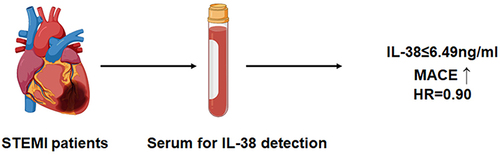
Abstract
Background
The relationship between serum IL-38 and major adverse cardiovascular events (MACE) in patients with ST elevation myocardial infarction (STEMI) remains unclear.
Methods
In the present study, 589 STEMI patients were included, the serum level of IL-38 was measured. The median follow-up time was 720 days, the STEMI patients were divided into high IL-38 (IL-38>6.49ng/mL) and low IL-38 groups (IL-38≤6.49ng/mL) to compare the probability of MACE.
Results
Plasma IL-38 levels were significantly lower in STEMI patients than in SAP patients (4.0±2.2 vs 6.9±3.2 ng/mL, P < 0.001). Ninety-three STEMI patients met the defined MACE study endpoint. The incidence of MACE was significantly lower in patients with high IL-38 group than in patients with low IL-38 group (7.8% vs 23.7%, P < 0.001). Low plasma IL-38 levels were independently associated with the occurrence of MACE (OR = 0.90, P < 0.001).
Conclusion
We get a conclusion that low plasma levels of IL-38 are independently associated with the occurrence of MACE.
Keywords:
Introduction
ST-segment elevation myocardial infarction (STEMI) is one of the most common causes of death in cardiovascular disease.Citation1 Although current lipid-lowering, antiplatelet and interventional therapies have significantly reduced morbidity and mortality in patients with STEMI, patients with STEMI are still at risk of recurrence after hospital discharge.Citation2 Therefore, the prediction of post-discharge major cardiovascular adverse events (MACE) is of great clinical importance for the construction of risk stratification and management of STEMI patients. Although some blood biomarkers have been reported in previous studies to predict the occurrence of MACE events in STEMI patients after hospital discharge, they have not been widely used in the clinic.Citation3,Citation4 Therefore, more studies are needed to focus on clinical biomarkers of MACE occurrence in STEMI patients.
Inflammatory cytokines are thought to be key factors in the development of atherosclerosis, and inflammatory factors secreted by immune cells in atherosclerotic plaque can increase plaque instability by recruiting immune cells and promoting smooth muscle cell proliferation.Citation5–7 Furthermore, previous studies have reported that pro-inflammatory factors can be used to predict offender plaque characteristics and adverse cardiovascular events after hospital discharge in patients with STEMI.Citation8–10 It was found that TNF-α expression in monocytes is highly correlated with plaque rupture, and TNF-α can be used to predict the occurrence of plaque rupture.Citation11 Evidence suggested that the IL-1β concentration in STEMI at admission was association with the risk of mortality and recurrent MACE, the increase of IL-1β concentration was the independent predictor of MACE.Citation12 Moreover, it was supported that serum IL-18 levels in STEMI patients were independent predictors of long-term MACE.Citation13
As we know, immune cells contribute to the progression of cardiovascular diseases. After myocardial infarction, CD4-positive T cells are activated and recruited to the injury site, these cells secrete characteristic inflammatory factors that exacerbate myocardial cell damage.Citation14,Citation15 Moreover, the gene set of immune cells after myocardial infarction has been extensively studied for the diagnosis and prediction of myocardial infarction.Citation16–19 Recently, a series of novel inflammatory factors, such as IL-36, IL-37 and IL-38, were identified here.Citation20 Current studies have confirmed that these novel inflammatory factors are closely associated with the progression of atherosclerosis.Citation21,Citation22 Sara et al found that specific over-expression of IL-37 in macrophages significantly attenuated atherosclerosis progression in hyperlipidemic mice.Citation23 Furthermore, IL-37 levels in patients with STEMI are highly associated with cardiovascular events in patients with atherosclerosis.Citation24 IL-38 is a newly identified IL-1 family cytokine that is expressed in a variety of tissues and secreted by a variety of cells. IL-38 has recently been reported to exert anti-inflammatory functions by binding to a variety of receptors, including IL-36 receptor, IL-1 receptor accessory protein-like 1 and IL-1 receptor 1, which block binding to other pro-inflammatory cytokines and inhibit subsequent signaling pathways.Citation25–27 However, the relationship between IL-38 levels and MACE in patients with ACS remains unclear.
