Abstract
Contact lenses (CL) have become an immensely popular means of vision correction, offering comfort to millions worldwide. However, the persistent issue of biofilm formation on lenses raises significant problems, leading to various ocular complications and discomfort. The aim of this review is to develop safer and more effective strategies for preventing and managing microbial biofilms on CL, improving the eye health and comfort of wearers. Taking these into consideration, the present study investigates the intricate mechanisms of biofilm formation, by exploring the interplay between microbial adhesion, the production of extracellular polymeric substances, and the properties of the lens material itself. Moreover, it emphasizes the diverse range of microorganisms involved, encompassing bacteria, fungi, and other opportunistic pathogens, elucidating their implications within lenses and other medical device-related infections and inflammatory responses. Going beyond the challenges posed by biofilms on CL, this work explores the advancements in biofilm detection techniques and their clinical relevance. It discusses diagnostic tools like confocal microscopy, genetic assays, and emerging technologies, assessing their capacity to identify and quantify biofilm-related infections. Finally, the paper delves into contemporary strategies and innovative approaches for managing and preventing biofilms development on CL. In Conclusion, this review provides insights for eye care practitioners, lens manufacturers, and microbiology researchers. It highlights the intricate interactions between biofilms and CL, serving as a foundation for the development of effective preventive measures and innovative solutions to enhance CL safety, comfort, and overall ocular health. Research into microbial biofilms on CL is continuously evolving, with several future directions being explored to address challenges and improve eye health outcomes as far as CL wearers are concerned.
Introduction
It has been discovered that the biofilms formation dates back more than three billion years, founded on the examination of microbial fossils. This demonstrates the ancient adaptation and the ubiquity of biofilm formation. As biofilms can form on biotic or abiotic surfaces, they are associated with a large variety of human infections, which has led to an increased interest in biofilm research.Citation1
Bacteria and fungi can exist as single, free-floating (planktonic) cells, or as groups of cells called biofilm. A structured consortium of microbial cells surrounded by a self-produced polymer matrix equals a microbial biofilm. In addition to microorganisms, the biofilm matrix may also contain elements from the host, such as fibrin, platelets, or immunoglobulins. Infections caused by biofilms can be either monomicrobial or polymicrobial.Citation2
Two-thirds of human illnesses are thought to involve biofilms. Gram-positive, gram-negative bacteria, fungi and protozoa can develop biofilms on medical devices, which is a major concern in this field. Pseudomonas, Enterococcus, Staphylococcus, Streptococcus, Klebsiella, and Serratia are the most prevalent bacteria species that create biofilms on medical equipment.Citation3
According to the Center for Disease Prevention and Control (CDC), biofilm-related pathology is one of the biggest safety issues facing healthcare systems. As a result, research is being done to better understand the intricate mechanisms underlying biofilm formation and operation. The scientific community can better comprehend the structure and operation of biofilms due to research using cutting-edge microscopy and molecular biology techniques, which have led to significant improvements in the treatment of illnesses brought on by biofilms.Citation4
The European Society of Clinical Microbiology and Infectious Diseases Biofilms Study Group (ESGB) claims that novel approaches are required for the investigation of illnesses within people that are connected with biofilms. However, only specialized research laboratories make use of such techniques.Citation2 Enhancing prophylaxis and therapy for biofilm-associated infections within people is the ultimate goal of ESGB. The ESGB understands that a multidisciplinary approach is required to achieve these goals, involving researchers from fundamental, environmental as well as molecular microbiology.Citation2,Citation5
On the other hand, eye infections can also be associated with biofilms. They can be mono- or polymicrobial and are mostly associated with risk factors, including contact lenses (CL) or other ocular devices, surgery, trauma, age, dry or previous eye disease. Bacteria can cause a variety of ocular infections such as conjunctivitis, keratitis, blepharitis, endophthalmitis, preseptal and orbital cellulitis, dacryocystitis manifestations. The ability of microbes to form biofilms has also increased the rate of antimicrobial resistance.Citation6
The conjunctiva and cornea are constantly washed by tears and are generally considered to be sterile environments. However, recent research has revealed the presence of a diverse microbiome on the ocular surface, albeit less abundant compared to other areas of the body (such as the skin or gut). The ocular microbiome consists mainly of bacteria, fungi, and viruses (Staphylococcus, Bacillus, Pseudomonas, and Corynebacterium being predominant). These microorganisms play an important role in maintaining ocular health by competing with potential pathogens for space and nutrients, modulating immune responses, and helping to produce antimicrobial peptides.Citation7–10
CL have become an immensely popular means of vision correction, offering convenience to millions worldwide. However, the persistent issue of biofilm formation on lenses poses significant concerns, leading to various ocular complications and discomfort for their wearers. The first description of CL-related corneal infiltrative events was made roughly 40 years ago.Citation11 The severity of microbial keratitis (MK), a dangerous condition that can cause blindness, is frequently determined by the buildup of biofilms on external CL. According to some studies the most frequent etiological agent of bacterial keratitis is Staphylococcus epidermidis, which is a commensal of the conjunctival sac.Citation12 When compared to CL surfaces, bacterial biofilm was present on storage case surfaces more frequently and the density of the biofilm was also significantly higher.Citation13
This review aims to synthesize existing literature on the etiology, development, composition, impact, and management of biofilms on CL and other medical devices. Its goal is to develop safer, more effective strategies for preventing and managing microbial biofilms, thereby improving eye health and comfort for CL wearers.
