedding was observed on the 7th and 8th PID and minimum on the 14th PID.
The oocyst reduction rate was affected variably among different tested treatments; it was the highest in zinc-treated mice (T6) (98.3%). Both Lactobacillus acidophilus LB and dill seed oil-treated groups (T4 and T5) are equally reduced oocysts shedding (95.77%). The oocysts reduction rate recorded in NTZ-treated group (T2) was 91.55%. Clove oil (T3) was the least effective, with a 74.65% reduction rate.
However, the difference in oocysts shedding found between the NTZ, dill seed oil, Lactobacillus acidophilus LB, and zinc was not a significant treatment, unlike the oocysts shedding reduction effect in the clove oil-treated group, which was statistically lower than other treated groups (P < 0.0001) .
Table 4 Comparison of Means Oocysts/gm Stool Detected in All Groups at the End of the Experiment and Reduction Rate of Each Treatment
Bodyweight (BW)
At the beginning of the study, there was no significant difference in the mice’s body weights of all groups compared to the negative control. By the end of the study, the following data were observed (): In the infected untreated group and clove oil-treated group (T1 and T3), there was a highly significant reduction in BW compared to the control group and other treated groups (P-value ≤0.0001). But this reduction was nearly equal between the two groups.
Table 5 Body Weights in All Groups Throughout the Experiment Compared to the Control Group
In the groups treated with NTZ and Lactobacillus acidophilus LB (T2 and T5), the body weight was decreased compared to the control group, which improved 5 days after NTZ and probiotic administration. The weight gain was more in the NTZ-treated group (P < 0.001).
In both dill seed oil- and zinc-treated groups (T4 and T6), BW decreased compared to the control group, which improved 6 days after dill and Zn administration. The weight gain was more in the zinc-treated group (P < 0.05).
IL10 Concentration Levels ()
Variable concentrations of IL10 serum level were recorded in different groups: levels were decreased in both the infected non-treated and the clove oil-treated groups compared to healthy controls; however, the reduction was significant only in the infected non-treated animals. The IL10 serum level was higher than the control in the NTZ, dill seed oil, Lactobacillus acidophilus LB, and zinc-treated groups; the increase was significant in both the NTZ and dill seed oil-treated groups.
Table 6 IL10 Difference Among Groups Compared to Control
TNF-α Concentration Levels ()
There was a significant reduction of TNF-α serum level in all tested groups compared to the healthy control. The reduction was remarkable in both zinc and dill seed oil-treated groups.
Table 7 TNF-α Difference Among Groups Compared to Control
Immunohistological Evaluation
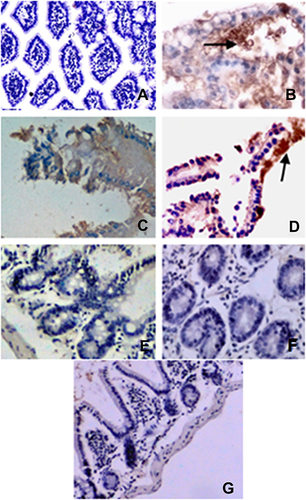
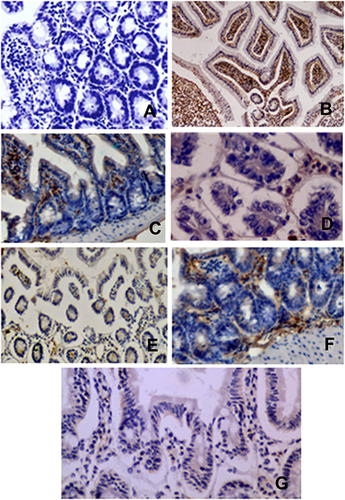
Intestinal tissue revealed the presence of the Cryptosporidium antigen in intestinal tissue only in the positive control group and the clove oil-treated group ( and ), compared to their absence in the healthy control, NTZ, dill seed oil, Lactobacillus acidophilus LB, and zinc-treated groups (, , , , and ).
The Expression of CD3 Antigens in Intestinal Tissue of Tested Mice
Immunohistological evaluation of the formalin-fixed paraffin revealed overexpression of the CD3 protein in lymphocytes that infiltrate the intestinal tissue of all tested groups versus healthy control (–).