In the present study, we compared the differences in peripheral blood IL-38 levels between patients with STEMI and patients with stable angina and investigated the relationship between IL-38 levels and MACE in STEMI patients after hospital discharge.
Method
Study Population and Design
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. The study was approved by the Harbin Medical University ethics committee, all the patients provided written informed consent.
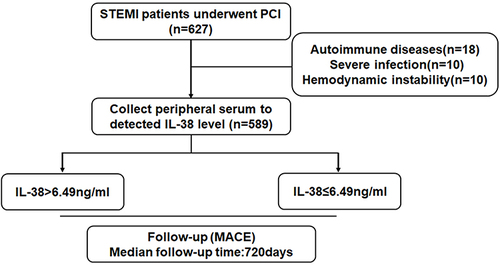
The study flow chart is shown in . Patients with a diagnosis of STEMI and were consecutively enrolled from January 2017 to April 2019 coming to the Department of Cardiology of the first Affiliated Hospital of Harbin Medical University. Patients with stable angina pectoris (SAP) were consecutively enrolled from January 2019 to March 2019 coming to the Department of Cardiology of the first Affiliated Hospital of Harbin Medical University. Among them, 627 patients with STEMI and 55 patients with SAP. The criteria for selection were 1) patients diagnosed with STEMI according to ESC guidelines,Citation27 2) time from the onset of chest pain symptoms to admission less than 6 hours, 3) receiving percutaneous coronary revascularization intervention, and 4) signing an informed consent. Exclusion criteria for STEMI and SAP patients were: 1) patients with malignancy; 2) autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis, and compulsory spondylitis; 3) co-infection with severe infection; 4) refusal to sign informed consent; and 5) hemodynamic instability during PCI. Thirty-eight patients with STEMI were excluded, and 589 patients with STEMI were included in this study. The criteria of selection for SAP patients: 1) The patient’s symptoms meet the diagnostic criteria of the American Heart Association SAP;Citation28 2) No history of ACS or unstable angina; 3) Coronary angiography stenosis is less than 50%; 4) Sign informed consent.
End Points
According to previous studies,Citation29,Citation30 MACE includes non-fatal myocardial infarction, target lesion revascularization (TLR), acute heart failure and cardiogenic death. Non-fatal myocardial infarction was defined as recurrent chest pain and/or the presence of new ECG changes (clinically guided ST-segment elevation or depression) with a 20% new increase in cardiac biomarkers (troponin as well as cardiac enzymes) measured after the recurrent event. Target vessel revascularization is defined as revascularization of the target vessel (including coronary artery bypass surgery and percutaneous coronary intervention) as required by the patient’s clinical symptoms and laboratory tests. Acute heart failure during the index hospitalization was defined as dyspnea, accompanied by physical signs of heart failure and need for additional/increased heart failure therapy. Cardiogenic death was defined as death due to MI or other cardiac causes. All adverse events were defined as occurring during follow-up after discharge.
Assessment of Serum IL-38 Levels
When the patient was just admitted, 5mL of venous blood was drawn with the patient’s consent, centrifuged using a cryogenic centrifuge (3000 rpm for 15 minutes), and the supernatant was collected and stored at −80 °C. We used Human IL-38/IL1F10 ELISA Kit (Boster, China) to measure plasma levels of IL-38 according to the manufacturer’s instructions. The experimenter was single-blind in the grouping of blood samples.
Statistical Analysis
Continuous data are presented as mean ± SD or median (interquartile range). The Student’s t-test or the Mann–Whitney U-test were used for statistical comparisons in two groups. Categorical variables are presented as count (percent), comparisons between groups were made with the χ2 or Fisher exact test. As mentioned in previous studies, we divided the patients into high IL-38 group (>6.49ng/mL) and low IL-38 group (≤6.49ng/mL) according to the median plasma IL-38 level of STEMI patients.Citation31,Citation32 For survival analysis, time-to-first event curves were generated by Kaplan–Meier analysis, and compared using the Log rank test. A COX regression model was used to evaluate the association between MACE and IL-38 level and was adjusted for all other baseline characteristics with p <0.10 and clinical cardiovascular risks on univariable analysis. Statistical analysis was performed with SPSS (Version 22.0). The sample size for the trial was estimated on the basis of the findings from our previous study.Citation33 We speculated that the incidence of MACE in the two groups was less than 9% and more than 20%, respectively. We calculated a power value of 0.88. According to this result, more than 200 patients per group are required, but taking into account the effect of covariates, we increased the number of patients in both groups by 30%.