Biofilm Development and Composition
Biofilms, intricate communities of microorganisms, represent a significant facet of life on Earth. Initially defined in the mid-1980s, our understanding of biofilms has since grown substantially, primarily through the elucidation of genetic pathways, signal transduction mechanisms, and physiological responses. These structures have been found to persistently infect various organisms, including plants, animals, and humans.Citation14 In medical contexts, concerns regarding contamination of medical devices and implants have highlighted the importance of comprehending biofilm dynamics.Citation1
Biofilms, functioning as microsystems, enable bacteria to thrive, either as isolated entities or clustered formations. Within this environment, nutrient availability is limited, resulting in a markedly slower rate of cellular division compared to planktonic conditions. Moreover, the accessibility of antibodies and bacteriophages to bacteria within biofilms is restricted, enhancing bacterial survival against immune responses, antibiotics, and other stressors.Citation15
The protective nature of biofilms serves as a defense mechanism against desiccation and host immune responses. Moreover, biofilms facilitate bacterial survival by fostering the production of virulence factors and promoting inter-bacterial communication. Certain bacterial species, like Staphylococcus epidermidis and Staphylococcus aureus, produce adhesive proteins known as “adhesins”, which strengthen the adhesion between the host surface and the biofilm, rendering them resistant to removal.Citation16
Structured as communities encased within a self-produced matrix of extracellular polymeric substances (EPS), biofilms adhere to surfaces and interfaces. The development of biofilms unfolds through a series of well-defined phases, each indispensable for the establishment and persistence of these complex structures.Citation17
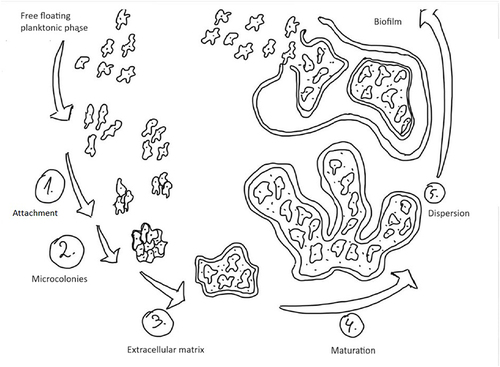
Attachment marks the initial phase, during which free-floating microorganisms adhere to surfaces, facilitated by factors such as hydrophobicity and surface charge. Microcolonies, formed subsequently, serve as the foundation for biofilm development, allowing for cooperative interactions between different microbial species.Citation18
The extracellular matrix plays a crucial role in biofilm architecture, offering structural support and protection against external stresses. As biofilms mature, they become increasingly resilient and complex, fostering microbial diversity and creating microenvironments with distinct metabolic activities.Citation1,Citation6
Despite their stability, mature biofilms exhibit dynamic behavior, as evidenced by the dispersion phase, wherein cells detach and disperse into the surrounding environment.Citation1,Citation14 This mechanism allows the colonization of new surfaces or return to planktonic states, facilitating biofilm survival and spread.Citation15 The development of biofilms is a dynamic and highly regulated process that progresses through distinct phases: attachment, microcolonies formation, extracellular matrix production, maturation, and dispersion (). Understanding the intricacies of biofilm development is paramount for devising effective strategies to control and manage biofilms in diverse contexts.Citation1,Citation6,Citation14 Targeted interventions at different stages of biofilm development hold promise for biofilm control, removal, or prevention, with far-reaching implications for healthcare, industry, and environmental management. Further exploration of the molecular and ecological underpinnings of biofilm dynamics is essential for advancing our knowledge and control strategies in this field.
The Variety of Bacteria That Cause Biofilms on Indwelling Medical Devices, Contact Lenses or Illnesses Linked to Biofilms
Microorganisms linked to biofilms seem to be responsible for a number of diseases, including cystic fibrosis, native valve endocarditis, otitis media, periodontitis, chronic prostatitis and keratitis. It has been demonstrated that a range of indwelling medical devices or other equipment used in the healthcare setting host biofilms, leading to detectable rates of device-associated infections.Citation19
Clinically significant bacteria known as ESKAPE, which stands for Enterococcus faecalis, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp., have come under increased attention in recent years due to their ability to form biofilms on medical devices. It has also been observed that biofilm growth has been subjected to a variety of ophthalmology-related materials and equipment. The required moisture for CL storage may provide a risk for biofilm formation and consequent corneal infections.Citation6 But the variety of pathogens involved in biofilm related infections, medical devices and CL contamination is much higher. ( and )
Table 1 Commonly Found Microorganisms in Biofilm Related Infection]sCitation4,Citation20–26
Table 2 Commonly Found Microorganisms in Biofilms on Medical Devices and Contact LensesCitation4,Citation20–22,Citation24–26
Clarifying Biofilm Implications Regarding Lens-Related Infections and Inflammatory Responses
Over 230 million people worldwide wear CL to correct functional or optical eye issues, or to alter the look of the eyes. Under ordinary circumstances, the corneal epithelial and stromal cells produce the innate defensive components that serve as the primary refractive structure for the eye, including chemokines, cytokines, antimicrobial peptides, proteins, and surfactants. Proteins, glycoproteins, and lipids from the tears quickly build up on the CL surface, once they are put into the eyes, providing a favorable habitat for microbes to flourish.Citation22
There are two types of CL: the soft ones, made of hydrogel or silicone hydrogel and the rigid ones, composed of silicone acrylates or fluorosilicone acrylates. The latter ones generally have a lower risk of infection compared to soft lenses. This is because rigid lenses have a less porous surface and are less susceptible to bacteria and deposit buildup compared to soft lenses.
