Abstract
Objective
The aim of this concise review is to summarize the use of silver nanomaterials for caries prevention.
Methods
Two researchers independently performed a literature search of publications in English using Embase, Medline, PubMed, and Scopus databases. The keywords used were (silver nanoparticles OR AgNPs OR nano silver OR nano-silver) AND (caries OR tooth decay OR remineralisation OR remineralization). They screened the title and abstract to identify potentially eligible publications. They then retrieved the full texts of the identified publications to select original research reporting silver nanomaterials for caries prevention.
Results
The search identified 376 publications, and 66 articles were included in this study. The silver nanomaterials studied were categorized as resin with silver nanoparticles (n=31), silver nanoparticles (n=21), glass ionomer cement with silver nanoparticles (n=7), and nano silver fluoride (n=7). Most (59/66, 89%) studies investigated the antibacterial properties, and they all found that silver nanomaterials inhibited the adhesion and growth of cariogenic bacteria, mainly Streptococcus mutans. Although silver nanomaterials were used as anti-caries agents, only 11 (11/66, 17%) studies reported the effects of nanomaterials on the mineral content of teeth. Eight of them are laboratory studies, and they found that silver nanomaterials prevented the demineralization of enamel and dentin under an acid or cariogenic biofilm challenge. The remaining three are clinical trials that reported that silver nanomaterials prevented and arrested caries in children.
Conclusion
Silver nanoparticles have been used alone or with resin, glass ionomer, or fluoride for caries prevention. Silver nanomaterials inhibit the adhesion and growth of cariogenic bacteria. They also impede the demineralization of enamel and dentin.
Introduction
Nanotechnology is science, engineering, and technology involves the synthesis, characterization, and application of materials by controlling the shape and size at the nanoscale.Citation1 The development of nanomaterials for medical care is an important part of nanotechnology. The European Commission defined “nanomaterial” in 2011 as a natural, incidental, or manufactured material containing particles in an unbound state, as an aggregate, or as an agglomerate. For 50% or more of the particles in the number size distribution, one or more external dimensions are in the size range of 1 nm – 100 nm.Citation2 Nanomaterials may possess unique physical properties, such as uniformity, conductance, or special optical properties, that make them desirable in biology and material science. Because of large surface area to volume ratio, they exhibit different biomedical activities to materials with normal size.Citation3 The application of nanomaterials can make a significant improvement in the health and daily lives of people during the past two decades.Citation4
Among the various kinds of nanomaterials, metallic nanomaterials—in particular, silver nanomaterials—have shown great promise in terms of biomedical applications. Silver nanomaterials in medicine allow researchers and clinicians to advance medical imaging by providing more detailed images of cellular processes.Citation5 They facilitate medical diagnosis by means of molecular contrast agents and materials to enable an earlier and more accurate initial diagnosis.Citation6 Silver nanomaterials also improve drug delivery by increasing solubility and thus enhanced bioavailability.Citation7 They are additionally used in cancer therapeutics to kill drug-resistant tumor cells and improve curative effect by low selective target and delivery of these anticancer drugs to tumor tissues.Citation8
Silver nanomaterials also draw public attention for their extraordinary antimicrobial activity with low toxicity and cost.Citation9 They are used in low concentrations and do not generate bacterial resistance. Various antibacterial actions of silver nanomaterials have been proposed although the exact mechanism of silver nanomaterials’ antibacterial effects has not been entirely clarified. Silver nanomaterials release silver ions that penetrate through microbial membranes and disrupt deoxyribonucleic acid replication and protein synthesis.Citation10 In addition, the silver ions can deactivate respiratory enzymes and ultimately cause cell lysis. Silver nanoparticles from nanomaterials can accumulate in the pits of the cell wall and cause membrane denaturation. They are also capable of penetrating bacterial cell walls and cytoplasmic membranes, causing the denaturation of the cell wall and cytoplasmic membrane.Citation11
Researchers investigated the use of silver nanomaterials in dental materials as an antimicrobial agent for clinical care. For example, silver nanoparticles modified implants were developed to prevent peri-implant infection.Citation12 Plenty of studies investigated silver nanomaterials for caries prevention. Silver nanoparticles are incorporated into adhesives and orthodontic brackets to prevent enamel caries, which is a common complication of orthodontic treatment.Citation13 Silver nanoparticles can also be added to restorative materials to prevent secondary caries, which is a common cause of the failure of a restoration.Citation14 The application of silver nanomaterials to combat dental caries, including the inhibition of biofilm formation and the regulation of the demineralization and remineralization balance, is a promising direction for the prevention and treatment of tooth decay. However, a search found rare review of silver nanomaterials for caries prevention. Thus, the purpose of this study is to perform a systematic review of the use of silver nanomaterials for caries prevention.
Materials and Methods
Search Strategy
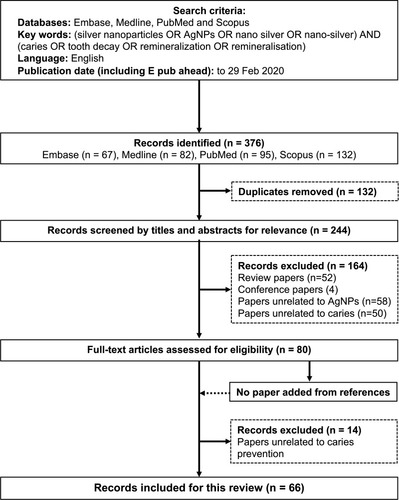
Two independent investigators (Yin and Zhao) performed a literature search to identify publications using four commonly used databases: Embase, Medline, PubMed, and Scopus. The search was restricted to publications in English. They searched publications using the keywords of ((silver nanoparticles) OR (AgNPs) OR (nano silver) OR (nano-silver)) AND ((caries) OR (tooth decay)). No publication-year limit was set, and the last search was made on 29 February 2020 ().
