Abstract
Background
Liver cirrhosis is a global health problem due to a large number of disability-associated life years and mortality. However, evidence is scarce on its causes in Eastern-Ethiopia, a place where there is a high prevalence of liver cirrhosis of unknown etiology. This study attempted to identify the risk factors related to liver cirrhosis in the area.
Methods
A case-control study was conducted at a tertiary care hospital from January 2020 to July 2021. Following diagnoses using an ultrasound-based cirrhosis scale, a total of 127 cases were identified and compared with 253 control patients. A structured questionnaire and data abstraction form were used to collect demographic, lifestyle, and clinical information. A blood sample was also taken from each participant for clinical chemistry, hepatitis B virus (HBV), and hepatitis C virus tests as well as for an aflatoxin B1 (AFB1) albumin adduct (AF-alb) assay. Binary logistic regression analysis was used to determine predictors of liver cirrhosis.
Results
AF-alb levels were detected in 75% of the cases and 64% of the controls, with a median (IQR) level of 11 pg/mg (5.5–25) and 7.0 pg/mg (4.3–20.5), respectively (p<0.05). Moreover, the number of subjects with high AF-alb levels (≥8.6 pg/mg) was greater in cases (45%, p<0.05)) than controls (28%). Age ≥55 years (adjusted odds ratio (AOR)=0.4; 95% CI: 0.2, 0.8), being a farmer (AOR= 3.0; 95% CI: 1.5, 6.0), family history of liver disease (AOR= 2.9; 95% CI: 1.1, 7.9), HBV seropositivity (AOR=4.0; 95% CI: 1.9, 8.8), and exposure to high levels of AF-alb (AOR=2.0; 95% CI: 1.1, 3.7) were significantly associated with liver cirrhosis.
Conclusion
This study found a strong link between AFB1 exposure and liver cirrhosis. Mitigation of aflatoxin exposure and a better understanding of additional environmental risk factors like pesticides may be necessary to reduce the disease burden in Ethiopia.
Introduction
Liver cirrhosis is characterized by distortion of the hepatic architecture and formation of regenerative nodules. It is a leading cause of morbidity and mortality in liver disease patients, accounting for 2.4% of all deaths globally in 2019.Citation1 In Ethiopia, it is considered the 7th leading cause of mortality, accounting for 24 deaths/100,000 population.Citation2 The treatment of liver cirrhosis has advanced dramatically in recent years. However, the prognosis remains bleak.Citation3 Thus, identifying and working against its preventable risk factors should be a priority agenda.
Liver cirrhosis has been known to be associated with obesity, non-alcoholic fatty liver disease, excessive alcohol intake, Hepatitis B or C virus (HBV or HCV) infection, autoimmune disorders, cholestatic diseases, and iron overload.Citation1,Citation4 Despite enormous progress in understanding the etiology of the disease, nearly one-third of the cases in sub-Saharan Africa are cryptogenic (cirrhosis of the liver of unknown etiology).Citation5 Indeed, a study done in the Eastern parts of Ethiopia revealed that up to 55% of liver cirrhosis cases were cryptogenic, and histopathological investigations indicated that these cases were characterized by toxic liver injury.Citation6
In this regard, dietary exposure to aflatoxin B1 (AFB1), a fungal toxin produced by Aspergillus flavus and Aspergillus parasiticus, has been implicated as a potential etiologic factor for liver cirrhosis in numerous epidemiological studies.Citation7–10 For instance, a recent study done in Guatemala, where maize is the primary staple cereal, known to be contaminated with AFB1, found a significant association between AFB1 exposure and liver cirrhosis.Citation9 In Eastern Ethiopia, several studies have shown severe contamination of principal dietary staple foods and cash crops including, sorghum, maize, and ground nut with AFB1.Citation11–15 Despite these studies, there has been little direct evidence linking AFB1 exposure to the etiology of liver cirrhosis. Human chronic exposure to AFB1 can be assessed by determining the biomarker, blood aflatoxin albumin adduct (AF-alb), using a competitive enzyme-linked immunosorbent assay (ELISA) method. To this end, this study sought to investigate aflatoxin exposure as a potential risk factor for liver cirrhosis in Eastern Ethiopia.
Materials and Method
Study Setting and Design
A case-control study was conducted in Hiwot Fana Comprehensive Specialized University Hospital (HFCSUH), Harar, Eastern Ethiopia, from 1 January 2020 to 31 July 2021. The teaching hospital is affiliated with Haramaya University and its catchment population is nearly six million, living in Eastern Oromia, Dire Dawa City Administration, Harari, and Somali Regional States.