Significant risk factors for corneal infection in lens carriers are P. aeruginosa and staphylococcal species. Microbial keratitis, a disorder caused by pathogens contacting lenses, can result in ulcers. Acute postoperative endophthalmitis caused by S. epidermidis, is connected to infections after surgically implanting intraocular lenses.Citation21
Microcolonies, an early stage of biofilm formation, are first created when planktonic bacteria interact with the CL surface. A microbial colony known as a biofilm can stick to both biotic and abiotic surfaces and generates extracellular polysaccharides. Antibiotics, disinfectants, and the host’s defensive mechanisms are less effective against the microbial cells that develop in a biofilm, because they are physiologically different from the planktonic cells of the same organism. In both human and animal models, keratitis has been linked to the maturation of biofilms on CL surfaces.Citation4,Citation19,Citation22
Keratitis, a generalized clinical term for the corneal inflammation, can alter the structure of the cornea and reduce its clarity. Numerous bacteria, fungus, and protozoa, are among the infectious organisms that can affect the cornea. Both Gram-negative and Gram-positive organisms, such as Pseudomonas aeruginosa and Staphylococcus aureus, can cause bacterial keratitis, particularly CL-associated infections. P. aeruginosa is the most prevalent and it is isolated from at least 70% of cases globally.Citation24 Although they are less common than bacteria, fungi like Candida albicans and Fusarium species are nevertheless often involved in the etiology of keratitis. In addition, the protozoan Acanthamoeba causes a rare but severe form of infectious keratitis.Citation22 Biofilms of both the CL cases and the CL of persons with MK contain these pathogens.Citation24 Despite being a rare side effect of wearing CLs, MK is one of the leading causes of blindness in both underdeveloped and developed nations.Citation22
Poor adherence to hygiene guidelines, disinfecting lenses with heat or chlorine, cleaning lenses infrequently or not at all, using a specific type of multipurpose CL disinfecting solution that contained only polyhexamethylene biguanide, are risk factors in the case of the development of CL-associated infections. Additionally, poor lens case hygiene (not air-drying lens cases after use) and failing to replace lens cases at least every three months are linked to an increased risk of MK while wearing CL on a daily basis.Citation24 According to Syed et al, CL-associated infections are typically caused by multi-drug-resistant (MDR) biofilm-forming P. aeruginosa.Citation25
In a different investigation, clinical and reference strains of P. aeruginosa, S. marcescens, and S. aureus have developed biofilms on lotrafilcon. While S. marcescens biofilm was resistant to a polyquaternium maintained care solution, but susceptible to hydrogen peroxide disinfection, P. aeruginosa and S. aureus biofilms were both susceptible to hydrogen peroxide and a polyquaternium preserved care solution, while the planktonic forms, however, were always vulnerable.Citation26
Advancements in Biofilm Detection Techniques and Their Clinical Relevance
The standard microbiological tests are insufficient in the detection of bacterial biofilms. The conventional method of identifying biofilms involves recovering live bacteria from the biofilm, detecting the biofilm using in vitro or in vivo techniques, and then identifying and imaging the microbial communities on the studied surfaces.Citation20 illustrates the main diagnostic tools for biofilm detection.
Table 3 Diagnostic Tools for Biofilm DetectionCitation20,Citation21,Citation27–34
Regarding the identification of biofilms from keratitis, there are numerous studies describing some of the above-mentioned methods, but also some different ones. A studyCitation35 that aimed to investigate 240 clinically suspected cases of acanthamoebic keratitis (AK) had their corneas scraped and swabbed for Acanthamoeba and microbiological cultures. For several samples, scanning electron microscopy was used. The capacity of biofilm to form was examined using a tissue culture plate approach. A modified version of the Kirby-Bauer disc diffusion method was used to identify the antibiotic resistance pattern. The results of the study presented the following data: 11 of the 102 AK patients with culture-proven infections had no co-infections, 74 had just one, and 17 had two. The most prevalent bacterial and fungal isolates were Enterobacterales and Aspergillus respectively. 64.7% of Enterobacterales, 50% of P. aeruginosa, 43.75% of S. aureus, 76.92% of Streptococcus pneumoniae, 28.57% of Corynebacterium spp., 60% of haemolytic streptococci, 40% of Acinetobacter, 100% of Candida spp. and 77.8% of Aspergillus isolates produced biofilms. When biofilm producers and non-biofilm producers were co-infected, severe symptoms were recorded more frequently. In general, a large proportion of the bacterial isolates that formed biofilms were sensitive to antibiotics in vitro.
Confocal microscopy enables non-invasive, in vivo imaging of mould and fungus in ocular tissue, surpassing the limitations of other methods. It boasts a sensitivity range of 66.7% to 95.0%, facilitating hyphae density measurement and aiding treatment response prediction. Compared to smear microscopy and inoculation, it offers quicker and often more accurate results, with 92.9% accuracy in diagnosing fungal keratitis. Dyed corneal tissue microscopy complemented these findings. Confocal microscopy can outperform culture and PCR in certain situations for early diagnosis. Limitations include species identification challenges, cost, and the need for skilled ophthalmologists. Photophobia and blepharospasm can hinder the process.Citation36
Anterior segment optical coherence tomography (OCT) enables the detection of corneal alterations indicative of the mycotic process. According to the OCT results, the cornea in the infiltrate region has thickened. The epithelium and endothelium are also more hyperreflective than the stroma. The posterior corneal surface changes because of the stroma’s widespread thickening and indications of oedema. The afflicted stroma may grow thinner than the healthy portions as a result of the protracted course of scarring processes that increase stroma reflectivity.Citation37 Specific OCT symptoms of aggressive forms of fungal keratitis include limited cystic formations of various diameters in the stroma matching necrotized tissues.Citation38 Over 85% of the endothelial plaques typical of fungal keratitis may be seen with OCT and confocal imaging.Citation39 This technique is more popular than confocal microscopy and is quick and non-intrusive. However, the OCT approach is useful for assessing the corneal condition in dynamics and permits to trace changes over the entire cornea. The indicators of corneal damage are merely indirect evidence of the fungal pathogen presence, making species identification impossible.