Study Selection and Data Extraction
The two investigators independently checked and excluded duplicate publications from the four databases to generate a list of publications. They screened the titles and abstracts of the publications to identify the potentially eligible list of articles. They excluded literature reviews, case reports, conference papers, publications on silver compounds that were not in nano scale, and studies not related to dental caries and abstracts without full papers. The two investigators retrieved full texts of the remaining publications for review. They selected publications that studied the use of silver nanomaterials on caries prevention, including the use of silver nanomaterials for inhibiting the growth of cariogenic bacteria and the demineralization of dental hard tissue. The two investigators performed a manual screening of the reference lists of the selected publications. They then discussed the inclusion of the selected publications with another investigator (Chu) to achieve an agreement of the list of publications included in this study.
For the publications included, the following information was recorded: the publication details (authors and year), the nanomaterials studied, the methods, the outcomes of the assessments, and other main findings.
Results
The initial literature search found 376 potentially eligible publications (95 articles in PubMed, 67 articles in Embase, 132 articles in Scopus, 82 articles in Medline). A total of 132 duplicate records of publications were removed (). Another 164 publications were removed after the screening of the titles and abstracts because these 164 publications were literature reviews, studies on other silver compounds, studies not related to dental caries, and other irrelevant studies. Full texts were obtained for the remaining 80 publications. A manual search of the references of these selected 80 publications did not identify any additional publications that met the inclusion criteria. Fourteen publications were excluded because they did not study silver nanomaterials’ effect on cariogenic bacteria or dental hard tissue. The remaining 66 publications that met the eligibility criteria were included in this review. The silver nanomaterials studied were categorized as silver nanoparticles (n=21), nano silver fluoride (n=7), resin with silver nanoparticles (n=31), and glass ionomer cement with silver nanoparticles (n=7).
Application of Silver Nanomaterials on Caries Prevention
Twenty-one publications investigated silver nanoparticles used for caries prevention. Eighteen of them reported the synthesis of silver nanoparticles and silver nanocomposites, which have the potential to manage caries. One study used silver nanoparticles in dentifrice.Citation15 One study reported coating silver nanoparticles on orthodontic brackets to prevent enamel caries.Citation13 Seven studies reported the use of nano silver fluoride solution for remineralizing early enamel caries and arresting dentin caries.Citation16–Citation22 Thirty-one studies used resins with silver nanoparticles to prevent caries. Silver nanoparticles were added into restorative materials, such as filling resins and adhesives, to prevent secondary caries.Citation23,Citation24 Silver nanoparticles were also incorporated into the resin of orthodontic materials, such as adhesives, elastomeric ligatures, and removable retainers, for caries prevention.Citation25 A clinical trial reported that a dental sealant with silver nanoparticles was better than a traditional sealant in preventing the enamel caries of first permanent molars.Citation26 Seven studies examined glass ionomer cements with silver nanoparticles for caries prevention. Six of them utilized glass ionomer with silver nanoparticles as orthodontic cements to prevent enamel caries. The remaining study applied it as restorative material to prevent secondary caries.Citation27
Effects of Silver Nanomaterials on Cariogenic Bacteria
Most of the publications (59/67) showed that silver nanomaterials have antibacterial effects mainly on Streptococcus mutans. summarizes 59 publications that studied the antibacterial effects of silver nanomaterials on cariogenic bacteria. Among them, 42 studies measured antibacterial properties using monospecies bacteria, such as Streptococcus, Lactobacillus, Enterococcus, and Pseudomonas, and 17 studies used oral microcosm. Most studies measured bactericidal properties in vitro, whereas only four studies were operated in vivo, including two animal studies and two clinical trials.Citation13,Citation28-30 The results of the minimum inhibitory concentration and the minimum bactericidal concentration of silver nanomaterials against cariogenic bacteria have high degrees of variation in different studies.Citation31–Citation33 The agar diffusion test demonstrated that disks treated with silver nanomaterials have larger inhibition zones compared with disks treated with water.Citation34 In addition, colony-forming unit counts proved that silver nanomaterials have the ability to inhibit the growth of bacteria.Citation29 They also revealed that biofilm treated with silver nanomaterials have less bacteria compared with that treated with water.Citation35 The live-to-dead ratios of the bacteria in biofilm were significantly lower after the application of silver nanomaterials compared with after the application of water.Citation36 Furthermore, biofilm treated with silver nanomaterials reduced the activity of metabolism, the production of lactic acid, and the expression of the glucosyltransferases gene.Citation37 It was evidenced that silver nanoparticles with smaller sizes have a stronger antibacterial effect.Citation38 Meanwhile, antibacterial properties were improved with the increased concentration of silver nanoparticles in materials.Citation39 Capping agents can also influence the bacterial effect of silver nanoparticles.Citation40
Table 1 Publications of Silver Nanomaterials and Cariogenic Bacteria (n = 59)
Effects of Silver Nanomaterials on Enamel and Dentin