Discussion
Syzygium aromaticum oil, Anethum graveolens seeds oil, Lactobacillus acidophilus LB, zinc, and NTZ commercial forms were supplied as treatment of Cryptosporidium infection. As HSE (2008) reported that the incubation period for cryptosporidiosis is 1–12 days (average, 7 days), they were supplied on the seventh PID, after oocysts discharge established and not for prophylaxis.Citation33
Although all the tested products failed to eradicate C. parvum infection, they modulated infection outcomes upon their administration in the experimental animal models. Oocysts shedding, body weight, local mucosal immunohistochemical changes, and systemic cytokines were affected by the investigated products with variable degrees.
NTZ, clove oil, dill seed oil, Lactobacillus acidophilus, and zinc had affected the multiplication of C. parvum, as they lowered the oocysts discharge rate. Oocysts discharge was probably decreased by hindering their multiplication nature in the animal models and regaining intestinal integrity. This decrease is supported by a marked difference in oocysts discharge between the untreated and treated groups, which reflects the good efficacy of all types of supplements in decreasing the in vivo ability of the parasite oocysts to multiply. This finding is consistent with numerous in vivo human and animal studies that clearly illustrated the preventative or therapeutic potential of one or more of these alternatives.Citation13,Citation18,Citation34 Logically recovery of intestinal integrity and decreased oocysts multiplication correlate with the ability of the parasite to cause the disease manifestations.
BW was improved after all treatments except for the clove oil-treated group; this could be postulated by simultaneous improvement of host immune status, oocysts shedding rate reduction. This correlated with previous preliminary researches showing the possibility of NTZ, dill, probiotics, and zinc to treat various forms of gastroenteritis; therefore, cryptosporidiosis manifestations improved by reducing both stool duration and frequency.Citation35–38 The current results oppose previous studies discussing the gastroprotective role of clove oil in different animal models.Citation39
According to Qin, Lactobacillus caused marked improvement of intestinal epithelia-infected animal intestines.Citation40 The intestinal integrity was completely restored after Lactobacilli treatment, probably through competing with the parasite for nutrient hindering or preventing the parasite colonization along the intestinal tract and improving the mucosal immunity to fight back the infection.Citation20 These could explain both negative C. parvum antigen and CD3 overexpression at the local mucosal level in Lactobacillus treated group.
Lactobacilli also prevent parasite colonization along the intestinal tract.Citation8 The other suggested mechanism is the effect on intestinal microflora and lowering intestinal pH, regulating intestinal motility, and mucus production.Citation41 According to Desouza et al, the non-pathogenic probiotic bacteria interact with the gut epithelial cells and immune cells to start the immune signals in the form of modulating immunoglobulin production. This interaction increases the number of IgA-producing cells at the level of mucosal immunity and increases certain cytokines profiles, including (TNF-α IFN-γ, IL-10, IL-12) to up or downregulate the immune responses and maintain intestinal homeostasis.Citation42 Furthermore, Lactobacillus acidophilus reduces the duration and number of C. parvum oocysts shed in feces of experimentally infected mice.Citation43,Citation44
Guitard et al had investigated the effects of Lactobacillus casei on C. parvum-infected rats, among many parameters including weight gain, parasite burden, mucosal histology, and mucosal cytokines’ production (IFNγ, IL10, and TNFα).Citation8 The authors’ findings agreed with the current study, rapid clearance of the parasite was noted in rats treated with probiotics, but it was unable to eradicate it. In contrast to the present study results, they could not find any significant effect of probiotic administration in weight gain or kinetics of mucosal cytokines during infection. Also, in a clinical trial performed in Peru, Salazar-Lindo et al found that the administration of a milk formula containing Lactobacillus is not useful in infants with acute diarrhea due to various microbial agents, including C. parvum. However, this low activity of probiotic treatment is surprising in light of previously published data on the beneficial effect of some probiotics on infectious diarrhea.Citation12,Citation45–47 The first case of successful resolution of prolonged cryptosporidiosis with probiotic treatment was a 12-year-old girl of previous good health supplied with a 4-week course of Lactobacilli; her clinical symptoms had completely resolved within 10 days of treatment inception.Citation48 Probiotics might excrete harmful substances to one of the developmental stages of the parasite leading to infection inhibition, offering new therapeutic agents for cryptosporidiosis treatment. Foster et al evaluated the in vivo effect of Lactobacillus acidophilus from human intestines on the oocyst stage of C. parvum using a flow cytometric viability assay. They found that Lactobacillus significantly reduced oocyst viability up to 81%. These results suggest antimicrobial substance(s) against the oocyst stage of C. parvum in Lactobacillus acidophilus cultures.Citation49
While Zn probably improves the nutritional state of the infected hosts, leading to improvement of immunity, making them able to win the infection battle.Citation22 This finding explains the marked improvement of BW reduction of oocysts discharge rate, negative C. parvum antigen, and CD3 overexpression at the local mucosal level at the end of the experiment.