Result
STEMI Patients Shown Significantly Lower IL-38 Level Than SAP
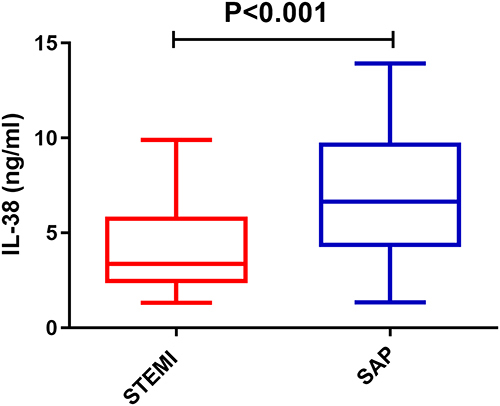
Clinical baseline of 589 patients with STEMI and 55 patients with SAP are shown in , where we observed no significant differences between the two groups in terms of age, gender, smoking history, ejection fraction, hemoglobin, platelets, and creatinine. The percentage of STEMI patients with hypertension and diabetes mellitus was significantly higher than that of patients in the SAP group. In addition, white blood cell count, lipid levels and Hypersensitive C-reactive protein (hs-CRP) levels were significantly higher in STEMI patients than in SAP patients. Correlation analysis of TNI, hs-CRP, BNP and IL-38 was performed, and the results are shown in Supplementary Table 1. Furthermore, shows that plasma levels of IL-38 were significantly lower in STEMI patients than in SAP patients (4.0±2.2 vs 6.9±3.2 ng/mL, P < 0.001).
Table 1 Clinical Data of the SAP and STEMI Patients
The Incidence of MACE Was Higher in STEMI Patients with Low IL-38
To investigate the relationship between IL-38 levels and the time to MACE in STEMI patients, we divided the patients into two groups (higher IL-38 group, IL-38>6.49ng/mL; lower IL-38 group, IL-38≤6.49ng/mL;) based on the median plasma IL-38 in STEMI patients. The clinical baseline of these patients is shown in . It can be observed that in higher IL-38 group, the level of LDL-C (105.3±21.3 vs 185.3±62.1mg/dL, P < 0.001) and platelet count (254.0±50.8 vs 271.7±50.8 10^9/L, P = 0.014) was significantly lower that lower IL-38 group. There is no significant difference in age, gender, hypertension, diabetes mellitus, culprit vessel, TIMI (Pre-PCI) and TIMI (Post-PCI) between the two groups. Also, we also found that the level of hs-CRP, creatinine, HbA1c, and hemoglobin did not differ between the two groups. However, the median follow-up period was 720 days, the incidence of MACE was significantly higher in the low-IL-38 group than in the high-IL-38 group during follow-up time (23.4% vs 7.2%, P < 0.001).
Table 2 Clinical, Angiographic, and Procedural Data of the Study Patients
Serum IL-38 Levels are an Independent Predictor of MACE in STEMI Patients
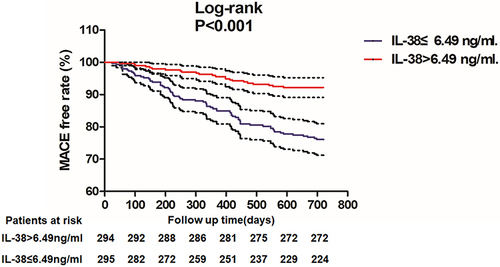
To further investigate the relationship between IL-38 and MACE, we analyzed the survival rate between the two groups using the KM survival curve. As shown in , the incidence of MACE was much higher in the group with low IL-38 levels than in the group with high IL-38 levels. The details of MACE in the two groups are shown in . Moreover, multivariable regression analysis was used to investigate the independent association between IL-38 and MACE (), and it was found that IL-38 levels remained independently associated with MACE occurrence after adjusted for gender, age, smoking, DM, lipid levels, culprit lesion, and TIMI blood flow classification (HR = 0.90, P = 0.040).
