Abstract
Background:
Food hypersensitivity is commonly suspected, but seldom verified. Patients with subjective food hypersensitivity suffer from both intestinal and extraintestinal health complaints. Abnormalities of the enterochromaffin cells may play a role in the pathogenesis. The aim of this study was to investigate enterochromaffin cell function in patients with subjective food hypersensitivity by measuring serum chromogranin A (CgA) and 5-hydroxytryptamine (5-HT, serotonin) in gut lavage fluid.
Methods:
Sixty-nine patients with subjective food hypersensitivity were examined. Twenty-three patients with inflammatory bowel disease and 35 healthy volunteers were included as comparison groups. CgA was measured in serum by enzyme-linked immunosorbent assay. Gut lavage fluid was obtained by administering 2 L of polyethylene glycol solution intraduodenally. The first clear fluid passed per rectum was collected and 5-HT was analyzed by liquid chromatography tandem mass spectrometry.
Results:
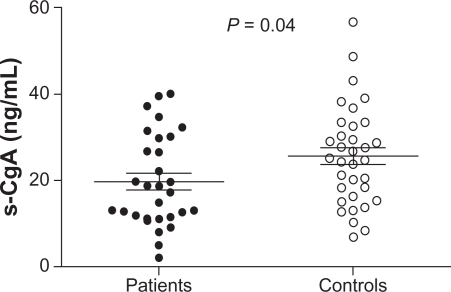
Serum levels of CgA were significantly lower in patients with subjective food hypersensitivity than in healthy controls (P = 0.04). No differences were found in 5-HT levels in gut lavage fluid between patients with subjective food hypersensitivity and the control groups. There was no correlation between serum CgA and gut lavage 5-HT.
Conclusion:
Decreased blood levels of CgA suggest neuroendocrine alterations in patients with subjective food hypersensitivity. However, 5-HT levels in gut lavage fluid were normal.
Introduction
Food hypersensitivity is commonly reported, but often remains unexplained, despite extensive medical examinations. Patients with such subjective food hypersensitivity attribute a number of somatic health complaints to the ingestion of specific foods, most often cow’s milk, wheat products, egg, fruits, and vegetables.Citation2 The abdominal symptoms are typically consistent with irritable bowel syndrome.Citation2 Psychological disturbances are often associated,Citation3 but do not seem to be major predictors of either gastrointestinal or extraintestinal symptom severity.Citation4 Hence, the etiology of subjective food hypersensitivity remains obscure.
Alterations of the gastrointestinal enterochromaffin cell population has been described in patients with functional gastrointestinal disorders,Citation5,Citation6 and are conceivably implicated in the pathogenesis of subjective food hypersensitivity. In a preliminary study, we recently observed low serum levels of chromogranin A (CgA) in patients with subjective food hypersensitivity.Citation7 CgA is stored and secreted together with amines and peptides from the diffuse neuroendocrine system, and serve as a precursor for several peptides with regulatory functions.Citation8 Enterochromaffin cells are a major source for circulating CgA. In addition, enterochromaffin cells secrete 5-hydroxytryptamine (5-HT, serotonin), a signaling molecule regulating several gut functions, including secretion, motility, and sensitivity. Indeed, most of the body’s 5-HT is contained within enterochromaffin cells. High systemic levels of 5-HT may cause symptoms resembling hypersensitivity reactions to food, such as diarrhea,Citation9,Citation10 nausea,Citation11 or flushing and heart palpitations.Citation12
5-HT is commonly measured in platelets or in plasma, the latter being a poor matrix due to rapid degradation of 5-HT into 5-hydroxyindolacetic acid. More than 95% of plasma 5-HT is present in platelets, and studies assessing platelet 5-HT content in patients with irritable bowel syndrome are somewhat conflicting.Citation13–Citation15 Alterations of 5-HT secretion within the gut are not necessarily reflected in the systemic circulation, and assessing 5-HT in gut tissue samples is often considered to be more accurate. However, the surface of the gastrointestinal tract is very large, and biopsies can only give information from a small fragment of this huge area. Indeed, enterochromaffin cell counts seem to vary a lot in different studies.Citation16,Citation17 Our group has developed a method for analyzing “whole gut 5-HT”, by analyzing gut lavage fluid.Citation18 Enterochromaffin cells release 5-HT mainly from granules at the basal border, but studies have shown that enterochromaffin cells are bipolar and are able to secrete 5-HT both basally, into the systemic circulation, and apically, into the gut lumen.Citation19–Citation21 Our method quantifies luminal enterochromaffin cell secretion along the entire length of the small and large bowel.