Although the benefits of zinc supplementation in the management of diarrhea have been established, the mechanisms by which zinc beneficially affects the pathological changes remain unknown in the current study. However, several postulations have been established for its specific effects in intestinal cryptosporidial infection, such as priming the immune system and tissue repair, as supported by Mathers et al. Zinc also plays a part in maintaining epithelial and tissue integrity through promoting cell growth and differentiation, suppressing apoptosis, improving immune function, and intestinal transport of water and electrolytesCitation22; thus, it may protect against Cryptosporidium infection. Zinc also has an antioxidant and an anti-inflammatory agent.Citation50 Zinc deficiency is associated with an increased risk of gastrointestinal infections, with its adverse effects on the structure and function of the gastrointestinal tract and impaired immune function.Citation22,Citation51,Citation52 According to Yones et al, a coincident decrease in serum Zn level was more prominent among Cryptosporidium sp. infected malnourished children.Citation23 The positive association between Cryptosporidium infection and zinc deficiency could be explained by the changes in the absorptive villous architecture that is often severely disrupted, inflamed, or destroyed by Cryptosporidium sp., which is observed in the present study and regained by probiotic and zinc treatment. There is currently an extensive clinical interest in Zn as a protective agent against various Cryptosporidium spp. infection aspects.
NTZ is reportedly effective against a wide spectrum of human microorganisms.Citation53 Over the years, through experimental and clinical trials, NTZ range of action extended to include enteric pathogens including protozoa (Cryptosporidium parvum, Giardia lamblia, Entamoeba, Blastocystis hominis, Cyclospora, Isospora), helminths (Taenia saginata, Hymenolepis nana), and bacteria (Clostridium, Bacteroides, Helicobacter).Citation54,Citation55 It reduces symptom severity and duration of gastroenteritis, mainly diarrhea.Citation38 Yet, its efficacy remains contested among immunocompromised patients. Moreover, Amadi et al found no significant benefit of using NTZ in HIV children infected with cryptosporidiosis despite high dose and longer treatment duration through a randomized, double-blind, placebo-controlled trial performed in Lusaka, Zambia.Citation56 The current study revealed the positive therapeutic effects of NTZ at the levels of oocysts shedding rate and local mucosal clearance, yet this effect did not reach a complete cure. This agrees with several previous studies targeting the same issue recorded variable degree of efficacy. NTZ reduced the oocysts shedding rate by 85% and 76.1% in drug-treated calf and mice in two studies performed by Ollivett et al and Sadek et al, respectively.Citation14,Citation57 Thus many authors recommended using NTZ with other drugs or herbal products to achieve more efficacy.Citation14,Citation58
Contrary to the current result, Theodos et al stated that NTZ did not show the expected positive effect on Cryptosporidium spp. burden in a gnotobiotic piglet diarrhea model.Citation59 Different experimental designs may explain these disagreements between studies, drug doses and diverse animal models used, and various diagnostic techniques used to evaluate the experimental outcomes.
Clove oil significantly reduced the oocysts discharge rate compared to the infected non-treated group in the present study. However, it showed the least reduction rate between all tested alternatives, including NTZ. This could be explained by the non-clearance of C. parvum infection from the intestinal mucosa, by finding the parasite antigen in the intestinal mucosa.
