Abstract
Background
Plant gums consist of polysaccharides which can be used in the preparation of nanocarriers and provide a wide application in pharmaceutical applications including as drug delivery agents and the matrices for drug release. The objectives of the study were to collect plant gums from Araucaria heterophylla L and Prosopis chilensis L and to extract and characterize their polysaccharides. Then to utilize these plant gum-derived polysaccharides for the formulation of nanocarriers to use for drug loading and to examine their purpose in drug delivery in vitro.
Methods
Plant gum was collected, polysaccharide was extracted, purified, characterized using UV-Vis, FTIR, TGA and GCMS and subjected to various bioactive studies. The purified polysaccharide was used for making curcumin-loaded nanocarriers using STMP (sodium trimetaphosphate). Bioactivities were performed on the crude, purified and drug-loaded nanocarriers. These polysaccharide-based nanocarriers were characterized using UV-Vis spectrophotometer, FTIR, SEM, and AFM. Drug release kinetics were performed for the drug-loaded nanocarriers.
Results
The presence of glucose, xylose and sucrose was studied from the UV-Vis and GCMS analysis. Purified polysaccharides of both the plants showed antioxidant activity and also antibacterial activity against Bacillus sp. Purified polysaccharides were used for nanocarrier synthesis, where the size and shape of the nanocarriers were studied using SEM analysis and AFM analysis. The size of the drug-loaded nanocarriers was found to be around 200 nm. The curcumin-loaded nanocarriers were releasing curcumin slow and steady.
Conclusion
The extracted pure polysaccharide of A. heterophylla and P. chilensis acted as good antioxidants and showed antibacterial activity against Bacillus sp. These polysaccharides were fabricated into curcumin-loaded nanocarriers whose size was below 200 nm. Both the drug-loaded nanocarriers synthesized using A. heterophylla and P. chilensis showed antibacterial activity with a steady drug release profile. Hence, these natural exudates can serve as biodegradable nanocarriers in drug delivery.
Introduction
Nanotechnology is becoming an increasingly important subject that highlights the variation of matter which ranges between 1 and 100 nm. The system is becoming the most interesting field of science due to their size and unique properties.Citation1,Citation2 Their surface area interaction of these nanoparticles leads to unique propertiesCitation3,Citation4 and it can be applied in electronics, biomedicine, imaging, environmental remediation etc.Citation5–Citation10 In nanobiotechnology, the recent decade has recorded extensive research on formulation of nanocarriers since they promote easy transport and availability of drug molecules to the targeted site. Nanocarriers are being formulated from both inorganic and organic resources.Citation11 They include metal nanoparticles like gold, silica, silver and magnetic iron oxide nanoparticles.Citation5,Citation12,Citation13 But the use of these metal nanoparticles as drug carriers can pose a threat of metal poisoning within the system. Some of the organically derived nanocarriers like polymeric micelles, liposomes, chitosan-based nanocarriers and polymeric nanogels are good for drug delivery.Citation14–Citation16 Likewise, plant gum can be used as readily accessible and non-noxious material for nanocarrier synthesis. Making nanocarriers as colloidal drug carriers helps in delivering drugs to targeted sites and also offers slow and constant delivery of drugs. This even improves the pharmacological activity of the drug inside the system.Citation17 Most drugs are cleared in the reticuloendothelial system (RES), where the nanocarriers of biopolymers helps for slower delivery.Citation18 Biopolymers are polymers derived from living organisms,Citation19 including DNA, proteins and polysaccharides.Citation20 Gum exudates are generally high molecular mass constituents which are produced by plants as a response to biotic and abiotic stress. These gums are hydrophilic in nature and consists of polysaccharides, fats and some proteins.Citation21,Citation22 These polysaccharides are composed of branched structures of monosaccharides with glycosidic linkages. These polysaccharides are highly biocompatible and biodegradable and can be used in pharmaceutical actions for drug carrier and drug release.Citation8,Citation23
Araucaria genus contains about 14 species and most species release gum through the bark of the plant.Citation24 These gums are brittle and irregular in shape. They are reported to have antioxidant, anticytotoxic, antibacterial and anti-ulcerogenic effects.Citation8,Citation25 The genus Prosopis belongs to the family Fabaceae with 45 species, consist of spines and grow into either trees or shrubs. This gum has been reported to have various bioactivities including anticancer, antioxidant etc.Citation26 There are reports where polysaccharides derived from plant gums have been made into nanocarriers in the presence of chelators like STMP.Citation4,Citation8 Polysaccharides make the nanocarrier biocompatible and also enhance the activity of drug loading by releasing the loaded drug slowly, whilst the polysaccharide itself has bioactivity. In this study, gum-derived polysaccharides of Araucaria heterophylla and Prosopis chilensis were purified, characterized and exploited for various bioactive studies including antibacterial and antioxidant. They were further used for nanocarrier synthesis and loaded with curcumin. These drug-laden nanocarriers were examined for in-vitro bioactivity like antibacterial activity and the drug release was also checked.
Materials and Methods
Chemicals, Reagents and Instruments
Chloroform (HmbG Chemicals), Ethanol (System Chemicals), Trichloroacetic acid (Merck Chemicals), Acetone (Friendemann Schmidt Chemicals), Sulphuric acid (HmbG Chemicals), Phenol (R&M Chemicals), Sodium Tri Meta Phosphate (SRL Chemicals), Crystal violet (Friendemann Schmidt Chemicals), Mueller Hinton broth (SRL Chemicals), Nutrient agar (SRL Chemicals), Nutrient broth (SRL Chemicals), DPPH reagent (SRL Chemicals), Ascorbic acid (SRL Chemicals), Methanol (R&M Chemicals), Curcumin (SRL Chemicals), Hydrochloric acid (R&M Chemicals) etc. Centrifuge (Thermo Scientific), ELISA microplate reader (Lab Tech), Weighing balance (A&D Company Ltd), UV-Vis spectrophotometer (Genesys IOS UV-VIS- Thermoscientific), FTIR (Parkin Elmer Spectrum Two), SEM (Scanvac, ZEISS Ultra55), AFM (Bruker, Dimension icon model), Magnetic stirrer (Lab Tech).