Table 3 MACE Stratified by IL-38>6.49 ng/ml and IL-38≤6.49 ng/ml
Table 4 Cox Regression Analyses of Plasma IL-38 Levels for MACE
Discussion
In the present study, we came to the following main conclusions, 1) plasma IL-38 levels were significantly lower in STEMI patients than in SAP patients. 2) MACE were significantly higher in patients with plasma IL-38 levels less than 6.49 ng/mL than in patients with IL-38 greater than 6.49 ng/mL. 3) Plasma IL-38 levels were independently associated with the occurrence of MACE.
Atherosclerosis is chronic inflammatory and inflammatory factors are involved in the whole process of atherosclerosis.Citation34,Citation35 The CANTOS study showed that the use of IL-1β monoclonal antibody was effective in reducing the occurrence of MACE in patients with acute myocardial infarction.Citation36 Monoclonal antibodies to IL-6 also significantly improved the long-term prognosis of STEMI patients.Citation37 Moreover, Colchicine has also been shown to improve the clinical prognosis of patients with acute coronary syndrome by inhibiting inflammasome.Citation38 These clinical trials suggested that inflammatory factors are closely associated with the progression of atherosclerosis-related diseases. However, previous studies have mostly focused on the role and potential mechanisms of classical inflammatory factors in atherosclerosis-related diseases. In recent years, more and more novel inflammatory factors have been identified, such as IL-37, and studies have confirmed that these novel inflammatory factors play an extremely important role in atherosclerosis.Citation39,Citation40 However, the role and value of these inflammatory factors for the diagnosis and prognostic evaluation of patients with atherosclerosis need to be further investigated.
We found that IL-38, a novel inflammatory factor, was significantly decreased in STEMI patients than SAP patients. Although our results do not match the clinical baseline of SAP and STEMI patients, these results suggest that IL-38 may play an important role in the progression of SAP to STEMI. Based on these findings, we considered whether IL-38 also had a poor prognostic effect in STEMI populations. Therefore, we divided STEMI patients into high and low IL-38 groups based on their IL-38 levels. In addition, the incidence of MACE was significantly lower in the higher IL-38 group than in the lower IL-38 group during follow-up time. To date, there are no data on the correlation between IL-38 levels and the diagnosis and prognosis of atherosclerosis-related diseases. Our results demonstrate that an independent correlation between plasma IL-38 levels and the occurrence of MACE after hospital discharge in STEMI patients. It was reported that IL-38 may be associated with patient sensitivity to lipid-lowering therapy and found that IL-38 levels in peripheral blood were significantly higher in statin-sensitive hyperlipidemic patients than in statin-resistant patients.Citation41 At the animal level, overexpression of IL-38 gene by adenovirus was found to be effective in ameliorating hyperlipidemia, inhibiting the secretion of inflammatory factors and attenuating the progression of atherosclerosis. These results can provide some theoretical basis for our conclusions. There are also studies of IL-38 and cardiovascular disease in obese patients. Dennis et al reported that in obese populations with impaired B-cell secretion of IL-38, aging, inflammation-related gene expression was significantly upregulated, and the incidence of cardiovascular disease was significantly higherCitation42 This study suggests that the role of IL-38 may be related to obesity. However, our results did not take into account obesity; therefore, further studies are needed to investigate the ability of IL-38 to evaluate the prognosis of patients with STEMI in a population with different height-to-weight ratios.
Ejection fraction proved to be an independent predictor of MACE events.Citation43 However, in our results, there was no significant difference in ejection fraction between the two groups of patients. The possible reasons are as follows: 1) The time from onset to admission was short, and the cardiac function was not seriously impaired; 2) Patients with hemodynamic instability have been excluded. Previous study reported that an elevated BNP level was associated with a higher risk of postoperative cardiogenic shock.Citation44 Further studies are needed to clarify the predictive value of combined BNP and IL-38 for MACE.
As we all know, atherosclerosis is a chronic inflammatory disease.Citation11,Citation45,Citation46 Several studies have found the role of other inflammatory factors in predicting poor outcomes in patients with STEMI. DNMT3A- and TET2-clonal haematopoiesis-driver mutations have been shown to drive inflammation to promote atherosclerosis. It was reported that DNMT3A and TET2 were independently associated with poor outcomes in STEMI patients.Citation33 Another study has found that the ratio fraction of IL-6/IL-8 can predict the occurrence of MACE, an IL-6-IL-8 score derived from PCA was found to independently predict MACE at one year. Further studies showed that the predictive value of IL-6/IL-8 scores was higher than that of inflammatory factors alone.Citation9 Although our results found that IL-38 can predict MACE, these results suggest that the combination of IL-38 with other inflammatory agents can effectively increase the predictive value of IL-38.