Some (11/67) studies reported the inhibition of the demineralization of enamel and dentin under acid or cariogenic challenge. summarizes the 11 publications that studied the effects of silver nanomaterials on the mineral content of enamel and dentin. Sound enamel treated with silver nanomaterials had a shallower lesion depth than did enamel treated with water after biofilm challenge.Citation23 In addition, enamel with artificial caries can be treated with silver nanoparticles to increase microhardness.Citation41 An orthodontic bracket coated with silver nanoparticles decreased the caries rate on an incisor’s surface in a rat’s mouth.Citation13 In addition, resin with silver nanoparticles can increase the microhardness of dentin caries after acid (pH-cycling) challenge.Citation14 A dental sealant with silver nanoparticles reduced the mineral loss of children’s first molars in a clinical trial.Citation26 Six studies investigated the remineralizing effect of nano silver fluoride. Sound enamel treated with nano silver fluoride has a similar value of microhardness to that of enamel treated with sodium fluoride.Citation18,Citation19 The microhardness value of enamel caries treated with nano silver fluoride is higher than that of enamel caries treated with sodium fluoride.Citation20 However, the difference in the mineral content between nano silver fluoride– and sodium fluoride–treated decayed enamel cannot be detected using optical coherence tomography.Citation16 Nano silver fluoride did arrest the dentin caries of children in two clinical trials.Citation17,Citation21
Table 2 Publications of Silver Nanomaterials and Mineral Content of Teeth (n = 11)
Discussion
Although no time limitation was used as part of the search strategy, the publications that used silver nanomaterials to control caries emerged in 2008. Almost half (32/67, 48%) of these publications were published in 2016 to 2019. Silver nanoparticles of different sizes, of different concentrations, and with different synthetic materials were synthesized to investigate their potential use for caries prevention. Researchers also combined silver nanoparticles with other nanoparticles, such as calcium glycerophosphate and zinc oxide, to develop multi-functional nanocomposites for caries prevention.Citation33 Sodium fluoride with silver nanoparticles can act as a strategy for preventing and arresting caries.Citation17,Citation18 Also, the addition of silver nanoparticles into restorative materials, such as adhesives and filling resins, can prevent secondary caries without compromising mechanical properties.Citation23,Citation24 Silver nanoparticles can furthermore be utilized in orthodontic treatment to prevent initial caries by cooperating with adhesives, brackets, elastomeric ligatures, and removable retainers.Citation25
Studies used cariogenic monospecies strains of genus Streptococcus, Lactobacillus, Enterococcus, Pseudomonas, and Candida to demonstrate the antimicrobial action of silver nanomaterials on microbial growth.Citation33,Citation34,Citation40 Streptococcus mutans are the mostly frequently used bacteria in these studies. These are the most common cariogenic bacteria found in a carious lesion. Streptococcus mutans are also associated with the initiation and progression of caries. In the oral environment, bacteria are collective in the extracellular matrix to form biofilm. The biofilm can increase the resistance of microorganisms to antimicrobial agents by hindering the transport of agents.Citation42 Therefore, the inhibition effect of biofilm adhesion is crucial for estimating the antibacterial effect of silver nanomaterials. Some researchers chose the single-species biofilm model in their studies because it is stable and easy to operate. However, single-species biofilm has a huge discrepancy with biological dental biofilm. Dental biofilm is a complicated ecosystem containing 1000 bacterial species. In addition, microcosm is cultured using materials including various bacteria and the extracellular matrix removed from the oral environment. It can effectively simulate the complexity and heterogeneity of the biological biofilm in the human mouth.Citation43 Hence, the microcosm model was frequently selected in reviewed publications to examine the antibacterial effect of silver nanomaterials.Citation39,Citation44 An in situ model is still necessary for examining the anti-biofilm efficacy because even the microcosm model cannot entirely imitate the actual oral environment. Four publications use an animal study or clinical trial to obtain the authentic antimicrobial efficiency of silver nanomaterial.Citation13,Citation28-30
Silver nanoparticles bring forth an antibacterial effect for silver nanomaterials. Although the exact mechanism of silver nanoparticles’ antibacterial effects has not been entirely clarified, various antibacterial actions have been proposed. Silver nanoparticles can release silver ions. The released silver ions can enhance the permeability of the cytoplasmic membrane and lead to the disruption of the bacterial envelope. After penetrating into the cell membrane, silver ions can deactivate respiratory enzymes and interrupt adenosine triphosphate production. Silver ions can also inhibit the replication of deoxyribonucleic acid and the synthesis of proteins.Citation45 In addition to the release of silver ions, silver nanoparticles can kill bacteria by themselves. Silver nanoparticles can accumulate in the pits that form on the cell wall after they anchor to the cell surface. Also, the accumulated silver nanoparticles can cause the denaturation of the cell membrane. Silver nanoparticles additionally have the ability to penetrate bacterial cell walls and to subsequently change the structure of the cell membrane due to their nanoscale size.Citation46
The antibacterial effectiveness of silver nanomaterials toward cariogenic bacteria is related to the characteristics of silver nanoparticles in nanomaterials. Literature reviews concluded that silver nanoparticles of smaller sizes expressed stronger antibacterial ability against the planktonic Streptococcus mutans.Citation31,Citation38,Citation47-49 Small silver nanoparticles are prone to releasing silver ions due to their larger ratios of surface to volume. A large number of released silver ions intensely increases the inhibition effect of silver nanoparticles. In addition, capping agents affect silver nanoparticles’ ability to inhibit cariogenic bacteria.Citation40 Capping agents modify the surface of silver nanoparticles and then change silver nanoparticles’ dissolution efficiency. The positively charged silver nanoparticles show strong bactericidal activity against Streptococcus mutans.Citation50 Cariogenic bacteria, such as Streptococcus mutans, on the other hand, have cellular membranes with negative charges. Positively charged silver nanoparticles are inclined to approach cariogenic bacteria due to the presence of the electrostatic attraction between them. All in all, silver nanoparticles showed effective inhibition on both gram-positive and gram-negative bacteria.Citation34 Note that gram-negative bacteria are generally more resistant to antibiotics than gram-positive bacteria are. The outer cell membrane of gram-negative bacteria acts as a unique barrier to prevent many antibiotics from entering cells. Also, silver nanoparticles are nanoscale size, which can contribute to penetrating into the outer cell membrane. Most studies used silver nanoparticles smaller than 50 nm, as it is harder for larger silver nanoparticles to penetrate biofilm.Citation51