Study Population and Eligibility
Patients diagnosed with liver cirrhosis through abdominal imaging among those attending the Internal Medicine unit of the hospital during the study period were identified as cases. Patients attending the same unit and without any history or clinical evidence of liver disease were identified as controls. In cases, patients aged ≥18 years old and having a score of ≥7 based on an ultrasound-based cirrhosis scaleCitation16,Citation17 were included, whilst those with evidence of space-occupying hepatic lesions, and unable or unwilling to provide informed consent were excluded. For controls, patients aged ≥18 years old and having ultrasound scores of ≤4 were included, while those with elevated liver function tests (ALT or AST above 40 IU/L) and unable or unwilling to provide informed consent were excluded.
Data Collection and Management
A structured questionnaire was used to gather information on sociodemographic characteristics, alcohol consumption, Catha edulis (Vahl) Forssk. Ex Endl. (khat) chewing, and tobacco usage habits. To ensure consistency, the original English version was translated into local languages (Afan Oromo and Amharic) and then back-translated into English by multilingual specialists. Alcohol consumption in the past and present was collected and quantified in grams. Alcohol abuse was defined as daily alcohol use of more than 20 g in women and more than 30 g in men for a minimum of 6 months.Citation18 The amount of khat, a herb chewed for its amphetamine-like effect, consumed was measured in grams using a visual analog scale. On average, 100–300 g of fresh khat leaves is chewed in a typical session. Thus, khat use refers to chewing at least 200 g of khat daily for one year.Citation6 Tobacco including cigarette, pipe, and shisha usageCitation19 in the past and present was recorded. Body Mass Index (BMI) was calculated to categorize as underweight (<18.5), normal (18.5–24.9), overweight (25.0–29.9), and obese (≥30.0).Citation20 Food consumption in the previous year was recorded using a food frequency questionnaire (FFQ).
Clinical Examination
A mobile digital color Doppler ultrasound machine (Model: SSI-8000, SonoScape Co. Ltd) was used to perform abdominal ultrasonography following a standard protocol. The diagnosis of liver cirrhosis was made by a physician and a radiologist based on an ultrasonography cirrhosis scale. The intrahepatic vascular type (score 1–3), liver parenchyma (score 1–3), liver surface (score 1–3), and spleen size index (score 1–2, 1 for a spleen size index of 20 cm2, and 2 for a greater spleen size index) are components of the scoring system. Liver cirrhosis was diagnosed using a score of ≥ 7 out of 11 and normal liver would have a maximum score of 4 in this scale.
Blood Sample Collection and Analysis
A blood sample was drawn via venipuncture using the BD Vacutainer® blood collection system (Becton Dickinson, NJ, USA). The blood was collected in a serum separator tube (3–5 mL) and two EDTA tubes (2 mL and 3 mL). The serum was then used for liver function tests (LFTs), Hepatitis surface antigen B (HBsAg), HCV antibody tests, and serum creatinine tests. The blood collected in the first EDTA tube was used for platelet count. The blood collected in the 2nd EDTA tube was centrifuged at 1100–1300 g for 10 min at 4 °C to separate the plasma and stored at −80°C using a cryotube. The plasma was transported in dry ice to the University of Leeds, United Kingdom for AF-alb analysis.
Clinical Laboratory Tests
Cobas C-311m, a fully automated and closed clinical chemistry analyzer (Roche/Hitachi Cobas C-311, Roche diagnostics GmbH, Mannheim, Germany) was used to perform LFTs including aspartate transaminase (AST), alanine transaminase (ALT), Alkaline phosphatase (ALP), serum bilirubin (total and direct), albumin, and serum creatinine tests. DxH 800 hematology analyzer (Beckman Coulter, Germany) was used to conduct platelet count. Moreover, advanced quality one-step HBsAg test kit (InTec PRODUCTS, INC.) and a one-step HCV test kit (Guangzhou Wondfo Biotech Co. Ltd) were used for HBsAg and anti-HCV screening, respectively.
Aflatoxin B1-Albumin (AF-Alb) Adduct Analysis
The levels of AF-alb in plasma were measured by albumin extraction, enzyme digestion, Sep-Pak C18 column purification, and finally quantification of the adduct using a competitive ELISA method as previously described.Citation21 Three positive and one negative control samples were analyzed with each batch of plasma samples for quality control. Samples were measured in duplicates on two separate days, and measurements with a coefficient of variations of less than 25% were taken. Moreover, 10% of the total sample was randomly selected and the whole process was repeated to ensure reproducibility of the test. The limit of detection (LOD) was 3 pg AFB1-lysine equivalents per mg of albumin (pg/mg). The median detectable adduct levels among the study population was measured and 8.6 pg/mg of albumin was used as a cutoff for low (<8.6 pg/mg;) and high (≥8.6 pg/mg) levels of AF-alb adduct.