Moreover, there are several studies reported that other inflammatory factors have the ability to predict the clinical outcome of patients with STEMI. Yang et al reported that the level of IL-18 on admission could predict 60-day adverse clinical outcome and contribute to the level of plasma IL-18 on admission may predict 60-day adverse clinical outcome in STEMI patients.Citation47 However, Individual inflammatory markers may have limited predictive power for MACE. It was reported that both IL-6 and IL-8 were associated with adverse outcome in acute myocardial infarction, while an IL-6-IL-8 score derived from principal component analysis showed a stronger ability to predict MACE.Citation9 Furthermore, Reza et al found that the levels of pro-inflammatory factors were consistently increased in the MACE group and that the combination of pro-inflammatory factor levels may serve as a useful predictor of MACE.Citation48 These results suggest that anti-inflammatory is an effective means to reduce the occurrence of MACE. In addition, multiple factors in combination with IL-38 are required to predict MACE in STEMI patients.
IL-38 belongs to the IL-36 family of cytokines, while IL-36 is part of the IL-1 family. It has been found that IL-38 can exert inflammatory and autoimmune suppressive effects by agonizing IL-36 receptors.Citation49,Citation50 Our results found that decreased IL-38 levels were independently associated with the occurrence of MACE in STEMI patients. However, the underlying mechanism for the protective role of IL-38 in atherosclerosis remains unclear. Only in-animal evidence alone suggests that IL-38 may slow the progression of atherosclerosis by improving lipid levels and inhibiting the inflammatory response.Citation41 There is some evidence that IL-38 inhibits cardiac remodeling as well as myocardial inflammation. Firstly, it was found that in mice with myocarditis, the gene levels of IL-36 receptor were significantly elevated in myocardial tissue, and plasma levels of IL-38 were also significantly elevated, which suggests that IL-38 may have a protective effect on myocardial inflammation. In addition, neutralizing IL-38 antibodies significantly exacerbated heart failure and significantly increased mortality in mice with myocarditis, and further study of the mechanism revealed that this may be achieved through inhibition of th17 cell differentiation.Citation51 Secondly, Wei et al reported that, in the anterior descending ligation mouse model, the level of IL-38 was significantly elevated. However, exogenous supplementation of IL-38 significantly improved cardiac ejection function and inhibited cardiac remodeling in mice, the underlying mechanism may be the inhibition of dendritic cell hyperactivation.Citation52 Therefore, combining our results with previous literature, we hypothesize that low levels of IL-38 are independently associated with poor prognosis in atherosclerosis-related diseases and that IL-38 supplementation may be a new potential target for the treatment of atherosclerosis-related diseases. Furthermore, IL-38 levels may be beneficial for risk stratification in STEMI patients, and more randomized controlled studies are needed to confirm our view.
Limitations
There are limitations to this study. First, this study is a single-center study with some geographical limitations of patients, and the findings may not apply to all STEMI patients. Our patients were all from northeast China, so our conclusions may only be applicable to part of the population. You cannot generalize to the whole population. Therefore, further large prospective cohort studies are needed to confirm the reliability of the conclusions. Second, the study enrolled a small number of patients, and the findings need to be further confirmed by a multicenter prospective study with a large sample size. Thirdly, in the IL-38≤6.49 group, the rate of smoking and hyperlipidemia was higher, although not significant. These may have affected the MACE rates in the group. Finally, IL-38 levels change continuously at different times of STEMI patient onset, and we only recorded IL-38 levels at the time of patient admission, without real-time testing of IL-38 levels in patients. Further studies are needed to observe the potential relationship between dynamic changes in IL-38 and the long-term prognosis of STEMI patients.
Conclusion
In STEMI patients, low plasma levels of IL-38 are independently associated with the occurrence of MACE. IL-38 facilitates clinical risk stratification in STEMI patients.
Data Sharing Statement
The data presented in this study are available on request from the corresponding author (Dr Yang Li). The data are not publicly available due to privacy or ethical restrictions.
Approval of Ethics and Consent to Participate
The ethics committee of Harbin Medical University has approved the study, which was conducted in view of the principles of the Declaration of Helsinki. Patient consents were obtained in writing by each of participants before participating in the study.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We would like to thank the members of the Department of Cardiology for their assistance in the preparation of this manuscript.