The aim of the present study was to explore whether patients with subjective food hypersensitivity display abnormal enterochromaffin cell functions. To evaluate this, we extended our preliminary observationsCitation7 and employed our recently developed liquid chromatography tandem mass spectrometry (LCMS) method for determination of 5-HT in gut lavage fluid.Citation18
Materials and methods
Patients
This study included 69 consecutive patients with subjective food hypersensitivity and abdominal complaints (55 females and 14 males, mean age 39 years, range 21–83 years). The patients went through an extensive medical examination, including detailed medical history-taking, physical examination, and routine laboratory tests, in addition to skin-prick tests, measurements of total and food-specific IgE levels in serum, and double-blind, placebo-controlled food challenge. Gastroenterological investigations included a gastroscopy with gastric and duodenal biopsy-taking to exclude Helicobacter pylori infection and celiac disease. Intestinal permeability was assessed as previously described,Citation22 and levels of calprotectin in gut lavage fluid samples were analyzed to exclude inflammatory bowel disease.Citation23 Patients with organic gastrointestinal diseases, IgE-mediated food allergies, and pregnant or lactating women were not included. A screening questionnaire based on the Rome II criteriaCitation24 was applied in all patients for the diagnosis of irritable bowel syndrome.
Control groups
Twenty-seven patients admitted to the Section of Gastroenterology at Haukeland University Hospital because of suspected inflammatory bowel disease were included as a “patient control group” (11 females and 16 males, mean age 35 years, range 21–65 years). A control group consisting of 35 healthy volunteers (24 females and 11 males, mean age 33 years, range 23–61 years) was also included. The participants in this group were mainly employees of National Institute of Nutrition and Seafood Research or Haukeland University Hospital and students at the University of Bergen. Pregnant or lactating women were not included. The study was performed in accordance with the Declaration of Helsinki and was approved by the Regional Committee for Medical Research Ethics.
Intestinal lavage
The procedure was performed in the morning and all the participants were fasting from midnight. The method of intestinal lavage has been described previously.Citation22 Briefly, 2 L of isotonic polyethylene glycol solution (MW 3350, Laxabon®, Tika, Sweden) was administered through a nasoduodenal feeding tube over a period of 40 minutes, using a peristaltic pump (505S/RL; Watson Marlow, Falmouth, UK). Approximately 50 μCi of 51Cr-labeled ethylenediaminetetra-acetic acid (51CrEDTA, Amersham, Little Chalfont, UK), was added to the solution to allow estimation of intestinal permeability.Citation22 A slightly increased intestinal permeability has been reported previously in patients with subjective food hypersensitivityCitation25 and this parameter was not included as a part of the present report. About one hour after the start of the polyethylene glycol infusion, the solution reached the distal colon and bowel movements started. The first clear fluid passed per rectum was collected and filtered through gauze, and a 4 mL aliquot was collected on tubes containing 0.5 mL of a solution with antiseptic and antiproteolytic activity prepared by adding 1 mL of 10% sodium azide (NaN3) to 50 mL of soybean trypsin inhibitor (Sigma, Taufkirchen, Germany). The samples were stored at −80°C until analysis.