The molecular genetic diagnosis technique known as PCR is superior to microscopy of stained preparations and cultural methods used in detecting fungal DNA fragments, even in circumstances when culturing results are negative.Citation40 The benefits of PCR are undeniable: the procedure is highly sensitive, allowing for the detection of a pathogen in a little scrape from a corneal ulcer or from samples from patients who have previously had antifungal therapy. Results can be achieved in 4 hours as opposed to 3–7 days in culture studies. PCR is intended for species identification, just like other molecular genetic techniques. However, because of its high price and restricted availability, it is not regarded as a preferred approach. Moreover, because PCR also detects non-viable organisms, there is a high probability of false-positive results.Citation41,Citation42
A metagenomics analysis with a focus on RNA assessment and species composition identification is a far more advanced and precise procedure. Shigeyasu et alCitation43 described a situation in which corneal specimens failed microbiological and histological examinations, and only metagenomics analysis was able to identify the Fusarium solani genes. In another study,Citation44 metagenomics analysis established the presence of a pathogen in 74% of cases, considering the fact that more than half of the patients have already received treatment, while culturing was positive in 52% of patients and showed a sensitivity of 70%.
Mass spectrometry, which relies on the study of ribosomal proteins from microorganisms, is another term for molecular genetic techniques. An indisputable benefit of laser-assisted desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) is the ability to identify an agent up to a species level.Citation45 Since the protein content of fungal lesions changes in a specific way, a tear from an infected and healthy eye was examined for its protein composition.Citation11 The results of the MALDI-TOF MS sensitivity research are conflicting, ranging from 51%Citation45 to 97%.Citation46 The drawbacks that prevent this technology from becoming the primary technique in the diagnosis of fungal keratitis are that it is not widely available, is expensive, and requires continual replenishment of the data bank with microorganism proteomes.Citation47
The initial outcomes of a novel approach of internal transcribed spacer (ITS) sequencing have been described by Ren et al.Citation48 The repeating rRNA genes are separated by a non-coding DNA region called ITS. This approach gives more thorough information concerning the eye microbiome since it is not constrained by a medium, time, fungal activity, or specimen size. The ITS has been proven to produce results that are comparable to those of confocal microscopy and traditional approaches. The average efficiency indicators combined with some ITS limitations (dependence on database integrity, need for multiple primers due to strict specificity for different types of fungi) allow one to draw the conclusion that this method appears to be an alternative to pathogen identification in the diagnosis of fungal keratitis.Citation48
Contemporary Strategies and Innovative Approaches to Managing and Preventing Biofilms on Contact Lenses
Development of Antimicrobial Coating for CL
In the ever-evolving landscape of ocular health and vision correction, the development of antimicrobial coatings for CL stands as a cutting-edge frontier, offering a proactive shield against microbial threats. This pursuit of innovation seeks not only to enhance the safety and comfort of CL wear, but also to redefine the standards of ocular care in an increasingly dynamic world.
Melimine, a brand-new cationic peptide, has been covalently added to silicone hydrogel lenses. Tests were conducted to confirm the presence of peptide and its antimicrobial activity. Next, the efficacy of cationic lenses in preventing CL induced acute red eye in the Pseudomonas aeruginosa guinea pig model and CL induced peripheral ulcers in the Staphylococcus aureus rabbit model was evaluated.Citation49 Antimicrobial CL and lens cases have employed many chemical techniques to address microorganisms (bacteria, amoeba, and fungi) implicated in a range of ocular disorders. Through four different mechanisms of action, direct penetration of microbial cells, alteration of microbial-substrate interfaces, disruption of microbial cells’ quorum-sensing mechanism, and production of reactive oxygen species, these antimicrobial drugs inhibit or stop microbial growth.Citation50 When combined with different types of biomaterials, silver has demonstrated efficacy as an antibacterial agent. The adhesion of several strains of P. aeruginosa could be reduced by at least 90% (ie, greater than one log unit reduction) by coating an endotracheal tube with silver, and when silver nanoparticles were added to poly(vinyl alcohol)-b-poly(acrylonitrile) micelles, they effectively killed S. aureus and P. aeruginosa. Likewise, perfluoropolyether-urethane coated with silver had antibacterial activity against S. aureus and P. aeruginosa. The effects of silver on Acanthamoeba sp. have not been thoroughly investigated up to this point.Citation51 Using a high-intensity ultrasonic horn, a sonochemical deposition process coated PureVision balafilcon lenses with Zn-CuO. Control experiments used non-coated lenses. Evaluating P. aeruginosa and S. epidermidis adhesion, the lowest effective coating concentration was determined. Subsequent experiments examined lens properties, revealing that the sonochemically aided nanocoating maintained antibacterial efficacy, while preserving essential silicone hydrogel contact lens attributes.Citation52 A straightforward, one-step sonochemical process was used to successfully create CLs that are biocompatible, antimicrobial, and antioxidant. These lenses showed notable antibacterial, antimycotic, and biofilm inhibition against pathogenic strains while maintaining their original physical characteristics. They also showed enhanced cytocompatibility, antifouling characteristics, and surface wettability, indicating possible uses in the management of ocular surface infections.Citation53 Silver and copper nanoparticles were synthesized in polyvinyl alcohol (PVA) polymers through metal salt incorporation and subsequent reduction with sodium hydroxide. Characterization via transmission electron microscopy, attenuated total reflection spectroscopy, and X-ray photoelectron spectroscopy confirmed nanoparticle integration. Evaluating physical properties, tensile strength doubled with nanoparticle addition, though elongation before fracture halved. Cytotoxicity tests revealed silver-containing PVA’s cytotoxic nature, while copper-containing PVA was non-cytotoxic. Antibacterial activity was observed in silver-containing lenses, while both silver and copper nanoparticles reduced bacterial adhesion.Citation54
Potential Use of Probiotics to Restore Ocular Surface Microbiota
In the dynamic landscape of ocular health research, a compelling exploration unfolds: the potential utilization of probiotics to restore and rejuvenate the ocular surface microbiota. In this review we tried to explore the complex field of probiotic therapies and look at how they can help maintain a balanced microbial community on the surface of the eye. By scrutinizing the intricate interplay between probiotics and ocular health, this analysis aims to shed light on their diverse mechanisms, therapeutic potential, and implications for preventing and addressing ocular surface dysbiosis.