Silver nanomaterials reduce the production of lactic acid in biofilm. It is circumstantial evidence to reveal that silver nanomaterials have the potential to reduce the demineralization of teeth. The assessment of mineral content is requisite to show that silver nanomaterials can inhibit the demineralization of teeth. Sound enamel treated with silver nanoparticles reduced mineral loss after biofilm challenge.Citation23 This proved that silver nanoparticles can prevent caries by reducing the demineralizing effect through decreasing the acids that biofilm produces. A study using a chemical model (ie, no bacteria) found that silver nanoparticles increased the microhardness of enamel caries.Citation41 Silver nanoparticles can penetrate into carious lesions and attach to hydroxyapatite crystals.Citation20 In addition, silver ions released from silver nanoparticles can generate insoluble silver chloride on dental hard tissue.Citation3 The precipitated silver nanoparticles and insoluble silver chloride increase the mineral density of dental hard tissue. In addition, silver nanomaterials can preserve exposed collagen in carious teeth. In the oral environment, the exposed collagen can be degraded by bacterial collagenases as well as proteinases in saliva and the dentin matrix, such as activated matrix metalloproteinases and cysteine cathepsins.Citation14 Silver nanoparticles can inhibit and deactivate these enzymes. Then, the preserved collagen can act as a scaffold for the deposition of a mineral crystal and for the prevention of calcium and phosphate’s further diffusion.
Despite the fact that silver nanoparticles display a positive effect on dental hard tissue, fluoride possesses a more profound remineralizing effect than silver nanoparticles do.Citation23,Citation41 Fluoride and its derivatives have been clinically applied for caries prevention. Fluoride can unite with calcium ions and hydrogen phosphate ions to form fluorapatite and fluorhydroxyapatite, which are more acid resistant than hydroxyapatite is. Fluoride also inhibits collagenases and therefore hinders dentin collagen degradation.Citation52 Therefore, researchers proposed using nano silver fluoride to promote the remineralization of enamel and dentin. Studies showed that nano silver fluoride has a remineralizing effect on enamel caries using optical coherence tomographyCitation16,Citation19 and the microhardness test.Citation20 Also, a clinical study demonstrated that nano silver fluoride is as effective as silver diamine fluoride is in arresting caries.Citation17 However, nano silver fluoride did not significantly stain a carious lesion, whereas silver diamine fluoride stained a carious lesion black. Still, many studies did not report the concentrations of silver nanoparticles and fluoride. Thus, further research is necessary to investigate the optimal concentrations of silver nanoparticles and fluoride for caries prevention.
The toxicity of silver nanomaterials mainly depends on the free silver ions released from silver nanoparticles. The silver ions can enter mammalian cells provoke the production of reactive oxygen species.Citation53 This increase the oxidative stress and produce deleterious effects.Citation54 Silver nanoparticles exhibit lower cytotoxicity to human oral cells, such as human gingival fibroblasts and dental pulp stem cells, than other silver compounds.Citation3 And the silver nanoparticles-containing resins did not harm the viability of fibroblasts when they were immersed into typical saliva flow for an average person.Citation37 There was no toxicity and adverse effects of 5% nano silver fluoride observed in clinical trial.Citation17
Although silver nanomaterials possess antibacterial and remineralizing properties, they are easily oxidized and aggregative. The stability of silver nanomaterials is a critical factor affecting the antibacterial effect of silver nanomaterials.Citation55 Nevertheless, no publication has reported their long-term stability, and few studies have investigated the long-term antibacterial effect of silver nanomaterials.
Conclusion
In this review, the silver nanomaterials used for caries prevention are categorized as silver nanoparticles, resin with silver nanoparticles, glass ionomer with silver nanoparticles, and nano silver fluoride. Silver nanomaterials can inhibit the growth of cariogenic bacteria and the adhesion of biofilm. They also inhibit collagenase activity and preserve the collagen matrix. In addition, they hinder the demineralization of enamel and dentin. Therefore, silver nanomaterials are promising materials for caries prevention. Because most studies are laboratory studies, further clinical studies are essential before they can be used for patient care.
Acknowledgments
This study is supported by the National Natural Science Foundation of China (NSFC) - General Program (2018) No. 81870812 and the Hong Kong University Grant Council General Research Fund (No. 17100218).
Disclosure
The authors declare that they have no conflicts of interest.
References
- Mazumder JA, Khatoon N, Batra P, Sardar M. Biosynthesized silver nanoparticles for orthodontic applications. Adv Sci Eng Med. 2018;10(12):1169–1173. doi:10.1166/asem.2018.2289
- Rauscher H, Sokull-Klüttgen B, Stamm H. The European Commission’s recommendation on the definition of nanomaterial makes an impact. Nanotoxicology. 2012;7(7):1195–1197. doi:10.3109/17435390.2012.72472422920756
- Yin IX, Yu OY, Zhao IS, et al. Developing biocompatible silver nanoparticles using epigallocatechin gallate for dental use. Arch Oral Biol. 2019;102:106–112. doi:10.1016/j.archoralbio.2019.03.02230999064
- Elkassas D, Arafa A. The innovative applications of therapeutic nanostructures in dentistry. Nanomedicine. 2017;13(4):1543–1562. doi:10.1016/j.nano.2017.01.01828232213
- Han X, Xu K, Taratula O, Farsad K. Applications of nanoparticles in biomedical imaging. Nanoscale. 2019;11(3):799–819. doi:10.1039/C8NR07769J30603750
- Lee SH, Jun BH. Silver nanoparticles: synthesis and application for nanomedicine. Int J Mol Sci. 2019;20(4):865.