Additional information
Funding
References
- Collet JP, Thiele H, Barbato E, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289–1367. doi:10.1093/eurheartj/ehaa575
- Bulluck H, Paradies V, Barbato E, et al. Prognostically relevant periprocedural myocardial injury and infarction associated with percutaneous coronary interventions: a Consensus Document of the ESC Working Group on Cellular Biology of the Heart and European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2021;42(27):2630–2642. doi:10.1093/eurheartj/ehab271
- Xiao S, Xue T, Pan Q, et al. MicroRNA-146a serves as a biomarker for adverse prognosis of ST-segment elevation myocardial infarction. Cardiovasc Ther. 2021;2021:2923441. doi:10.1155/2021/2923441
- Shon HS, Bae JW, Kim KO, Cha EJ, Kim KA. Biomarker for the prediction of major adverse cardiac events in patients with non-ST-segment elevation myocardial infarction. Osong Public Health Res Perspect. 2017;8(4):237–246. doi:10.24171/j.phrp.2017.8.4.02
- Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res. 2019;124(2):315–327. doi:10.1161/CIRCRESAHA.118.313591
- Soehnlein O, Libby P. Targeting inflammation in atherosclerosis - from experimental insights to the clinic. Nat Rev Drug Discov. 2021;20(8):589–610. doi:10.1038/s41573-021-00198-1
- Li B, Li W, Li X, Zhou H. Inflammation: a novel therapeutic target/direction in atherosclerosis. Curr Pharm Des. 2017;23(8):1216–1227. doi:10.2174/1381612822666161230142931
- Shou X, Lin J, Xie C, Wang Y, Sun C. Plasma IL-37 elevated in patients with chronic heart failure and predicted major adverse cardiac events: a 1-year follow-up study. Dis Markers. 2017;2017:9134079. doi:10.1155/2017/9134079
- Kristono GA, Holley AS, Hally KE, et al. An IL-6-IL-8 score derived from principal component analysis is predictive of adverse outcome in acute myocardial infarction. Cytokine: X. 2020;2(4):100037. doi:10.1016/j.cytox.2020.100037
- Anroedh SS, Akkerhuis KM, Oemrawsingh RM, et al. Associations of 26 circulating inflammatory and renal biomarkers with near-infrared spectroscopy and long-term cardiovascular outcome in patients undergoing coronary angiography (ATHEROREMO-NIRS substudy). Curr Atheroscler Rep. 2018;20(10):52. doi:10.1007/s11883-018-0752-8
- Luo X, Zhao C, Wang S, Jia H, Yu B. TNF-α is a novel biomarker for predicting plaque rupture in patients with ST-segment elevation myocardial infarction. J Inflamm Res. 2022;15:1889–1898. doi:10.2147/JIR.S352509
- Silvain J, Kerneis M, Zeitouni M, et al. Interleukin-1β and risk of premature death in patients with myocardial infarction. J Am Coll Cardiol. 2020;76(15):1763–1773. doi:10.1016/j.jacc.2020.08.026
- Furtado MV, Rossini AP, Campani RB, et al. Interleukin-18: an independent predictor of cardiovascular events in patients with acute coronary syndrome after 6 months of follow-up. Coron Artery Dis. 2009;20(5):327–331. doi:10.1097/MCA.0b013e32832e5c73
- Kumar V, Prabhu SD, Bansal SS. CD4(+) T-lymphocytes exhibit biphasic kinetics post-myocardial infarction. Front Cardiovasc Med. 2022;9:992653. doi:10.3389/fcvm.2022.992653
- Kumar V, Rosenzweig R, Asalla S, Nehra S, Prabhu SD, Bansal SS. TNFR1 contributes to activation-induced cell death of pathological CD4(+) T lymphocytes during ischemic heart failure. JACC Basic Transl Sci. 2022;7(10):1038–1049. doi:10.1016/j.jacbts.2022.05.005
- Zhang J, Xu M, Chen T, Zhou Y. Bioinformatics analysis of common differential genes of viral myocarditis and dilated cardiomyopathy: screening for potential pharmacological compounds. J Cardiovasc Dev Dis. 2022;9(10). doi:10.3390/jcdd9100353