Statistical Analysis
Statistical analysis was performed using Statistical Package for Social Sciences (SPSS) version 26.0. All continuous variables were deemed to have non-normal distribution and expressed as median (IQR). Whereas, percentages were used for categorical variables. To examine differences in the characteristics between cases and controls, the Mann–Whitney U-test was used for continuous variables, but χ2 or Fisher exact tests for categorical variables. Variables with a p-value less than 0.25 in the univariate analysis and also biologically relevant variables including gender and age were fitted to the multivariable logistic regression model to determine risk factors associated with liver cirrhosis. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated in the final logistic regression model. The level of significance was set at p < 0.05.
Ethical Considerations
This study was conducted following the Declaration of Helsinki. Ethical approval was obtained from both the Institutional Review Board of the College of Health Sciences, Addis Ababa University (Protocol No. 064/19/SOP and Reference No. CHS/RTTD/229/2020) and the National Ethical Review Committee of the Ministry of Science and Higher Education (Reference No. 04/246/680/21). Written informed consent was obtained from participants before the survey.
Results
A total of 163 patients with probable liver disease were initially identified as potential cases. After excluding 32 patients who did not fulfill the inclusion criteria (26 for ultrasound criteria and 6 for having hepatic focal lesions), 131 patients were enrolled in the study. Four patients were further excluded, as three of them refused to provide blood samples and it was impossible to draw blood from one patient. Regarding controls, 287 patients without any signs of liver disease were identified as potential controls. After excluding twenty-nine patients who did not fulfill the inclusion criteria (1 for ultrasound and 28 for LFT), 258 patients were enrolled in the study. Five patients were further excluded as they refused to provide blood samples. As a result, data for 127 cases and 253 controls were used for analysis.
Demographic Characteristics
The demographic characteristics of cases and controls are presented in . There was a preponderance of males in both cases (66%) and controls (57%), but no significant difference in gender was observed between the two groups (p=0.08). The median age of cases and controls was not statistically different (p=0.73) [32 years (IQR: 28–45) vs 35 years (IQR: 25–50)]. Majority (80%) of the cases and more than half (55%) of the controls had no formal education. A large proportion of cases (82%) were farmers compared to controls (53%). There were significant differences (p<0.05) between cases and controls in terms of residence, education, and occupation ().
Table 1 Demographic Characteristics of Study Participants, Eastern Ethiopia, 2020/21
Lifestyle Characteristics
Khat and tobacco use were more commonly observed in cases (72% and 22%) than controls (58% and 13%), with the use being significantly higher (p<0.05) in cases than controls. It is of note that khat was a substance largely used more than tobacco in both groups (). Almost all (98%) of the cases and controls did not have a history of alcohol use. About 1/3 of the participants were underweight, and only a small proportion of cases (7%) and controls (5%) were overweight.
Table 2 Lifestyle Characteristics of Study Participants, Eastern Ethiopia, 2020/21
Medical and Aflatoxin B1 Exposure Characteristics
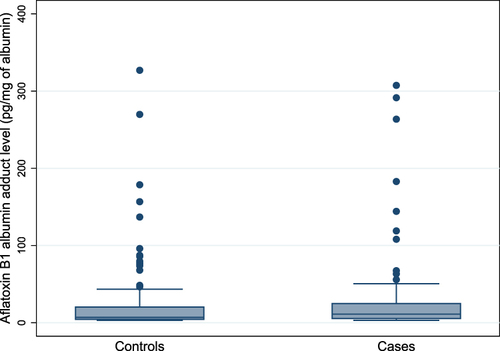
As shown in , a significant proportion of cases were HBV seropositive (23%) and had a family history of liver disease (13%) compared with controls (5.5% and 3%, respectively). HCV infections were found to be less common in both cases (0.8%) as well as controls (0.4%). AF-alb adducts were detected in 257 (68%) of the total 380 samples with a median level of 8.6 (4.7–21.9) pg/mg of albumin. AF-alb positive rate was significantly higher (p<0.05) in cases (75%) than in controls (64%). As shown in and , the median AF-alb adduct of cases [11 pg/mg (5.5–25)] was significantly higher (p<0.05) than controls [7.0 pg/mg (4.3–20.5)]. Moreover, based on the cut-off value used in the present study, a significant proportion of cases (45%) had a higher level (p<0.05) of AF-alb compared to controls (28%).