Among the limited studies published, addressed the effects of clove oil and or its major compounds on parasitic infection, Tanghort et al stated that clove essential oil resulted in a significant decrease in the number of C. baileyi and C. galli oocysts by about 85% up to 98% in time and dose-dependent manners.Citation60 In another study conducted by Fang et al, clove oil has shown 100% acaricidal efficacy by killing all Sarcoptes scabiei mites subjected to it within 20.Citation61 Eugenol, one of the main clove oil components, has the potential to precipitate in the treatments of many parasites as Giardia lamblia, Leishmania infantum, Trypanosoma cruzi, and Plasmodium falciparum at high doses as stated in many in-vitro studies.Citation62–65 Eugenol also proved to be effective against Schistosoma mansoni. It reduced the tested worm burden by 19.2%, but it did not affect egg development.Citation66
Based on previous studies investigating the possible effects of clove oil on different bacteria and protozoa other than Cryptosporidium sp., the mechanism of action hypothesized the ability of essential oil to disturb the cell function and morphology. This can be explained by the lipophilic properties of the plant elements, so they cross the cell membrane and disrupt the phospholipid layer structure. It also has the cytotoxic ability, leading to membrane damage, leakage of macromolecules, depolarization of the action of the mitochondrial membrane in the cytoplasm and nucleus, and finally, lysis.Citation67
As far as we know, no previous study addressed the potential antiparasitic effects of dill seed oil against Cryptosporidium sp., even though its anti-inflammatory proprietaries are widely discussed in different studies. Essential oil of dill has been reported to have a good inhibitory action on a broad spectrum of microorganisms, especially gram-positive bacteria were generally more liable to be affected by oils.Citation68 Two studies examined the effects of dill seed extracts on the parasitic population. First, the study investigated the effect of aqueous dill extract in children infected with giardiasis. The study revealed that 5 days’ consummation of the extract and the regular dose of metronidazole improved symptoms earlier than treatment with metronidazole alone.Citation69 The second study evaluated the protective effects of dill extracts on amoebic liver abscess through in vivo and in vitro studies at multiple concentrations. The study revealed very promising anti-protozoal effects in comparison to metronidazole effects.Citation13
The thorough mechanism by which dill seed oil exhibited antiparasitic effects is undetermined and needs further studies. However, it could be the dill chemical structure and its major components, such as dillapiole and anethole, that form hydrogen bonds with the active site of the target enzymes.Citation70 Dill is also popular for being mucosal protective, antioxidant, and anti-secretory action at the intestinal mucosa.Citation71
The precise cause explains the discharge of oocysts in mouse feces at end of the experiment while Cryptosporidium antigen was not visualized on ileal sections at the same period is still unknown and need further investigations, perhaps it may be attributed to the single antigen of Cryptosporidium oocysts that the Immunohistological analysis targeted in the current study, as there are other endogenous developmental stages: meront and gamonts beside oocysts. They reproduce within the intestinal epithelial cells.Citation72
Conclusion
Our work provided pieces of evidence for the eminent antiprotozoal activity of Anethum graveolens seeds oil, zinc, and Lactobacillus acidophilus LB commercial forms against Cryptosporidium infection by clearance of the parasite from intestinal mucosa and significant reduction of oocysts shedding rate. Collectively, our results provide a rational basis for using these safe, cheap, and commercially available products to treat cryptosporidiosis. We recommend more investigatory studies on a larger scale in both experimental animals and calves, as well as clinical trials to set a determined valid accredited therapy strategy for cryptosporidiosis including single and combined alternatives.
Author Contributions
Gaber and Galal contributed to the study concept and design, acquisition of data, analysis and interpretation of data, drafting and revision of the manuscript. Farrag, Badary, Alkhalil and Elossily contributed to the acquisition of data, analysis and interpretation of data, and revision of the article. All authors gave final approval of the version to be published, agreed on the journal to which the article has been submitted and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- Almeida A, Moreira MJ, Soares S, et al. Presence of Cryptosporidium spp. and Giardia duodenalis in drinking water samples in the north of Portugal. Korean J Parasitol. 2010;48(1):43–48. doi:10.3347/kjp.2010.48.1.43
- Bitton G. Pathogens and parasites in domestic wastewater. Wastewater Microbiol. 2005:107–151. DOI:10.1002/0471717967.ch4
- El-Shazly A, Gabor A, Mohmoud M, Abdel Aziz S, Saleh W. The use of Ziehl-Neelsen stain, Enzyme Linked immunosorbent assay and nested polymerase chain reaction in diagnosis of cryptosporidiosis in immuno competent, compromised patients. J Egypt Soc Parasitol. 2002;32(1):1155–1166.
- Shoukry N, Dawoud H, Haridy F. Studies on zoonotic cryptosporidiosis parvum in Ismailia Governorate, Egypt. J Egypt Soc Parasitol. 2009;39:479–488.