- Rizzello L, Pompa PP. Nanosilver-based antibacterial drugs and devices: mechanisms, methodological drawbacks, and guidelines. Chem Soc Rev. 2014;43(5):1501–1518. doi:10.1039/C3CS60218D24292075
- Perinot A, Kshirsagar P, Malvindi MA, Pompa PP, Fiammengo R, Caironi M. Direct-written polymer field-effect transistors operating at 20 MHz. Sci Rep. 2016;6(1):38941. doi:10.1038/srep3894127941844
- Xiao S, Wang H, Liang K, et al. Novel multifunctional nanocomposite for root caries restorations to inhibit periodontitis-related pathogens. J Dent. 2018.
- Bapat R, Chaubal T, Joshi C, et al. An overview of application of silver nanoparticles for biomaterials in dentistry. Mat Sci Eng C. 2018;91:881. doi:10.1016/j.msec.2018.05.069
- Samberg ME, Orndorff PE, Monteiro-Riviere NA. Antibacterial efficacy of silver nanoparticles of different sizes, surface conditions and synthesis methods. Nanotoxicology. 2011;5(2):244–253. doi:10.3109/17435390.2010.52566921034371
- Yang Y, Ren S, Zhang X, et al. Safety and efficacy of PLGA(Ag-Fe 3 O 4)-coated dental implants in inhibiting bacteria adherence and osteogenic inducement under a magnetic field. Int J Nanomed. 2018;13:3751–3762. doi:10.2147/IJN.S159860
- Metin-Gursoy G, Taner L, Akca G. Nanosilver coated orthodontic brackets: in vivo antibacterial properties and ion release. Eur J Orthod. 2017;39(1):9–16. doi:10.1093/ejo/cjv09726787659
- Xiao S, Liang K, Weir MD, et al. Combining bioactive multifunctional dental composite with PAMAM for root dentin remineralization. Materials. 2017;10(1):89.
- Ahmed F, Prashanth ST, Sindhu K, Nayak A, Chaturvedi S. Antimicrobial efficacy of nanosilver and chitosan against Streptococcus mutans, as an ingredient of toothpaste formulation: an in vitro study. J Indian Soc Pedod Prev Dent. 2019;37(1):46–54. doi:10.4103/JISPPD.JISPPD_239_1830804307
- Silva A, JdA T, Melo Júnior P, et al. Remineralizing potential of nano-silver-fluoride for tooth enamel: an optical coherence tomography analysis. Pesquisa Brasileira em Odontopediatria e Clínica Integrada. 2019;19(1):1–13.
- Tirupathi S, Svsg N, Rajasekhar S, Nuvvula S. Comparative cariostatic efficacy of a novel nano-silver fluoride varnish with 38% silver diamine fluoride varnish a double-blind randomized clinical trial. J Clin Exp Dent. 2019;e105–e112. doi:10.4317/jced.5499530805113
- Vieira Costa e Silva A, Teixeira JA, Mota CCBO, et al. In vitro morphological, optical and microbiological evaluation of nanosilver fluoride in the remineralization of deciduous teeth enamel. Nanotechnol Rev. 2018;7(6):509–520. doi:10.1515/ntrev-2018-0083
- Teixeira JA, Silva AVCE, Dos Santos Júnior VE, et al. Effects of a new nano-silver fluoride-containing dentifrice on demineralization of enamel and streptococcus mutans adhesion and acidogenicity. Int J Dent. 2018;2018:1351925. doi:10.1155/2018/135192529853891
- Nozari A, Ajami S, Rafiei A, Niazi E. Impact of nano hydroxyapatite, nano silver fluoride and sodium fluoride varnish on primary teeth enamel remineralization: an in vitro study. J Clin Diagn Res. 2017;11(9):Zc97–Zc100. doi:10.7860/JCDR/2017/30108.10694
- Dos Santos VE, Vasconcelos Filho A, Targino AGR, et al. A new “silver-bullet” to treat caries in children–nano silver fluoride: a randomised clinical trial. J Dent. 2014;42(8):945–951. doi:10.1016/j.jdent.2014.05.01724930870
- Targino AGR, Flores MAP, Dos Santos Junior VE, et al. An innovative approach to treating dental decay in children. A new anti-caries agent. J Mater Sci Mater Med. 2014;25(8):2041–2047. doi:10.1007/s10856-014-5221-524818873
- Wu R, Zhao Q, Lu S, Fu Y, Yu D, Zhao W. Inhibitory effect of reduced graphene oxide-silver nanocomposite on progression of artificial enamel caries. J Appl Oral Sci. 2018;27:e20180042. doi:10.1590/1678-7757-2018-004230540069
- Zhang K, Li F, Imazato S, et al. Dual antibacterial agents of nano-silver and 12-methacryloyloxydodecylpyridinium bromide in dental adhesive to inhibit caries. J Biomed Mater Res B Appl Biomater. 2013;101(6):929–938. doi:10.1002/jbm.b.3289823529901
- Hernandez-Gomora AE, Lara-Carrillo E, Robles-Navarro JB, et al. Biosynthesis of silver nanoparticles on orthodontic elastomeric modules: evaluation of mechanical and antibacterial properties. Molecules. 2017;22(9):1407. doi:10.3390/molecules22091407
- Salas-Lopez EK, Pierdant-Perez M, Hernandez-Sierra JF, Ruiz F, Mandeville P, Pozos-Guillen AJ. Effect of silver nanoparticle-added pit and fissure sealant in the prevention of dental caries in children. J Clin Pediatr Dent. 2017;41(1):48–52. doi:10.17796/1053-4628-41.1.4828052214
