Abstract
In view of the worldwide serious health threat of type 2 diabetes mellitus (T2DM), natural sources of chemotherapies have been corroborated as the promising alternatives, with the excellent antidiabetic activities, bio-safety, and more cost-effective properties. However, their clinical application is somewhat limited, because of the poor solubility, instability in the gastrointestinal tract (GIT), low bioavailability, and so on. Nowadays, to develop nanoscaled systems has become a prominent strategy to improve the drug delivery of phytochemicals. In this review, we primarily summarized the intervention mechanisms of phytocompounds against T2DM and presented the recent advances in various nanosystems of antidiabetic phytocompounds. Selected nanosystems were grouped depending on their classification and structures, including polymeric NPs, lipid-based nanosystems, vesicular systems, inorganic nanocarriers, and so on. Based on this review, the state-of-the-art nanosystems for phytocompounds in T2DM treatment have been presented, suggesting the preponderance and potential of nanotechnologies.
Introduction
Diabetes mellitus, a chronic lifelong metabolic disorder, is a major global health issue that has reached alarming levels.Citation1 According to the latest data from the International Diabetes Federation, approximately 463 million adults worldwide are currently suffering from diabetes. Without sufficient action to control the pandemic, 578 million people will live with diabetes by 2030. By 2045, the number will jump to an astonishing 700 million.Citation2 T2DM is the most common type of diabetes. It has been estimated that nearly 95% of diabetic patients in the world have T2DM.Citation3,Citation4 The major pathogenesis of T2DM is insufficient insulin secretion from β cells under the background of insulin resistance. After insulin being produced by the pancreas, it cannot be efficiently utilized by the cells, which is named “insulin resistance”.Citation5 T2DM is characterized by high levels of blood glucose called hyperglycemia. Long-term hyperglycemia results in the glycation of proteins that in turn leads to secondary complications,Citation6 including retinopathy, cardiovascular disease, diabetic foot, neuropathy, and nephropathy. As a consequence, the quality of life decreases and the risk of disability or even mortality increases.Citation7,Citation8 Frustratingly, there is no cure for T2DM.Citation9 At present, the therapeutic strategies for T2DM mainly include regular physical exercise, proper low-carbohydrate diets, and adherence to long-term medication therapy, like the oral administration of chemical hypoglycemic drugs and the injection of insulin.Citation4 However, most diabetes medications promote weight gain, gastrointestinal disturbances,Citation10 diarrhea, renal failure, hypersensitivity,Citation11 and there is a proportionate risk of hypoglycemia using them to achieve tight glycemic control.Citation12,Citation13 Drug resistance is another serious obstacle. For example, sulfonylureas lose their effectiveness after treatment for 6 years in approximately 44% of diabetics.Citation14 Therefore, it is momentous to discover new antidiabetic candidates with greater safety but fewer side effects.
Plants have always been an exemplary source of ethnic medicines or natural drugs. Phytocompounds are natural components isolated from plants, which have been paid increased attention with progressively in-depth research of modern medicine in the field of alternative medicine.Citation15 Nowadays, approximately 50% of the drugs approved by the Food and Drug Administration (FDA) are plant-derived compounds or their derivatives.Citation16 For instance, metformin, a biguanidine-type antidiabetic drug, is developed from galegine which is isolated from Galega officinalis L. (Fabaceae)Citation17 and is currently used as the first-line oral medication for T2DM treatment.Citation18,Citation19 Compared with conventional therapies, natural plant-derived agents are more affordable and accessible, with fewer side-effects, so pharmaceutical research is increasingly inclined to discover new antidiabetic drugs from plants.Citation20,Citation21 About 1200 plants have been claimed to contain antidiabetic compounds, and more than 400 plants and their bioactive compounds have been scientifically evaluated for T2DM treatment.Citation21,Citation22 Phytocompound-based therapies are able to be developed as new approaches to treat T2DM or as adjuvants to support the existing monotherapies.
Generally, oral administration is recognized as the easiest and most convenient way. It is preferred by the patients with chronic diseases who require repeated dosing,Citation23 like T2DM patients, since the oral administration of therapeutic drugs shows good patient compliance without any pain and risk of needlestick injuries. However, the shortcomings of orally delivered phytocompounds, such as poor solubility, instability in the GIT, extremely low bioavailability, short half-life, and so on, restrain the translation of biological activities from in vitro evaluations to in vivo applications,Citation24,Citation25 so it is of paramount importance to design innovative oral delivery systems for diabetic patients. Nanomedicine has been proven to be able to effectively improve the oral delivery efficacy of compounds through circumventing various delivery restrictions.Citation26 It has been reported that the uptake of nanostructures is 15~250 times greater than that of microparticles.Citation27 Besides, nanostructures are always employed to sustain the release of encapsulated compounds in order to reduce dose and dosing frequency, thereby increasing patient compliance and minimizing side effects.Citation28 At present, a comprehensive review covering various oral nano delivery systems of phytocompounds for T2DM treatment in the preclinical stage is not available. In this review, we aimed to systematically summarize and critically analyze phytocompound-based oral nano delivery systems for T2DM treatment in recent years, and identify their therapeutic effects supported by experimental evidence in vitro or/and in vivo. On this basis, researchers could apply excellent delivery systems to undeveloped antidiabetic phytocompounds to explore more therapeutic possibilities in the future.
Main Antidiabetic Mechanisms of Phytocompounds
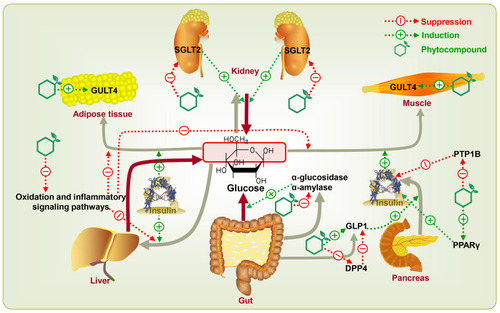
It is common for people to use natural plant medicine to prevent and/or treat diabetes in Central America, Asia, and West Africa.Citation29 According to an estimate published by the World Health Organization in 2008, about 80% of diabetic patients currently rely on medicinal plants for their successive treatments.Citation29,Citation30 A wide variety of phytocompounds derived from medicinal plants, including flavonoids, polyphenols, terpenoids, alkaloids, saponins, quinones, polysaccharides, etc., have been intensively investigated for their antidiabetic effects.Citation14,Citation29,Citation31–Citation34 The main mechanisms of phytocompounds for T2DM therapy might be based on four aspects involving certain key targets ().Citation35
Reduction of Carbohydrate Decomposition and Glucose Absorption
Polyphenols, terpenoids, and tannins display inhibitory effects on α-glucosidase and α-amylase, the major carbohydrate hydrolyzing digestive enzymes.Citation36 α-Amylase hydrolyzes macromolecules like starch into oligoglucans. At the brush border of the small intestine, these substances are further degraded by α-glucosidase into absorbable glucose and then permeate into blood circulation.Citation37 Under these conditions, phytocompounds can significantly reduce postprandial blood glucose levels by inhibiting α-glucosidase and α-amylase.Citation36 Hyperglycemia may make the kidney reabsorb glucose above the normal level.Citation38 Sodium-glucose co-transporter 2 (SGLT2) is the principal co-transporter of glucose reabsorption in the proximal convoluted tubule of the kidney. Phlorizin, chlorogenic acid, quercetin, kaempferol, kurarinone, and sophoraflavanone, with SGLT2 inhibitory activity, can promote the excretion of glucose in urine and suppress the reabsorption of glucose in the kidney. The excretion of glucose leads to a decrease of glucose in plasma levels, thus ameliorating all glycemic parameters. Typically, some synthetic compounds derived from phlorizin, including dapagliflozin, canagliflozin, ertugliflozin, and empagliflozin, have been approved by the FDA for T2DM treatment.Citation39,Citation40
Promotion of Glucose Uptake and Metabolism
Flavonoids and polyphenolic compounds can potentiate glucose transporter isoform 4 (GLUT4) expression as well as glucose uptake.Citation41,Citation42 GLUT4 is located at adipose tissue and skeletal muscle, which is mainly controlled by insulin secretion. Insulin stimulates the translocation of GLUT4 from an intracellular location to the cell surface, which can transport glucose into the inner and in turn decrease blood glucose levels. Pathologically, diabetic patients have lower expression of GLUT4.Citation43 The abnormality of GLUT4 expression causes a malfunction in the glucose uptake system and eventually results in insulin resistance in T2DM. Sayem et alCitation44 reviewed the action of phytocompounds on the modulation of GLUT4 translocation through insulin signaling pathways. For instance, berberine and vanillic acid improved the translocation of GLUT4 via adenosine 5‘-monophosphate-activated protein kinase (AMPK)-dependent pathway whereas resveratrol via phosphatidylinositol 3-kinase (PI3K)-protein kinase B (Akt) pathway.
Improvement of Insulin Action and Sensitivity
Glucagon-like peptide 1 (GLP1), an incretin hormone, can stimulate postprandial insulin secretion. Dipeptidyl peptidase 4 (DPP4) is a GLP1-degrading enzyme.Citation45,Citation46 The evidence suggests that polyphenols such as chlorogenic acid, epicatechin, and syringic acid can stimulate the secretion of GLP1 from intestinal L-cells, increase the half-life of GLP1 by inhibiting DPP4, stimulate the secretion of insulin from β-cells, irritate the peripheral response to insulin, and ultimately result in improving the overall effects of the GLP1-insulin axis.Citation45 Phytocompounds can also regulate insulin sensitivity via the blockage of protein tyrosine phosphatase 1B (PTP1B) and the stimulation of peroxisome proliferator-activated receptor γ (PPARγ). PTP1B is an enzyme responsible for reversing the autophosphorylation of the insulin receptor. The inhibition of PTP1B results in a prolonged insulin signaling cascade, thereby increasing insulin sensitivity.Citation47 Researches have reported approximately 500 PTP1B inhibitors (248 phenolics, 159 terpenoids, 40 alkaloids, 24 fatty acids, and 17 steroids) isolated from 100 species of natural sources. These phytocompounds could be developed as antidiabetic drugs or at least as promising drug candidates in the near future.Citation48 PPARγ is the predominant molecular target of the thiazolidinedione class of insulin-sensitizing drugs like pioglitazone, troglitazone, and rosiglitazone.Citation49 Many in vivo studies have implied that some natural product activators of PPARγ like honokiol, amorphastilbol, amorfrutin 1, and amorfrutin B have similar effects on stimulating glucose uptake as full thiazolidinedione agonists, but with reduced side effects.Citation50,Citation51
Antioxidant and Antiinflammatory Actions
The oxidation and inflammatory responses can establish a causal relationship in the occurrence of T2DM, which further leads to insulin resistance, or oxidation and inflammatory responses may be augmented by the hyperglycemic state, which results in T2DM-related complications.Citation52–Citation58 Recently, Ahangarpour et alCitation58 and Gothai et alCitation57 reviewed the research progress of phytocompounds, including chlorogenic acid, ellagic acid, curcumin, resveratrol, apigenin, quercetin, naringenin, catechin, etc., ameliorating insulin resistance and diabetic complications by suppressing oxidation or/and inflammatory signaling pathways. Phytocompounds can not only treat diabetes but also reduce the risk of T2DM-associated complications, which is a very attractive feature.
The Necessity of Developing Antidiabetic Phytocompounds-Loaded Nano Drug Delivery Systems
Despite the promising pharmacological activities of various phytocompounds, there are still some difficulties in translating their beneficial effects via oral route in clinical practice.Citation59 A review of clinical trials showed that the dose of phytocompounds for diabetes treatment was a crucial variable affecting clinical response.Citation60 In most studies, higher doses of phytocompounds exhibited better curative activity which may be due to the low bioavailability. Improving oral bioavailability plays a key role in further clinical applications. The way to overcome the low bioavailability of phytocompounds through higher doses showed better efficacy but simultaneously caused toxicity in several organs.Citation60 This is because the apparent volume of distribution of phytocompounds is quite large, which results in a large accumulation of drugs in normal organs, thus increasing undesired side effects.Citation61 There is another problem with antidiabetic phytocompounds. Because of the low stability of phytocompounds in the process of absorption, they have a lower potential antidiabetic effect in vivo.Citation62 Great attempts have been made to develop delivery systems in order to overcome these critical shortcomings.
Conventional drug delivery systems like microspheres,Citation63,Citation64 microemulsions,Citation65 amorphous solid dispersion,Citation66,Citation67 β-Cyclodextrin,Citation68 pH-sensitive hydrogelsCitation69 have been used to deliver antidiabetic phytocompounds for T2DM treatment. However, conventional drug delivery systems always have some restrictions, such as lack of curative effect because of ineffective or improper dose, diminished potency or altered effects because of drug metabolism, and lack of precise target specificity. More and more researches are focused on nano delivery systems.Citation70 In the past two decades, several nanotherapeutics have been approved by the FDA for the treatment of diabetes, high cholesterol, cancer, hepatitis, neurological diseases, autoimmune diseases, cardiovascular diseases, Parkinson’s disease, and certain infectious diseases.Citation71 Oral nano delivery systems can not only protect antidiabetic phytocompounds from enzymatic and/or chemical degradation in GIT but also provide other benefits, such as avoidance of first-pass metabolism, improvement of pharmacokinetic and pharmacodynamic profile, fast onset of action, targeted drug delivery, sustained drug release, lower dose and dosing frequency, and fewer side effects.Citation2
Oral Nano Drug Delivery Systems of Antidiabetic Phytocompounds
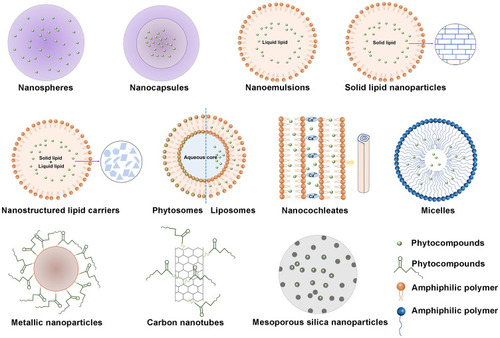
This section will focus on different types of oral nano delivery systems of antidiabetic phytocompounds () (), their impact on pharmacokinetic parameters, and their therapeutic effects.