- Khalifa R, Ahmed A, Abdel-hafeez E, Mosllem F. Present status of protozoan pathogens causing water-borne disease in northern part of EL-Minia Governorate, Egypt. J Egypt Soc Parasitol. 2014;44(3):559–566. doi:10.21608/jesp.2014.90143
- Khalifa A, Ibrahim I, Said D, Abdel-Aleem E, Nabil R. Cryptosporidium and Giardia in water in Alexandria: detection and evaluation of viability by flow cytometry and different stains. PUJ. 2011;4(2):155–164.
- Sayed FG, Hamza AI, Galal LA, Sayed DM, Gaber M. Virulence of geographically different Cryptosporidium parvum isolates in experimental animal model. Ann Parasitol. 2016;62(3):221–232. doi:10.17420/ap6203.56
- Guitard J, Menotti J, Desveaux A, et al. Experimental study of the effects of probiotics on Cryptosporidium parvum infection in neonatal rats. Parasitol Res. 2006;99(5):522–527. doi:10.1007/s00436-006-0181-4
- Vohra P, Sharma M, Chaudhary U. A comprehensive review of diagnostic techniques for detection of cryptosporidium parvum in stool samples. J Pharm. 2012;2(5):15–26.
- Mederle O, Mederle N, Dărăbuş G, Imre K, Olariu T. Epidemiological investigations in human cryptosporidiosis. Preliminary study. Sci Parasitol. 2010;11:20–23.
- Vaarala O. Immunological effects of probiotics with special reference to lactobacilli. Clin Exper Allergy. 2003;33(12):1634–1640. doi:10.1111/j.1365-2222.2003.01835.x
- Picard C, Fioramonti J, Francois A, Robinson T, Neant F, Matuchansky C. Review article: bifidobacteria as probiotic agents - physiological effects and clinical benefits. Aliment Pharmacol Ther. 2005;22(6):495–512. doi:10.1111/j.1365-2036.2005.02615.x
- Abas ASM, Elagib SM. Antiparasitic activity of aqueous extract of Anethum graveolens against Entamoeba histolytica: in vitro and in vivo study. Biocatal Agric Biotechnol. 2021;34:1–7. doi:10.1016/j.bcab.2021.102026
- Sadek HA, Abdel-Rahman SM, Bakir HY, et al. The potential convention of garlic and black seed. J Egypt Soc Parasitol. 2020;50(3):613–621. doi:10.21608/jesp.2020.131094
- Al-Snafi AE. Antiparasitic effects of medicinal plants (part 1)-A review. IOSR J Pharm. 2016;6(3):51–66.
- Batiha GES, Alkazmi LM, Wasef LG, Beshbishy AM, Nadwa EH, Rashwan EK. Syzygium aromaticum l. (Myrtaceae): traditional uses, bioactive chemical constituents, pharmacological and toxicological activities. Biomolecules. 2020;10(2). doi:10.3390/biom10020202
- Altameme HJ, Hameed IH, Hamza LF. Anethum graveolens: physicochemical properties, medicinal uses, antimicrobial effects, antioxidant effect, anti-inflammatory and analgesic effects: a review. Int J Pharm Qual Assur. 2017;8(3):88–91. doi:10.25258/ijpqa.v8i03.9569
- Ajiboye TO, Mohammed AO, Bello SA, et al. Antibacterial activity of Syzygium aromaticum seed: studies on oxidative stress biomarkers and membrane permeability. Microb Pathog. 2016;95:208–215. doi:10.1016/j.micpath.2016.03.011
- Carey CM, Lee H, Trevors JT. Biology, persistence and detection of Cryptosporidium parvum and Cryptosporidium hominis oocyst. Water Res. 2004;38(4):818–862. doi:10.1016/j.watres.2003.10.012
- Travers M-A, Florent I, Kohl L, Grellier P. Probiotics for the control of parasites: an overview. J Parasitol Res. 2011;2011:610769. doi:10.1155/2011/610769
- Mathers CD, Ezzati M, Lopez AD. Measuring the burden of neglected tropical diseases: the global burden of disease framework. PLoS Negl Trop Dis. 2007;1(2):e114–e114. doi:10.1371/journal.pntd.0000114
- Khan W, Sellen D. Zinc Supplementation in the Management of Diarrhea Biological, Behavioural and Contextual Rationale. Toronto, Canada:University of Toronto; 2011.