Collection of the Plant Gums
Plant gum from A. heterophylla was obtained from Melaka, Malaysia and plant gum from P. chilensis was obtained from Chennai, Tamil Nadu, India ().
Extraction and Purification of the Polysaccharides
The gum was crushed using a pestle and mortar. Water extract was obtained by mixing the gum with distilled water, centrifuged and the supernatant was dried. In the purification process, gum was soaked in 100% ethanol to de-fat, followed with drying at 60ºC. Then the gum was allowed to dissolve in 50 mL water and continuously stirred in a magnetic stirrer. The solution was boiled to increase its viscosity and filtrated using filter paper. Equal volume of 10% TCA (Trichloroacetic acid) was added to precipitate the proteinsCitation27 and proteins were removed as pellets after subjecting to centrifugation at 7000 rpm. The collected supernatant was added with half the volume with acetone on continuous stirring to form a white mass. The obtained white mass was dialyzed against milli Q water for 3 days, then subjected to lyophilization and stored at 4°C.Citation28
Characterization of the Polysaccharides
The polysaccharides were characterized using UV-Vis spectroscopy analysis which was measured at 200–800 nm.Citation29 The polysaccharides were then subjected for FT-IR analysis at the frequency range of 450–4000 cm−1. Phenol sulphuric assay and GC-MS were performed to find any monosaccharides present. Phenol sulfuric acid assay was done by adding 1 mg/mL of purified samples with 5% aqueous phenol and 2 mL of concentrated sulphuric acid, kept undisturbed for about 10 minutes, followed by vortex, left at room temperature and the solution was now measured between 200 nm and 800 nm.Citation30 TGA (thermogravimetric analysis) was performed for purified polysaccharides.
Pure gum exudate consists of complex hydrophilic polysaccharides, therefore, the purified polysaccharides were derivatized to their simple sugar moieties before detecting them in GC analysis as described earlier.Citation31 1 μL of the derivatized sample was loaded and the analysis was performed using JEOL GC MATE II HP 5 MS (5% phenyl-methylpolysiloxane) fitted with a Quadrupole double focusing Mass analyzer. The mobile phase used here was helium gas with a flow rate of 1mL/min. The column temperature was raised from 50 ºC to 250 ºC at 10 ºC/min and the detection temperature was maintained as 250 ºC in a scan range of 50–600 amu. Ionization was achieved by electron impact mode at 70 eV.
Bioactivities of Polysaccharides
Antibacterial Activity
Purified dried polysaccharide was weighed and mixed with sterile distilled water and used for the antibacterial activity studies against Klebsiella sp, Bacillus sp, Staphylococcus aureus, and Escherichia coliCitation32 which were swabbed all over the nutrient agar plate. The sample which showed activity was subjected for MIC (Minimal inhibition concentration) and biofilm inhibition assay in 96-well plates (micro dilution method).Citation33 Swarming motility was performed as described before.Citation10
Antioxidant Assay (DPPH)
DPPH solution (0.1 mg/mL) in methanol was prepared. Then, 3 mL of purified polysaccharide (different concentration) was added with 1 mL of DPPH solution. After vigorously shaking, it was left in the dark for 1 hour. Absorbance was recorded at 517 nm. Ascorbic acid was used as standard. % DPPH radical scavenging activity was determined as mentioned by Shen et al.Citation34
Preparation of Nanocarriers and Drug-Loading Nanocarriers
Purified gum was carboxymethylated with 0.1 M NaOH and 2.36 g of chloroacetic acid as described earlier.Citation4 The obtained 0.5 g of polysaccharide was added to 10 mL of distilled water and stirred for 2 hours using a magnetic stirrer. 100 mL of 0.01% of STMP (Tri Sodium Tri Meta Phosphate) was added dropwise into the solution under continuous stirring. The mixture was left stirring overnight. The solution was then centrifuged at 12,000 rpm for 15 minutes. The supernatant was removed and the pellet was lyophilized. 15 mg curcumin/10 mL of ethanol: water (1:1) was added to the nanocarrier formed. The mixture was stirred for 3 hours. The solution was adjusted to pH 5 using 0.01 M hydrochloric acid and magnetic stirred at room temperate overnight. The solution was then centrifuged and the pellet was lyophilized.Citation4
Characterization of Synthesized Nanocarriers
Both the loaded and unloaded nanocarriers were subjected for UV-Vis, FTIR, Atomic force microscopy (AFM) and Scanning electron microscopy (SEM).Citation9
Antibacterial Activity of Loaded Nanocarriers
A total of 1 mg of nanocarrier was dispersed in 1 mL of water and ethanol separately. Antibacterial activity of drug-loaded nanocarriers was performed against both Gram-positive (S. aureus and Bacillus sp.) and Gram-negative bacteria (E. coli and Klebsiella sp). The solvent used and unloaded nanocarrier were used as controls.
Drug Release Kinetics
A total of 1 mg of drug-loaded nanocarrier was added to 1 mL of distilled water in a dialysis tube and was dialyzed against distilled water. Absorbance was taken at every 15 minutes using UV-Vis spectrophotometer at the wavelength of 420 nm. The analysis was done for 3 hours.Citation35
Statistical Analysis
All the data obtained in this study were performed thrice and illustrated as mean ± standard error.