- El-Wassefy NA, El-Mahdy RH, El-Kholany NR. The impact of silver nanoparticles integration on biofilm formation and mechanical properties of glass ionomer cement. J Esthet Restor Dent. 2018;30(2):146–152. doi:10.1111/jerd.1235329197139
- Ghorbanzadeh R, Pourakbari B, Bahador A. Effects of baseplates of orthodontic appliances with in situ generated silver nanoparticles on cariogenic bacteria: a randomized, double-blind cross-over clinical trial. J Contemp Dent Pract. 2015;16(4):291. doi:10.5005/jp-journals-10024-167826067732
- Farhadian N, Usefi Mashoof R, Khanizadeh S, Ghaderi E, Farhadian M, Miresmaeili A. Streptococcus mutans counts in patients wearing removable retainers with silver nanoparticles vs those wearing conventional retainers: a randomized clinical trial. Am J Orthod Dentofacial Orthop. 2016;149(2):155–160. doi:10.1016/j.ajodo.2015.07.03126827971
- Li F, Fang M, Peng Y, Zhang J. Antibacterial properties of nano silver-containing orthodontic cements in the rat caries disease model. J. Wuhan Univ Technol Mater Sci Ed. 2015;30(6):1291–1296. doi:10.1007/s11595-015-1310-7
- Espinosa-Cristóbal LF, Martínez-Castañón GA, Martínez-Martínez RE, et al. Antibacterial effect of silver nanoparticles against Streptococcus mutans. Mater Lett. 2009;63(29):2603–2606. doi:10.1016/j.matlet.2009.09.018
- Dos Santos Junior VE, Targino AGR, Flores MAP, et al. Antimicrobial activity of silver nanoparticle colloids of different sizes and shapes against Streptococcus mutans. Res Chem Intermed. 2017;43(10):5889–5899. doi:10.1007/s11164-017-2969-5
- Fernandes GL, Delbem ACB, Do Amaral JG, et al. Nanosynthesis of silver-calcium glycerophosphate: promising association against oral pathogens. Antibiotics. 2018;7(3). doi:10.3390/antibiotics7030077
- Schwass DR, Lyons KM, Love R, Tompkins GR, Meledandri CJ. Antimicrobial activity of a colloidal AgNP suspension demonstrated in vitro against monoculture biofilms: toward a novel tooth disinfectant for treating dental caries. Adv Dent Res. 2018;29(1):117–123. doi:10.1177/002203451773649529355416
- Zhang N, Weir MD, Chen C, Melo MAS, Bai Y, Xu HHK. Orthodontic cement with protein-repellent and antibacterial properties and the release of calcium and phosphate ions. J Dent. 2016;50:51–59. doi:10.1016/j.jdent.2016.05.00127157089
- Freire PL, Stamford TC, Albuquerque AJ, et al. Action of silver nanoparticles towards biological systems: cytotoxicity evaluation using hen’s egg test and inhibition of Streptococcus mutans biofilm formation. Int J Antimicrob Agents. 2015;45(2):183–187. doi:10.1016/j.ijantimicag.2014.09.00725455849
- Li F, Weir MD, Chen J, Xu HHK. Comparison of quaternary ammonium-containing with nano-silver-containing adhesive in antibacterial properties and cytotoxicity. Dent Mater. 2013;29(4):450–461. doi:10.1016/j.dental.2013.01.01223428077
- Espinosa-Cristóbal LF, López-Ruiz N, Cabada-Tarín D, et al. Antiadherence and antimicrobial properties of silver nanoparticles against Streptococcus mutans on brackets and wires used for orthodontic treatments. J Nanomater. 2018;2018:1–11. doi:10.1155/2018/9248527
- Wang X, Wang B, Wang Y. Antibacterial orthodontic cement to combat biofilm and white spot lesions. Am J Orthod Dentofacial Orthop. 2015;148(6):974–981. doi:10.1016/j.ajodo.2015.06.01726672703
- Jesse JT, Shobini J. A plausible antibacterial green synthesized AgNPs from Tridax procumbens leaf-flower extract. J Pure Appl Microbiol. 2018;12(4):2135–2142. doi:10.22207/JPAM.12.4.51
- Scarpelli BB, Punhagui MF, Hoeppner MG, et al. In vitro evaluation of the remineralizing potential and antimicrobial activity of a cariostatic agent with silver nanoparticles. Braz Dent J. 2017;28(6):738–743. doi:10.1590/0103-644020170136529211131
- Sodagar A, Akhavan A, Hashemi E, et al. Evaluation of the antibacterial activity of a conventional orthodontic composite containing silver/hydroxyapatite nanoparticles. Prog Orthod. 2016;17(1):40. doi:10.1186/s40510-016-0153-x27819127
- Cheng L, Zhang K, Zhou CC, Weir MD, Zhou XD, Xu HH. One-year water-ageing of calcium phosphate composite containing nano-silver and quaternary ammonium to inhibit biofilms. Int J Oral Sci. 2016;8(3):172–181. doi:10.1038/ijos.2016.1327281037
- Zhang N, Melo MAS, Antonucci JM, et al. Novel dental cement to combat biofilms and reduce acids for orthodontic applications to avoid enamel demineralization. Materials. 2016;9(6):413.