The various commensal bacterial flora that coexists in the human cornea and conjunctiva and create a protective microbiome against pathogenic colonization are revealed by molecular techniques, such as next-generation sequencing. Pseudomonas, Propionibacterium, Acinetobacter and Corynebacterium are among the most common genera. An imbalance in the ocular microbiota is caused by disruptions brought on by illnesses, CL wear, environmental variables, and antibiotic use. This mismatch connects changes in the oral and intestinal microbiota to disorders like glaucoma, uveitis, and other ophthalmic diseases, and it may also be a contributing factor in ocular diseases. Researching probiotic-based treatment interventions for ocular diseases and developing preventive measures require an understanding of the ocular microbiota.Citation55
Ming Cheng Chiang et alCitation56 have illustrated that a total of 17 positive swabs were taken from individuals who had bacterial conjunctivitis (four isolates of S. aureus and thirteen isolates of S. epidermidis). After having done an antibiotic susceptibility profile, it was discovered that all strains of Staphylococcus were resistant to cephalexin, oxacillin, and penicillin G (that is, they were methicillin resistant). To assess their antimicrobial capabilities, a total of six probiotic strains from the Lactobacillus and Bifidobacterium species were produced. It’s interesting to note that, even against species of bacteria resistant to antibiotics, every probiotic in their investigation showed encouraging reduction of bacterial growth.
Neisseria gonorrhoeae colonizing in the mother’s birth canal causes gonococcal conjunctivitis in newborns, which can cause blindness or severe visual impairment. The L. rhamnosus strain L60’s biogenic material had the ability to inhibit Neisseria gonorrhoeae proliferation. The development and course of ocular surface diseases appeared to be significantly influenced by oxidative stress and ageing. Chisari et alCitation57 illustrate that probiotic strains were administered, and the symptoms of dry eye syndrome improved. Based on these findings, the authors have determined that the activity of the probiotics (Enterococcus faecium LMG S-28935 and Saccharomyces boulardii MUCL 53837) integrates with the action of tear substitutes. Additionally, they have standardised the clinical parameters of the tear film and microbiological activity in restoring the ocular surface microbiota in subjects suffering from dry eye syndrome. When comparing the ocular surface microbiome profile of patients with unilateral keratitis to controls, differences were found in both eyes. There was an increase in suspected pathogens and a lower density of commensal species beyond the causal bacterium. Unilateral keratitis patients have different ocular surface microbiome profiles in both eyes, which may help explain how the microbiome contributes to the pathogenesis of this condition.Citation58
Probiotics have been shown to exhibit antimicrobial properties, including the production of ribosomally synthesised peptides, bacteriocin, or proteins with antimicrobial activity and compete for nutrients and space. It was discovered that Lactobacillus species: L. rhamnosus, L. brevis, L. plantarum, L. acidophilus reduced the biofilm formation of Bacillus spp.Citation59
CL wear correlates with increased P. aeruginosa-induced keratitis, prompting an investigation into the associated factors. Genomics-based studies revealed altered ocular commensal communities in lens wearers, raising questions about lens contamination versus ocular microbiome immune functions. There is research proving the pivotal role of ocular microbiome in regulating protection against P. aeruginosa infections, particularly in modulating neutrophil recruitment at the ocular surface. Notably, this study highlighted the impact of a common gram-positive commensal coagulase-negative staphylococci (CNS), and emphasized the contribution of gut microbiota in stimulating neutrophil development, influencing susceptibility to P. aeruginosa-induced keratitis.Citation60
Discussion
International bodies and the scientific communities have been paying more attention, lately, to the challenges posed by biofilm-related pathology, as well as to the currently available diagnostic tools and solutions needed for their prevention and control. We do not refer only to microbial biofilms associated with medical devices in general, but also to those associated with CL. To better understand these issues, a multidisciplinary approach comprising researchers from molecular, environmental, and fundamental microbiology and clinical specialties is needed.
Using a synthesis of previous research findings and literature, the goal of this review was to provide a thorough presentation of the biofilm development, composition, variety of pathogens involved, impact and management of biofilms on CL.
Millions of people use CL globally to correct functional or refractive abnormalities or to change the color of their eyes. But CL wear also represents a major risk factor for MK, infectious corneal ulcer and also lid margin disease. Microbes can attach to the lenses and within lens storage cases, often in association with biofilms that will serve as reservoirs to determine eye infections. From 24% to 81% of CL cases are contaminated with microbial multi-species biofilms. Lens storage cases are more frequently associated with biofilms and with more numerous organisms than CL, but the microbes attached to CL correlate more closely with ocular infections.Citation1,Citation14,Citation16
Under normal conditions, the corneal cells synthesize factors of innate defense. But, when the CL is inserted on the surface of the cornea, some substances such as glycoproteins, proteins and lipids in the tear easily accumulate on its surface, creating a suitable environment for pathogens.Citation15,Citation16 For CL related eye infections, MDR strains of P. aeruginosa, S. aureus, CNS, or Candida albicans are the most frequently involved pathogens.Citation16
Corneal infections are initiated when CL modifies or compromises the epithelial surface, allowing the microbes to adhere and invade. Despite improvements in CL solutions, materials and wearing habits, latest epidemiological studies find that incidence of keratitis has not changed in the last two decades among CL wearers. That suggested microbes are able to adapt to any local modifications designed to intervene in corneal protection.Citation14 In lid margin diseases such as blepharitis, biofilms accumulate and enlarge microscopically year after year, without any removal. This process starts much earlier in CL wearers, because these lenses are abiotic foreign bodies, producing an early biofilm that provides protection for bacteria.