Table 3 Medical and Aflatoxin B1 Exposure Characteristics of Study Participants, Eastern Ethiopia, 2020/21
Determinants of Liver Cirrhosis
Age, gender, residence, marital status, formal education, occupation, khat use, tobacco use, BMI, family history of liver disease, HBV status, and AF-alb levels were fitted in the multivariable logistic regression analysis model. Age 55 years and above, being a farmer, family history of liver disease, HBV seropositivity, and high level of AF-alb were found to be significantly associated with liver cirrhosis (). The odds of developing liver cirrhosis decreased by 60% (AOR=0.4; 95% CI: 0.2, 0.8) in patients with age 55 years and above compared with patients younger than 35 years. Patients who were farmers by occupation had 3 times higher odds of having liver cirrhosis than those who were not farmers (AOR= 3.0; 95% CI: 1.5, 6.0). The odds of liver cirrhosis were 2.9 times higher among patients with a family history of liver disease compared to patients without a family history (AOR= 2.9; 95% CI: 1.1, 7.9). HBV seropositive patients had 4 times higher odds of developing liver cirrhosis than HBV seronegative patients (AOR=4.0; 95% CI: 1.9, 8.8). Likewise, the odds of liver cirrhosis were 2 times higher among patients who were exposed to a high level of AF-alb adduct compared to patients with undetectable levels (AOR=2.0; 95% CI: 1.1, 3.7).
Table 4 Multivariable Binary Logistic Regression Analysis of Predictive Factors of Liver Cirrhosis in Eastern Ethiopia, 2020/21
Discussion
Regardless of the substantial burden of the disease, limited knowledge of the risk factors or etiologic data have been available on liver cirrhosis or chronic liver disease from the Eastern Ethiopian population.Citation6,Citation22 This study aims to provide a drive for further inquiry and action regarding AFB1 exposure as an etiologic factor to liver cirrhosis in a region with a high burden of unexplained liver cirrhosis. The current study found that age 55 years and above, being a farmer, family history of liver disease, HBV seropositivity, and exposure to high levels of AF-alb adduct were significantly associated with liver cirrhosis.
In our study, the odds of developing liver cirrhosis were decreased in patients with age 55 years and above compared with patients younger than 35 years. In general, cirrhosis of any etiology is a major risk factor for hepatocellular carcinoma (HCC).Citation23 Currently, it is believed that HCC in sub-Saharan Africa is a disease of young adults, unlike in North Africa, Japan, Western Europe, and North America, where it is common in the elderly.Citation24 In this regard, it is well known that sub-Saharan Africa has a relatively high incidence of HBV, with the majority of cases of exposure occurring during childhood through vertical or horizontal transmission.Citation25,Citation26 Aflatoxin exposure is also known to have started in the earliest years of life.Citation27 The combination of these findings may thus partially explain why liver cirrhosis, or HCC, manifests itself at a younger age in sub-Saharan Africa than in the rest of the globe. Indeed, studies also revealed the influence of age at infection for the establishment of HBV infection and development of liver cirrhosis or HCC.Citation28,Citation29
On the other hand, patients who were farmers by occupation had higher odds of having liver cirrhosis. In this context, in addition to dietary exposure, farmers face a substantial risk of AFB1 airborne exposure while managing infected grains as well as processing and handling animal feed.Citation30,Citation31 This is further fueled by lack of awareness about mycotoxins, especially in resource-limited settings.Citation32,Citation33 In support of this, our study demonstrated a significant (p<0.01) difference in the median levels of AF-alb adduct between patients who were farmers by occupation compared with patients who were not farmers (10.0 pg/mg, 5.3–29.4 vs 6.7 pg/mg, 4.3–14.7) (Supplementary Table 1). These findings together suggest that AFB1 exposure might be one possible factor that made farmers to be at high risk for developing liver cirrhosis than non-farmers.
Moreover, numerous studies reported the link between hepatic toxicity and occupational exposure to pesticides in farmers residing in Africa and Asia due to poor safety practices.Citation34–36 In Ethiopia, the use of pesticides in small-scale farms has increased dramatically in the last decades and the situation of inappropriate use of pesticides due to poor knowledge and management of pesticide products is much more serious than commonly visualized.Citation37–39 In Eastern Ethiopia, the insecticide, Dichlorodiphenyltrichloroethane (DDT), has been extensively used to control insect pests in cash crop production including khat and its inappropriate use and management is of concern.Citation40,Citation41 To this effect, a study conducted in rural northern Ethiopia to find out the cause of liver disease with unknown etiology detected high levels of DDT in urine and serum samples and inferred its possible association with the disease.Citation42 Interestingly, DDT exposure has been shown to enhance the toxic effect of environmental toxins, including AFB1 through induction of CYP3A4.Citation43,Citation44
Our study also revealed that patients with a family history of liver disease were more likely to have liver cirrhosis than patients without a family history. Studies indicated that inflammation and oxidative stress play a pathogenic role in liver injury that progresses to liver cirrhosis due to exposure to viral and environmental factors.Citation45,Citation46 This could be explained by genetic alterations in different cellular signaling pathways linked with DNA repair,Citation47 and immune response.Citation48 Furthermore, liver metabolic enzymes, both Phase I and Phase II, have a range of genetic polymorphisms that cause genetic predisposition in activation and/or detoxification functions, influence metabolism, and subsequently increase the toxicity of environmental chemical toxins like AFB1.Citation49,Citation50 Thus, it is not surprising that patients with a family history of liver disease would have a higher risk of experiencing liver cirrhosis.