Table 1 Oral Nano Delivery Systems Of Antidiabetic Phytocompounds For T2DM Treatment Investigated in vivo (↑) Increase, (↓) Decrease
Figure 2 Different types of oral nano delivery systems of antidiabetic phytocompounds for T2DM treatment.
Polymeric NPs
NPs are colloidal drug delivery systems that cover particles ranging from 10 nm to 1000 nm in diameter. They can be a reservoir system in which drugs are enclosed in a cavity surrounded by polymeric membranes called nanocapsules or as a matrix system in which drugs are dispersed throughout particles called nanospheres ().Citation2 Polymers have attracted much attention in therapeutic applications because of the design flexibility based on functionalization, polymer diversity, and macromolecular synthesis methods.Citation117 According to the different materials used to construct them, polymer-based delivery systems can be divided into synthetic polymer NPs and inartificial polymer NPs.Citation2
NPs Based Upon Inartificial Polymers
Inartificial polymers have the characteristics of low cost in processing and high abundance in nature.Citation118 Non-toxicity and biocompatibility are the primary advantages of inartificial polymers as compared to synthetic polymers.Citation119 Sodium alginate, chitosan, gum arabica, gum rosin, and dextran are the inartificially occurring polymers that have been developed for the delivery of antidiabetic phytocompounds.
Chitosan-Based NPs
Chitosan, a kind of polycationic polysaccharide, is produced by alkaline deacetylation of chitinCitation120 which is derived from the cell walls of fungi, the cuticle of insects, and the exoskeleton of crustaceans.Citation121,Citation122 By far, chitosan is the most extensively used for the delivery of antidiabetic phytocompounds due to its easy surface modification, property to blend with multitudinous polymers, non-immunogenicity as well as non-toxicity, and significant compatibility with cells and tissues.Citation123 A bottom-up ionic gelation method was applied to prepare chitosan NPs encapsulating ferulic acidCitation72 or curcumin.Citation124 Chauhan et alCitation124 reported, in L6 rat skeletal muscle cells in vitro, curcumin chitosan NPs exhibited a superior effect on the translocation of GLUT4 to the cell surface as compared to free curcumin. In another study of Panwar et al,Citation72 ferulic acid chitosan NPs showed four-fold enhanced oral bioavailability in vivo compared with free ferulic acid and displayed better antidiabetic potential in streptozotocin (STZ)-induced diabetic rats than ferulic acid. This might be attributed to the unique ability of chitosan to temporarily open the tight junctions between epithelial cells.Citation125 Moreover, the high positive charge of chitosan makes it well suitable for oral drug delivery because it enhances the adhesion to negatively charged mucosal surfaces, thereby increasing the uptake of cells.Citation126 Besides, the −OH and −NH2 active groups on the chitosan molecule are prone to chemical reactions.Citation127 Zhu et al investigated the grafting of catechin onto chitosan using hydrogen peroxide (H2O2) and ascorbic acid (Vc) as redox initiator in acetic acid solution.Citation128 In vitro antidiabetic activity revealed that the inhibitory effect of α-glucosidase decreased in the order of catechin-g-chitosan > catechin > acarbose > chitosan, and the inhibitory effect of α-amylase decreased in the order of acarbose > catechin-g-chitosan > catechin > chitosan. Although chitosan possesses favorable biological properties, it is rarely used alone for oral drug administration because chitosan as a carrier is subject to leak out the entrapped drugs as it dissolves easily in acidic conditions.Citation125,Citation129
Alginate/Chitosan-Based NPs
Another inartificial polymer sought with chitosan is alginate. Alginate is a hydrophilic anionic copolymer broadly distributed in the cell walls of brown algae. The wide pharmaceutical applicability of alginate is owing to its unique ability to form gels in aqueous medium or at low pH under the presence of divalent cations like calcium ions.Citation130 Alginate and chitosan are able to form polyelectrolyte complexes through electrostatic interactions between oppositely charged groups.Citation125 The poor solubility of alginate network at low pH decreases the high solubility of chitosan at low pH, while chitosan which is less soluble at high pH stabilizes alginate which is more soluble at high pH.Citation118 The alginate/chitosan complex protects the encapsulated phytocompounds and effectively slows down the release than either chitosan or alginate alone. For instance, in vitro assays, the chitosan-alginate polyelectrolyte complex prolonged the average release time of curcumin by 40 min in simulated gastric fluid and concurrently reduced the loss of curcumin by 20%. Besides, the curcumin nanoformulation prepared by chitosan-alginate complex had a 30% higher glucose-lowering effect in vivo than that prepared by chitosan only.Citation73 Maity et alCitation74 prepared a novel alginate coated chitosan core-shell nanocarrier system which was effectively used in the oral delivery of naringenin to STZ-induced diabetic rats. The innovation spot lied in its structural chemistry because the core-shell construction was designed to decrease the particle size and assure efficient prevention of embedded naringenin from fast-pass metabolism through sheltering it within the core of NPs. Similarly, in another study of Mukhopadhyay et al,Citation75 a pH-sensitive polymeric NPs with core-shell-corona morphology for encapsulating quercetin were prepared by the use of succinyl chitosan and alginate. Succinyl chitosan and alginate show excellent pH sensitivity due to the presence of carboxyl groups (-COOH) on their structure. The spherical nanoformulation could perform a pH-sensitive controlled release of quercetin and follow an anomalous (non-Fickian) trend, no matter in vitro or in vivo studies. Compared with native phytocompounds, both core-shell NPs exerted pronounced hypoglycemic effects and effective maintenance of glucose homeostasis in STZ-induced diabetic rats. Besides, both NPs displayed no toxicity in vivo.
Gum-Based NPs and Gum/Chitosan-Based NPs
Natural gums, like guar gum, gellan gum, karaya gum, gum acacia, locust bean gum, konjac gel, or xanthan gums have been extensively employed to develop friendly drug delivery systems. They have the capacity to form hydrogels when exposed to water and show high stability in a broad pH range.Citation131 Gum rosin is an inartificial anionic polymer derived from pine trees.Citation132 Rani et alCitation76 developed thymoquinone-loaded gum rosin nanocapsules using the nanoprecipitation method, also called the solvent displacement method. Thymoquinone nanoformulations contained only half the amount administrated as native thymoquinone but performed a better antihyperglycemic activity in type 2 diabetic rats. Rani et alCitation77 chose gum arabica and chitosan as basic polymers for the preparation of the glycyrrhizin-loaded NPs which were synthesized by the electrostatic interaction of negatively charged carboxylic groups (−COO) of gum arabica with positively charged amine group (−NH2) of chitosan. Glycyrrhizin-loaded NPs exerted striking antihyperglycemic and antihyperlipidemic effects in type 2 diabetic rats, which were comparable to the standard antidiabetic drug, metformin. In another study of Rani et al,Citation133 two kinds of nanoformulations discussed above were mixed to explore their combined effects. Compared with the single nanoformulation, even at a decreased drug load, combined nanoformulations exhibited significant improvement on antidiabetic activity in vivo as proven by encouraging responses in all eight tested parameters.
Dextran-Based NPs
Dextran is a highly water-soluble polysaccharide with negatively charged. It predominantly contains linear α-1,6-linked glucopyranose units and some degree of 1.3-branching.Citation134 Dextran mainly comes from the sucrose-rich environment of Lactobacillus, Streptococcus, and Leuconostoc.Citation135 Because of the low affinity between hydrophilic polymeric matrix and lipophilic drugs, drug encapsulation is difficult. Kapoor et alCitation136 modified dextran by connecting hexadecyl chains with ether bonds to make it amphiphilic. This synthesized O-hexadecyl-dextran could self-assemble in aqueous media, enclosing berberine into the hydrophobic pockets, fabricating NPs. In vitro study on primary hepatocytes, berberine-loaded O-hexadecyl-dextran NPs, even at a 20-fold reduced concentration, were as efficient as berberine in preventing high glucose-induced oxidative stress, mitochondrial depolarization as well as downstream events of apoptotic cell death.
Despite their highly biodegradable, the limitation of inartificial polymers is their batch-to-batch variability because they are usually extracted from different species, regions, and under various climatic conditions, which makes them less attractive than synthetic polymers that are more versatile and reproducible.Citation137
NPs Based Upon Synthetic Polymers
Unlike the inartificial polymers, synthetic polymers are available in a large variety compositions with desirable properties, the ease of control over the synthetic processes, and batch-to-batch reproducibility.Citation2,Citation135,Citation138 Synthetic polymer NPs usually consist of poly (vinyl alcohol) (PVA), poly (ε-caprolactone) (PCL), poly (lactic acid) (PLA), poly (lactic-co-glycolic acid) (PLGA), and poly (methyl methacrylate) (PMMA), which are approved by the FDA due to their biodegradability and biocompatibility.Citation1,Citation139 Polyesters are applied in amphiphilic diblock copolymers where the versatility provided by changing the compositions and adjusting the block lengths can make changes in drug loading capacity, carrier size, and drug release.Citation140
PLGA-Based NPs
At first, PLGA acted as clinical suture materials because they could be thoroughly absorbed by the body. PLGA-based particles have been utilized as carriers for more than 15 small molecular drugs approved by the FDA.Citation137 PLGA polymers, with the capability to encapsulate a wide range of hydrophilic or hydrophobic drugs, are usually made by the ring-opening co-polymerization of glycolide and lactide. One of the most notable characteristics of PLGA polymers as drug delivery systems is the possibility of adjusting the physicochemical properties like lactide/glycolide ratio in order to obtain the desired release profile.Citation141 By following the solvent displacement technique, pelargonidin was encapsulated in PLGA to form nano pelargonidin which was found to protect alloxan-induced hyperglycemic L6 cells against mitochondrial dysfunction, oxidative stress, DNA damage, imbalance in glucose homeostasis in vitro and exhibited better protective effect than native pelargonidin with a ~10-fold reduced dose or showed a ~10-fold greater protective effect with an equivalent dose.Citation141,Citation142 Chitkara et al adopted the emulsion diffusion evaporation method to produce quercetin-loaded PLGA NPs for diabetes care with the aim to reduce dose and dosing frequency. In a pharmacokinetic study, the relative oral bioavailability of nano quercetin was increased by 523% as compared with quercetin suspension. The therapeutic effect on STZ-induced diabetic rats implied that the same dose of quercetin nanoformulation every 5 days was enough to produce a similar effect to the daily dose of quercetin suspension.Citation78 Using the same preparation method as quercetin, Kozuka et alCitation79 successfully fabricated γ-oryzanol-loaded PLGA NPs. Compared with regular γ-oryzanol, nano γ-oryzanol markedly ameliorated lipid and blood glucose metabolism in obese diabetic ob/ob mice with an unexpected amount (about 1000-fold lower dose). Even under the condition of treatment once every 2 weeks, such a prominent impact was also accomplishable. Marked efficacy differences between antidiabetic phytocompounds and their PLGA-based nanoformulations can be ascribed to the specialized uptake of NPs through payer’s patches via M-Cells into lymphatic system and then directly into systemic circulation and thus avoiding first-pass metabolism.Citation143 Furthermore, the PLGA matrix provided a barrier for phytocompounds to prevent them from enzymatic degradation in the GIT.Citation144
PLA-Based NPs, PCL-Based NPs, and PVA-Based NPs
PLA is often used with PGA to form PLGA. It is formed by the condensation polymerization of lactic acid, which is produced by the fermentation of sugars from carbohydrate sources like sugarcane, corn, or tapioca.Citation145 PLA is a hydrophobic polymer because of the presence of –CH3 side groups.Citation146 The stevioside was encapsulated into Pluronic-F-68-PLA through the nanoprecipitation method.Citation147 The use of Pluronic F-68 surfactants in NPs synthesis can usually improve drug loading capacity, stability as well as cell–nanoparticle interaction.Citation148,Citation149 In this research, Pluronic-F-68 copolymer increased the encapsulation and bioavailability of stevioside and provided a longer sustained-release activity. The half release and complete release were respectively observed at 25 ± 4 h and 200 ± 10 h in vitro.Citation147 Another compound, rebaudioside A, with one more extra glucose molecule in its chemical structure than stevioside, also possesses strong antidiabetic activity. To assess the effect of nano rebaudioside A and compare it with nano stevioside, the entirely same methodology as stevioside was applied to fabricate rebaudioside A PLA NPs. The antidiabetic effect of rebaudioside A NPs was claimed to be superior than that of stevioside NPs on account of achieved higher drug loading ability and similar in vitro release properties.Citation150
PCL is a hydrophobic, semi-crystalline polymer.Citation151 It is achiral and has the ability to resist chemical hydrolysis, which improves the structural stability of polymeric chains in the nanoencapsulation.Citation148 Kamaraj et alCitation152 made 14-deoxy, 11, 12-didehydroandrographolide-loaded PCL NPs using the solvent evaporation technique. The results obtained from the study demonstrated that after nanoencapsulation, the glucose uptake effect in L6 myotubes in vitro was found to be improved because of its prolonged and sustained drug delivery. A biphasic pattern of drug release in vitro exerted an initial burst release at 24 h followed by a sustained release for up to 11 days.
PVA is a polymer with -OH hydrophilicity. It is produced by the radical polymerization of vinyl acetate and then followed with partial hydrolysis. Because of its unique properties like water solubility and multiple -OH groups for further chemical modification, it has been broadly used in drug delivery applications.Citation153 Mishra et alCitation80 completed the preparation of lutein PVA NPs by the precipitation method. The results showed that lutein PVA NPs at much lower doses caused a significant blood glucose-lowering effect along with prominent antihyperlipidemic and antioxidant effects compared with native lutein in non-insulin-dependent diabetic rats.