Results
Characterization of the Polysaccharides
UV Vis Analysis
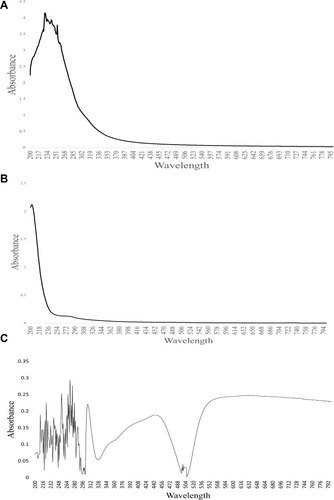
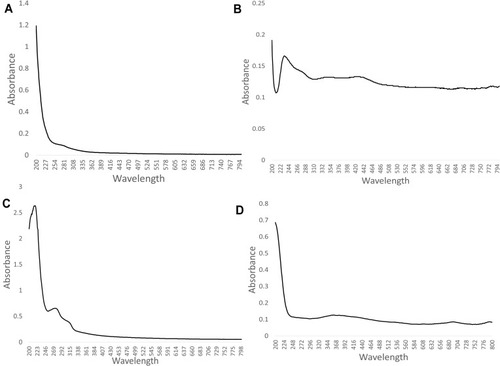
and shows UV-Vis analysis of crude, purified polysaccharide and phenol sulphuric assay of A. heterophylla and P. chilensis. For A. heterophylla, the maximum absorbance for the analysis of crude was found between 234 and 300 nm (). For the purified sample, there was absorbance around 210 nm which indicated the presence of xylose (). For P. chilensis, the maximum absorbance for the analysis of the purified sample was found between 264 and 290 nm (). For the crude sample, absorbance around 210 nm was indicative of xylose and the absorbance peak around 265 nm was because of glucose (), whilst phenol sulfuric acid assay showed peaks from 255 nm indicating the presence of xylose ().Citation36
FTIR Analysis
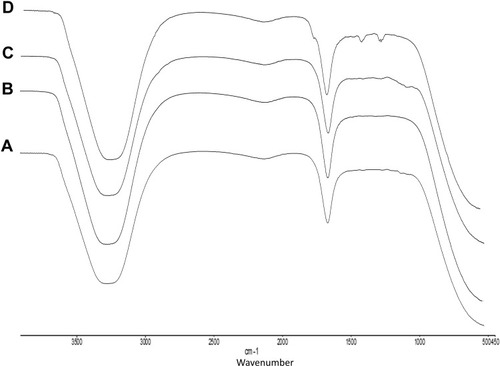
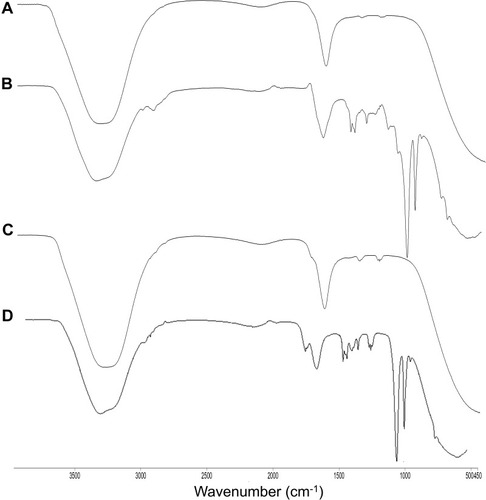
shows FTIR analysis of A. heterophylla and P. chilensis. In the study, all polysaccharides showed a broad peak at 3417 cm−1 for OH stretching, 1635 cm−1 peak was for C=C stretching of alkenes.Citation4 Both the polysaccharides showed peaks at 1384 cm−1, 1260 cm−1, and 1751 cm−1 which correspond to esters of C = O stretching, CH3CH stretching, and C-H stretching.Citation37
TGA Analysis
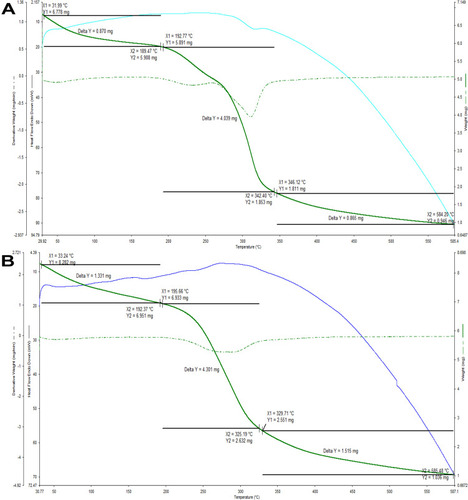
Thermogravimetric analysis of both the polysaccharides found that they were losing weight to around 70% between the temperatures 55 and 500°C (). Likewise, polysaccharides of A. heterophylla gum had the same kind of thermogravimetry.Citation8
GC-MS Analysis
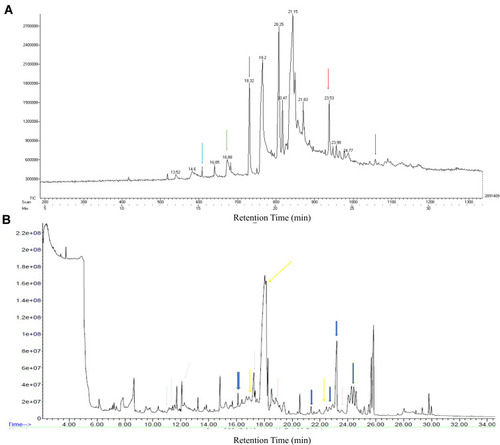
The gums exudate of Araucaria heterophylla was previously reported by Anderson and MunroCitation38 to contain galactose, uronic acid, arabinose, rhamnose and xylose by molecular sieve and TLC chromatography. For GC-MS analysis, the polysaccharides are generally hydrolyzed to their simple sugars. Hydrolysis with TFA and acetic anhydride convert these sugars to aldononitrile acetate derivatives.Citation39 In GC-MS, the spectra were obtained for the aldononitrile acetate derivates of aldose sugars. The peaks were identified by comparing the retention time and mass spectra of previous reports. represents the GC-MS chromatogram of monosaccharides of Araucaria heterophylla. The peaks recorded at 15.4, 16.88, 18.32, 23.53, 26.5 correspond to erythrose, ribose, xylose, arabinose and rhamnose, respectively.Citation40,Citation41 In the earlier studies of Samrot et al,Citation8 he also reported rhamnose, allose, glucosinolate, threose, idosan, galactose and arabinose as the monosaccharide residues of Araucaria heterophylla gum polysaccharide. Sugar derivatives seen in P. chilensis were: Peak at 11.169, 18.516, 23.571 were for xylose, peak at 11.726, 12.086 were for erythrose, D-Erythronic acid, 16.112 for a derivative of ribose, 17.252, 18.045, 22.456 for arabinose, 17.339 for rhamnose, 21.353, 22.666, 23.199 for galactose, 24.413 for a derivative of mannose ().