Serotonin analysis
5-HT was analyzed by LCMS as described previously.Citation18 Briefly, a sample of gut lavage fluid was thawed at room temperature and centrifuged. The supernatant was collected and filtered using a hydrophilic nylon membrane syringe filter, 4 mm diameter and 0.45 μm pore size (Chromacol Ltd, Trumbull, CT). Aliquots of 50 μL of the filtered supernatants were transferred into tubes containing internal standard, which was evaporated to dryness in advance. The tubes were vortex-mixed for 1 minute, transferred to an autosampler vial and submitted to LCMS/MS analysis. The internal standard 5-methoxytryptamine (5-CH3O-HT) was purchased from Sigma.
The LCMS system used in this study was an Agilent 1100 series LC/MSD trap, SL model with an electrospray interface, a quaternary pump, degasser, autosampler, thermostated column compartment, variable wavelength ultraviolet light detector, and 25 μL injection volume. The column was a Zorbax Eclipse-C8 RP 150 × 4.6 mm, 5 μm (Agilent Technologies, Palo Alto, CA). The solvent system operated in gradient mode at 0.2 mL/min and consisted of water with formic acid 0.1% v/v and acetonitrile, and ultraviolet detection at 254 nm. Complete system control, data acquisition and processing were done using the ChemStation for LC/MSD version 4.2 from Agilent. The transitions monitored were 177 → 160 m/z for 5-HT and 191 → 174 m/z for 5-CH3O-HT.
Chromogranin A analysis
Fasting venous blood samples were collected at 8.30 am using gel vials with no added anticoagulants. The patients were fasting for 10 hours. Serum CgA was measured by enzyme-linked immunosorbent assay following the manufacturer’s instructions (ALPCO Diagnostics, Salem, NH).
Statistics
Data were analyzed and displayed using the GraphPad Prism statistical software package (v 5.00 for Windows; GraphPad Software, San Diego, CA). Log-transformed serotonin levels were evaluated by analysis of variance, whereas CgA levels were evaluated by using unpaired t-tests. All tests were two-tailed; P values of less than 5% were considered statistically significant.
Results
Subjects
Organic gastrointestinal diseases were not demonstrated in any of the patients with subjective food hypersensitivity. Sixty-four (95%) of the 69 included patients with subjective food hypersensitivity had irritable bowel syndrome according to Rome II criteria, and all completed the lavage procedure. Blood samples for CgA analysis were obtained from 32 of the patients. All of the 27 control patients with suspected inflammatory bowel disease completed the lavage procedure. However, blood samples were not obtained from this group. Based on the medical examination, including endoscopy and histological assessment of mucosal biopsies, 23 of 27 was diagnosed with inflammatory bowel disease, 19 had Crohn’s disease, and four had ulcerative colitis. The remaining patients (n = 4) were excluded from the study. Thirty-five healthy volunteers were included, and blood samples for CgA analysis were obtained from all of them. Twenty of the 35 controls agreed to participate in the lavage procedure.
Chromogranin analysis
Serum CgA samples from two of the 32 patients with subjective food hypersensitivity and one of the 35 healthy controls were excluded due to use of proton pump inhibitors, which elevate CgA in blood. Serum levels of CgA were significantly lower in patients with subjective food hypersensitivity compared with healthy controls (P = 0.04, ).
Serotonin analysis
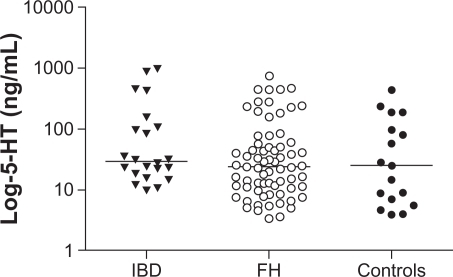
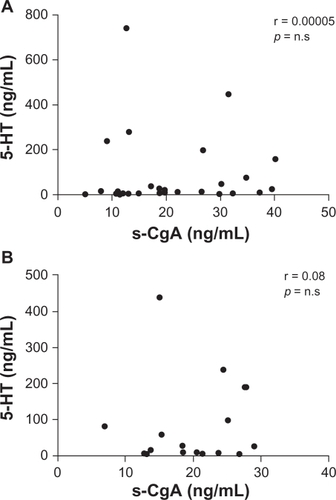
No significant differences in 5-HT levels were demonstrated between patients with subjective food hypersensitivity and the control groups. No differences in 5-HT levels were found between the two control groups either. In the patient group and the healthy volunteer group, two of 69 samples and three of 20 samples, respectively, were nondetectable. The 5-HT analysis revealed a high degree of variance within all three groups. There was a trend towards a higher level of 5-HT in the inflammatory bowel disease group, albeit not statistically significant (). There was no correlation between serum CgA levels and whole gut lavage 5-HT levels, neither in patients (r = 0.00005, not statistically significant) nor in controls (r = 0.08, P = not statistically significant, ).