Poly (Ethylene Glycol) (PEG) Surface Modification
However, there are certain limitations of synthetic polymers such as opsonization by plasma proteins and subsequent recognition and clearance by mononuclear phagocyte system because of high surface hydrophobicity.Citation154 These drawbacks can be overcome by PEGylation approach. PEG is a hydrophilic, nontoxic, blood-compatible polymer that is the most widely used material for the surface modification of NPs.Citation155 PEG is applied to endow nanocarriers with a hydrophilic camouflage surface, which can provide several advantages in vivo applications, such as improving the hydrophilicity of synthetic polymer, minimizing immunogenicity and phagocytosis as related to rapid reticuloendothelial system, reducing intermolecular aggregation, and increasing aqueous solubility and stability in blood, eventually contributing to the prolonged circulation of NPs.Citation156
El-Naggar et alCitation81 successfully developed curcumin-loaded PLA–PEG NPs using the emulsion-diffusion evaporation technique. In this amphiphilic system, PLA represented the hydrophobic segment of the copolymer and PEG, the hydrophilic one.Citation59 Hydrophobic curcumin was encapsulated into hydrophobic polymer’s portion and stabilized by cationic surfactant (cetyltrimethylammonium bromide). The results validated the superiority of curcumin encapsulated into PLA–PEG NPs over both polymer NPs itself and free curcumin in elevating the level of plasma insulin, decreasing the level of plasma glucose, protecting the liver from inflammation, and ameliorating hepatic functioning in STZ-induced diabetic rats.Citation81 Used PLGA-PEG-COOH and PCL conjugate as carriers, novel polymeric NPs, which efficiently encapsulated fisetin, were fabricated by the nanoprecipitation technique. In the drug delivery system, PCL exhibited extraordinary capability to form blends with other polymers, which made it possible to design degradation kinetics and mechanical properties.Citation157 During the preparation of NPs, the PCL block could interact with PLGA to build a hydrophobic core, while the hydrophilic PEG-COOH chains could protrude from the particle surface to stabilize the core.Citation158 In vitro release assays demonstrated that NPs could preserve and protect the release of fisetin under gastric-simulated conditions, along with controlling release in the intestinal medium. What’s more, fisetin nanoformulation obtained an approximately 20-fold higher α-glucosidase inhibitory effect than commercial acarbose.Citation157 Recent reports implied that PEG could cause an immunogenic response in mammals. However, the extent of this response elicited by the binding of anti-PEG antibodies is not clear. PEG remains the FDA approved polymer and is still the most extensively utilized polymeric coating of nanomedicines, either in industrial use or academic research.Citation159
Despite synthetic polymers-based NPs with numerous benefits, there are still a couple of concerns about potential toxicity. Polymer toxicity can be a result of residual monomer in the prepared polymer product and/or physical interactions with biological homeostatic mechanisms, that is, gill oxygen transport.Citation160 Besides, NPs also have certain other weaknesses including high manufacturing costs, unpredictable stability, and short shelf life.Citation2,Citation161
Lipid-Based Nano Systems
Lipid-based nano delivery systems are comprised of biocompatible/biodegradable lipid ingredients generally recognized as safe (GRAS), which have attracted considerable research attention over the last 15 years after the discovery that the oral bioavailability of poorly water-soluble drugs could be improved when they are co-administered with a diet rich in fat.Citation162 Lipids are absorption enhancers because they can increase oral bioavailability of poorly water-soluble drugs in multitudinous ways like promoting dissolution as a micellar solution, acting as inhibitors of efflux transporters, and increasing the lymphatic uptake.Citation162–Citation164 Besides, the structural and chemical richness of lipids provides multiple possibilities to form delivery systems with different characteristics and contain various active compounds.Citation165
Nanoemulsions (NEs) and Self-Nanoemulsifying Drug Delivery Systems (SNEDDS)
NEs
NEs are colloidal dispersions consisting of an oily phase, surfactant, and a water phase.Citation166 It remains controversial in defined size values of NEs, with the upper limit fixed at 100 nm, 200 nm,or 500 nm.Citation167 Generally, as oil droplets’ size decreases, the energy density required to break down oil droplets increases, which signifies that high energy input is necessary to form NEs. Mechanical devices are often used, like ultrasonification, microfluidizers, and high-pressure homogenizers.Citation168 NEs are drug delivery systems with the ability to entrap hydrophobic and hydrophilic molecules.Citation166 It markedly enhanced the absorption of antidiabetic phytocompounds entrapped in NEs, which can be realized by the small particle size and high surface-to-volume ratio of NEs, the surfactant-induced membrane fluidity then permeability improvement, the stimulation of various lipid sensing mechanisms in GIT, and the initiation of intestinal lymphatic transport pathway.Citation168–Citation170
Bitter gourd seed oil was emulsified by the two-stage homogenization process for NEs preparation with a low surfactant to oil ratio (0.65). Bitter gourd seed oil NEs significantly ameliorated hyperglycemia and oxidative stressed state in alloxan-induced type 2 diabetic rats. Interestingly, compared with equivalent doses of bitter gourd seed oil conventional emulsion and 1% (w/v) bitter gourd seed oil NEs, 0.5% (w/v) bitter gourd seed oil NEs displayed maximum therapeutic efficiency possibly due to increased bioavailability.Citation82 Further studies on pharmacokinetics are needed to conduct. Xu et alCitation83 reported that NEs decreased the P-gp efflux of berberine by 2-fold and increased its permeability by 5.5-fold in vitro Caco-2 cells transport and in situ single-pass intestinal perfusion investigations. Compared with berberine control, NEs enhanced the oral bioavailability of berberine in vivo by 212.02% and reduced the blood glucose level of diabetic mice by 3-fold. Moreover, the therapeutic effect of berberine NEs on diabetes was better than that of metformin. Cyclodextrin-based nanosponges, an innovative delivery system, are made up of hyper-cross-linked cyclodextrins connected in a three-dimensional network.Citation171 They can surpass the limitations of native cyclodextrins.Citation172 Superior properties are ascribed to their nanoporous, sponge-like structure, beneficial for encapsulation of complex lipophilic and hydrophilic phytocompounds.Citation173 Nait Bachir et al synthesized two engineered NEs in order to enhance the bioactivity and stability of sage essential oil.Citation84 The first one was stabilized by native β-cyclodextrin employing physical method and the second one was stabilized by β-cyclodextrin nanosponges using naphthalene dicarboxylic acid as a cross-linking agent and employing polycondensation method. The experiment results revealed the stability of the latter was higher than that of the former. The antidiabetic activity in vivo of NEs stabilized by β-cyclodextrin nanosponges performed better curative efficacy than that of NEs stabilized by natural β-cyclodextrin and free sage essential oil.Citation84 Hatanaka et al prepared three NEs of α-tocopherol at different loading amounts (10%, 30%, and 50%) via the mechanochemical method using a homomixer and microfluidizer. By comparison with the control mixture of oil and α-tocopherol, 10% α-tocopherol-loaded NEs exhibited a 2.6-fold increased bioavailability in vivo and a more significant antioxidative effect on several organs, especially the liver, in STZ-induced diabetic rats. However, when the content of α-tocopherol of NEs was 30% or higher, severe droplet aggregation occurred during long-time storage.Citation85 The main restriction that reduces the wide application of NEs is stability.Citation174 NEs are thermodynamically unstable and kinetically stable systems.Citation175 In other words, if NEs are given sufficient time, phase separation eventually occurs. Ostwald ripening is the main destabilization mechanism of NEs.Citation176 It was reported that during storage and applications, the stability of NEs could be maintained against environmental factors including pH and temperature by controlling size, surfactant, and oil concentrations.Citation177
SNEDDS
In contrast to NEs, SNEDDS do not incorporate any water, so they are much more chemically as well as physically stable, and therefore can be easily stored for a longer time.Citation178 Besides, free energy required to produce SNEDDS is very low, and the formulation is thermodynamically spontaneous.Citation179 SNEDDS (pre-emulsion concentrates) are anhydrous isotropic mixtures of oil, drug, surfactant, and/or co-surfactant. Such systems are diluted by aqueous phase (gastrointestinal fluids) in vivo, and then under gentle agitation provided by the digestive motility of the intestine and stomach, they can form fine oil-in-water NEs, providing a large interfacial surface area for improving drug absorption. So, one of the most crucial features of SNEDDS is the change that occurs when the system is diluted by body fluids after administration.Citation162,Citation180,Citation181 Hence, it is crucial to identify efficient self-emulsification regions and decide on the most suitable concentrations of surfactant, cosurfactant, and oil for the formulation of SNEDDS with good stability. Garg et alCitation87 prepared SNEDDS of polypeptide-k and curcumin for better antidiabetic efficacy in STZ-induced diabetic rats. A pseudo-ternary phase diagram was constructed through oil (labrafil M 1944 CS), surfactant (tween-80), cosurfactant (transcutol P) to select the efficient self-emulsification region. Box–Behnken design was utilized to optimize the liquid formulation based on the results of zeta potential, percentage drug loading, polydispersity index, and mean droplet size. Under the condition of variation in pH, dilution, and temperature, the absence of phase separation and drug precipitation suggested that the optimized formulation was stable. The rate of emulsification is a crucial index for the evaluation of the efficiency of emulsification. The self-emulsification time of optimal formula resveratrol SNEDDS (propylene glycol, tween 80, and olive oil in the ratio 533.3:266.7:200) was only 27 ± 0.8 s without precipitation in vitro. Ten milligram/kilogram resveratrol nanoformulation displayed significant hypolipidemic and hypoglycemic effects on STZ and glucose induced-diabetic rats, similar to the high dose (20 mg/kg) of free resveratrol.Citation88 In another study, the relative oral bioavailability in vivo of trans-cinnamic acid SNEDDS (10% PEG 400, 30% isopropyl myristate, and 60% Kolliphor EL) was approximately 246% as compared with trans-cinnamic acid suspension, indicating that SNEDDS have a remarkable capability to improve bioavailability.Citation89 These phenomena can be attributed to multi-concerted mechanisms like reduced intra-enterocyte metabolism through cytochrome P450 enzymes, decreased P-gp efflux activity, and bypassed hepatic first-pass metabolism through lymphatic absorption.Citation182 SNEDDS of trans-cinnamic acid enhanced the antidiabetic efficacy of trans-cinnamic acid in alloxan-induced diabetic rats, which could be comparable to that of metformin.Citation89 SNEDDS are related with many merits, but they still have some defects. Characterization- and formulation-associated issues include the correlation of in vitro model with in vivo studies, the usage of a high amount of surfactant, the precipitation of drug in vivo, the oxidation potential of lipid components, the difficulty of low encapsulation, etc.Citation183 Nowadays, research is moving towards some novel applications of SNEDDS to make up for their shortcomings, such as self-double emulsions (w/o/w), solid SNEDDS, controlled release SNEDDS, supersaturated SNEDDS, targeted SNEDDS.Citation175,Citation184,Citation185 For example, solid SNEDDS are more stable and amenable to compress into tablets as compared to their liquid counterparts.Citation186 Garg et alCitation86 solidified liquid SNEDDS of polypeptide-k using Aerosil 200 as hydrophobic carrier through the spray drying technique. The biochemical, hematological, and histopathological results of STZ-induced diabetic rats revealed better antidiabetic potential of polypeptide-k loaded in SNEDDS than that of naive form.
Solid Lipid NPs (SLNs) and Nanostructured Lipid Carriers (NLCs)
Lipid-based NPs possess an inner solid lipid phase. According to their internal structure, lipid-based NPs are divided into solid lipid NPs (SLNs) and nanostructured lipid carriers (NLCs).Citation187 As the first generation of lipid-based NPs, SLNs are only composed of solid lipids, whereas NLCs, as the upgrade of SLNs, are composed of a mixture of liquid and solid lipids, but the solid lipid is in a relatively high amount to fabricate nanoparticles. This solid matrix realizes the controlled release of enclosed either lipophilic or hydrophilic molecules, protects them from degradation, and increases the long-time stability of the system.Citation188 Numerous methods have been reported for the preparation of lipid NPs like hot and cold high-pressure homogenization, microemulsion-based technique, solvent emulsification/evaporation, solvent diffusion method, etc.Citation163 Probably the most vital reasons for lipid NPs as a suitable alternative to previous polymeric NPs, are low toxicity potential and the ease of large-scale production.Citation189
SLNs
Because myricitrin is susceptible to high temperature, the cold homogenization method has been employed to prepare myricitrin SLNs. SLNs of myricitrin exhibited antioxidant, antidiabetic, and antiapoptotic activities in STZ-nicotinamide-induced diabetes in mice and hyperglycemic myotubes.Citation90 In another study, a solvent injection method was utilized to develop the resveratrol SLNs. Compared with pure resveratrol, oral administration of resveratrol SLNs to rats with T2DM showed better hypoglycemic effects and more significantly reduced the expression of SNARE proteins associated with insulin resistance in muscle and adipose tissue.Citation91 Xue et alCitation92 prepared berberine SLNs using their patented method, the solvent diffusion method (No 201210495674.4, People’s Republic of China patent). Oral pharmacokinetic studies in vivo showed AUC0~t, Cmax, t1/2, and VRT0~t of berberine SLNs were 113.57 ± 72.93 µg·h/L, 44.65 ± 4.77 µg/L, 11.50 ± 10.78 h, and 42.58 ± 21.82 h, respectively, in contrast to 56.48 ± 29.61 µg·h/L, 11.06 ± 6.24 µg/L, 9.228 ± 5.13 h, and 23.40 ± 13.92 h of pure berberine. The above results indicated that nanoformulations could promote absorption, possess a slow-release character, and reduce fluctuations in drug concentrations. Consequently, berberine SLNs exerted more powerful effectiveness than an equivalent dose of berberine in db/db mice, especially for the effect on improving insulin sensitivity and glucose tolerance. After further research, Xue et alCitation93 found that the drug concentration of berberine SLNs group in the liver was approximately 2-fold higher than that of berberine group. The maximum drug concentration in the liver was 20-fold higher than that in the blood after oral administration of berberine SLNs, suggesting a predominant accumulation of berberine SLNs in the liver. However, the capability of SLNs to transform crystalline phases-low-energetic form results in an increased degree of order which decreases the imperfections in the crystal lattice followed by drug expulsion phenomena and low encapsulation efficiency ().Citation71,Citation190 For example, the encapsulation efficiency of the discussed SLNs of myricitrin,Citation90 resveratrol,Citation91 berberineCitation92,Citation93 is 56.2%, 79.9%, 58%, respectively.