- Yones DA, Galal LA, Abdallah AM, Zaghlol KS. Effect of enteric parasitic infection on serum trace elements and nutritional status in upper Egyptian children. Trop Parasitol. 2015;5(1):29–35. doi:10.4103/2229-5070.145581
- National Research Council. The Development of Science-Based Guidelines for Laboratory Animal Care. Washington, DC: The National Academies Press; 2003. Available from: http://www.nap.edu/catalog/11138.html.
- Hassan D, Farghali M, Eldeek H, Gaber M, Elossily N, Ismail T. Antiprotozoal activity of silver nanoparticles against Cryptosporidium parvum oocysts: new insights on their feasibility as a water disinfectant. J Microbiol Methods. 2019;165:105698. doi:10.1016/j.mimet.2019.105698
- Suresh P, Rehg JE. Comparative evaluation of several techniques for purification of Cryptosporidium parvum oocysts from rat feces. J Clin Microbiol. 1996;34(1):38–40.
- Shahiduzzaman M, Daugschies A. Therapy and prevention of cryptosporidiosis in animals. Vet Parasitol. 2012;188(3–4):203–214. doi:10.1016/j.vetpar.2012.03.052
- Taher YA, Samud AM, El-Taher FE, et al. Experimental evaluation of anti-inflammatory, antinociceptive and antipyretic activities of clove oil in mice. Libyan J Med. 2015;10(1):1–7. doi:10.3402/ljm.v10.28685
- Zeng H, Tian J, Zheng Y, et al. In vitro and in vivo activities of essential oil from the seed of Anethum graveolens L. against Candida spp. Evid Based Complement Alternat Med. 2011;2011:1–8. doi:10.1155/2011/659704
- EPA. Toxicological review of. Rev Lit Arts Am. 2010;39(110):759–786
- Henriksen SA, Pohlenz JFL. Staining of Cryptosporidia by a modified Ziehl-Neelsen technique. Acta Vet Scand. 1981;22(3):594–596. doi:10.1186/BF03548684
- Egerton JR, Ott WH, Cuckler AC. Methods for evaluating anthelmintics in the laboratory and their applications to field conditions. In: Ejl S, editor. The Evaluation of Anthelmintics. 1st ed. Merck and Sharpe; 1963:46–53.
- HSE. Drinking water and health. A review and guide for population Health Service Executive. “Health Services Executive in Ireland’s”; 2008. Available from: www.hse.ie. Accessed December 31, 2021.
- Isolauri E, Sütas Y, Kankaanpää P, Arvilommi H, Salminen S. Probiotics: effects on immunity. Am J Clin Nutr. 2001;73(2):444s–450s. doi:10.1093/ajcn/73.2.444s
- Mao Y, Nobaek S, Kasravi B, et al. The effects of Lactobacillus strains and oat fiber on methotrexate- induced enterocolitis in rats. Gastroenterology. 1996;111(2):334–344. doi:10.1053/gast.1996.v111.pm8690198
- Mutlu-Ingok A, Karbancioglu-Guler F. Cardamom, cumin, and dill weed essential oils: chemical compositions, antimicrobial activities, and mechanisms of action against Campylobacter spp. Molecules. 2017;22(7):1191. doi:10.3390/molecules22071191
- Goldman RD. Zinc supplementation for acute gastroenteritis. Can Fam Physician. 2013;59(4):363–364. doi:10.1097/MOG.0b013e32833fd48a.8
- Bolia R. Nitazoxanide: jack of all, master of none? Indian J Pediatr. 2020;87(1):4–5. doi:10.1007/s12098-019-03131-y
- Santin JR, Lemos M, Klein-Júnior LC, et al. Gastroprotective activity of essential oil of the Syzygium aromaticum and its major component eugenol in different animal models. Naunyn Schmiedebergs Arch Pharmacol. 2011;383(2):149–158. doi:10.1007/s00210-010-0582-x
- Qin H-L, Shen T-Y, Gao Z-G, et al. Effect of lactobacillus on the gut microflora and barrier function of the rats with abdominal infection. World J Gastroenterol. 2005;11(17):2591–2596. doi:10.3748/wjg.v11.i17.2591
- Wohlgemuth S, Loh G, Blaut M. Recent developments and perspectives in the investigation of probiotic effects. Int J Med Microbiol. 2010;300(1):3–10. doi:10.1016/j.ijmm.2009.08.003
- Desouza A, Rajkumar C, Cooke J, Bulpitt C. Probiotic in prevention of antibiotic associated diarrhea: meta-analysis. BMJ. 2003;324(7350):1361–1370. doi:10.1136/bmj.324.7350.1361
- Alak JB, Wolf B, Mdurvwa E, Pimentel-Smith G, Adeyemo O. Effect of Lactobacillus reuteri on intestinal resistance to Cryptosporidium parvum infection in a murine model of acquired immunodeficiency syndrome. J Infect Dis. 1997;175(1):218–221. doi:10.1093/infdis/175.1.218
- Alak J, Wolf B, Mdurvwa E, et al. Supplementation with Lactobacillus reuteri or L. acidophilus reduced intestinal shedding of Cryptosporidium parvum oocysts in immunodeficient C57Bl/6 mice. Cell Mol Biol. 1999;45(6):855–863.