- Cheng L, Li R, Liu G, et al. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int J Nanomed. 2018;13:3311–3327. doi:10.2147/IJN.S165125
- Dias HB, Bernardi MIB, Marangoni VS, de Abreu Bernardi AC, de Souza Rastelli AN, Hernandes AC. Synthesis, characterization and application of Ag doped ZnO nanoparticles in a composite resin. Mater Sci Eng C Mater Biol Appl. 2019;96:391–401. doi:10.1016/j.msec.2018.10.06330606547
- Espinosa-Cristóbal LF, Martinez-Castanon GA, Martínez-Martínez RE, et al. Antimicrobial sensibility of Streptococcus mutans serotypes to silver nanoparticles. Mater Sci Eng C. 2012;32(4):896–901. doi:10.1016/j.msec.2012.02.009
- Lu Z, Rong K, Li J, Yang H, Chen R. Size-dependent antibacterial activities of silver nanoparticles against oral anaerobic pathogenic bacteria. J Mater Sci Mater Med. 2013;24(6):1465–1471. doi:10.1007/s10856-013-4894-523440430
- Pérez-Díaz MA, Boegli L, James G, et al. Silver nanoparticles with antimicrobial activities against Streptococcus mutans and their cytotoxic effect. Mater Sci Eng C. 2015;55:360–366. doi:10.1016/j.msec.2015.05.036
- Abbaszadegan A, Ghahramani Y, Gholami A, et al. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: a preliminary study. J Nanomater. 2015;2015.
- Espinosa-Cristóbal L, Martínez-Castañón G, Téllez-Déctor E, Niño-Martínez N, Zavala-Alonso N, Loyola-Rodríguez J. Adherence inhibition of Streptococcus mutans on dental enamel surface using silver nanoparticles. Mater Sci Eng C. 2013;33(4):2197–2202. doi:10.1016/j.msec.2013.01.039
- Mei ML, Li QL, Chu CH, Yiu CKY, Lo ECM. The inhibitory effects of silver diamine fluoride at different concentrations on matrix metalloproteinases. Dent Mater. 2012;28(8):903–908. doi:10.1016/j.dental.2012.04.01122578660
- De Matteis V, Malvindi MA, Galeone A, et al. Negligible particle-specific toxicity mechanism of silver nanoparticles: the role of Ag+ ion release in the cytosol. Nanomedicine. 2015;11(3):731–739. doi:10.1016/j.nano.2014.11.00225546848
- Kshirsagar P, Sangaru SS, Brunetti V, Malvindi MA, Pompa PP. Synthesis of fluorescent metal nanoparticles in aqueous solution by photochemical reduction. Nanotechnology. 2014;25(4):045601. doi:10.1088/0957-4484/25/4/04560124394346
- Leo B, Chen S, Kyo Y, et al. The stability of silver nanoparticles in a model of pulmonary surfactant. Environ Sci Technol. 2013;47(19):11232. doi:10.1021/es403377p23988335
- Emmanuel R, Palanisamy S, Chen SM, et al. Antimicrobial efficacy of green synthesized drug blended silver nanoparticles against dental caries and periodontal disease causing microorganisms. Mater Sci Eng C Mater Biol Appl. 2015;56:374–379. doi:10.1016/j.msec.2015.06.03326249603
- Wang S, Wu J, Yang H, Liu X, Huang Q, Lu Z. Antibacterial activity and mechanism of Ag/ZnO nanocomposite against anaerobic oral pathogen Streptococcus mutans. J Mater Sci Mater Med. 2017;28(1):23. doi:10.1007/s10856-016-5837-828044252
- Kumar A, Majumdar RS, Dhewa T. In vitro efficacy of biosynthesized AgNPs against Streptococcus mutans causing dental plaque formation. J Sci Ind Res. 2018;77(4):225–228.