However, this review brings useful information related to the antimicrobial coverage of CL, the antimicrobial activity of cationic peptides or silver and copper nanoparticles, providing protection against microbial threats, the creation of biocompatible, antimicrobial and antioxidant CLs, as well as the potential use of probiotics to restore and rejuvenate the ocular microbiome.
But beyond the innovative methods described above, considering ISO standards for CL disinfection is essential to develop safer, more effective strategies to manage microbial biofilms, thus improving eye health and comfort for wearers. ISO microbiological requirements are crucial for ensuring the safety and efficacy of CL disinfection solutions before they are brought to market. These standards establish guidelines and testing protocols to assess the antimicrobial effectiveness of disinfectants against microorganisms commonly found in CL care systems and provide crucial definitions and criteria for evaluating the effectiveness of disinfection products and regimens. Firstly, a “CL disinfecting product” is defined as a product capable of killing, destroying, or inactivating microorganisms. The product must pass the primary criteria outlined in the ISO standard stand-alone test. Secondly, a “CL disinfecting regimen” encompasses the entire CL care routine, specifically designed to effectively disinfect the lenses. This regimen must meet both the secondary criteria of the stand-alone test and the regimen test outlined in the International Standard. Lastly, “CL disinfection” refers to the process of reducing the number of viable microorganisms on the lenses. This process can be achieved through chemical or physical methods, with performance requirements specified in the relevant sections of the ISO standard.Citation61
Study Limitation
Similar to other narrative evaluations, this one has its share of limitations. This kind of studies always carry some bias risk, sample size related constraints, and methodological limitations which could be mitigated by producing a systematic review or a meta-analysis. Studies that are observational and non-randomized have inherent biases and limitations as well. Furthermore, data can be presented in different ways in different articles.
Conclusion
This review provides insights for eye care practitioners, lens manufacturers, clinical microbiology specialists and researchers. It seeks to clarify the intricate interactions between biofilms and CL, serving as a foundation for the development of effective preventive measures and innovative solutions to enhance CL safety, comfort, and overall ocular health. Research into microbial biofilms on CLs is continuously evolving, exploring future directions such as developing advanced anti-biofilm materials, innovative disinfection solutions, improved cleaning technologies, personalized lens care regimens, and even enhancing standardization efforts to improve ocular health for CL wearers.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We would like to acknowledge “VICTOR BABES” UNIVERSITY OF MEDICINE AND PHARMACY TIMISOARA for their support in covering the costs of publication for this research paper.
References
- Bispo P, Haas W, Gilmore M. Biofilms in infections of the eye. Pathogens. 2015;4(1):111–136. doi:10.3390/pathogens4010111
- Hoiby N, Bjarnsholt T, Moser C, et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect. 2015;21(Suppl 1):S1–25. doi:10.1016/j.cmi.2014.10.024
- Campolo A, Pifer R, Shannon P, Crary M. Microbial adherence to contact lenses and pseudomonas aeruginosa as a model organism for microbial keratitis. Pathogens. 2022;11(11):1383. doi:10.3390/pathogens11111383
- Donlan RM. Biofilms and device-associated infections. Emerg Infect Dis. 2001;7(2):277–281. doi:10.3201/eid0702.010226
- ESCMID Study Group for Biofilms – ESGB. Available from: http://www.escmid.org/research_projects/study_groups/biofilms/presentations_publications/. Accesed October 15, 2023.
- Teweldemedhin M, Gebreyesus H, Atsbaha AH, Asgedom SW, Saravanan M. Bacterial profile of ocular infections: a systematic review. BMC Ophthalmol. 2017;17(1). doi:10.1186/s12886-017-0612-2
- Borroni D, Paytuví-Gallart A, Sanseverino W, et al. Exploring the healthy eye microbiota niche in a multicenter study. Int J Mol Sci. 2022;23(18):10229. doi:10.3390/ijms231810229
- Rocha-de-Lossada C, Mazzotta C, Gabrielli F, et al. Ocular surface microbiota in naïve keratoconus: a multicenter validation study. J Clin Med. 2023;12(19):6354. doi:10.3390/jcm12196354
- Borroni D, Rocha de Lossada C, Mazzotta C, Sánchez-González JM, Papa F, Gabrielli F. Ocular microbiome evaluation in dry eye disease and meibomian gland dysfunction: values of variables. Exp Eye Res. 2023;236:109656. doi:10.1016/j.exer.2023.109656
- Ballesteros-Sánchez A, Sánchez-González JM, Borrone MA, Borroni D, Rocha-de-lossada C. The influence of lid-parallel conjunctival folds and conjunctivochalasis on dry eye symptoms with and without contact lens wear: a review of the literature. Ophthalmol Ther. 2024;13(3):651–670. doi:10.1007/s40123-023-00877-9
- Moriyana AS, Hofling-Lima AL. Contact lens-associated microbial keratitis. Arq Bras Oftalmol. 2008;71(6 Supl):32–36.
- Nayak N, Satpathy G, Vajpayee RB, Mrudula S. Phenotypic and plasmid pattern analysis of Staphylococcus epidermidis in bacterial keratitis. Indian J Ophthalmol. 2007;55(1):9–13. PMID: 17189880. doi:10.4103/0301-4738.29488
- Borlace L, Stapleton F, Matheson M, Dart JK. Bacterial biofilm on contact lenses and lens storage cases in wearers with microbial keratitis. J Appl Microbiol. 1998;84(5):827–838. PMID: 9674137. doi:10.1046/j.1365-2672.1998.00418.x
- Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol. 2016;14(9):563–575. doi:10.1038/nrmicro.2016.94
- Elder MJ, Tapleton FS, Evans E, Dart JKG. Biofilm-related infections in ophthalmology. Eye. 1995;9:102–109.