Figure 6 GC-MS analysis of: (A) Araucaria heterophylla, erythrose – 15.4 (blue arrow), ribose – 16.88 (green arrow), xylose – 18.32 (black arrow), arabinose – 23.53 (red arrow), rhamnose – 26.5 (purple arrow). (B) Prosopis chilensis peak at 11.169, 18.516, 23.571 (red arrow) – derivative of xylose (xylitol), peak at 11.726, 12.086 (blue arrow) – erythrose, D-erythronic acid, peak at 16.112 (green arrow) – derivative of ribose, peak at 17.252, 18.045, 22.456 (yellow arrow) – arabinose, peak at 17.339 (black dotted arrow) – rhamnose, peak at 21.353, 22.666, 23.199 (blue big arrow) – galactose, peak at 24.413 (orange arrow) - derivative of mannose.
Bioactivities of the Polysaccharides
Antibacterial Activity
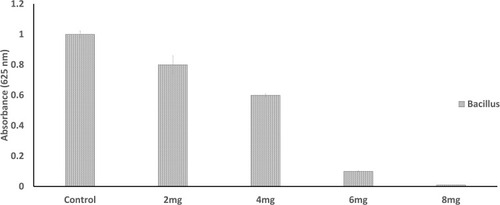
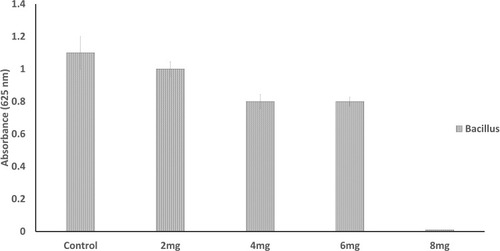
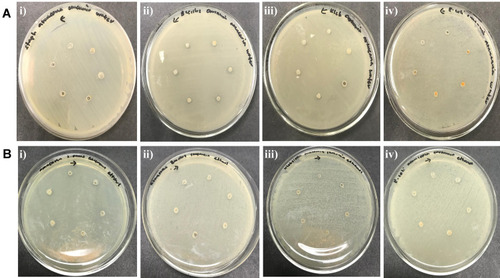
There was no zone of inhibition found for water extract. For chloroform extract, the zone of inhibition can only be seen against S. aureus, E. coli and Bacillus. For the purified polysaccharide of A. heterophylla antibacterial activity was found against Bacillus sp (). Water extract of P. chilensis showed a zone of inhibition against S. aureus and Bacillus sp. For the purified polysaccharide of P. chilensis, antibacterial activity was found against Bacillus sp alone (). The presence of zone of inhibition shows that the extract was able to inhibit the bacterial growth.Citation42 Gum of plants like Acacia gum and gum-derived silver nanoparticles were reported to have antibacterial activity.Citation9 Minimal inhibitory concentration (MIC) of purified A. heterophylla and P. chilensis polysaccharide extract against Bacillus sp was found to be 8 mg ( and ). Thus, both the polysaccharides were tested further against Bacillus sp alone.
Table 1 Antibacterial Activity of A. heterophylla
Table 2 Antibacterial Activity of P. chilensis
Biofilm Inhibition Assay
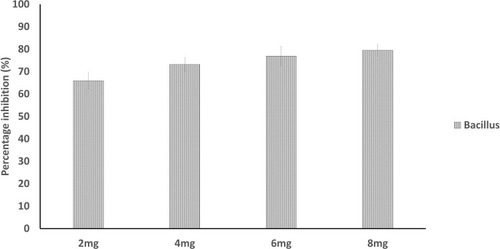
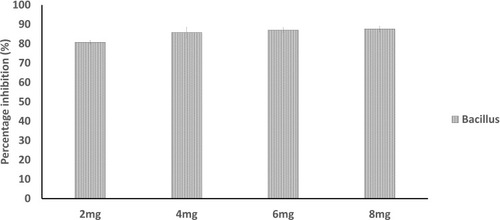
The onset of bacterial infection is achieved by biofilm formation.Citation43 Plant polysaccharides are notably reported to show anti-biofilm action by preventing the adhesion of various bacteria.Citation44 The extracted polysaccharide was also found to be interfering in biofilm formation of Bacillus sp. The purified gum of A. heterophylla (8 mg) was inhibiting the development of biofilm to 79.49% () whereas it was 87.6% for P. chilensis (8 mg) against Bacillus sp. (). The result showed that increase of polysaccharide extract concentration causes an increase of biofilm inhibition percentage. Grishin et al.Citation45 evaluated the impact of various plant polysaccharides in biofilm formation of Pseudomonas aeruginosa. They concluded that the polysaccharides galactan and galactomannan greatly reduced the biofilm formation of clinical isolate, P. aeruginosa 216.