Discussion
Most intestinal enterochromaffin cells are of the “open” type with apical cytoplasmic extensions which project into the gut lumen and short microvilli,Citation26 where they sense and respond to the luminal contents by releasing regulatory compounds like 5-HT and CgA, both into the circulation and in the intestinal lumen. Hyperplasia of enterochromaffin cells together with an increase in 5-HT containing granules has been reported in several studies of both functional disorders and inflammatory diseases of the gut,Citation14,Citation27,Citation28 and 5-HT is supposed to be involved in the pathogenesis of various gastrointestinal disorders.Citation29–Citation31 However, in the present study, gut lavage 5-HT were found to be normal in patients with subjective food hypersensitivity. Patients with inflammatory bowel disease had slightly higher mean levels of 5-HT, but the difference was not significant. The high degree of variance in the 5-HT data may be due to high individual differences, as well as a consequence of the preanalytical sampling procedure. The use of gut lavage fluid for analytical purposes is unique. Based on our previous experience with the procedure,Citation22,Citation23 the first clear fluid was chosen because it was desirable to have as few particles as possible to avoid analytical problems, but we acknowledge that the time point of sampling was not necessarily the same for each participant.
Wang et al demonstrated an interesting immunoendocrine axis in the gut, where 5-HT production in enterochromaffin cells increases in response to secretory products from CD4+ T cells.Citation32 Production and secretion of 5-HT from enterochromaffin cells may thus depend on the immunological profile of the immune response,Citation33 and there is also evidence that mucosal 5-HT modulates the immune response via serotonergic receptors expressed by immune cells.Citation34 Indeed, identification of multiple 5-HT receptor subtypes in the gut, especially 5-HT3 and 5-HT4 receptors, suggests multifaceted actions of 5-HT, and have led to the development of several therapeutic agents for functional gastrointestinal disorders.Citation35
Serum CgA was significantly lower in the patients with subjective food hypersensitivity compared with the healthy controls, which is consistent with our previous results.Citation7 Sidhu et al have reported abnormally elevated levels of serum CgA in a small proportion of their patients with diarrhea-predominant irritable bowel syndrome.Citation36 However, they included no healthy controls, and in many of the patients with elevated CgA, the levels declined with time, which the authors explain as short-lived enterochromaffin cell hyperplasia, as previously reported by Dunlop et al.Citation6 Thus, it would be of interest to perform serial measurements of CgA during a day, and also for several continuous days, in future studies to verify possible fluctuations in circulating CgA levels.
Although CgA is a major enteroendocrine secretion product, little is known about its potential role in gastrointestinal pathophysiology. CgA serves as a prohormone for shorter peptide fragments with regulatory properties.Citation8 Fragments of CgA exert antimicrobial effects and may modulate gastrointestinal motility, sensitivity, and barrier function.Citation37,Citation38 The low levels of CgA demonstrated in the present study could be a consequence of alterations in the gut microbial flora in patients with subjective food hypersensitivity.Citation39,Citation40 Intriguingly, Dlugoz et al have recently demonstrated Chlamydia trachomatis antigens in enteroendocrine cells and macrophages of the small bowel in patients with irritable bowel syndrome.Citation41 Although the clinical significance is yet unknown, this is an exciting finding that, together with the present finding of low serum levels of CgA, could imply impairment of enterochromaffin cell function in patients with subjective food hypersensitivity and irritable bowel syndrome. El-Salhy et al recently demonstrated decreased CgA-positive cells in biopsies from patients with irritable bowel syndrome.Citation42 Thus, a decrease in numbers of CgA secretory cells could be an explanation for the low serum CgA found in the present study.