- Vincent M, Quintero J, Perry HD, Rynerson JM. Biofilm Theory for Lid Margin and Dry Eye Disease. IntechOpen; 2019. doi:10.5772/intechopen.89969
- Kim J, Park HD, Chung S. Microfluidic approaches to bacterial biofilm formation. Molecules. 2012;17(8):9818–9834. doi:10.3390/molecules17089818
- Wang Y, Bian Z, Wang Y. Biofilm formation and inhibition mediated by bacterial quorum sensing. Appl Microbiol Biotechnol. 2022;106(19–20):6365–6381. PMID: 36089638. doi:10.1007/s00253-022-12150-3
- Donlan RM. Biofilms: microbial Life on Surfaces. Emerg Infect Dis. 2002;8(9):881–890. doi:10.3201/eid0809.020063
- Licker M, Moldovan R, Hogea E, et al. Microbial biofilm in human health - an updated theoretical and practical insight. Rev Rom Med Lab. 2017;25(1):9–26. doi:10.1515/rrlm-2017-0001
- Stoica P, Chifiriuc MC, Rapa M, Lazăr V. Overview of biofilm-related problems in medical devices. In: Biofilms and Implantable Medical Devices. Copyright © 2017 Elsevier Ltd. (nu gasesc revista in pub med); 2017. doi10.1016/B978-0-08-100382-4.00001-0
- Dosler S, Hacioglu M, Yilmaz FN, Oyardi O. Biofilm modelling on the contact lenses and comparison of the in vitro activities of multipurpose lens solutions and antibiotics. PeerJ. 2020;8:e9419. doi:10.7717/peerj.9419
- Maslova E, Eisaiankhongi L, Sjöberg F, McCarthy RR. Burns and biofilms: priority pathogens and in vivo. NPJ Biofilms Microbiomes. 2021;7(73). doi:10.1038/s41522-021-00243-2
- Willcox MDP, Bahatheg G, Carnt N, et al. Biofilms and contact lenses: problems and solutions. Microbiol Aust. 2023;44(2):96–99. doi:10.1071/MA23027
- Syed HA, Sherwani SK, Siddiqui TR, Bashir A, Shahana U. Drug resistance profile and biofilm forming potential of Pseudomonas aeruginosa isolated from contact lenses in Karachi-Pakistan. BMC Ophthalmol. 2013;13:57.
- Szczotka-Flynn LB, Imamura Y, Chandra J, et al. Increased resistance of contact lens-related bacterial biofilms to antimicrobial activity of soft contact lens care solutions. Cornea. 2009;28(8):918–926. doi:10.1097/ICO.0b013e3181a81835
- Silva NBS, Marques LA, Roder DDB. Diagnosis of biofilm infections: current methods used, challenges and perspectives for the future. J Appl Microbiol. 2021;131:2148–2160. doi:10.1111/jam.15049
- Toc DA, Csapai A, Popa F, et al. Easy and affordable: a new method for the studying of bacterial biofilm formation. Cells. 2022;11:4119. doi:10.3390/cells11244119
- Peeters E, Nelis HJ, Coenye T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J Microbiol Methods. 2008;72:157–165.
- Fischer M, Triggs GJ, Krauss TF. Optical sensing of microbial life on surfaces. Appl Environ Microbiol. 2016;82:1362–1371. doi:10.1128/AEM.03001-15
- Xu Z, Liang Y, Lin S, et al. Crystal violet and XTT assay on staphylococcus aureus biofilm quantification. Curr Microbiol. 2016. doi:10.1007/s00284-016-1081-1
- Allkja J, Bjarnsholt T, Coenye T, et al. Minimum information guideline for spectrophotometric and fluorometric methods to assess biofilm formation in microplates. Biofilm. 2020;2:100010. doi:10.1016/j.bioflm.2019.100010
- Coenye T, Goeres D, Van Bambeke F, Bjarnsholt T. Should standardized susceptibility testing for microbial biofilms be introduced in clinical practice? Clin Microbiol Infect. 2018;24. doi:10.1016/j.cmi.2018.01.003
- Coenye T, Kjellerup B, Stoodley P, Bjarnsholt T. the 2019 biofilm bash participants. The future of biofilm research – report on the “2019 Biofilm Bash”. Biofilm. 2020;2:100012. doi:10.1016/j.bioflm.2019.100012
- Hasby Saad MA, Khalil HSM. Biofilm testing of microbiota: an essential step during corneal scrap examination in Egyptian acanthamoebic keratitis cases. Parasitol Int. 2018;67(5):556–564. doi:10.1016/j.parint.2018.05.001
- Sitnova AV, Svetozarskiy SN. Modern technologies in diagnosis of fungal keratitis (Review). Sovrem Tekhnologii Med. 2023;15(2):73–84. doi:10.17691/stm2023.15.2.07
- Skryabina YV, Astakhov YS, Konenkova YS, et al. Diagnosis and treatment of fungal keratitis. Part I. Ophthal Rep. 2018;11(3):63–73. doi:10.3892/or.2017.6107
- Soliman W, Fathalla AM, El-Sebaity DM, Al-Hussaini AK. Spectral domain anterior segment optical coherence tomography in microbial keratitis. Graefes Arch Clin Exp Ophthalmol. 2013;251(2):549–553. doi:10.1007/s00417-012-2086-5
- Jin X, Jin H, Shi Y, Zhang N, Zhang H. Clinical observation of corneal endothelial plaques with fungal and bacterial keratitis by anterior segment optical coherence tomography and in vivo confocal microscopy. Cornea. 2022;41(11):1426–1432. doi:10.1097/ICO.0000000000002912