Patients with HBV seropositive had 4 times higher odds of developing liver cirrhosis than HBV seronegative patients. This was consistent with previous findings done in different areas of the world, including Ethiopia.Citation51,Citation52 The mechanism by which chronic HBV infection predisposes to liver cirrhosis is still enigmatic. However, in several models of HBV infection, it has been identified that liver injuries in chronic infection are considered to be associated with the activity of HBV-specific T cells, as HBV is not directly cytopathic.Citation53,Citation54 Moreover, its immuno-pathogenesis depends on an intricate interaction of host factors (such as age, gender, immune status, and genetics),Citation55 viral,Citation56 and environmental factors, including AFB1 exposure.Citation8
Our study demonstrated that the median AF-alb level was significantly higher among cases (11.0 pg/mg) than controls (7.0 pg/ mg), and patients exposed to high levels of AF-alb adduct were also at significantly higher risk of developing liver cirrhosis. The results of the present study are consistent with other studies reported from Africa,Citation7 Asia,Citation9 and South America,Citation9 where there is a high burden of aflatoxin exposure. The Gambian study also showed a synergy between AFB1 exposure and HBV infection on the risk of liver cirrhosis.Citation7 Moreover, other studies done in Egypt,Citation57 Turkey,Citation58 and TaiwanCitation59 reported a significantly higher proportion of AFB1 signature mutation in TP53, a higher mean level of AF-alb adduct, and a positive association of AF-alb adduct level and ultrasonographic parenchyma scores among patients with chronic liver disease or liver cirrhosis. As reviewed by Mekuria et al,Citation10 the toxic effects of AFB1 against the liver are related to its intermediate metabolic product, AFB1-exo-8,9-epoxide, formed by CYP3A4 and CYP1A2 enzymes in the hepatocytes and the associated formation of reactive oxygen species, leading to oxidative stress and induction of apoptosis through the mitochondrial signaling pathways.
Cases and controls were recruited using ultrasound examination that has up to 80% and 97% sensitivity and specificity, respectively, versus liver biopsy to diagnose liver cirrhosis.Citation60,Citation61 Moreover, the ultrasound examination reduced the chance of including study participants with undiagnosed liver disease in the control group. Nevertheless, we acknowledge the following limitations of our study. First, due to the inherent limitation of the study design, this study cannot establish the temporal sequence between exposure and outcome. Second, because participants were asked about their previous experiences, our findings could be influenced by recall bias.
Conclusions
The detection of higher levels of AF-alb adduct in cases and its strong association with liver cirrhosis suggests that this fungal toxin could, at least, in part explain the missing etiologic factor for the high burden of unexplained liver cirrhosis in the region. Interventions to limit aflatoxin exposure, as well as efforts to identify the role of other risk factors for liver cirrhosis, may be essential in reducing the high burden of liver cirrhosis in Eastern Ethiopia.
Ethics Statement
This study was conducted following the Declaration of Helsinki. Ethical approval was obtained from both the Institutional Review Board of the College of Health Sciences, Addis Ababa University (Protocol No. 064/19/SOP and Reference No. CHS/RTTD/229/2020) and the National Ethical Review Committee of the Ministry of Science and Higher Education (Reference No. 04/246/680/21).
Author Contributions
All authors made a significant contribution to the work reported whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there are no conflicts of interest in this work.
Acknowledgments
We would like to express our gratitude to those patients who participated in the study. We also would like to thank the staff of HFCSUH for their unreserved support and collaboration during data collection. The authors would like to acknowledge Dr. Afework Fanta (Radiologist, Bolodia Medium Clinic, Harar) for his immense support in ultrasound image readings. The authors would also like to appreciate and acknowledge Addis Ababa University and Haramaya University (HURG-2020-02-01-76) for their financial support to carry out this study. Moreover, the authors would like to thank the Center for Innovative Drug Development and Therapeutic Trials for Africa (CDT Africa) for the travel grant and the British Council Partnership grant on food safety to the University of Leeds, School of Food Science and Nutrition for Laboratory access to determine the AF-alb adduct.
References
- Huang DQ, Terrault NA, Tacke F, et al. Global epidemiology of cirrhosis — aetiology, trends and predictions. Nat Rev Gastroenterol Hepatol. 2023;20 (6) :388–398.
- World Health Organization. Global health estimates 2020: deaths by cause, age, sex, by country and by region, 2000–2019. Available from: https://who.int/data/gho/data/themes/mortality-andglobal-health-estimates/ghe-leadingcauses-of-death. Accessed May 28, 2023.