Conclusion
We conclude that measurements of systemic CgA reveal significantly lower levels in patients with subjective food hypersensitivity than in healthy controls, whereas “whole gut 5-HT” levels seem to be normal. Impaired secretion of CgA may play a role in functional gastrointestinal disorders, and warrants further investigation.
Acknowledgements
KG, JV, KL, and GAL have research grants from the Western Norway Regional Health Authority and the University of Bergen. The authors are indebted to the patients for their participation.
Disclosure
The authors report no conflicts of interest in this work.
References
- LindRArslanGEriksenHRA Subjective health complaints and modern health worries in patients with subjective food hypersensitivityDig Dis Sci2005501245125116047467
- ArslanGLindROlafssonSFlorvaagBerstadAQuality of life in patients with subjective food hypersensitivity: applicability of the 10-item short form of the Nepean Dyspepsia IndexDig Dis Sci20044968068715185878
- LillestølKBerstadALindRFlorvaagELiedGATangenTAnxiety and depression in patients with self-reported food hypersensitivityGen Hosp Psychiatry201032424820114127
- LindRLiedGALillestølKValeurJBerstadADo psychological factors predict symptom severity in patients with subjective food hypersensitivity?Scand J Gastroenterol20104583584320433401
- LiXChenHLuHThe study on the role of inflammatory cells and mediators in post-infectious functional dyspepsiaScand J Gastroenterol20104557358120163288
- DunlopSPJenkinsDNealKSpillerRRelative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBSGastroenterology20031251651165914724817
- ValeurJMildeAMHelleKBBerstadALow serum chromogranin A in patients with self-reported food hypersensitivityScand J Gastroenterol2008431403140418654936
- HelleKBChromogranins A and B and secretogranin II as prohormones for regulatory peptides from the diffuse neuroendocrine systemResults Probl Cell Differ201050214420217490
- LundgrenO5-Hydroxytryptamine, enterotoxins, and intestinal fluid secretionGastroenterology1998115100910129786724
- TurvillJLConnorPFarthingMJThe inhibition of cholera toxin-induced 5-HT release by the 5-HT(3) receptor antagonist, granisetron, in the ratBr J Pharmacol20001301031103610882387
- SangerGJNew antiemetic drugsCan J Physiol Pharmacol1990683143242178755
- McCormickDCarcinoid tumors and syndromeGastroenterol Nurs20022510511112055378
- AtkinsonWLockhartSWhorwellPJKeevilBHoughtonLAAltered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndromeGastroenterology2006130344316401466
- DunlopSPColemanNSBlackshawEAbnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndromeClin Gastroenterol Hepatol2005334935715822040
- HoughtonLAAtkinsonWWhitakerRPWhorwellPJRimmerMJIncreased platelet depleted plasma 5-hydroxytryptamine concentration following meal ingestion in symptomatic female subjects with diarrhoea predominant irritable bowel syndromeGut20035266367012692050
- WheatcroftJWakelinDSmithAMahoneyCRMaweGSpillerREnterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunctionNeurogastroenetrol Motil200517863870
- WangSHDongLLuoJYDecreased expression of serotonin in the jejunum and increased numbers of mast cells in the terminal ileum in patients with irritable bowel syndromeWorld J Gastroenterol2007136041604718023097
- GregersenKFrøylandLBerstadAAraujoPDirect determination of serotonin in gut lavage fluid by liquid chromatographic ion trap tandem mass spectrometryTalanta20087546647218371908
- NilssonOAhlmanHGeffardMDahlströmAEricsonLEBipolarity of duodenal enterochromaffin cells in the ratCell Tissue Res198724849543552241
- AhlmanHBhargavaHNDahlströmALarssonINewsonBPetterssonGOn the presence of serotonin in the gut lumen and possible release mechanismsActa Physiol Scand19811122632696974954