Swarming Motility Assay
Swarming motility activity of A. heterophylla and P. chilensis against Bacillus sp was determined () and both the polysaccharides were found to inhibit swarming motility and having biofilm inhibition. A poor biofilm development over the substrate layer on the plate showed that the polysaccharide was able to prevent the motility of the organism.Citation46
Table 3 Swarming Motility
Antioxidant Assay (DPPH)
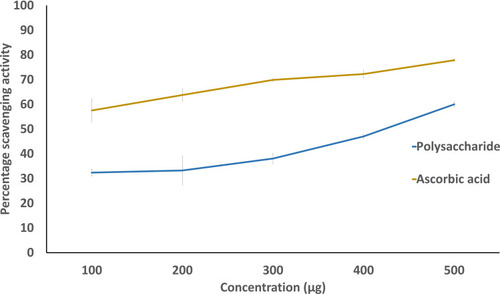
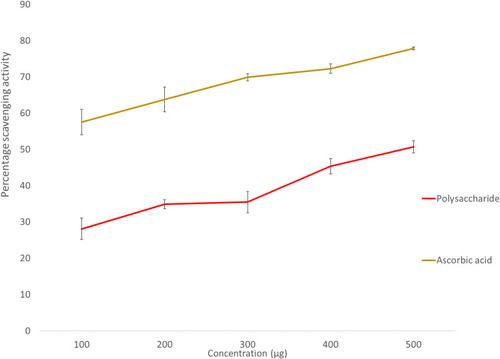
As the concentration of the extract increased, the percentage of scavenging activity increased. The percentage of scavenging activity was 47.03% and 45.34% at 500 µg concentration for A. heterophylla and P. chilensis respectively ( and ). As the concentration increases, the amount of free radical scavenged was increased. From the results obtained, both the polysaccharides were found to have an increased radical scavenging activity against DPPH with the increase of the concentration.Citation4,Citation47
Characterization of Loaded and Unloaded Nanocarrier
The fabrication of carboxymethylated gum into nanoparticles was attained by using STMP (sodium trimetaphosphate). STMP acted as crosslinker which initiated the carboxymethylated gum to form nanoparticles. Here, the reaction was brought by formation of phosphoester linkage between two carboxymethylated biopolymer chains (with STMP as a crosslinker) and release of sodium pyrophosphate as the by-product. This mechanism of crosslinking the carboxymethylated biopolymers resulted in a 3-dimensional network of nanoparticulates which are used as nanocarriers to load drugs. The size of these nanocarriers can be controlled with the ratio of STMP and carboxymethyl agents used.Citation48 Loading of the drug is achieved by a simple precipitation of curcumin onto the nanocarriers in acidic pH.Citation49,Citation50
UV-Vis Analysis
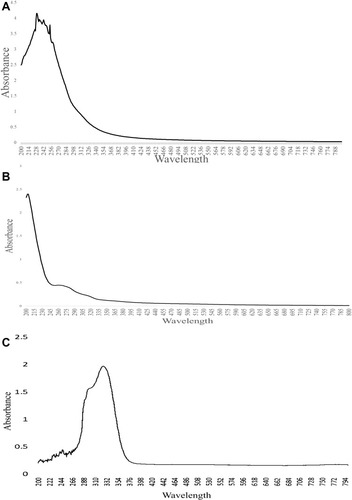
shows the UV-Vis analysis of unloaded nanocarrier and loaded nanocarrier of A. heterophylla and P. chilensis respectively. For both the drug-unloaded and -loaded nanocarriers of A. heterophylla and P. chilensis showed absorbance around 210 nm and 265 nm, which indicated the xylose and glucose of polysaccharide ( , B, C and ).Citation51 The absorbance peak for drug-loaded nanocarriers were showing around 400 nm, a characteristic of curcumin ().Citation3
FTIR Analysis
The unloaded nanocarriers showed peaks at 3307 cm−1, 2144 cm−1, 1635 cm−1, and 1217 cm−1 for polysaccharides and STMP (, B, C and ). For the loaded sample, carbonyl group and enolic C-O group of curcumin was observed in loaded nanocarrier at peaks 1645 cm−1 and 1217 cm−1 () which were absent in the unloaded nanocarrier.Citation52 Peaks at 3447 cm−1 represent the OH stretching of the polysaccharides, and 870 cm−1 for C-H bending.Citation4
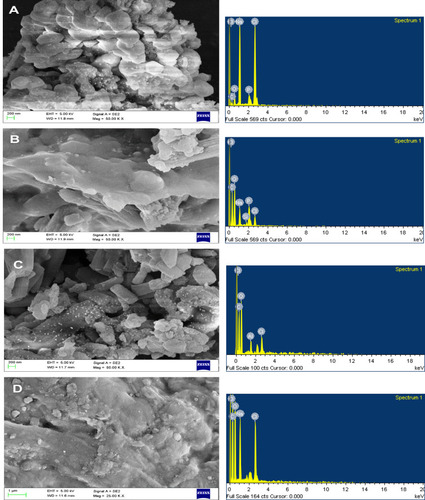
Scanning Electron Microscopy
The size of unloaded nanocarriers was found to be below 100 nm but the shape was irregular (), where the drug-loaded nanocarrier of A. heterophylla and P. chilensis was around 200–500 nm respectively. Increase in size happened as the drug was encapsulated (). Even Samrot et al,Citation8 found increased size in drug-loaded nanocarriers. EDX analysis for both unloaded and loaded nanocarriers showed peaks for carbon, oxygen and other trace elements which was contributed to by the polysaccharide nature of the nanocarrier, water, and chelator used.Citation8
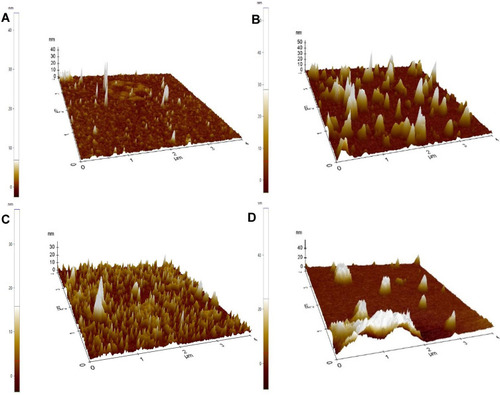
Atomic Force Microscopy
The size of unloaded nanocarriers of A. heterophylla, was below 100 nm and the size for loaded nanocarriers was about 200 nm () whereas the unloaded nanocarriers of P. chilensis were below 100 nm, however size increased to 400 nm in drug-loaded nanocarriers (). Thus, the results that were found were on a par with the SEM analysis.