- Guo N, Zhang N, Yan L, et al. Weighted gene co‑expression network analysis in identification of key genes and networks for ischemic‑reperfusion remodeling myocardium. Mol Med Rep. 2018;18(2):1955–1962. doi:10.3892/mmr.2018.9161
- Wang L, Zhang Y, Yu M, Yuan W. Identification of hub genes in the remodeling of non-infarcted myocardium following acute myocardial infarction. J Cardiovasc Dev Dis. 2022;9(12):409. doi:10.3390/jcdd9120409
- Acet H, Güzel T, Aslan B, Isik MA, Ertas F, Catalkaya S. Predictive value of C-reactive protein to albumin ratio in ST-segment elevation myocardial infarction patients treated with primary percutaneous coronary intervention. Angiology. 2021;72(3):244–251. doi:10.1177/0003319720963697
- Bao S, Hu R, Hambly BD. IL-34, IL-36 and IL-38 in colorectal cancer-key immunoregulators of carcinogenesis. Biophys Rev. 2020;12(4):925–930. doi:10.1007/s12551-020-00726-0
- McCurdy S, Liu CA, Yap J, Boisvert WA. Potential role of IL-37 in atherosclerosis. Cytokine. 2019;122:154169. doi:10.1016/j.cyto.2017.09.025
- Law CC, Puranik R, Fan J, Fei J, Hambly BD, Bao S. Clinical implications of IL-32, IL-34 and IL-37 in atherosclerosis: speculative role in cardiovascular manifestations of COVID-19. Front Cardiovasc Med. 2021;8:630767. doi:10.3389/fcvm.2021.630767
- McCurdy S, Baumer Y, Toulmin E, Lee BH, Boisvert WA. Macrophage-specific expression of IL-37 in hyperlipidemic mice attenuates atherosclerosis. J Immunol. 2017;199(10):3604–3613. doi:10.4049/jimmunol.1601907
- Zhu R, Zhang F, Pan C, Yu K, Zhong Y, Zeng Q. Role of IL-37- and IL-37-treated dendritic cells in acute coronary syndrome. Oxid Med Cell Longev. 2021;2021:6454177. doi:10.1155/2021/6454177
- van de Veerdonk FL, de Graaf DM, Joosten LA, Dinarello CA. Biology of IL-38 and its role in disease. Immunol Rev. 2018;281(1):191–196. doi:10.1111/imr.12612
- Boutet MA, Nerviani A, Pitzalis C. IL-36, IL-37, and IL-38 cytokines in skin and joint inflammation: a comprehensive review of their therapeutic potential. Int J Mol Sci. 2019;20(6):1257. doi:10.3390/ijms20061257
- Han MM, Yuan XR, Shi X, et al. The pathological mechanism and potential application of IL-38 in autoimmune diseases. Front Pharmacol. 2021;12:732790. doi:10.3389/fphar.2021.732790
- Arnold SV, Bhatt DL, Barsness GW, et al. Clinical management of stable coronary artery disease in patients with type 2 diabetes mellitus: a scientific statement from the American Heart Association. Circulation. 2020;141(19):e779–e806. doi:10.1161/CIR.0000000000000766
- Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta-analysis of prospective studies. J Am Heart Assoc. 2017;6(7). doi:10.1161/JAHA.116.004947
- van Kranenburg M, Magro M, Thiele H, et al. Prognostic value of microvascular obstruction and infarct size, as measured by CMR in STEMI patients. JACC Cardiovasc Imaging. 2014;7(9):930–939. doi:10.1016/j.jcmg.2014.05.010
- Sardu C, Consiglia Trotta M, Santella B, et al. Microbiota thrombus colonization may influence athero-thrombosis in hyperglycemic patients with ST segment elevation myocardial infarction (STEMI). Marianella study. Diabetes Res Clin Pract. 2021;173:108670. doi:10.1016/j.diabres.2021.108670
- Gao J, Yan KT, Wang JX, et al. Gut microbial taxa as potential predictive biomarkers for acute coronary syndrome and post-STEMI cardiovascular events. Sci Rep. 2020;10(1):2639. doi:10.1038/s41598-020-59235-5
- Wang S, Hu S, Luo X, et al. Prevalence and prognostic significance of DNMT3A- and TET2- clonal haematopoiesis-driver mutations in patients presenting with ST-segment elevation myocardial infarction. EBioMedicine. 2022;78:103964. doi:10.1016/j.ebiom.2022.103964
- Geovanini GR, Libby P. Atherosclerosis and inflammation: overview and updates. Clin Sci. 2018;132(12):1243–1252. doi:10.1042/CS20180306