- Tavaf Z, Tabatabaei M, Khalafi-Nezhad A, Panahi F. Evaluation of antibacterial, antibofilm and antioxidant activities of synthesized silver nanoparticles (AgNPs) and casein peptide fragments against Streptococcus mutans. Eur J Integr Med. 2017;12:163–171. doi:10.1016/j.eujim.2017.05.011
- Besinis A, De Peralta T, Handy RD. Inhibition of biofilm formation and antibacterial properties of a silver nano-coating on human dentine. Nanotoxicology. 2014;8(7):745–754. doi:10.3109/17435390.2013.82534323875717
- Saafan A, Zaazou MH, Sallam MK, Mosallam O, El Danaf HA. Assessment of photodynamic therapy and nanoparticles effects on caries models. Open Access Maced J Med Sci. 2018;6(7):1289–1295. doi:10.3889/oamjms.2018.24130087739
- Hernandez-Sierra JF, Salas-Lopez EK, Martinez-Gutierrez F, et al. Bactericidal capacity of silver nanoparticles associated with Gantrez S-97 on Streptococcus mutans. J Clin Pediatr Dent. 2010;35(2):183–185. doi:10.17796/jcpd.35.2.c61l421mj0655lgm21417121
- Ahn S-J, Lee S-J, Kook J-K, Lim B-S. Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles. Dent Mater. 2009;25(2):206–213. doi:10.1016/j.dental.2008.06.00218632145
- Fan C, Chu L, Rawls HR, Norling BK, Cardenas HL, Whang K. Development of an antimicrobial resin–a pilot study. Dent Mater. 2011;27(4):322–328. doi:10.1016/j.dental.2010.11.00821112619
- Azarsina M, Kasraei S, Yousefi-Mashouf R, Dehghani N, Shirinzad M. The antibacterial properties of composite resin containing nanosilver against Streptococcus mutans and Lactobacillus. J Contemp Dent Pract. 2013;14(6):1014–1018. doi:10.5005/jp-journals-10024-144224858742
- Kasraei S, Sami L, Hendi S, Alikhani MY, Rezaei-Soufi L, Khamverdi Z. Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and Lactobacillus. Restor Dent Endod. 2014;39(2):109–114.24790923
- Mohammed HF, Riad MI. The effect of silver nanoparticles incorporation in the self-etch adhesive system on its antibacterial activity and degree of conversion: an in-vitro study [version 1; peer review: awaiting peer review]. F1000Research. 2019;8:244. doi:10.12688/f1000research.17687.1
- Cheng L, Zhang K, Weir MD, Liu H, Zhou X, Xu HHK. Effects of antibacterial primers with quaternary ammonium and nano-silver on Streptococcus mutans impregnated in human dentin blocks. Dent Mater. 2013;29(4):462–472. doi:10.1016/j.dental.2013.01.01123422420
- Ionescu AC, Brambilla E, Travan A, et al. Silver-polysaccharide antimicrobial nanocomposite coating for methacrylic surfaces reduces Streptococcus mutans biofilm formation in vitro. J Dent. 2015;43(12):1483–1490. doi:10.1016/j.jdent.2015.10.00626477347
- Melo MA, Orrego S, Weir MD, Xu HH, Arola DD. Designing multiagent dental materials for enhanced resistance to biofilm damage at the bonded interface. ACS Appl Mater Interfaces. 2016;8(18):11779–11787. doi:10.1021/acsami.6b0192327081913
- Cheng L, Weir MD, Xu HHK, et al. Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dent Mater. 2012;28(5):561–572. doi:10.1016/j.dental.2012.01.00522305716
- Cheng L, Zhang K, Melo MAS, Weir MD, Zhou X, Xu HHK. Anti-biofilm dentin primer with quaternary ammonium and silver nanoparticles. J Dent Res. 2012;91(6):598–604. doi:10.1177/002203451244412822492276
- Cheng L, Weir MD, Xu HH, et al. Effect of amorphous calcium phosphate and silver nanocomposites on dental plaque microcosm biofilms. J Biomed Mater Res B Appl Biomater. 2012;100(5):1378–1386. doi:10.1002/jbm.b.3270922566464
- Zhang K, Cheng L, Imazato S, et al. Effects of dual antibacterial agents MDPB and nano-silver in primer on microcosm biofilm, cytotoxicity and dentine bond properties. J Dent. 2013;41(5):464–474. doi:10.1016/j.jdent.2013.02.00123402889
- Cheng L, Weir MD, Zhang K, Arola DD, Zhou X, Xu HHK. Dental primer and adhesive containing a new antibacterial quaternary ammonium monomer dimethylaminododecyl methacrylate. J Dent. 2013;41(4):345–355. doi:10.1016/j.jdent.2013.01.00423353068
- Zhang K, Melo MAS, Cheng L, Weir MD, Bai Y, Xu HHK. Effect of quaternary ammonium and silver nanoparticle-containing adhesives on dentin bond strength and dental plaque microcosm biofilms. Dent Mater. 2012;28(8):842–852. doi:10.1016/j.dental.2012.04.02722592165
- Melo MAS, Cheng L, Zhang K, Weir MD, Rodrigues LKA, Xu HHK. Novel dental adhesives containing nanoparticles of silver and amorphous calcium phosphate. Dent Mater. 2013;29(2):199–210. doi:10.1016/j.dental.2012.10.00523138046
- Melo MA, Cheng L, Weir MD, Hsia RC, Rodrigues LK, Xu HH. Novel dental adhesive containing antibacterial agents and calcium phosphate nanoparticles. J Biomed Mater Res B Appl Biomater. 2013;101(4):620–629. doi:10.1002/jbm.b.3286423281264
- Zhang K, Cheng L, Wu EJ, Weir MD, Bai Y, Xu HHK. Effect of water-ageing on dentine bond strength and anti-biofilm activity of bonding agent containing new monomer dimethylaminododecyl methacrylate. J Dent. 2013;41(6):504–513. doi:10.1016/j.jdent.2013.03.01123583528
- Li F, Weir MD, Fouad AF, Xu HHK. Effect of salivary pellicle on antibacterial activity of novel antibacterial dental adhesives using a dental plaque microcosm biofilm model. Dent Mater. 2014;30(2):182–191. doi:10.1016/j.dental.2013.11.00424332270
- Cao W, Zhang Y, Wang X, et al. Development of a novel resin-based dental material with dual biocidal modes and sustained release of Ag(+) ions based on photocurable core-shell AgBr/cationic polymer nanocomposites. J Mater Sci Mater Med. 2017;28(7):103. doi:10.1007/s10856-017-5918-328534286
- Li F, Li Z, Liu G, He H. Long-term antibacterial properties and bond strength of experimental nano silver-containing orthodontic cements. J. Wuhan Univ Technol Mater Sci Ed. 2013;28(4):849–855. doi:10.1007/s11595-013-0781-7
- Zhang N, Chen C, Weir MD, Bai Y, Xu HHK. Antibacterial and protein-repellent orthodontic cement to combat biofilms and white spot lesions. J Dent. 2015;43(12):1529–1538. doi:10.1016/j.jdent.2015.09.00626427311