NLCs
NLCs are designed to triumph over SLNs shortcomings. NLCs are also maintained in solid state at room and body temperature.Citation165 However, instead of only a solid lipid, a liquid lipid or a mixture of liquid lipids are used to replace the part of a solid lipid, leading to a less ordered lipid matrix-imperfect crystal lattice () which contributes to increased loading efficiencies, enhanced stability along with the prevention of drug expulsion during storage,Citation190,Citation191 as illustrated by the following studies.
Two lipid-based nanocarriers for oral delivery of silymarin were prepared through the method of emulsion/evaporation/solidifying.Citation94,Citation95 The first one was produced with lauroglycol 90 as liquid lipid, cetyl palmitate as solid lipid, and Brij S20 as surfactant,Citation94 the second one with capryol 90 as liquid lipid, stearic acid as solid lipid, and Brij S20 as surfactant.Citation95 Both encapsulation efficiency was more than 92% with excellent chemical and physical stability. In vitro release studies showed that NLCs may improve the passive permeation of silymarin via the Caco-2 cell layer. In vivo, both silymarin-loaded NLCs exhibited a more significant down-regulation of triglyceride and blood glucose levels compared with native silymarin. Besides, both nanoformulations performed a remarkable antihyperalgesic activity on STZ-induced neuropathy. However, the second nanoformulation showed more pronounced and longer lasting therapeutic effects.Citation94,Citation95 Similarly, in another study, baicalin NLCs were formed by the hot melting high-pressure homogenization method using miglyol as liquid lipid and precirol as solid lipid. The results suggested that baicalin NLCs had a better capability to retain drugs and possessed relatively good physical stability. To be specific, during 1 month of storage at 4°C, aggregation and gelation were not found by visual observation and no significant change of zeta potential, particle size, and polydispersity index was discovered by data analysis, indicating relative good physical stability of baicalin NLCs; the average encapsulation efficiency of freshly prepared baicalin NLCs was 85.29 ± 3.42%, indicating their better ability to retain drugs. Compared with pure baicalin, baicalin NLCs had better hypoglycemic and hypolipidemic effects in vivo.Citation96 In the past few years, at the academic level, the potential of NLCs as drug delivery systems has been broadly researched. However, to the best of our knowledge, no NLCs for therapeutical use have obtained the regulatory approval or even have reached the clinical study stage until now.Citation191
Vesicular Systems
Vesicles are colloidal systems with a size of less than a micrometer.Citation192 Vesicular delivery systems are composed of an aqueous core generally surrounded by one or more lipidic bilayers.Citation193 The hydrophilic agents are enclosed in the inner aqueous core, while lipophilic drugs in the lipid bilayer.Citation194 The agents encapsulated in lipid vesicles can easily cross the cell membrane, which alters the rate and extent of the absorption of agents and their disposition.Citation195 Vesicular delivery systems of antidiabetic phytocompounds that have been studied so far are discussed below.
Liposomes
Phospholipids are the major component of all biological membranes.Citation196 Liposomes are phospholipid bilayer vesicles. It has been over 50 years that liposomes explored in pharmaceutical research as drug delivery systems.Citation197 Due to the use of phospholipids, liposomes possess a biofilm similar structure and exhibit excellent biocompatibility.Citation198,Citation199 More than 40 liposomes loaded with drugs are under different clinical research stages or have been successfully marketed.Citation199
Amjadi et al used the ultrasonic-mechanical method for the fabrication of betanin liposomes. In vitro release studies, betanin-loaded liposomes exhibited a relatively favorable sustained release profile in simulated intestinal and gastric fluids. Liposomal encapsulation improved the physicochemical stability of betanin during in vitro digestion and more effectively regulated hyperlipidemia, hyperglycemia, and oxidative stress than free betanin in STZ-induced rats.Citation97 Yucel et al prepared nanoliposomal formulations containing resveratrol using the dry film hydration method. It was concluded that nanoformulation significantly decreased high glucose levels along with increasing insulin levels in glucose and STZ-induced diabetic β-TC3 cells, and exerted prolonged antioxidant activity for 24 h as compared with resveratrol solution.Citation200 However, the therapeutical use of liposomes exists some restrictions, predominantly their high formulation cost and poor stability under harsh conditions, typically exposed to the GIT. Some studies have been reported on the use of cochleates as an alternative platform to liposomes in order to surpass these limitations.Citation201
Nanocochleates
Nanocochleates are stable phospholipid precipitates derived from the physical interaction of divalent cation with anionic lipid vesicles,Citation202 generally phosphatidylserine and calcium.Citation203 Nanocochleates have cylindrical (cigar-like) microstructures made up of solid, lipid bilayer sheet which was rolled up in a spiral or in stacked sheets to minimize their interaction with water, consequently, nanocochleates with no or little internal aqueous space.Citation203,Citation204 This unique structure provides protection for the encapsulated drugs from biodegradation, even if they are exposed to hazardous environmental conditions in the GIT.Citation205 Yucel et alCitation206 developed resveratrol-loaded nanocochleates by the trapping method and assessed their therapeutic efficiency in diabetic pancreatic β TC cell line in vitro. Compared with native resveratrol, the lower dose of nanocochleates loaded with resveratrol better improved the decreased insulin levels rendered with glucose and STZ, markedly reduced the increased glucose levels, and also exhibited prolonged antioxidant activity for 24 h. However, self-aggregation, which usually happens during production and storage, is currently the major obstacle hindering the development of efficient cochleate-based drug delivery systems.Citation207
Niosomes
Niosomes are such hydrated vesicular systems mainly consisting of cholesterol along with nonionic surfactants. Niosomes can keep stable over a longer period of time in different conditions and require less cost of production, so break through major restrictions of liposomes.Citation208 Sharma et alCitation209 prepared lycopene-loaded niosomes by developing a novel method called adsorption-hydration technique. The release mechanism of lycopene was Fickian type and followed zero-order release kinetics in the first 10 h, after that the regression equation best matched Korsmeyer-Peppas model. Thus, the lycopene release from niosomes in vitro was sustained and prolonged profile, which was beneficial to reduce dosing frequency. Antidiabetic studies in vivo showed lycopene-loaded niosomes had an activity similar to a standard antidiabetic drug, glibenclamide.Citation98 Similarly, embelin-loaded niosomes, produced by the thin-film hydration process, displayed a hypoglycemic effect in vivo which was comparable to another standard antidiabetic agent, repaglinide. Moreover, lipid peroxidation decreased and glutathione (GSH), catalase (CAT), and superoxide dismutase (SOD) increased significantly, which verified the antioxidant efficacy of the nanoformulation.Citation99 In another study of Singhal et al,Citation100 researchers used the thin-film hydration technique to prepare gymnemic acid-loaded niosomes which revealed antioxidant, antihyperglycemic, antihyperlipidemic, and antiglycation (AGEs) effects and eventually, exerted a protective action in STZ-nicotinamide-induced diabetic nephropathy in Wistar rats.
Phytosomes
Phytosomes, also known as phytophospholipid complexes, are prepared by interactions between active phytocompounds and the polar part of phospholipids.Citation210,Citation211 The biggest difference between liposomes and phytosomes is that, in liposomes, the active phytocompound is distributed in lipid layers of the membrane or in the medium containing a cavity,Citation211 whereas, in phytosomes, it is an integral part of the membrane, which gives the greater stability and better bioavailability because of the chemical links between active phytocompounds and phospholipid molecules ().Citation213,Citation214 Yu et alCitation101 prepared phytosomes loaded with berberine phospholipid complex using the rapid solvent evaporation method followed by self-assembly technique. In vivo studies, this nanoformulation could produce a 3-fold enhanced oral bioavailability of berberine. More importantly, the antidiabetic efficacy was improved remarkably in db/db mice by ameliorating hyperlipidemia and lowering fasting glucose levels. Besides, the types of phospholipid matrix applied in the preparation of phytosomes influenced their performance. For instance, compared with pure chrysin and chrysin prepared with soya phosphatidylcholine, chrysin prepared with egg phospholipid exhibited a greater glucose uptake promoting effect in C2C12 muscle cells through upregulating gene expression of PPARγ and GLUT4.Citation214
Micelles
Micelles have been extensively studied as nanocarriers for hydrophobic drugs with core/shell design. They commonly have a particle size within 5–50 nm range.Citation215 Micelles are made by the self-assembly of amphiphilic molecules in aqueous media above the critical micelle concentration (CMC).Citation216 The hydrophobic portion of amphiphilic molecules constitutes the core of the micelle, which serves as a reservoir and protects the agent, while the hydrophilic portion constitutes the micelles’ shell, which confers steric stability and aqueous solubility to the micellar structure.Citation217,Citation218
The transplantation of islet β-cells is an efficient therapy for type 1 and 2 diabetes. However, the isolated islets are under hypoxic conditions and then undergo the apoptotic process. Therefore, it is necessary to protect islets against hypoxia for enhancing the efficacy of islet transplantation. A peptide micelle-mediated curcumin delivery system was demonstrated to be effective for islet β-cells protection in the process of transplantation in vitro. This delivery system was developed by Han et al through an oil-in-water (O/W) emulsion/solvent evaporation method. In addition, the peptides consisted of a 3-arginine hydrophilic stretch and a 6-valine hydrophobic stretch.Citation219 Zhang et al used the dialysis method to fabricate an amentoflavone-loaded micelle system composed of amphiphilic copolymer (N-vinyl pyrrolidone and maleic acid guerbet alcohol monoester). Owing to the micelle formation, the oral bioavailability of amentoflavone was increased by nearly 3.2 times in vivo. The antidiabetic effect of amentoflavone nanoformulation is comparable to that of metformin in insulin-resistant diabetic KKAy mice.Citation102 Soluplus® is one such commercially triblock copolymer composed of PCL-Polyvinyl acetate (PVAc)-PEG units with the capability to enhance the solubility and bioavailability of poorly water-soluble drugs.Citation220 Singh et alCitation103 prepared quercetin-loaded soluplus®/poloxamer 407 micelles by the cosolvent evaporation method. In this system, poloxamer 407 was used as a surfactant in order to enhance the solubilization of quercetin and increase the stability of nanomicelles. In vivo pharmacokinetic studies, the relative oral bioavailability of quercetin nanoformulation was 1676% as compared to pure drug suspension. Moreover, quercetin nanoformulation showed significantly lower glucose levels, higher catalase and SOD levels in STZ-induced diabetic rats. Pluronics is another type of triblock copolymers with a central hydrophobic poly (propylene oxide) (PPO) chain and two hydrophilic poly (ethylene oxide) (PEO) on each side, arranged in PEO-PPO-PEO structure.Citation221 Pluronic micelles are capable to increase the stability and solubility of incorporated drugs and improve their pharmacokinetics and biodistribution.Citation222 El-Far et al developed silymarin-loaded pluronic nanomicellesCitation104 and curcumin-loaded pluronic nanomicellesCitation105 using the nanoprecipitation technique. Nanoformulations were found to significantly improve the antihyperlipidemic, antioxidant, and antihyperglycemic properties in STZ-induced diabetic rats when compared with their native candidates. As we discussed, both synthetic and inartificial polymers have their own merits and defects. Combinations of these polymers could make the best use of the advantages and bypass the disadvantages. Using alginate, chitosan, maltodextrin, pluronic P123, pluronic F127, and tween 80, Akbar et alCitation106 developed curcumin-loaded mixed polymeric micelles by the thin-film hydration method. The achieved results showed that the antidiabetic activity of curcumin-loaded mixed polymeric micelles was comparable to that of metformin in bisphenol A-induced diabetics rats. Besides, curcumin-loaded mixed polymeric micelles administered topically on the surface of wound performed superior wound healing potential (fast wound closure) with the reduction of scar formation in vivo. However, the primary challenge faced by micelles is that micelles tend to disintegrate and cannot keep entrapped drugs stabilized when they are diluted below the CMC. This phenomenon typically occurs when the drug/micelle formulation is infused into body and will lead to severely decrease drugs’ bioavailability and deteriorate therapeutic performance.Citation223
Inorganic Nanocarriers
Inorganic materials have been employed to explore nanocarriers with controlled morphology and size.Citation224 Recent breakthroughs on the surface functionalization and structural control of inorganic nanocarriers have brought more possibilities for drug delivery.Citation225
Metallic NPs
Metallic NPs such as selenium,Citation226,Citation227 gold,Citation228 silver,Citation229,Citation230 and zinc oxide NPsCitation107,Citation231 appear very promising for the treatment of T2DM.Citation232 However, metallic NPs are synthesized by physical and chemical methods with many shortcomings, including high energy consumption, the use of toxic solvents, and the generation of hazardous by-products.Citation233 The biosynthesis of metallic NPs through medicinal plants has obtained widespread attention as a proper alternative to hazardous chemical synthetic technique.Citation234 Antidiabetic phytocompounds act as reducing and stabilizing agents during the synthesis of metallic NPs. Currently, there have been many papers reporting the use of antidiabetic phytocompounds as stabilizing and reducing agents to synthesize metallic NPs, such as gymnemic acid gold NPs,Citation235 vicenin gold NPs,Citation236 escin gold NPs,Citation237 docosahexaenoic acid zinc oxide NPs,Citation107 and guavanoic acid gold NPs.Citation238 In these studies, all of them showed good antidiabetic effects in vitro or/and in vivo. Besides, phytocompounds with antidiabetic effects can enhance the diabetic efficacy of metal NPs. For instance, Catathelasma ventricosum polysaccharides SeNPs exhibited significantly higher antidiabetic effects than other selenium preparations like SeNPs, selenocysteine, sodium selenite.Citation108 On the base of berberine-loaded NLCs, Yin et alCitation109 used an in-situ reduction technique to fabricate berberine-loaded SeNLCs, as Se4+ was reduced to Se which precipitated on the surface of NPs. In vivo studies reported berberine SeNLCs yielded the highest Cmax and AUC0–t, up to 172.88 ng/mL and 1,107.80 ng·h/mL, when compared with berberine solution (Cmax=45.06 ng/mL, AUC0–t=173.74 ng·h/mL) and berberine NLCs (Cmax=148.21 ng/mL, AUC0–t=689.54 ng·h/mL). Accordingly, the hypoglycemic activity of berberine SeNLCs was also significantly superior to that of berberine solution and berberine NLCs. It turned out that Se coating, plus the synergy of selenium, was basically responsible for increased oral bioavailability and enhanced hypoglycemic activity in vivo.