- ForsbergEJMillerRJCholinergic agonists induce vectorial release of serotonin from duodenal enterochromaffin cellsScience19822173553567089569
- BerstadAArslanGFolvikGRelationship between intestinal permeability and calprotectin concentration in gut lavage fluidScand J Gastroenterol20001646910672837
- ArslanGKahrsGELindRFrøylandLFlorvaagEBerstadAPatients with subjective food hypersensitivity: the value of analyzing intestinal permeability and inflammation markers in gut lavage fluidDigestion200470263515297775
- ThompsonWGLongstrethGFDrossmanDAHeatonKWIrvineEJMuller-LissnerSAFunctional bowel disorders and functional abdominal painGut199945II43II4710457044
- LillestølKHelgelandLILiedAGIndications of ‘atopic bowel’ in patients with self-reported food hypersensitivityAliment Pharmacol Ther2010311112112220163379
- GustafssonBIBakkeITømmeråsKWaldumHLA new method for visualization of gut mucosal cells, describing the enterochromaffin cell in the rat gastrointestinal tractScand J Gastroenterol20064139039516635905
- GershonMDThe serotonin signaling system: from basic understanding to drug development for functional GI disordersGastroenterology200713239741417241888
- KimHSLimJHParkHLeeSIIncreased immunoendocrine cells in intestinal mucosa of postinfectious irritable bowel syndrome patients 3 years after acute Shigella infection – an observation in a small case control studyYonsei Med J201051455120046513
- CoatesMDMahoneyCRLindenDRMolecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndromeGastroenterology20041261657166415188158
- BishopAEPietrolettiRTaatCWBrummelkampWHPolakJMIncreased populations of endocrine cells in Crohn’s ileitisVirchows Arch A Pathol Anat Histopathol19874103913963103321
- El-SalhyMDanielssonAStenlingRGrimeliusLColonic endocrine cells in inflammatory bowel diseaseJ Intern Med19972424134199408072
- WangHSteedsJMotomuraYCD4+ T cell-mediated immunological control of enterochromaffin cell hyperplasia and 5-hydroxytryptamine production in enteric infectionGut20075694995717303597
- MotomuraYGhiaJEWangHEnterochromaffin cell and 5-hydroxytryptamine responses to the same infectious agent differ in Th1 and Th2 dominant environmentsGut20085747548118198200
- Cloëz-TayaraniIChangeuxJPNicotine and serotonin in immune regulation and inflammatory processes: a perspectiveJ Leukoc Biol20078159960617108054
- LadabaumUSafety, efficacy and costs of pharmacotherapy for functional gastrointestinal disorders: the case of alosetron and its implicationsAliment Pharmacol Ther2003171021103012694084
- SidhuRMcAlindonMELeedsJSSkillingJSandersDSThe role of serum chromogranin a in diarrhoea predominant irritable bowel syndromeJ Gastrointestin Liver Dis200918232619337629
- ShooshtarizadehPZhangDChichJFThe antimicrobial peptides derived from chromogranin/secretogranin family, new actors of innate immunityRegul Pept201016510211019932135
- KhanWIGhiaJEGut hormones: emerging role in immune activation and inflammationClin Exp Immunol2010161192720408856
- UribeAAlamMJohanssonOMidtvedtTTheodorssonEMicroflora modulates endocrine cells in the gastrointestinal mucosa of the ratGastroenterology1994107125912697926490
- ValeurJMorkenMHNorinEMidtvedtTBerstadAIntestinal fermentation in patients with self-reported food hypersensitivity: painful, but protective?Clin Exp Gastroenterol20103657021694848
- DlugoszATörnblomHMohammadianGChlamydia trachomatis antigens in enteroendocrine cells and macrophages of the small bowel in patients with severe irritable bowel syndromeBMJ Gastroenterol20101019
- El-SalhyMLomholt-BeckBHauskenTChromogranin A as a possible tool in the diagnosis of irritable bowel syndromeScand J Gastroenterol2010451435143920602602