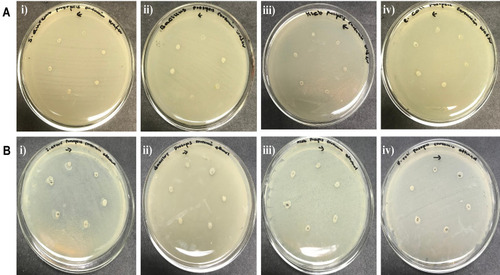
Antibacterial Activity of Loaded Nanocarriers
The antibacterial activity for unloaded and drug-loaded nanocarriers was performed using water and ethanol as solvents ( and ). and show the antibacterial activity of unloaded and loaded nanocarriers of A. heterophylla and P. chilensis. There was antibacterial activity against the bacteria while water and ethanol were used as the solvent. When just ethanol was used as the solvent, there was increased antibacterial activity which may be because of its bipolar solvent nature.
Table 4 Antibacterial Activity of Unloaded and Drug-Loaded Nanocarrier of A. heterophylla
Table 5 Antibacterial Activity of Unloaded and Drug-Loaded Nanocarrier of P. chilensis
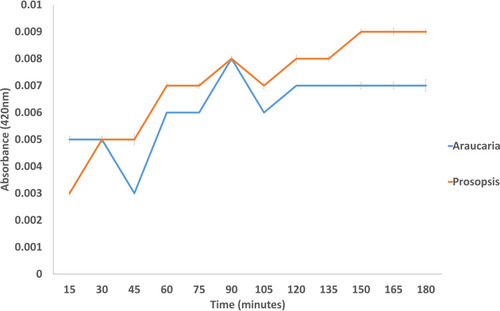
Drug Release Kinetics
The release of drug molecules from nanocarriers were detected spectrophotometrically at 420 nm for every aliquot sample with a 15-minute time interval. The absorbance was found to increase as the incubation period was extended. This confirmed that the curcumin molecules were gradually released from the nanocarriers. Bashir et alCitation53 also photometrically analyzed the activity of theophylline from loaded hydrogels. shows the result for drug release kinetics of loaded polysaccharides A. heterophylla and P. chilensis. Throughout the incubation time (a 3-hour period), curcumin was released slowly. The curcumin release was steady with the case of loaded P. chilensis nanocarriers comparatively. Polysaccharide-based nanoparticles and hydrogels were reported to release drugs efficiently.Citation8,Citation54–Citation57
Conclusion
In this study, polysaccharides from A. heterophylla and P. chilensis gum were extracted, purified and characterized. Bioactivities were performed for both of the gums. Purified polysaccharides were found to have a good antioxidant property and also antibacterial activity against Bacillus sp. Purified polysaccharide was made to favor nanocarrier synthesis, where STMP chelator was used for the synthesis. Thus, the produced nanocarrier was loaded with curcumin and characterized by Fourier transform infra-red spectroscopic (FTIR), Scanning electron microscopy (SEM) and Atomic force microscopy (AFM) analysis. The size of drug-loaded nanocarriers was found to be below 200–500 nm for A. heterophylla and P. chilensis respectively. There was efficient drug release even against bacterial activity in vitro. Plant gum-based polysaccharides are natural components which are biocompatible and biodegradable. Hence, formulation of nanocarriers from these non-toxic natural materials can be a favorable option for drug delivery agents.
Disclosure
The authors report no conflicts of interest in this work.
References
- Zheng Y, Monty J, Linhard RJ. Polysaccharide-based nanocomposites and their applications. Carbohydr Res. 2015;405:23–32. doi:10.1016/j.carres.2014.07.01625498200
- Safari J, Zarnegar Z. Advanced drug delivery systems: nanotechnology of health design A review. J Saudi Chem Soc. 2014;18(2):85–99. doi:10.1016/j.jscs.2012.12.009
- Samrot AV, Akanksha, Jahnavi T, et al. Chelators influenced synthesis of chitosan-carboxymethyl cellulose microparticles for controlled drug delivery. Appl Nanosci. 2016;6:1219. doi:10.1007/s13204-016-0536-9
- Samrot AV, Suvedhaa B, Sahithya CS, Madankumar A. Purification and utilization of gum from Terminalia catappa L. for synthesis of curcumin loaded nanoparticle and its in vitro bioactivity studies. J Clust Sci. 2018;29(6):989–1002. doi:10.1007/s10876-018-1412-4
- Sruthi PD, Sahithya CS, Justin C, et al. Utilization of chemically synthesized superparamagnetic iron oxide nanoparticles in drug delivery, imaging and heavy metal removal. J Clust Sci. 2018. doi:10.1007/s10876-018-1454-7
- Justin C, Philip SA, Samrot AV. Synthesis and characterization of superparamagnetic iron-oxide nanoparticles (SPIONs) and utilization of SPIONs in X-ray imaging. Appl Nanosci. 2018. doi:10.1007/s13204-017-0583-x
- Raji P, Samrot AV, Keerthana D, Karishma S. Antibacterial activity of alkaloids, flavonoids, saponins and tannins mediated green synthesised silver nanoparticles against Pseudomonas aeruginosa and Bacillus subtilis. J Clust Sci. doi:10.1007/s10876-019-01547-2
- Samrot AV, Angalene JLA, Roshini SM, et al. Purification, characterization and utilization of polysaccharide of Araucaria heterophylla gum for the synthesis of curcumin loaded nanocarrier. Int J Biol Macromol. 2019;140:393–400. doi:10.1016/j.ijbiomac.2019.08.12131425761
- Samrot AV, Angalene JLA, Roshini SM, et al. Bioactivity and heavy metal removal using plant gum mediated green synthesized silver nanoparticles. J Clust Sci. 2019;30:1599–1610. doi:10.1007/s10876-019-01602-y
- Samrot AV, Sahithya CS, Selvarani AJ, Pachiyappan S, Kumar SS. Surface-engineered superparamagnetic iron oxide nanoparticles for chromium removal. Int J Nanomed. 2019;2019:8105–8119.