Carbon Nanotubes
Carbon nanotubes are tiny tubes approximately 10,000 times thinner than a human hair. They consist of rolled-up sheets of carbon hexagons.Citation239 Either by noncovalent interactions or covalent attachment, drugs can be loaded within the interior core or onto the surface of carbon nanotubes.Citation240 In comparison with spherical NPs, carbon nanotubes’ needlelike shape gives them superior flow dynamics and enhanced capacity to penetrate cellular membranes.Citation241 Ilie et alCitation242 firstly prepared nanotubes with oxidation properties using 1:3 (v/v) concentrated nitric acid and sulfuric acid, which gave them stability and hydrophilicity in aqueous systems because of the formation of -OH and -COOH groups on the lateral sides of or at the end of the tubes. And then, Ilie et alCitation242 covalently conjugated nicotinamide onto the surfaces of oxidized multiwalled carbon nanotubes. In vitro study, 1.4E7 cells administrated with nicotinamide-functionalized multiwalled carbon nanotubes better regulated insulin secretion and increased insulin production as compared with nicotinamide or multiwalled carbon nanotubes.
Mesoporous Silica NPs (MSNs)
Based on unique intrinsic properties of MSNs such as large pore volume, high surface area, facile functionalization of surface, uniform and tunable pore size, and stable framework, they have been utilized extensively as drug carriers.Citation225,Citation243 It is widely agreed in published literature that endocytosis is a common mechanism for the translocation of MSNs.Citation243 Huang et alCitation110 designed the surface functionalization of MSNs with amine groups to act as reservoirs for efficient immobilization of phytocompounds into pores for antidiabetic therapies. The results revealed that 16-Hydroxycleroda-3,13-Dine-16,15-Olid incorporated into MSNs caused a reduction of DPP4 activity in a dose- and time-dependent fashion in vitro and simultaneously possessed less adverse effects and reliable efficacy in down-regulation of hyperglycemia in diet-induced diabetic mice.
Although inorganic NPs can be metabolized by the kidney after their degradation into small-sized fragments,Citation244 most inorganic nanomaterials still have high bioaccumulation risks.Citation245 Moreover, the generated ions, especially heavy metals, may lead to toxicity or damage to the related organs during the excretion.Citation244 Therefore, it is essential to systematically study the toxicity of these nanomaterials to different organs in the process of metabolism.Citation246
Nanosuspensions
Drug nanocrystals are nanosized drug particles often formed as nanosuspensions, namely submicron dispersions in liquid media where surfactants and/or polymers act as stabilizers. Although, without any carriers, nanosuspensions are an excellent delivery platform for poorly water-soluble drugs.Citation247 Reducing the drug size to nanoscale usually causes a significant increase in solubility and dissolution rate along with a distinct improvement in oral bioavailability.Citation248 Up to now, several studies have been reported that nanosuspensions enhanced the therapeutic effects of antidiabetic phytocompounds on diabetes, such as ursolic acid,Citation113 berberine,Citation114 curcumin,Citation111 gymnemic acids.Citation115 Chen et alCitation249 prepared Fructus Mori polysaccharides with spherical particles by the antisolvent precipitation method. According to the research results, the smaller the particle, the higher the bioavailability. We can deduce that the smaller size after spheroidization is able to be beneficial for the improved bioavailability. In comparison with the corresponding native polysaccharide, the spheroidization improved the hypoglycemic activity and antioxidant ability in vitro. Zhao et alCitation116 developed betulin nanocrystallization by the antisolvent precipitation technique. The results exhibited that nano betulin possessed the same chemical structure as raw betulin, but have smaller crystal size and lower crystallinity. The solubility and dissolution rate of nano betulin were, respectively, 1.54 and 3.12 times of raw drug. Compared with raw betulin, betulin nanosuspensions showed a 1.21-fold increased bioavailability in vivo and an excellent hypoglycemic effect in STZ-induced diabetic rats. However, instability is the most disadvantage of nanosuspensions and restricts their application to pharmaceutical industry.Citation250 Stabilizers are crucial to prevent the aggregation of high energy nanosuspensions. Nonetheless, a suitable stabilizer is often selected by a trial and error method.Citation251 Meanwhile, the potential raised toxicity concerns if the stabilizers are used in large quantities for a long term.Citation252
Conclusion
As antidiabetic agents, phytocompounds are potential candidates with abundant sources, significant curative effects, and low side-effects. Generally, there are four hypoglycemic mechanisms of phytocompounds, including reduction of carbohydrate decomposition and glucose absorption, promotion of glucose uptake and metabolism, improvement of insulin action and sensitivity, and antioxidant and antiinflammatory actions. However, conventional oral administration of antidiabetic phytocompounds has some inherent defects. Oral nano drug delivery systems for phytocompounds to treat T2DM not only maintain the advantages of oral administration but also overcome the shortcomings of conventional oral drug delivery.
In this review, we discussed phytocompounds-based oral nano delivery systems for T2DM treatment, including polymeric NPs, lipid-based nanosystems, vesicular systems, micelles, inorganic nanocarriers, and nanosuspensions. As observed in studies, oral nano drug delivery systems for T2DM treatment have the following advantages: 1) phytocompounds encapsulated into nano delivery systems can improve the stability of the former and protect them from enzymatic and/or chemical degradation in GIT. 2) nano delivery systems act at the molecular level to increase the cellular drug uptake or block drug efflux mechanisms like P-glycoprotein (P-gp) pump, which further improves the pharmacokinetic and pharmacodynamic profile of antidiabetic molecules.Citation2 3) oral drug bioavailability can be significantly limited by first-pass hepatic metabolism. Intestinal lymphatic drug transport is regarded as the best for improving oral drug delivery through bypassing first-pass metabolism. Nano delivery systems can be transferred into the lymph, arrive at lymphatic system through M cells, and increase the subsequent release of drugs in systemic circulation.Citation253 4) tailored engineering of nanocarriers fulfills controlling drug release, preventing opsonization, and targeting drug delivery.Citation2 Due to the above advantages, phytocompounds have clearly shown better antidiabetic efficacy in oral nano drug delivery systems with increased bioavailability, decreased toxicity, targeted specific site, and reduced dose and dosing frequency.
However, there are still some deficiencies in the existing research. On the one hand, it is worth noting that a control group administered with non-loaded carrier is not conducted in the vast majority of works so that the drug-independent effect of the carrier cannot be ignored. Rho et alCitation254 reported that empty self-assembled hyaluronic acid NPs without any drug could be used as a therapeutic agent for T2DM treatment. On the other hand, most of those materials used for the preparation of nano delivery systems are usually obtained from inorganic matter or synthetic polymers through a rather complex and tedious synthesis process, which may inevitably result in potential toxicity. Even if materials from natural sources are employed as nanocarriers, organic chemicals may be introduced into the process due to the requirement for the preparation. Security is a prime concern. However, the majority of published data come from cellular and animal models but not a clinical study. Since toxicological research in animals has limitations, clinical trials are ultimately required. There are few clinical trials on potential antidiabetic nano phytocompounds, only curcumin nanomicelle,Citation255 curcumin nanocapsules.Citation256 Despite few severe adverse effects were reported during the treatment, long-term human toxicology research is still lacking. The FDA recently issued guidance to help promote the safe development of nanotechnology-based products for clinical use.Citation257 More clinical research is worth conducting.
Above all, oral nano drug delivery systems for T2DM treatment are an available administration strategy for antidiabetic phytocompounds. This review could provide researchers with promising antidiabetic phytocompounds and excellent oral delivery systems to explore more therapeutic possibilities. With the extensive development of the pharmacological activity research of antidiabetic phytocompounds and the continuous progress of material science and technology, more and more excellent oral nano phytocompounds will be employed in clinical pharmaceutical intervention for T2DM treatment.
Acknowledgments
The authors would like to express their gratitude to Chuanhong Luo, Jiaying Long for proof reading the article.
Disclosure
Xin Nie, Zhejie Chen are co-first authors. The authors report no conflicts of interest in this work.
References
- Fangueiro JF, Silva AM, Garcia ML, Souto EB. Current nanotechnology approaches for the treatment and management of diabetic retinopathy. Eur J Pharm Biopharm. 2015;95(Pt B):307–322. doi:10.1016/j.ejpb.2014.12.02325536109
- Uppal S, Italiya KS, Chitkara D, Mittal A. Nanoparticulate-based drug delivery systems for small molecule anti-diabetic drugs: an emerging paradigm for effective therapy. Acta Biomaterialia. 2018;81:20–42. doi:10.1016/j.actbio.2018.09.04930268916
- Thomas CC, Philipson LH. Update on diabetes classification. Med Clin North Am. 2015;99(1):1–16.25456640
- Ran Q, Wang J, Wang L, Zeng HR, Yang XB, Huang QW. Rhizoma coptidis as a Potential Treatment Agent for Type 2 Diabetes Mellitus and the Underlying Mechanisms: A Review. Front Pharmacol. 2019;10:805.31396083
- Sun Z, Sun X, Li J, et al. Using probiotics for type 2 diabetes mellitus intervention: advances, questions, and potential. Crit Rev Food Sci Nutr. 2020;60(4):670–683.30632770
- Manukumar HM, Shiva Kumar J, Chandrasekhar B, Raghava S, Umesha S. Evidences for diabetes and insulin mimetic activity of medicinal plants: present status and future prospects. Crit Rev Food Sci Nutr. 2017;57(12):2712–2729.26857927
- Rios JL, Francini F, Schinella GR. Natural Products for the Treatment of Type 2 Diabetes Mellitus. Planta Med. 2015;81(12–13):975–994.26132858
- Choudhury H, Pandey M, Hua CK, et al. An update on natural compounds in the remedy of diabetes mellitus: A systematic review. J Tradit Complement Med. 2018;8(3):361–376.29992107
- Habtemariam S. The Quest to Enhance the Efficacy of Berberine for Type-2 Diabetes and Associated Diseases: physicochemical Modification Approaches. Biomedicines. 2020;8:4.
- Adisakwattana S. Cinnamic Acid and Its Derivatives: mechanisms for Prevention and Management of Diabetes and Its Complications. Nutrients. 2017;9(2):2. doi:10.3390/nu9020163
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of Hyperglycemia in Type 2 Diabetes, 2015: A Patient-Centered Approach: update to a Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–149. doi:10.2337/dc14-244125538310
- Rubino F, Schauer PR, Kaplan LM, Cummings DE. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu Rev Med. 2010;61(1):393–411. doi:10.1146/annurev.med.051308.10514820059345
- Yanai H, Adachi H, Katsuyama H, Moriyama S, Hamasaki H, Sako A. Causative anti-diabetic drugs and the underlying clinical factors for hypoglycemia in patients with diabetes. World J Diabetes. 2015;6(1):30–36.25685276
- Salehi B, Ata A, Vak N, et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules. 2019;9:10.
- Petrovska BB. Historical review of medicinal plants′ usage. Pharmacogn Rev. 2012;6(11):1–5. doi:10.4103/0973-7847.9584922654398
- Oh YS. Plant-Derived Compounds Targeting Pancreatic Beta Cells for the Treatment of Diabetes. Evid Based Complement Alternat Med. 2015;2015:629863. doi:10.1155/2015/62986326587047
- Munhoz ACM, Frode TS. Isolated Compounds from Natural Products with Potential Antidiabetic Activity - A Systematic Review. Curr Diabetes Rev. 2018;14(1):36–106.28474555
- Qaseem A, Barry MJ, Humphrey LL, Forciea MA. Clinical Guidelines Committee of the American College of P. Oral Pharmacologic Treatment of Type 2 Diabetes Mellitus: A Clinical Practice Guideline Update From the American College of Physicians. Ann Intern Med. 2017;166(4):279–290. doi:10.7326/M16-186028055075
- Apostolova N, Iannantuoni F, Gruevska A, Muntane J, Rocha M, Victor VM. Mechanisms of action of metformin in type 2 diabetes: effects on mitochondria and leukocyte-endothelium interactions. Redox Biol. 2020;34:101517. doi:10.1016/j.redox.2020.10151732535544
- Alam F, Islam MA, Kamal MA, Gan SH. Updates on Managing Type 2 Diabetes Mellitus with Natural Products: towards Antidiabetic Drug Development. Curr Med Chem. 2019;25(39):5395–5431. doi:10.2174/0929867323666160813222436
- Chang CLT, Lin Y, Bartolome AP, Chen Y-C, Chiu S-C, Yang W-C. Herbal therapies for type 2 diabetes mellitus: chemistry, biology, and potential application of selected plants and compounds. Evid Based Complement Alternat Med. 2013;2013:378657. doi:10.1155/2013/37865723662132
- Singh J, Cumming E, Manoharan G, Kalasz H, Adeghate E. Medicinal chemistry of the anti-diabetic effects of momordica charantia: active constituents and modes of actions. Open Med Chem J. 2011;5(Suppl 2):70–77. doi:10.2174/187410450110501007021966327
- Chen H. Oral particulate delivery: status and future trends. Adv Drug Deliv Rev. 1998;34(2–3):339–350. doi:10.1016/S0169-409X(98)00047-710837685
- Nouri Z, Hajialyani M, Izadi Z, Bahramsoltani R, Farzaei MH, Abdollahi M. Nanophytomedicines for the Prevention of Metabolic Syndrome: A Pharmacological and Biopharmaceutical Review. Front Bioeng Biotechnol. 2020;8:425.32478050
- Long J, Song J, Zhang X, et al. Tea saponins as natural stabilizers for the production of hesperidin nanosuspensions. Int J Pharm. 2020;583:119406.32387309
- Dening TJ, Rao S, Thomas N, Prestidge CA. Oral nanomedicine approaches for the treatment of psychiatric illnesses. J Control Release. 2016;223:137–156.26739547
- Ochubiojo M, Chinwude I, Ibanga E, Ifianyi S. Nanotechnology in Drug Delivery. Recent Advances in Novel Drug Carrier Systems. 2012.