- Salazar-Lindo E, Miranda-Langschwager P, Campos-Sanchez M, Chea-Woo E, Sack RB. Lactobacillus casei strain GG in the treatment of infants with acute watery diarrhea: a randomized, double-blind, placebo controlled clinical trial [ISRCTN67363048]. BMC Pediatr. 2004;4(1):18. doi:10.1186/1471-2431-4-18
- Szajewska H, Mrukowicz JZ. Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: a systematic review of published randomized, double-blind, placebo-controlled trials. J Pediatr Gastroenterol Nutr. 2001;33:S17–S25. doi:10.1097/00005176-200110002-00004
- Allen SJ, Okoko B, Martinez E, Gregorio G, Dans LF. Probiotics for treating infectious diarrhoea. In: The Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd; 2000. doi10.1002/14651858.cd003048
- Pickerd N, Tuthill D. Resolution of cryptosporidiosis with probiotic treatment. Postgrad Med J. 2004;80(940):112–113. doi:10.1136/pmj.2003.014175
- Foster JC, Glass MD, Courtney PD, Ward LA. Effect of Lactobacillus and Bifidobacterium on Cryptosporidium parvum oocyst viability. Food Microbiol. 2003;20(3):351–357. doi:10.1016/s0740-0020(02)00120-x
- Medema G, Teunis P, Blokker M, et al. Guidelines for Drinking Water Quality: Cryptosporidium. New York:WHO;2009.
- Lukacik M, Thomas RL, Aranda JVA. Meta-analysis of the effects of oral zinc in the treatment of acute and persistent diarrhea. Pediatrics. 2008;121(2):326–336. doi:10.1542/peds.2007-0921
- Aggarwal R. Reactogenicity of a combined hepatitis A and hepatitis B vaccine in healthy Indian children and adults. Indian J Gastrol. 2007;26(5):248–249.
- Rossignol J. Nitazoxanide: A First-In-Class Broad-Spectrum Antiviral Agent. Antivir Res. 2014;110:94–103.
- Rodriguez-Morales AJ, Martinez-Pulgarin DF, Muñoz-Urbano M, Gómez-Suta D, Sánchez-Duque JA, Machado-Alba JE. Bibliometric assessment of the global scientific production of nitazoxanide. Cureus. 2017;9(5):1–12. doi:10.7759/cureus.1204
- Aslam S, Musher DM. Nitazoxanide: clinical studies of a broad-spectrum anti-infective agent. Future Microbiol. 2007;2(6):583–590. doi:10.2217/17460913.2.6.583
- Amadi B, Mwiya M, Sianongo S, et al. High dose prolonged treatment with nitazoxanide is not effective for cryptosporidiosis in HIV positive Zambian children: a randomised controlled trial. BMC Infect Dis. 2009;7:1–7. doi:10.1186/1471-2334-9-195
- Ollivett TL, Nydam DV, Bowman DD, et al. Effect of nitazoxanide on cryptosporidiosis in experimentally infected neonatal dairy calves. J Dairy Sci. 2009;92(4):1643–1648. doi:10.3168/jds.2008-1474
- El Shafei OK, Gawad A, Saad E, et al. Therapeutic effect of phenyl vinyl sulfone and nitazoxanide on experimentally infected mice with cryptosporidiosis. Menoufia Med J. 2018;31(3):786–794. doi:10.4103/mmj.mmj
- Theodos CM, Griffiths JK, D’Onfro J, Fairfield A, Tzipori S. Efficacy of nitazoxanide against Cryptosporidium parvum in cell culture and in animal models. Antimicrob Agents Chemother. 1998;42(8):1959–1965. doi:10.1128/aac.42.8.1959
- Tanghort M, Chefchaou H, Mzabi A, et al. Oocysticidal Effect of Essential Oils (EOs) and their major components on Cryptosporidium baileyi and Cryptosporidium galli. Int J Poult Sci. 2019;18(10):475–482. doi:10.3923/ijps.2019.475.482