- Tapper EB, Ufere NN, Huang DQ, et al. Review article: current and emerging therapies for the management of cirrhosis and its complications. Aliment Pharmacol Ther. 2022;55(9):1099–1115. doi:10.1111/apt.16831
- Wang K, Yang F, Zhang P, et al. Genetic effects of iron levels on liver injury and risk of liver diseases: a two-sample Mendelian randomization analysis. Front Nutr. 2022;9:964163.
- Mokdad AA, Lopez AD, Shahraz S, et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. 2014;12(1):145. doi:10.1186/s12916-014-0145-y
- Orlien SMS, Ismael NY, Ahmed TA, et al. Unexplained chronic liver disease in Ethiopia: a cross sectional study. BMC Gastroenterol. 2018;18(1):27. doi:10.1186/s12876-018-0755-5
- Kuniholm MH, Lesi OA, Mendy M, et al. Aflatoxin exposure and viral hepatitis in the etiology of liver cirrhosis in the Gambia, West Africa. Environ Health Perspect. 2008;116(11):1553–1557. doi:10.1289/ehp.11661
- Chu YJ, Yang HI, Wu HC, et al. Aflatoxin B1 exposure increases the risk of cirrhosis and hepatocellular carcinoma in chronic hepatitis B virus carriers. Int J Cancer. 2017;141(4):711–720. doi:10.1002/ijc.30782
- Alvarez CS, Hernández E, Escobar K, et al. Aflatoxin B1exposure and liver cirrhosis in Guatemala: a case–control study. BMJ Open Gastroenterol. 2020;7(1):e000380. doi:10.1136/bmjgast-2020-000380
- Mekuria AN, Routledge MN, Gong YY, et al. Aflatoxins as a risk factor for liver cirrhosis: a systematic review and meta-analysis. BMC Pharmacol Toxicol. 2020;21(1):39.
- Chala A, Mohammed A, Ayalew A, et al. Natural occurrence of aflatoxins in groundnut (Arachis hypogaea L.) from eastern Ethiopia. Food Control. 2013;30(2):602–605. doi:10.1016/j.foodcont.2012.08.023
- Chala A, Taye W, Ayalew A, et al. Multimycotoxin analysis of sorghum (Sorghum bicolor L. Moench) and finger millet (Eleusine coracana L. Garten) from Ethiopia. Food Control. 2014;45:29–35. doi:10.1016/j.foodcont.2014.04.018
- Taye W, Ayalew A, Chala A, et al. Aflatoxin B 1 and total fumonisin contamination and their producing fungi in fresh and stored sorghum grain in East Hararghe, Ethiopia. Food Addit Contam Part B Surveill. 2016;9(4):237–245. doi:10.1080/19393210.2016.1184190
- Mamo FT, Abate BA, Tesfaye K, et al. Mycotoxins in Ethiopia: a review on prevalence, economic and health impacts. Toxins. 2020;12(10):648. doi:10.3390/toxins12100648
- Mohammed A, Bekeko Z, Yusufe M, et al. Fungal species and multi-mycotoxin associated with post-harvest Sorghum (Sorghum bicolor (L.) Moench) Grain in Eastern Ethiopia. Toxins. 2022;14(7):473. doi:10.3390/toxins14070473
- Lin D-Y, Sheen I-S, Chiu C-T, et al. Ultrasonographic changes of early liver cirrhosis in chronic hepatitis B: a longitudinal study. J Clin Ultrasound. 1993;21(5):303–308. doi:10.1002/jcu.1870210502
- Shen L, Li JQ, Zeng MD, et al. Correlation between ultrasonographic and pathologic diagnosis of liver fibrosis due to chronic virus hepatitis. World J Gastroenterol. 2006;12(8):1292–1295. doi:10.3748/wjg.v12.i8.1292
- Hagström H. Alcohol consumption in concomitant liver disease: how much is too much? Curr Hepatol Rep. 2017;16(2):152–157. doi:10.1007/s11901-017-0343-0
- Heikkinen H, Jallinoja P, Saarni SI, et al. The impact of smoking on health-related and overall quality of life: a general population survey in Finland. Nicotine Tob Res. 2008;10(7):1199–1207. doi:10.1080/14622200802163142
- Hidese S, Ota M, Matsuo J, et al. Association of body mass index and its classifications with gray matter volume in individuals with a wide range of body mass index group: a whole-brain magnetic resonance imaging study. Original Research. Front Human Neurosci. 2022;16. doi:10.3389/fnhum.2022.926804
- Chapot B, Wild CP. ELISA for quantification of aflatoxin-albumin adducts and their application to human exposure assessment. In: Warhol M, van Velzen D, Bullock GR, editors. Techniques in Diagnostic Pathology. San Diego CA: Academic Press; 1991:135–155.