- Gutierrez RMP, Mendez JVM, Vazquez IA. Chapter 2 - A novel approach to the oral delivery of bionanostructures for systemic disease In: Andronescu E, Grumezescu AM, editors. Nanostructures for Oral Medicine. Elsevier; 2017:27–59.
- Bacanli M, Dilsiz SA, Basaran N, Basaran AA. Effects of phytochemicals against diabetes. Adv Food Nutr Res. 2019;89:209–238.31351526
- Ezuruike UF, Prieto JM. The use of plants in the traditional management of diabetes in Nigeria: pharmacological and toxicological considerations. J Ethnopharmacol. 2014;155(2):857–924.24929108
- Xu L, Li Y, Dai Y, Peng J. Natural products for the treatment of type 2 diabetes mellitus: pharmacology and mechanisms. Pharmacol Res. 2018;130:451–465.29395440
- Li R, Zhang Y, Rasool S, Geetha T, Babu JR. Effects and Underlying Mechanisms of Bioactive Compounds on Type 2 Diabetes Mellitus and Alzheimer’s Disease. Oxid Med Cell Longev. 2019;2019:8165707.30800211
- Bai L, Li X, He L, et al. Antidiabetic Potential of Flavonoids from Traditional Chinese Medicine: A Review. Am J Chin Med. 2019;47(5):933–957.31248265
- Wang PC, Zhao S, Yang BY, Wang QH, Kuang HX. Anti-diabetic polysaccharides from natural sources: A review. Carbohydr Polym. 2016;148:86–97.27185119
- He JH, Chen LX, Li H. Progress in the discovery of naturally occurring anti-diabetic drugs and in the identification of their molecular targets. Fitoterapia. 2019;134:270–289.30840917
- Tundis R, Loizzo MR, Menichini F. Natural products as alpha-amylase and alpha-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: an update. Mini Rev Med Chem. 2010;10(4):315–331.20470247
- Gray GM. Carbohydrate digestion and absorption. Role of the small intestine. N Engl J Med. 1975;292(23):1225–1230.1093023
- Abbas G, Al Harrasi A, Hussain H, Hamaed A, Supuran CT. The management of diabetes mellitus-imperative role of natural products against dipeptidyl peptidase-4, alpha-glucosidase and sodium-dependent glucose co-transporter 2 (SGLT2). Bioorg Chem. 2019;86:305–315.30738330
- Blaschek W. Natural Products as Lead Compounds for Sodium Glucose Cotransporter (SGLT) Inhibitors. Planta Med. 2017;83(12–13):985–993.28395363
- Moradi-Marjaneh R, Paseban M, Sahebkar A. Natural products with SGLT2 inhibitory activity: possibilities of application for the treatment of diabetes. Phytother Res. 2019;33(10):2518–2530.31359514
- Gannon NP, Conn CA, Vaughan RA. Dietary stimulators of GLUT4 expression and translocation in skeletal muscle: a mini-review. Mol Nutr Food Res. 2015;59(1):48–64.25215442
- Hussain T, Tan B, Murtaza G, et al. Flavonoids and type 2 diabetes: evidence of efficacy in clinical and animal studies and delivery strategies to enhance their therapeutic efficacy. Pharmacol Res. 2020;152:104629.31918019
- Cline GW, Petersen KF, Krssak M, et al. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N Engl J Med. 1999;341(4):240–246.10413736
- Sayem ASM, Arya A, Karimian H, Krishnasamy N, Ashok Hasamnis A, Hossain CF. Action of Phytochemicals on Insulin Signaling Pathways Accelerating Glucose Transporter (GLUT4) Protein Translocation. Molecules. 2018;23:2.
- Dominguez Avila JA, Rodrigo Garcia J, Gonzalez Aguilar GA. de la Rosa LA. The Antidiabetic Mechanisms of Polyphenols Related to Increased Glucagon-Like Peptide-1 (GLP1) and Insulin Signaling. Molecules. 2017;22:6.
- Wani JH, John-Kalarickal J, Fonseca VA. Dipeptidyl Peptidase-4 as a New Target of Action for Type 2 Diabetes Mellitus: A Systematic Review. Cardiology Clinics. 2008;26(4):639–648.18929237
- Duarte AM, Guarino MP, Barroso S, Gil MM. Phytopharmacological Strategies in the Management of Type 2 Diabetes Mellitus. Foods. 2020;9:3.
- Jiang CS, Liang LF, Guo YW. Natural products possessing protein tyrosine phosphatase 1B (PTP1B) inhibitory activity found in the last decades. Acta Pharmacol Sin. 2012;33(10):1217–1245.22941286
- Fukunaga T, Zou W, Rohatgi N, Colca JR, Teitelbaum SL. An insulin-sensitizing thiazolidinedione, which minimally activates PPARγ, does not cause bone loss. J Bone Miner Res. 2015;30(3):481–488.25257948
- Wang L, Waltenberger B, Pferschy-Wenzig EM, et al. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): a review. Biochem Pharmacol. 2014;92(1):73–89.25083916
- Matsuda H, Nakamura S, Yoshikawa M. Search for new type of PPARγ agonist-like anti-diabetic compounds from medicinal plants. Biol Pharm Bull. 2014;37(6):884–891.24882400
- Dembinska-Kiec A, Mykkanen O, Kiec-Wilk B, Mykkanen H. Antioxidant phytochemicals against type 2 diabetes. Br J Nutr. 2008;99 E Suppl 1:ES109–117.18503731
- Halim M, Halim A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab Syndr. 2019;13(2):1165–1172.31336460
- Rahimi-Madiseh M, Malekpour-Tehrani A, Bahmani M, Rafieian-Kopaei M. The research and development on the antioxidants in prevention of diabetic complications. Asian Pac J Trop Med. 2016;9(9):825–831.27633293
- Dal S, Sigrist S. The Protective Effect of Antioxidants Consumption on Diabetes and Vascular Complications. Diseases. 2016;4:3.
- Leiherer A, Mundlein A, Drexel H. Phytochemicals and their impact on adipose tissue inflammation and diabetes. Vascul Pharmacol. 2013;58(1–2):3–20.22982056
- Gothai S, Ganesan P, Park SY, Fakurazi S, Choi DK. Natural Phyto-Bioactive Compounds for the Treatment of Type 2 Diabetes: inflammation as a Target. Nutrients. 2016;8:8.
- Ahangarpour A, Sayahi M, Sayahi M. The antidiabetic and antioxidant properties of some phenolic phytochemicals: A review study. Diabetes Metab Syndr. 2019;13(1):854–857.30641821
- Lagoa R, Silva J, Rodrigues JR, Bishayee A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnol Adv. 2020;38:107382.30978386
- Rezaeiamiri E, Bahramsoltani R, Rahimi R. Plant-derived natural agents as dietary supplements for the regulation of glycosylated hemoglobin: A review of clinical trials. Clin Nutr. 2020;39(2):331–342.30797623
- Xie J, Yang Z, Zhou C, Zhu J, Lee RJ, Teng L. Nanotechnology for the delivery of phytochemicals in cancer therapy. Biotechnol Adv. 2016;34(4):343–353.27071534
- Ganesan P, Arulselvan P, Choi DK. Phytobioactive compound-based nanodelivery systems for the treatment of type 2 diabetes mellitus - current status. Int J Nanomedicine. 2017;12:1097–1111.28223801
- Pathak S, Regmi S, Nguyen TT, et al. Polymeric microsphere-facilitated site-specific delivery of quercetin prevents senescence of pancreatic islets in vivo and improves transplantation outcomes in mouse model of diabetes. Acta Biomater. 2018;75:287–299.29883808
- Meena KP, Vijayakumar MR, Dwibedy PS. Catechin-loaded Eudragit microparticles for the management of diabetes: formulation, characterization and in vivo evaluation of antidiabetic efficacy. J Microencapsul. 2017;34(4):342–350.28562190
- Chen L, Lin X, Teng H. Emulsions loaded with dihydromyricetin enhance its transport through Caco-2 monolayer and improve anti-diabetic effect in insulin resistant HepG2 cell. Journal of Functional Foods. 2020;64.
- Zhaojie M, Ming Z, Shengnan W, et al. Amorphous solid dispersion of berberine with absorption enhancer demonstrates a remarkable hypoglycemic effect via improving its bioavailability. Int J Pharm. 2014;467(1–2):50–59.24607213
- Li J, Du H, Zhang M, et al. Amorphous solid dispersion of Berberine mitigates apoptosis via iPLA2beta/Cardiolipin/Opa1 pathway in db/db mice and in Palmitate-treated MIN6 beta-cells. Int J Biol Sci. 2019;15(7):1533–1545.31337982
- Shulman M, Cohen M, Soto-Gutierrez A, et al. Enhancement of naringenin bioavailability by complexation with hydroxypropyl-beta-cyclodextrin. [corrected]. PLoS One. 2011;6(4):e18033.21494673
- Demirdirek B, Uhrich KE. Salicylic acid-based pH-sensitive hydrogels as potential oral insulin delivery systems. J Drug Target. 2015;23(7–8):716–724.26453167
- Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16(1):71.30231877
- Prasad M, Lambe UP, Brar B, et al. Nanotherapeutics: an insight into healthcare and multi-dimensional applications in medical sector of the modern world. Biomed Pharmacother. 2018;97:1521–1537.29793315
- Panwar R, Raghuwanshi N, Srivastava AK, Sharma AK, Pruthi V. In-vivo sustained release of nanoencapsulated ferulic acid and its impact in induced diabetes. Mater Sci Eng C Mater Biol Appl. 2018;92:381–392.30184764
- Akolade JO, Oloyede HOB, Onyenekwe PC. Encapsulation in chitosan-based polyelectrolyte complexes enhances antidiabetic activity of curcumin. Journal of Functional Foods. 2017;35:584–594.
- Maity S, Mukhopadhyay P, Kundu PP, Chakraborti AS. Alginate coated chitosan core-shell nanoparticles for efficient oral delivery of naringenin in diabetic animals-An in vitro and in vivo approach. Carbohydr Polym. 2017;170:124–132.28521977
- Mukhopadhyay P, Maity S, Mandal S, Chakraborti AS, Prajapati AK, Kundu PP. Preparation, characterization and in vivo evaluation of pH sensitive, safe quercetin-succinylated chitosan-alginate core-shell-corona nanoparticle for diabetes treatment. Carbohydr Polym. 2018;182:42–51.29279124
- Rani R, Dahiya S, Dhingra D, Dilbaghi N, Kim KH, Kumar S. Improvement of antihyperglycemic activity of nano-thymoquinone in rat model of type-2 diabetes. Chem Biol Interact. 2018;295:119–132.29421519
- Rani R, Dahiya S, Dhingra D, Dilbaghi N, Kim KH, Kumar S. Evaluation of anti-diabetic activity of glycyrrhizin-loaded nanoparticles in nicotinamide-streptozotocin-induced diabetic rats. Eur J Pharm Sci. 2017;106:220–230.28595874
- Chitkara D, Nikalaje SK, Mittal A, Chand M, Kumar N. Development of quercetin nanoformulation and in vivo evaluation using streptozotocin induced diabetic rat model. Drug Deliv Transl Res. 2012;2(2):112–123.25786720
- Kozuka C, Shimizu-Okabe C, Takayama C, et al. Marked augmentation of PLGA nanoparticle-induced metabolically beneficial impact of gamma-oryzanol on fuel dyshomeostasis in genetically obese-diabetic ob/ob mice. Drug Deliv. 2017;24(1):558–568.28181829
- Mishra SB, Malaviya J, Mukerjee A. Attenuation of Oxidative Stress and Glucose Toxicity by Lutein Loaded Nanoparticles from Spinacia oleracea Leaves. Journal of Pharmaceutical Sciences and Pharmacology. 2015;2(3):242–249.
- El-Naggar ME, Al-Joufi F, Anwar M, Attia MF, El-Bana MA, Curcumin-loaded PLA. PEG copolymer nanoparticles for treatment of liver inflammation in streptozotocin-induced diabetic rats. Colloids Surf B Biointerfaces. 2019;177:389–398.30785036
- Paul D, Dey TK, Mukherjee S, Ghosh M, Dhar P. Comparative prophylactic effects of α-eleostearic acid rich nano and conventional emulsions in induced diabetic rats. J Food Sci Technol. 2014;51(9):1724–1736.25190828
- Xu HY, Liu CS, Huang CL, et al. Nanoemulsion improves hypoglycemic efficacy of berberine by overcoming its gastrointestinal challenge. Colloids Surf B Biointerfaces. 2019;181:927–934.31382342
- Nait Bachir Y, Nait Bachir R, Hadj-Ziane-Zafour A. Nanodispersions stabilized by β-cyclodextrin nanosponges: application for simultaneous enhancement of bioactivity and stability of sage essential oil. Drug Dev Ind Pharm. 2019;45(2):333–347.30388376
- Hatanaka J, Chikamori H, Sato H, et al. Physicochemical and pharmacological characterization of α-tocopherol-loaded nano-emulsion system. Int J Pharm. 2010;396(1–2):188–193.20558261
- Garg V, Kaur P, Singh SK, et al. Solid self-nanoemulsifying drug delivery systems for oral delivery of polypeptide-k: formulation, optimization, in-vitro and in-vivo antidiabetic evaluation. Eur J Pharm Sci. 2017;109:297–315.28842349
- Garg V, Kaur P, Gulati M, et al. Coadministration of Polypeptide-k and Curcumin Through Solid Self-Nanoemulsifying Drug Delivery System for Better Therapeutic Effect Against Diabetes Mellitus: formulation, Optimization, Biopharmaceutical Characterization, and Pharmacodynamic Assessment. Assay Drug Dev Technol. 2019;17(4):201–221.31100018
- Balata GF, Essa EA, Shamardl HA, Zaidan SH, Abourehab MA. Self-emulsifying drug delivery systems as a tool to improve solubility and bioavailability of resveratrol. Drug Des Devel Ther. 2016;10:117–128.