- Lombardo D, Kiselev MA, Caccamo MT. Smart nanoparticles for drug delivery application: development of versatile nanocarrier platforms in biotechnology and nanomedicine. J Nanomater. 2019;3702518. doi:10.1155/2019/3702518
- Khandelia R, Jaiswal A, Ghosh SS, Chattopadhyay A. Polymer coated gold nanoparticle–protein agglomerates as nanocarriers for hydrophobic drug delivery. J Mater Chem B. 2014;2(38):6472–6477. doi:10.1039/c4tb00800f32261808
- Kanwal Z, Raza MA, Riaz S, et al. Synthesis and characterization of silver nanoparticle-decorated cobalt nanocomposites (Co@AgNPs) and their density-dependent antibacterial activity. R Soc Open Sci. 2019;6(5):182135. doi:10.1098/rsos.18213531218038
- Daglar B, Ozgur E, Corman ME, Uzun L, Demirel GB. Polymeric nanocarriers for expected nanomedicine: current challenges and future prospects. RSC Adv. 2014;4(89):48639–48659. doi:10.1039/c4ra06406b
- Amin MCIM, Butt AM, Amjad MW, Kesharwani P. Polymeric micelles for drug targeting and delivery. Nanotechnology-Based Approaches Targeting Delivery Drugs Genes. 2017;167–202. doi:10.1016/b978-0-12-809717-5.00006-3
- Suhail M, Rosenholm JM, Minhas MU, et al. Nanogels as drug delivery systems: a comprehensive overview. Ther Deliv. 2019;10:697–717. doi:10.4155/tde-2019-001031789106
- Din FU, Aman W, Ullah I, et al. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int J Nanomed. 2017;12:7291–7309. doi:10.2147/IJN.S146315
- Khodabandehloo H, Zahednasab H, Hafez AA. Nanocarriers usage for drug delivery in cancer therapy. Iran J Cancer Prev. 2016;9(2):e3966.27482328
- Ayeldeen MK, Negm AM, Sawwaf MA. Evaluating the physical characteristics of biopolymer/soil mixtures. Arab J Geosci. 2016;9:371. doi:10.1007/s12517-016-2366-1
- Yadav P, Yadav H, Shah VG, Shah G, Dhaka G. Biomedical biopolymers, their origin and evolution in biomedical sciences: a systematic review. J Clin Diagn Res. 2015;9(9):ZE21–ZE25. doi:10.7860/JCDR/2015/13907.656526501034
- Suchithra BG. Plant-derived bioadhesives for wound dressing and drug delivery system. Fitoterapia. 2015;137:104241.
- Granzotto C, Arslanoglu J, Rolando C, Tokarski C. Plant gum identification in historic artworks. Scientific. 2017;7.
- Mirhosseini H, Amid BT. A review study on chemical composition and molecular structure of newly plant gum exudates and seed gums. Food Res Int. 2012;46(1):387–398. doi:10.1016/j.foodres.2011.11.017
- Maiden JH. The gums, resins and other vegetable exudation of Australia. J Proc R Soc N S W. 1901;35:161–212.
- Abdel-Sattar E, Monem ARA, Ezzat SM, El-Halawany AM, Mouneir SM. Chemical and biological investigation of Araucaria heterophylla Salisb. Resin. Zeitschrift Fur Naturforschung C. 2009;64(1112):819–823. doi:10.1515/znc-2009-11-1211
- Henciya S, Seturaman P, Jamesa AR, et al. Biopharmaceutical potentials of Prosopis spp. (Mimosaceae, Leguminosa). J Food Drug Anal. 2017;25(1):187–196. doi:10.1016/j.jfda.2016.11.00128911536
- Zou P, Yang X, Huang W, et al. Characterization and bioactivity of polysaccharides obtained from pine cones of Pinus koraiensis by graded ethanol precipitation. Molecules. 2013;18:9933–9948. doi:10.3390/molecules1808993323966080
- Wasiak I, Kulikowska A, Janczewska M, et al. Dextran nanoparticle synthesis and properties. PLoS One. 2016;11(1):e0146237. doi:10.1371/journal.pone.014623726752182
- Jiang Y, Zi W, Pei Z, Liu S. Characterization of polysaccharides and their antioxidant properties from Plumula nelumbinis. Saudi Pharm J. 2018;26(5):656–664. doi:10.1016/j.jsps.2018.02.02629989035
- Jain VM, Karibasappa GN, Dodamani AS, Mali GV. Estimating the carbohydrate content of various forms of tobacco by phenol-sulfuric acid method. J Edu Health Promot. 2017;6:90. doi:10.4103/jehp.jehp_41_17
- Raguraman V, Abraham SL, Jyotsna J, et al. Sulfated polysaccharide from Sargassum tenerrimum attenuates oxidative stress induced reactive oxygen species production in in vitro and in zebrafish model. Carbohydr Polym. 2019;203:441–449. doi:10.1016/j.carbpol.2018.09.05630318233
- Shubha HS, Hiremath RS. Evaluation of antimicrobial activity of rasaka bhasma. Ayu. 2010;31(2):260–262. doi:10.4103/0974-8520.7241222131722
- Daly SM, Sturge CR, Greenberg DE. Inhibition of bacterial growth by peptide-conjugated morpholino oligomers. Methods Mol Biol. 2017;1565:115–122.28364238
- Shen Q, Zhang B, Xu R, Wang Y, Ding X, Li P. Antioxidant activity in vitro of the selenium- contained protein from the Se- enriched Bifidobacterium animalis 01. Anaerobe. 2010;16(4):380–386. doi:10.1016/j.anaerobe.2010.06.00620601030
- Gomez-Ballesteros M, Andres-Guerrero V, Parra FJ, et al. Amphiphilic acrylic nanoparticles containing the poloxamer star bayfit® 10WF15 as ophthalmic drug carriers. Polymers. 2019;11(7):1213. doi:10.3390/polym11071213
- Kaijanen L, Paakkunainen M, Pietarinen S, Jernstrom E, Reinikainen S. Ultraviolet detection of monosaccharides: multiple wavelength strategy to evaluate results after capillary zone electrophoretic separation. Int J Electrochem. 2015;10:2950–2961.