- Orlien SMS, Sandven I, Berhe NB, et al. Khat chewing increases the risk for developing chronic liver disease: a hospital-based case-control study. Hepatol. 2018;68(1):248–257. doi:10.1002/hep.29809
- Hung TH, Liang CM, Hsu CN, et al. Association between complicated liver cirrhosis and the risk of hepatocellular carcinoma in Taiwan. PLoS One. 2017;12(7):e0181858. doi:10.1371/journal.pone.0181858
- Kedar Mukthinuthalapati VVP, Sewram V, Ndlovu N, et al. Hepatocellular carcinoma in Sub- Saharan Africa. JCO Glob Oncol. 2021;7:756–766. doi:10.1200/GO.20.00425
- Sonderup MW, Spearman CW. Global disparities in hepatitis B elimination-a focus on Africa. Viruses. 2022;14(1):82. doi:10.3390/v14010082
- Ansari A, Vincent JP, Moorhouse L, et al. Risk of early horizontal transmission of hepatitis B virus in children of uninfected mothers in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health. 2023;11(5):e715–e728. doi:10.1016/S2214-109X(23)00131-6
- Ismail A, Naeem I, Gong YY, et al. Early life exposure to dietary aflatoxins, health impact and control perspectives: a review. Trends Food Sci Technol. 2021;112:212–224. doi:10.1016/j.tifs.2021.04.002
- Edmunds WJ, Medley GF, Nokes DJ, et al. The influence of age on the development of the hepatitis B carrier state. Proc Biol Sci. 1993;253:197–201.
- Shimakawa Y, Yan H-J, Tsuchiya N, et al. Association of early age at establishment of chronic hepatitis B infection with persistent viral replication, liver cirrhosis and hepatocellular carcinoma: a systematic review. PLoS One. 2013;8(7):e69430. doi:10.1371/journal.pone.0069430
- Malik A, Ali S, Shahid M, et al. Occupational exposure to Aspergillus and aflatoxins among food-grain workers in India. Int J Occup Environ Health. 2014;20(3):189–193. doi:10.1179/2049396714Y.0000000055
- Wangia RN, Tang L, Wang J-S. Occupational exposure to aflatoxins and health outcomes: a review. J Environ Sci Health C. 2019;37(4):215–234. doi:10.1080/10590501.2019.1664836
- Udomkun P, Wossen T, Nabahungu NL, et al. Incidence and farmers’ knowledge of aflatoxin contamination and control in Eastern Democratic Republic of Congo. Food Sci Nutr. 2018;6(6):1607–1620. doi:10.1002/fsn3.735
- Gichohi-Wainaina WN, Kumwenda N, Zulu R, et al. Aflatoxin contamination: knowledge disparities among agriculture extension officers, frontline health workers and small holder farming households in Malawi. Food Control. 2021;121:107672. doi:10.1016/j.foodcont.2020.107672
- Manfo FPT, Mboe SA, Nantia EA, et al. Evaluation of the effects of agro pesticides use on liver and kidney function in farmers from Buea, Cameroon. J Toxicol. 2020;2020:2305764. doi:10.1155/2020/2305764
- Huang X, Zhang C, Hu R, et al. Association between occupational exposures to pesticides with heterogeneous chemical structures and farmer health in China. Sci Rep. 2016;6(1):25190. doi:10.1038/srep25190
- Prado-Lu JL D. Pesticide exposure, risk factors, and health problems among cut-flower farmers: a cross-sectional study. J Occup Med Toxicol. 2007;2:9. doi:10.1186/1745-6673-2-9
- Nigatu AW, Bråtveit M, Moen BE. Self-reported acute pesticide intoxications in Ethiopia. BMC Public Health. 2016;16(1):575. doi:10.1186/s12889-016-3196-5
- Tessema RA, Nagy K, Ádám B. Pesticide use, perceived health risks and management in Ethiopia and in Hungary: a comparative analysis. Int J Environ Res Public Health. 2021;18(19):10431. doi:10.3390/ijerph181910431
- Alebachew F, Azage M, Kassie GG, et al. Pesticide use safety practices and associated factors among farmers in Fogera district wetland areas, south Gondar zone, Northwest Ethiopia. PLoS One. 2023;18(1):e0280185. doi:10.1371/journal.pone.0280185
- Derso AG, Dagnew GG. Exposure and health risk assessment of farmers to DDT during khat production in Chiro Woreda, West Hararghe Zone: Ethiopia. World J Agri Res. 2019;7:29–35.
- Regassa G, Regassa C. Knowledge and attitude of khat growing farmers on the safe use and handling of pesticides in Haromaya Wereda, Oromia Regional State, Eastern Ethiopia. Afr J Environ Sci Technol. 2021;15:16–26. doi:10.5897/AJEST2020.2916
- Robinson O. Aetiology of an Unknown Liver Disease in Northern Ethiopia. Imperial College London; 2012.