- Wang H, Li Q, Deng W, et al. Self-nanoemulsifying drug delivery system of trans-cinnamic acid: formulation development and pharmacodynamic evaluation in alloxan-induced type 2 diabetic rat model. Drug Dev Res. 2015;76(2):82–93.25847843
- Ahangarpour A, Oroojan AA, Khorsandi L, Kouchak M, Badavi M. Solid Lipid Nanoparticles of Myricitrin Have Antioxidant and Antidiabetic Effects on Streptozotocin-Nicotinamide-Induced Diabetic Model and Myotube Cell of Male Mouse. Oxid Med Cell Longev. 2018;2018:7496936.30116491
- Mohseni R, ArabSadeghabadi Z, Ziamajidi N, Abbasalipourkabir R, RezaeiFarimani A. Oral Administration of Resveratrol-Loaded Solid Lipid Nanoparticle Improves Insulin Resistance Through Targeting Expression of SNARE Proteins in Adipose and Muscle Tissue in Rats with Type 2 Diabetes. Nanoscale Res Lett. 2019;14(1):227.31290033
- Xue M, Yang MX, Zhang W, et al. Characterization, pharmacokinetics, and hypoglycemic effect of berberine loaded solid lipid nanoparticles. Int J Nanomedicine. 2013;8:4677–4687.24353417
- Xue M, Zhang L, Yang MX, et al. Berberine-loaded solid lipid nanoparticles are concentrated in the liver and ameliorate hepatosteatosis in db/db mice. Int J Nanomedicine. 2015;10:5049–5057.26346310
- Piazzini V, Micheli L, Luceri C, et al. Nanostructured lipid carriers for oral delivery of silymarin: improving its absorption and in vivo efficacy in type 2 diabetes and metabolic syndrome model. Int J Pharm. 2019;572:118838.31715362
- Piazzini V, Lemmi B, D’Ambrosio M, et al. Nanostructured Lipid Carriers as Promising Delivery Systems for Plant Extracts: the Case of Silymarin. Applied Sciences. 2018;8:7.
- Shi F, Wei Z, Zhao Y, Xu X. Nanostructured Lipid Carriers Loaded with Baicalin: an Efficient Carrier for Enhanced Antidiabetic Effects. Pharmacogn Mag. 2016;12(47):198–202.27601850
- Amjadi S, Mesgari Abbasi M, Shokouhi B, Ghorbani M, Hamishehkar H. Enhancement of therapeutic efficacy of betanin for diabetes treatment by liposomal nanocarriers. Journal of Functional Foods. 2019;59:119–128.
- Pk S. P S, A J, M C, A B. Anti-Diabetic Activity of Lycopene Niosomes: experimental Observation. J Pharm Drug Dev. 2017;4:1.
- Alam MS, Ahad A, Abidin L, Aqil M, Mir SR, Mujeeb M. Embelin-loaded oral niosomes ameliorate streptozotocin-induced diabetes in Wistar rats. Biomed Pharmacother. 2018;97:1514–1520.29793314
- Singhal T, Mujeeb M, Ahad A, et al. Preparation, optimization and biological evaluation of gymnemic acid loaded niosomes against streptozotocin-nicotinamide induced diabetic-nephropathy in Wistar rats. J Drug Deliv Sci Technol. 2019;54.
- Yu F, Li Y, Chen Q, et al. Monodisperse microparticles loaded with the self-assembled berberine-phospholipid complex-based phytosomes for improving oral bioavailability and enhancing hypoglycemic efficiency. Eur J Pharm Biopharm. 2016;103:136–148. doi:10.1016/j.ejpb.2016.03.01927020531
- Zhang J, Zhou J, Zhang T, et al. Facile Fabrication of an Amentoflavone-Loaded Micelle System for Oral Delivery To Improve Bioavailability and Hypoglycemic Effects in KKAy Mice. ACS Appl Mater Interfaces. 2019;11(13):12904–12913. doi:10.1021/acsami.9b0327530860811
- Singh J, Mittal P, Vasant Bonde G, Ajmal G, Mishra B. Design, optimization, characterization and in-vivo evaluation of Quercetin enveloped Soluplus®/P407 micelles in diabetes treatment. Artif Cells Nanomed Biotechnol. 2018;46(sup3):S546–S555. doi:10.1080/21691401.2018.150137930322273
- El-Far YM, Zakaria MM, Gabr MM, El Gayar AM, El-Sherbiny IM, Eissa LA. A newly developed silymarin nanoformulation as a potential antidiabetic agent in experimental diabetes. Nanomedicine. 2016;11(19):2581–2602. doi:10.2217/nnm-2016-020427623396
- El-Far YM, Zakaria MM, Gabr MM, El Gayar AM, Eissa LA, El-Sherbiny IM. Nanoformulated natural therapeutics for management of streptozotocin-induced diabetes: potential use of curcumin nanoformulation. Nanomedicine. 2017;12(14):1689–1711. doi:10.2217/nnm-2017-010628635562
- Akbar MU, Zia KM, Akash MSH, Nazir A, Zuber M, Ibrahim M. In-vivo anti-diabetic and wound healing potential of chitosan/alginate/maltodextrin/pluronic-based mixed polymeric micelles: curcumin therapeutic potential. Int J Biol Macromol. 2018;120(Pt B):2418–2430. doi:10.1016/j.ijbiomac.2018.09.01030195611
- Hussein J, Attia MF, El Bana M, et al. Solid state synthesis of docosahexaenoic acid-loaded zinc oxide nanoparticles as a potential antidiabetic agent in rats. Int J Biol Macromol. 2019;140:1305–1314. doi:10.1016/j.ijbiomac.2019.08.20131449866
- Liu Y, Zeng S, Liu Y, et al. Synthesis and antidiabetic activity of selenium nanoparticles in the presence of polysaccharides from Catathelasma ventricosum. Int J Biol Macromol. 2018;114:632–639. doi:10.1016/j.ijbiomac.2018.03.16129601883
- Yin J, Hou Y, Yin Y, Song X. Selenium-coated nanostructured lipid carriers used for oral delivery of berberine to accomplish a synergic hypoglycemic effect. Int J Nanomedicine. 2017;12:8671–8680. doi:10.2147/IJN.S14461529263662
- Huang P-K, Lin S-X, Tsai M-J, et al. Encapsulation of 16-Hydroxycleroda-3,13-Dine-16,15-Olide in Mesoporous Silica Nanoparticles as a Natural Dipeptidyl Peptidase-4 Inhibitor Potentiated Hypoglycemia in Diabetic Mice. Nanomaterials. 2017;7(5):5. doi:10.3390/nano7050112
- Gouda W, Hafiz NA, Mageed L, et al. Effects of nano-curcumin on gene expression of insulin and insulin receptor. Bulletin Nat Res Centre. 2019;43(1):1. doi:10.1186/s42269-019-0164-0
- Abu-Taweel GM, Attia MF, Hussein J, et al. Curcumin nanoparticles have potential antioxidant effect and restore tetrahydrobiopterin levels in experimental diabetes. Biomed Pharm. 2020;131.
- Singh AK, Pandey H, Ramteke PW, Mishra SB. Nano-suspension of ursolic acid for improving oral bioavailability and attenuation of type II diabetes: A histopathological investigation. Biocatalysis and Agricultural Biotechnology. 2019;2:22.
- Wang Z, Wu J, Zhou Q, Wang Y, Chen T. Berberine nanosuspension enhances hypoglycemic efficacy on streptozotocin induced diabetic C57BL/6 mice. Evid Based Complement Alternat Med. 2015;2015:239749.25866534
- Ravichandran R. Studies on Gymnemic Acids Nanoparticulate Formulations Against Diabetes Mellitus. Int J Biomed Clin Eng. 2012;1(2):1–12. doi:10.4018/ijbce.2012070101
- Zhao X, Wang W, Zu Y, et al. Preparation and characterization of betulin nanoparticles for oral hypoglycemic drug by antisolvent precipitation. Drug Deliv. 2014;21(6):467–479. doi:10.3109/10717544.2014.88143824479653
- Banik BL, Fattahi P, Brown JL. Polymeric nanoparticles: the future of nanomedicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8(2):271–299. doi:10.1002/wnan.136426314803
- Katuwavila NP, Perera ADLC, Samarakoon SR, et al. Chitosan-Alginate Nanoparticle System Efficiently Delivers Doxorubicin to MCF-7 Cells. Journal of Nanomaterials. 2016;2016:1–12.
- Jao D, Xue Y, Medina J, Protein-Based Drug-Delivery HX. Materials. Materials (Basel. 2017;10:5.
- Fonte P, Araujo F, Silva C, et al. Polymer-based nanoparticles for oral insulin delivery: revisited approaches. Biotechnol Adv. 2015;33(6):1342–1354. doi:10.1016/j.biotechadv.2015.02.01025728065
- Sami El-banna F, Mahfouz ME, Leporatti S, El-Kemary M, Hanafy AN. N. Chitosan as a Natural Copolymer with Unique Properties for the Development of Hydrogels. Applied Sciences. 2019;9(11):11. doi:10.3390/app9112193
- Rizeq BR, Younes NN, Rasool K, Nasrallah GK. Synthesis, Bioapplications, and Toxicity Evaluation of Chitosan-Based Nanoparticles. Int J Mol Sci. 2019;20(22):22. doi:10.3390/ijms20225776
- George A, Shah PA, Shrivastav PS. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int J Pharm. 2019;561:244–264. doi:10.1016/j.ijpharm.2019.03.01130851391
- Chauhan P, Tamrakar AK, Mahajan S, Prasad G. Chitosan encapsulated nanocurcumin induces GLUT-4 translocation and exhibits enhanced anti-hyperglycemic function. Life Sci. 2018;213:226–235. doi:10.1016/j.lfs.2018.10.02730343126
- Sonia TA, Sharma CP. An overview of natural polymers for oral insulin delivery. Drug Discov Today. 2012;17(13–14):784–792. doi:10.1016/j.drudis.2012.03.01922521664
- Karlsson J, Vaughan HJ, Green JJ. Biodegradable Polymeric Nanoparticles for Therapeutic Cancer Treatments. Annu Rev Chem Biomol Eng. 2018;9(1):105–127. doi:10.1146/annurev-chembioeng-060817-08405529579402
- Wang W, Meng Q, Li Q, et al. Chitosan Derivatives and Their Application in Biomedicine. Int J Mol Sci. 2020;21:2.
- Zhu W, Zhang Z. Preparation and characterization of catechin-grafted chitosan with antioxidant and antidiabetic potential. Int J Biol Macromol. 2014;70:150–155. doi:10.1016/j.ijbiomac.2014.06.04724995632
- Muzzarelli RAA, Chitosan Chemistry MC. Relevance to the Biomedical Sciences. In: Polysaccharides I. 2005;2:151–209.
- Severino P, da Silva CF, Andrade LN, de Lima Oliveira D, Campos J, Souto EB. Alginate Nanoparticles for Drug Delivery and Targeting. Curr Pharm Des. 2019;25(11):1312–1334.31465282
- Bassas-Galia M, Follonier S, Pusnik M, Zinn M. 2 - Natural polymers: A source of inspiration In: Perale G, Hilborn J, editors. Bioresorbable Polymers for Biomedical Applications. Woodhead Publishing; 2017:31–64.