- Fang F, Candy K, Melloul E, et al. In vitro activity of ten essential oils against Sarcoptes scabiei. Parasit Vectors. 2016;9(1):1–7. doi:10.1186/s13071-016-1889-3
- Machado M, Dinis A, Salgueiro L, Custódio JBA, Cavaleiro C, Sousa M. Anti-Giardia activity of Syzygium aromaticum essential oil and eugenol: effects on growth, viability, adherence and ultrastructure. Exp Parasitol. 2011;127(4):732–739. doi:10.1016/j.exppara.2011.01.011
- Dutra FL, Oliveira MM, Santos RS, et al. Effects of linalool and eugenol on the survival of Leishmania (L.) infantum chagasi within macrophages. Acta Trop. 2016;164:69–76. doi:10.1016/j.actatropica.2016.08.026
- Pontes KAO, Silva LS, Santos EC, et al. Eugenol disrupts Plasmodium falciparum intracellular development during the erythrocytic cycle and protects against cerebral malaria. Biochim Biophys Acta Gen Subj. 2021;1865(3):129813. doi:10.1016/j.bbagen.2020.129813
- Azeredo CMO, Soares MJ. Combination of the essential oil constituents citral, eugenol and thymol enhance their inhibitory effect on Crithidia fasciculata and Trypanosoma cruzi growth. Rev Bras Farmacogn. 2013;23(5):762–768. doi:10.1590/S0102-695X2013000500007
- El-kady AM, Abdelraouf AA, Hassan TM, El-Deek HE, Fouad SS, Althagfan SS. Eugenol, a potential schistosomicidal agent with anti-inflammatory and antifibrotic effects against Schistosoma mansoni, induced liver pathology. Infect Drug Resist. 2019;12:709–719. doi:10.2147/IDR.S196544
- Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils - A review. Food Chem Toxicol. 2008;46(2):446–475. doi:10.1016/j.fct.2007.09.106
- Delaquis PJ, Stanich K, Girard B, Mazza G. Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int J Food Microbiol. 2002;74(1–2):101–109. doi:10.1016/S0168-1605(01)00734-6
- Sahib AS, Mohammed IH, Sloo SA. Antigiardial eff ect of Anethum graveolens aqueous extract in children. J Intercult Ethnopharmacol. 2014;3(3):5–8. doi:10.5455/jice.20140523104104
- Farag RS, Daw ZY, Abo-Raya SH. Influence of some spice essential oils on aspergillus parasiticus growth and production of aflatoxins in a synthetic medium. J Food Sci. 1989;54(1):74–76. doi:10.1111/j.1365-2621.1989.tb08571.x
- Shekhawat S, Jana GS. Anethum graveolens: an Indian traditional medicinal herb and spice. Pharmacogn Rev. 2010;4(8):179–184. doi:10.4103/0973-7847.70915
- Mele R, Gomez Morales MA, Tosini F, Pozio E. Cryptosporidium parvum at different developmental stages modulates host cell apoptosis in vitro. Infect Immun. 2004;72(10):6061–6067. doi:10.1128/IAI.72.10.6061-6067.2004
- Pedraza-Díaz S, Amar C, Nichols GL, McLauchlin J. Nested polymerase chain reaction for amplification of the Cryptosporidium oocyst wall protein gene. Emerg Infect Dis. 2001;7(1):49–56. doi:10.3201/eid0701.700049
- Spano F, Putignani L, McLauchlin J, Casemore DP, Crisanti A. PCR-RFLP analysis of the Cryptosporidium oocyst wall protein (COWP) gene discriminates between C. wrairi and C. parvum, and between C. parvum isolates of human and animal origin. FEMS Microbiol Lett. 1997;150(2):209–217. doi:10.1016/S0378-1097(97)00115-8