- Munajad A, Subroto C. Suwarno. Fourier transform infrared (FTIR) spectroscopy analysis of transformer paper in mineral oil-paper composite insulation under accelerated thermal aging. Energies. 2018;11(364):5–12.
- Anderson DMW, Munro AC. An analytical study of gum exudates from the genus Araucaria jussieu (gymnospermae). Carbohydr Res. 1969;11:43–51. doi:10.1016/S0008-6215(00)80640-0
- Ruiz-Matute AI, Hernández-Hernández O, Rodríguez-Sánchez S, Sanz ML, Martínez-Castro I. Derivatization of carbohydrates for GC and GC–MS analyses. J Chromatogr B. 2011;879:1226–1240. doi:10.1016/j.jchromb.2010.11.013
- Poinsot V, Carpéné MA, Couderc F. Coupled mass spectrometric strategies for the determination of carbohydrates at very low concentrations: the case of polysaccharides involved in the molecular dialogue between plants and rhizobia. Complex World Polysaccharides. 2012.
- Ye F, Yan X, Xu J, Chen H. Determination of Aldoses and Ketoses by GC-MS using Differential derivatisation. Phytochem Anal. 2006;17:379–383. doi:10.1002/pca.92817144244
- Haghgoo R, Mehran M, Afshari E, Zadeh HF, Ahmadvand M. Antibacterial effects of different concentrations of Althaea officinalis root extract versus 0.2% chlorhexidine and penicillin on streptococcus mutans and lactobacillus (in vitro). J Int Soc Prevent Communit Dent. 2017;7(4):180–185.
- Gingichashvili S, Duanis-Assaf D, Shemesh M, Featherstone JDB, Feuerstein O, Steinberg D. Bacillus subtilis biofilm development a computerized study of morphology and kinetics. Front Microbiol. 2017. doi:10.3389/fmicb.2017.02072
- Thomas R, Brooks T. Common oligosaccharide moieties inhibit the adherence of typical and atypical respiratory pathogens. J Med Microbiol. 2004;53:833–840. doi:10.1099/jmm.0.45643-015314189
- Grishin A, Karyagina AS, Tiganova IG, et al. Inhibition of Pseudomonas aeruginosa biofilm formation by LecA-binding polysaccharides. Int J Antimicrob Agents. 2013;42(5):471–472. doi:10.1016/j.ijantimicag.2013.07.00323988717
- Glasenapp Y, Catto C, Villa F, Saracchi M, Cappitelli F, Papenbrock J. Promoting beneficial and inhibiting undesirable biofilm formation with mangrove extracts. Int J Mol Sci. 2019;20(14):3549. doi:10.3390/ijms20143549
- Song H, He M, Gu C, et al. Extraction optimization, purification, antioxidant activity, and preliminary structural characterization of crude polysaccharide from an arctic chlorella sp. Polymers. 2018;10(3):292.
- Dodi G, Pala A, Barbu E, et al. Carboxymethyl guar gum nanoparticles for drug delivery applications: preparation and preliminary in-vitro investigations. Mater Sci Eng C. 2016;63:628–636. doi:10.1016/j.msec.2016.03.032
- Manna PJ, Mitra T, Pramanik N, Kavitha V, Gnanamani A, Kundu PP. Potential use of curcumin loaded carboxymethylated guar gum grafted gelatin film for biomedical applications. Int J Biol Macromol. 2015;75:437–446. doi:10.1016/j.ijbiomac.2015.01.04725661877
- Chin SF, Mohd Yazid SNA, Pang SC. Preparation and characterization of starch nanoparticles for controlled release of curcumin. Int J Polym Sci. 2014;2014:1–8. doi:10.1155/2014/340121
- Ishola MM, Ylitervo P, Taherzadeh MJ. Co-utilization of glucose and xylose for enhanced lignocellulosic ethanol production with reverse membrane bioreactors. Membranes. 2015;5(4):844–856. doi:10.3390/membranes504084426633530
- Athira GK, Jyothi AN. Preparation and characterization of curcumin loaded cassava starch nanoparticles with improved cellular absorption. Int j Pharm Pharm Sci. 2014;6(10):171–176.
- Bashir S, Teo YY, Ramesh S, Ramesh K. Physico-chemical characterization of pH-sensitive N -Succinyl chitosan- g -poly (acrylamide- co -acrylic acid) hydrogels and in vitro drug release studies. Polym Degrad. 2017;139:38–54. doi:10.1016/j.polymdegradstab.2017.03.014
- Hu X, Wang Y, Zhang L, Xu M. Construction of self-assembled polyelectrolyte complex hydrogel based on oppositely charged polysaccharides for sustained delivery of green tea polyphenols. Food Chem. 2019;125632. doi:10.1016/j.foodchem.2019.12563231606634
- Hu X, Wang Y, Zhang L, Xu M. Formation of self-assembled polyelectrolyte complex hydrogel derived from salecan and chitosan for sustained release of Vitamin C. Carbohydr Polym. 2020;234:115920. doi:10.1016/j.carbpol.2020.11592032070539
- Bashir S, Teo YY, Ramesh S, Ramesh K. Synthesis and characterization of karaya gum-g- poly (acrylic acid) hydrogels and in vitro release of hydrophobic quercetin. Polymer. 2018;147:108–120. doi:10.1016/j.polymer.2018.05.071
- Samrot AV, Angalene JLA, Roshini SM, et al. Purification, characterization and exploitation of Azadirachta indica gum for the production of drug loaded nanocarrier. Mater Res Express. 2020;7:055007. doi:10.1088/2053-1591/ab8b16