- Coumoul X, Diry M, Barouki R. PXR-dependent induction of human CYP3A4 gene expression by organochlorine pesticides. Biochem Pharmacol. 2002;64(10):473–479. doi:10.1016/S0006-2952(02)01298-4
- Zhao B, Shen H, Liu F, et al. Exposure to organochlorine pesticides is independent risk factor of hepatocellular carcinoma: a case–control study. J Expo Sci Environ Epidemiol. 2011;21(6):601–608. doi:10.1038/jes.2011.24
- Seen S. Chronic liver disease and oxidative stress – a narrative review. Expert Rev Gastroenterol Hepatol. 2021;15(9):1021–1035.
- Conde de la Rosa L, Goicoechea L, Torres S, et al. Role of oxidative stress in liver disorders. Livers. 2022;2(4):283–314.
- Rybicka M, Woziwodzka A, Sznarkowska A, et al. Liver cirrhosis in chronic hepatitis B patients is associated with genetic variations in DNA repair pathway genes. Cancers. 2020;12(11):3295. doi:10.3390/cancers12113295
- Ashouri S, Khor SS, Hitomi Y, et al. Genome-wide association study for chronic hepatitis B infection in the Thai Population. Front Genet. 2022;13:887121. doi:10.3389/fgene.2022.887121
- Deng J, Zhao L, Zhang NY, et al. Aflatoxin B1 metabolism: regulation by phase I and II metabolizing enzymes and chemoprotective agents. Mutat Res/Rev Mutat Res. 2018;778:79–89. doi:10.1016/j.mrrev.2018.10.002
- Eom SY, Yim DH, Zhang Y, et al. Dietary aflatoxin B1 intake, genetic polymorphisms of CYP1A2, CYP2E1, EPHX1, GSTM1, and GSTT1, and gastric cancer risk in Korean. Cancer Causes Control. 2013;24(11):1963–1972. doi:10.1007/s10552-013-0272-3
- Alberts CJ, Clifford GM, Georges D, et al. Worldwide prevalence of hepatitis B virus and hepatitis C virus among patients with cirrhosis at country, region, and global levels: a systematic review. Lancet Gastroenterol Hepatol. 2022;7(8):724–735. doi:10.1016/S2468-1253(22)00050-4
- Tesfaye BT, Feyissa TM, Workneh AB, et al. Chronic liver disease in Ethiopia with a particular focus on the etiological spectrums: a systematic review and meta-analysis of observational studies. Can J Gastroenterol Hepatol. 2021;2021:8740157. doi:10.1155/2021/8740157
- Chuang YC, Tsai K-N, Ou JHJ. Pathogenicity and virulence of Hepatitis B virus. Virulence. 2022;13(1):258–296. doi:10.1080/21505594.2022.2028483
- Suhail M, Abdel-Hafiz H, Ali A, et al. Potential mechanisms of hepatitis B virus induced liver injury. World J Gastroenterol. 2014;20(35):12462–12472. doi:10.3748/wjg.v20.i35.12462
- Zhang Z, Wang C, Liu Z, et al. Host genetic determinants of hepatitis B virus infection. Front Genet. 2019;10:696. doi:10.3389/fgene.2019.00696
- Lin J, Wu JF, Zhang Q, et al. Virus-related liver cirrhosis: molecular basis and therapeutic options. World J Gastroenterol. 2014;20(21):6457–6469. doi:10.3748/wjg.v20.i21.6457
- Hosny G, Farahat N, Tayel H, et al. Ser-249 TP53 and CTNNB1 mutations in circulating free DNA of Egyptian patients with hepatocellular carcinoma versus chronic liver diseases. Cancer Lett. 2008;264(2):201–208. doi:10.1016/j.canlet.2008.01.031
- Aydın M, Aydın S, Bacanlı M, et al. Aflatoxin levels in chronic hepatitis B patients with cirrhosis or hepatocellular carcinoma in Balıkesir, Turkey. J Viral Hepat. 2015;22(11):926–935. doi:10.1111/jvh.12410
- Chen CH, Wang MH, Wang JH, et al. Aflatoxin exposure and hepatitis C virus in advanced liver disease in a hepatitis C virus endemic area in Taiwan. Am J Trop Med Hyg. 2007;77(4):747–752. doi:10.4269/ajtmh.2007.77.747
- Yen YH, Kuo FY, Chen CH, et al. Ultrasound is highly specific in diagnosing compensated cirrhosis in chronic hepatitis C patients in real world clinical practice. Med. 2019;98(27):e16270. doi:10.1097/MD.0000000000016270
- Kelly EMM, Feldstein VA, Parks M, et al. An assessment of the clinical accuracy of ultrasound in diagnosing cirrhosis in the absence of portal hypertension. Gastroenterol Hepatol. 2018;14(6):367–373.