- Behera HS, Srigyan D. Evaluation of polymerase chain reaction over routine microbial diagnosis for the diagnosis of fungal keratitis. Optom Vis Sci. 2021;98(3):280–284. doi:10.1097/OPX.0000000000001652
- Pouyeh B, Galor A, Miller D, Alfonso EC. New horizons in one of ophthalmology’s challenges: fungal keratitis. Expert Rev Ophthalmol. 2011;6(5):529–540. doi:10.1586/eop.11.58
- Thomas PA. Fungal infections of the cornea. Eye. 2003;17(8):852–862. doi:10.1038/sj.eye.6700557
- Shigeyasu C, Yamada M, Aoki K, et al. Metagenomic analysis for detecting Fusarium solani in a case of fungal keratitis. J Infect Chemother. 2018;24(8):664–668. doi:10.1016/j.jiac.2017.12.019
- Lalitha P, Prajna NV, Sikha M, et al. Evaluation of metagenomic deep sequencing as a diagnostic test for infectious keratitis. Ophthalmology. 2021;128(3):473–475. doi:10.1016/j.ophtha.2020.07.030
- Rohilla R, Meena S, Mohanty A, et al. Etiological spectrum of infectious keratitis in the era of MALDI-TOF-MS at a tertiary care hospital. J Family Med Prim Care. 2020;9(9):4576–4581. doi:10.4103/jfmpc.jfmpc_630_20
- Sharma S, Kunimoto DY, Gopinathan U, et al. Evaluation of corneal scraping smear examination methods in the diagnosis of bacterial and fungal keratitis: a survey of eight years of laboratory experience. Cornea. 2002;21(7):643–647.
- Ananthi S, Chitra T, Bini R, Prajna NV, Lalitha P, Dharmalingam K. Comparative analysis of the tear protein profile in mycotic keratitis patients. Mol Vis. 2008;14:500–507.
- Ren Z, Liu Q, Wang Y, Dong Y, Huang Y. Diagnostic information profiling and evaluation of causative fungi of fungal keratitis using high-throughput internal transcribed spacer sequencing. Sci Rep. 2020;10(1):1640. doi:10.1038/s41598-020-58245-7
- Cole N, Hume EBH, Vijay AK, Sankaridurg P, Kumar N, Willcox MDP. In vivo performance of melimine as an antimicrobial coating for contact lenses in models of CLARE and CLPU. Invest Ophthalmol Vis Sci. 2010;51(1):390–395. doi:10.1167/iovs.09-4068
- Khan SA, Lee CS. Recent progress and strategies to develop antimicrobial contact lenses and lens cases for different types of microbial keratitis. Acta Biomater. 2020;113:101–118. doi:10.1016/j.actbio.2020.06.039
- Willcox MDP, Hume EBH, Vijay AK, Petcavich R. Ability of silver-impregnated contact lenses to control microbial growth and colonization. J Optom. 2010;3(3):143–148. doi:10.1016/S1888-4296(10)70020-0
- Nahum Y, Israeli R, Mircus G, et al. Antibacterial and physical properties of a novel sonochemical-assisted Zn-CuO contact lens nanocoating. Graefes Arch Clin Exp Ophthalmol. 2019;257:95–100. doi:10.1007/s00417-018-4172-9
- Khan SA, Shahid S, Mahmood T, Lee CS. Contact lenses coated with hybrid multifunctional ternary nanocoatings (Phytomolecule-coated ZnO nanoparticles: Gallic Acid: tobramycin) for the treatment of bacterial and fungal keratitis. Acta Biomater. 2021;128:262–276. doi:10.1016/j.actbio.2021.04.014
- Kharaghani D, Dutta D, Gitigard P, et al. Development of antibacterial contact lenses containing metallic nanoparticles. Polym Test. 2019;79:106034. doi:10.1016/j.polymertesting.2019.106034
- Petrillo F, Pignataro D, Lavano MA, et al. Current evidence on the ocular surface microbiota and related diseases. Microorganisms. 2020;8(7):1033. doi:10.3390/microorganisms8071033
- Chiang MC, Chern E. Ocular surface microbiota: ophthalmic infectious disease and probiotics. Front Microbiol. 2022. doi:10.3389/fmicb.2022.952473
- Chisari G, Chisari EM, Borzi AM, et al. Aging eye microbiota in dry eye syndrome in patients treated with Enterococcus faecium and Saccharomyces boulardii. Curr Clin Pharmacol. 2017;12(2):99–105. doi:10.2174/1574884712666170704145046
- Cavuoto KM, Galor A, Banerjee S. Ocular surface microbiome alterations are found in both eyes of individuals with unilateral infectious keratitis. Transl Vis Sci Technol. 2021;10(2):19. doi:10.1167/tvst.10.2.19
- Chiang M-C, Chern E. More than antibiotics: latest therapeutics in the treatment and prevention of ocular surface infections. J Clin Med. 2022;11:4195. doi:10.3390/jcm11144195
- Kugadas A, Christiansen SH, Sankaranarayanan S, et al. Impact of microbiota on resistance to ocular pseudomonas aeruginosa-induced keratitis. PLoS Pathog. 2016;12(9):e1005855. doi:10.1371/journal.ppat.1005855
- Ophthalmic optics — Contact lens care products — Microbiological requirements and test methods for products and regimens for hygienic management of contact lenses. Available from: https://www.iso.org/obp/ui/#iso:std:iso:14729:ed-1:v1:en. Accessed May 8, 2024.