- Bäck M, Yurdagul A, Tabas I, Öörni K, Kovanen PT. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol. 2019;16(7):389–406. doi:10.1038/s41569-019-0169-2
- Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10105):1833–1842. doi:10.1016/S0140-6736(17)32247-X
- Ridker PM, Devalaraja M, Baeres FMM, et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, Phase 2 trial. Lancet. 2021;397(10289):2060–2069. doi:10.1016/S0140-6736(21)00520-1
- Martínez GJ, Celermajer DS, Patel S. The NLRP3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis-associated inflammation. Atherosclerosis. 2018;269:262–271. doi:10.1016/j.atherosclerosis.2017.12.027
- Tian Y, Ling XY, Chen DL, Zhang XQ, Qiu CM. Interleukin-36 receptor antagonist attenuates atherosclerosis development by inhibiting NLRP3 inflammasome. J Cell Physiol. 2020;235(12):9992–9996. doi:10.1002/jcp.29813
- Mukherjee P, Chattopadhyay A, Grijalva V, et al. Oxidized phospholipids cause changes in jejunum mucus that induce dysbiosis and systemic inflammation. J Lipid Res. 2022;63(1):100153. doi:10.1016/j.jlr.2021.100153
- Yang N, Song Y, Dong B, et al. Elevated interleukin-38 level associates with clinical response to atorvastatin in patients with hyperlipidemia. Cell Physiol Biochem. 2018;49(2):653–661. doi:10.1159/000493029
- de Graaf DM, Jaeger M, van den Munckhof ICL, et al. Reduced concentrations of the B cell cytokine interleukin 38 are associated with cardiovascular disease risk in overweight subjects. Eur J Immunol. 2021;51(3):662–671. doi:10.1002/eji.201948390
- Janjani P, Motevaseli S, Salehi N, Heidari Moghadam R, Siabani S, Nalini M. Predictors of 1-year major cardiovascular events after ST-elevation myocardial infarction in a specialized cardiovascular center in Western Iran. J Tehran Heart Cent. 2022;17(2):62–70. doi:10.18502/jthc.v17i2.9839
- Duchnowski P. N-terminal of the prohormone brain natriuretic peptide predicts postoperative cardiogenic shock requiring extracorporeal membrane oxygenation. J Clin Med. 2022;11(19):254.
- Luo X, Weng X, Bao X, et al. A novel anti-atherosclerotic mechanism of quercetin: competitive binding to KEAP1 via Arg483 to inhibit macrophage pyroptosis. Redox Biol. 2022;57:102511. doi:10.1016/j.redox.2022.102511
- Luo X, Lv Y, Bai X, et al. Plaque erosion: a distinctive pathological mechanism of acute coronary syndrome. Front Cardiovasc Med. 2021;8:711453. doi:10.3389/fcvm.2021.711453
- Gao Y, Tong GX, Zhang XW, et al. Interleukin-18 levels on admission are associated with mid-term adverse clinical events in patients with ST-segment elevation acute myocardial infarction undergoing percutaneous coronary intervention. Int Heart J. 2010;51(2):75–81. doi:10.1536/ihj.51.75
- Mohebi R, McCarthy CP, Gaggin HK, van Kimmenade RRJ, Januzzi JL. Inflammatory biomarkers and risk of cardiovascular events in patients undergoing coronary angiography. Am Heart J. 2022;252:51–59. doi:10.1016/j.ahj.2022.06.004
- Dinarello CA. The IL-1 family of cytokines and receptors in rheumatic diseases. Nat Rev Rheumatol. 2019;15(10):612–632. doi:10.1038/s41584-019-0277-8
- Boutet MA, Najm A, Bart G, et al. IL-38 overexpression induces anti-inflammatory effects in mice arthritis models and in human macrophages in vitro. Ann Rheum Dis. 2017;76(7):1304–1312. doi:10.1136/annrheumdis-2016-210630
- Xue Y, Chen M, Chen Q, et al. Neutralization of interleukin-38 exacerbates coxsackievirus B3-induced acute myocarditis in mice. Virol J. 2021;18(1):220. doi:10.1186/s12985-021-01687-w
- Wei Y, Lan Y, Zhong Y, et al. Interleukin-38 alleviates cardiac remodelling after myocardial infarction. J Cell Mol Med. 2020;24(1):371–384. doi:10.1111/jcmm.14741