- Mandaogade PM, Satturwar PM, Fulzele SV, Gogte BB, Dorle AK. Rosin derivatives: novel film forming materials for controlled drug delivery. Reactive and Functional Polymers. 2002;50(3):233–242. doi:10.1016/S1381-5148(01)00117-1
- Rani R, Dahiya S, Dhingra D, et al. <p>Antidiabetic activity enhancement in streptozotocin + nicotinamide–induced diabetic rats through combinational polymeric nanoformulation. Int J Nanomedicine. 2019;14:4383–4395. doi:10.2147/IJN.S20531931354267
- Fan Z, Cheng P, Liu M, et al. Dynamic crosslinked and injectable biohydrogels as extracellular matrix mimics for the delivery of antibiotics and 3D cell culture. RSC Advances. 2020;10(33):19587–19599. doi:10.1039/D0RA02218G
- Hamid Akash MS, Rehman K, Chen CS. Natural and Synthetic Polymers as Drug Carriers for Delivery of Therapeutic Proteins. Polymer Rev. 2015;55(3):371–406. doi:10.1080/15583724.2014.995806
- Kapoor R, Singh S, Tripathi M, Bhatnagar P, Kakkar P, Gupta KC. O-hexadecyl-dextran entrapped berberine nanoparticles abrogate high glucose stress induced apoptosis in primary rat hepatocytes. PLoS One. 2014;9(2):e89124. doi:10.1371/journal.pone.008912424586539
- Kamaly N, Yameen B, Wu J, Farokhzad OC. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: mechanisms of Controlling Drug Release. Chem Rev. 2016;116(4):2602–2663. doi:10.1021/acs.chemrev.5b0034626854975
- Angelova N, Hunkeler D. Rationalizing the design of polymeric biomaterials. Trends in Biotechnology. 1999;17(10):409–421. doi:10.1016/S0167-7799(99)01356-610481173
- Obayemi JD, Jusu SM, Salifu AA, et al. Degradable porous drug-loaded polymer scaffolds for localized cancer drug delivery and breast cell/tissue growth. Mater Sci Eng C Mater Biol Appl. 2020;112:110794. doi:10.1016/j.msec.2020.11079432409024
- Washington KE, Kularatne RN, Karmegam V, Biewer MC, Stefan MC. Recent advances in aliphatic polyesters for drug delivery applications. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(4):4. doi:10.1002/wnan.1446
- Mir M, Ahmed N, Rehman AU. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf B Biointerfaces. 2017;159:217–231. doi:10.1016/j.colsurfb.2017.07.03828797972
- Samadder A, Abraham SK, Khuda-Bukhsh AR. Nanopharmaceutical approach using pelargonidin towards enhancement of efficacy for prevention of alloxan-induced DNA damage in L6 cells via activation of PARP and p53. Environ Toxicol Pharmacol. 2016;43:27–37. doi:10.1016/j.etap.2016.02.01026943895
- Torché A-M, Jouan H, Le Corre P, et al. Ex vivo and in situ PLGA microspheres uptake by pig ileal Peyer’s patch segment. Int J Pharm. 2000;201(1):15–27. doi:10.1016/S0378-5173(00)00364-110867261
- Ismail R, Bocsik A, Katona G, Grof I, Deli MA, Csoka I. Encapsulation in Polymeric Nanoparticles Enhances the Enzymatic Stability and the Permeability of the GLP-1 Analog, Liraglutide, Across a Culture Model of Intestinal Permeability. Pharmaceutics. 2019;11(11):11. doi:10.3390/pharmaceutics11110599
- Nagarajan V, Mohanty AK, Misra M. Perspective on Polylactic Acid (PLA) based Sustainable Materials for Durable Applications: focus on Toughness and Heat Resistance. ACS Sustainable Chemistry & Engineering. 2016;4(6):2899–2916. doi:10.1021/acssuschemeng.6b00321
- Vroman I, Tighzert L. Biodegradable Polymers. Materials. 2009;2(2):307–344. doi:10.3390/ma2020307
- Barwal I, Sood A, Sharma M, Singh B, Yadav SC. Development of stevioside Pluronic-F-68 copolymer based PLA-nanoparticles as an antidiabetic nanomedicine. Colloids Surf B Biointerfaces. 2013;101:510–516. doi:10.1016/j.colsurfb.2012.07.00523022553
- Ponsart S, Coudane J, Vert M. A novel route to poly(ε-caprolactone)-based copolymers via anionic derivatization. Biomacromolecules. 2000;1(2):275–281. doi:10.1021/bm005521t11710111
- Weissenbock A, Wirth M, Gabor F. Pluronic® F-68 Enhances the Nanoparticle-Cell Interaction. J Control Release. 2004;99(3):383–392.15451596
- Barwal I, Yadav YS. Rebaudioside A Loaded Poly-D, L-Lactide-Nanoparticles as an Anti-Diabetic Nanomedicine. Journal of Bionanoscience. 2014;8(2):137–140. doi:10.1166/jbns.2014.1212
- Gholipour Kanani A, Bahrami SH. Effect of Changing Solvents on Poly(-Caprolactone) Nanofibrous Webs Morphology. Journal of Nanomaterials. 2011;2011:1–10. doi:10.1155/2011/72415321808638
- Kamaraj N, Rajaguru PY, Issac PK, Sundaresan S. Fabrication, characterization, in vitro drug release and glucose uptake activity of 14-deoxy, 11, 12-didehydroandrographolide loaded polycaprolactone nanoparticles. Asian J Pharm Sci. 2017;12(4):353–362. doi:10.1016/j.ajps.2017.02.00332104346
- Chen W, Hou Y, Tu Z, Gao L, Haag R. pH-degradable PVA-based nanogels via photo-crosslinking of thermo-preinduced nanoaggregates for controlled drug delivery. Journal of Controlled Release. 2017;259:160–167. doi:10.1016/j.jconrel.2016.10.03227810557
- Sharma P, Sen D, Neelakantan V, Shankar V, Jhunjhunwala S. Disparate effects of PEG or albumin based surface modification on the uptake of nano- and micro-particles. Biomater Sci. 2019;7(4):1411–1421. doi:10.1039/C8BM01545G30663741
- Masood F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater Sci Eng C Mater Biol Appl. 2016;60:569–578. doi:10.1016/j.msec.2015.11.06726706565
- Ahlawat J, Henriquez G, Narayan M. Enhancing the Delivery of Chemotherapeutics: role of Biodegradable Polymeric Nanoparticles. Molecules. 2018;23(9):9. doi:10.3390/molecules23092157
- Sechi M, Syed DN, Pala N, et al. Nanoencapsulation of dietary flavonoid fisetin: formulation and in vitro antioxidant and α-glucosidase inhibition activities. Mater Sci Eng C Mater Biol Appl. 2016;68:594–602. doi:10.1016/j.msec.2016.06.04227524059
- Sanna V, Siddiqui IA, Sechi M, Mukhtar H. Resveratrol-Loaded Nanoparticles Based on Poly(epsilon-caprolactone) and Poly(d, l-lactic-glycolic acid)–Poly(ethylene glycol) Blend for Prostate Cancer Treatment. Mol Pharm. 2013;10(10):3871–3881. doi:10.1021/mp400342f23968375
- Kong L, Campbell F, Kros A. DePEGylation strategies to increase cancer nanomedicine efficacy. Nanoscale Horiz. 2019;4(2):378–387. doi:10.1039/C8NH00417J32254090
- Carbone JP, Reinert KH. Synthetic Polymers In: Reference Module in Earth Systems and Environmental Sciences. Elsevier; 2015.
- Davatgaran-Taghipour Y, Masoomzadeh S, Farzaei MH, et al. Polyphenol nanoformulations for cancer therapy: experimental evidence and clinical perspective. Int J Nanomedicine. 2017;12:2689–2702. doi:10.2147/IJN.S13197328435252
- Cerpnjak K, Zvonar A, Gasperlin M, Vrecer F. Lipid-based systems as a promising approach for enhancing the bioavailability of poorly water-soluble drugs. Acta Pharm. 2013;63(4):427–445. doi:10.2478/acph-2013-004024451070
- Talegaonkar S, Bhattacharyya A. Potential of Lipid Nanoparticles (SLNs and NLCs) in Enhancing Oral Bioavailability of Drugs with Poor Intestinal Permeability. AAPS PharmSciTech. 2019;20(3):121. doi:10.1208/s12249-019-1337-830805893
- Jia JL. Nanoparticle Formulation Increases Oral Bioavailability of Poorly Soluble Drugs: approaches, Experimental Evidences and Theory. Curr Nanosci. 2005;1(3):237–243. doi:10.2174/15734130577464293919865587
- Gordillo-Galeano A, Mora-Huertas CE. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. Eur J Pharm Biopharm. 2018;133:285–308. doi:10.1016/j.ejpb.2018.10.01730463794
- Sanchez-Lopez E, Guerra M, Dias-Ferreira J, et al. Current Applications of Nanoemulsions in Cancer Therapeutics. Nanomaterials. 2019;9(6):6. doi:10.3390/nano9060821
- Pavoni L, Perinelli DR, Bonacucina G, Cespi M, Palmieri GF. An Overview of Micro- and Nanoemulsions as Vehicles for Essential Oils: formulation, Preparation and Stability. Nanomaterials. 2020;10(1):1. doi:10.3390/nano10010135
- Salvia-Trujillo L, Soliva-Fortuny R, Rojas-Grau MA, McClements DJ, Martín-Belloso M-BO. Edible Nanoemulsions as Carriers of Active Ingredients: A Review. Annu Rev Food Sci Technol. 2017;8(1):439–466. doi:10.1146/annurev-food-030216-02590828125342
- Jiang X-C, Gao J-Q. Exosomes as novel bio-carriers for gene and drug delivery. Int J Pharm. 2017;521(1–2):167–175. doi:10.1016/j.ijpharm.2017.02.03828216464
- Singh Y, Meher JG, Raval K, et al. Nanoemulsion: concepts, development and applications in drug delivery. J Control Release. 2017;252:28–49. doi:10.1016/j.jconrel.2017.03.00828279798
- Trotta F, Dianzani C, Caldera F, Mognetti B, Cavalli R. The application of nanosponges to cancer drug delivery. Expert Opin Drug Deliv. 2014;11(6):931–941. doi:10.1517/17425247.2014.91172924811423
- Krabicova I, Appleton SL, Tannous M, et al. History of Cyclodextrin Nanosponges. Polymers (Basel. 2020;12(5):5. doi:10.3390/polym12051122
- Pawar S, Shende P, Trotta F. Diversity of β-cyclodextrin-based nanosponges for transformation of actives. Int J Pharm. 2019;565:333–350. doi:10.1016/j.ijpharm.2019.05.01531082468
- Karthik P, Ezhilarasi PN, Anandharamakrishnan C. Challenges associated in stability of food grade nanoemulsions. Crit Rev Food Sci Nutr. 2017;57(7):1435–1450. doi:10.1080/10408398.2015.100676726114624
- Rehman FU, Shah KU, Shah SU, Khan IU, Khan GM, Khan A. From nanoemulsions to self-nanoemulsions, with recent advances in self-nanoemulsifying drug delivery systems (SNEDDS). Expert Opin Drug Deliv. 2017;14(11):1325–1340. doi:10.1080/17425247.2016.121846227485144
- Gupta A, Eral HB, Hatton TA, Doyle PS. Nanoemulsions: formation, properties and applications. Soft Matter. 2016;12(11):2826–2841. doi:10.1039/C5SM02958A26924445
- Tayeb HH, Sainsbury F. Nanoemulsions in drug delivery: formulation to medical application. Nanomedicine. 2018;13(19):2507–2525. doi:10.2217/nnm-2018-008830265218
- Dokania S, Joshi AK. Self-microemulsifying drug delivery system (SMEDDS) – challenges and road ahead. Drug Deliv. 2015;22(6):675–690. doi:10.3109/10717544.2014.89605824670091
- Alghananim A, Ozalp Y, Mesut B, Serakinci N, Ozsoy Y, Gungor S. A Solid Ultra Fine Self-Nanoemulsifying Drug Delivery System (S-SNEDDS) of Deferasirox for Improved Solubility: optimization, Characterization, and In Vitro Cytotoxicity Studies. Pharmaceuticals. 2020;13(8):8. doi:10.3390/ph13080162
- Date AA, Desai N, Dixit R, Nagarsenker M. Self-nanoemulsifying drug delivery systems: formulation insights, applications and advances. Nanomedicine. 2010;5(10):1595–1616. doi:10.2217/nnm.10.12621143036
- Date AA, Nagarsenker MS. Design and evaluation of self-nanoemulsifying drug delivery systems (SNEDDS) for cefpodoxime proxetil. Int J Pharm. 2007;329(1–2):166–172. doi:10.1016/j.ijpharm.2006.08.03817010543
- Cherniakov I, Domb AJ, Hoffman A. Self-nano-emulsifying drug delivery systems: an update of the biopharmaceutical aspects. Expert Opin Drug Deliv. 2015;12(7):1121–1133. doi:10.1517/17425247.2015.99903825556987
- Chatterjee B, Hamed Almurisi S, Ahmed Mahdi Dukhan A, Mandal UK, Sengupta P. Ahmed Mahdi Dukhan A, Mandal UK, Sengupta P. Controversies with self-emulsifying drug delivery system from pharmacokinetic point of view. Drug Deliv. 2016;23(9):3639–3652. doi:10.1080/10717544.2016.121499027685505
- Kumar R, Khursheed R, Kumar R, et al. Self-nanoemulsifying drug delivery system of fisetin: formulation, optimization, characterization and cytotoxicity assessment. J Drug Deliv Sci Technol. 2019;54.
- Kumar B, Garg V, Singh S, et al. Impact of spray drying over conventional surface adsorption technique for improvement in micromeritic and biopharmaceutical characteristics of self-nanoemulsifying powder loaded with two lipophilic as well as gastrointestinal labile drugs. Powder Technology. 2018;326:425–442. doi:10.1016/j.powtec.2017.12.005
- Khursheed R, Singh SK, Wadhwa S, et al. Exploring role of probiotics and Ganoderma lucidum extract powder as solid carriers to solidify liquid self-nanoemulsifying delivery systems loaded with curcumin. Carbohydr Polym. 2020;250:116996. doi:10.1016/j.carbpol.2020.11699633049905
- Sadegh Malvajerd S, Azadi A, Izadi Z, et al. Brain Delivery of Curcumin Using Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: preparation, Optimization, and Pharmacokinetic Evaluation. ACS Chem Neurosci. 2019;10(1):728–739. doi:10.1021/acschemneuro.8b0051030335941
- Garces A, Amaral MH, Sousa Lobo JM, Silva AC. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: A review. Eur J Pharm Sci. 2018;112:159–167. doi:10.1016/j.ejps.2017.11.02329183800
- Ghasemiyeh P, Mohammadi-Samani S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res Pharm Sci. 2018;13(4):288–303. doi:10.4103/1735-5362.23515630065762
- Tapeinos C, Battaglini M, Ciofani G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J Control Release. 2017;264:306–332. doi:10.1016/j.jconrel.2017.08.03328844756
- Khosa A, Reddi S, Saha RN. Nanostructured lipid carriers for site-specific drug delivery. Biomed Pharmacother. 2018;103:598–613. doi:10.1016/j.biopha.2018.04.05529677547
- Soussan E, Cassel S, Blanzat M, Rico-Lattes I. Drug delivery by soft matter: matrix and vesicular carriers. Angew Chem Int Ed Engl. 2009;48(2):274–288. doi:10.1002/anie.20080245319072808
- Elizondo E, Moreno E, Cabrera I, et al. Liposomes and other vesicular systems: structural characteristics, methods of preparation, and use in nanomedicine. Prog Mol Biol Transl Sci. 2011;104:1–52.22093216
- Kapoor B, Gupta R, Singh SK, Gulati M, Singh S. Prodrugs, phospholipids and vesicular delivery - An effective triumvirate of pharmacosomes. Adv Colloid Interface Sci. 2018;253:35–65. doi:10.1016/j.cis.2018.01.00329454464
- Kapoor B, Gupta R, Gulati M, Singh SK, Khursheed R, Gupta M. The Why, Where, Who, How, and What of the vesicular delivery systems. Adv Colloid Interface Sci. 2019;271:101985. doi:10.1016/j.cis.2019.07.00631351415
- Aloulou A, Ali YB, Bezzine S, Gargouri Y, Gelb MH. Phospholipases: an overview. Methods Mol Biol. 2012;861:63–85.22426712
- Li T, Cipolla D, Rades T, Boyd BJ. Drug nanocrystallisation within liposomes. J Control Release. 2018;288:96–110. doi:10.1016/j.jconrel.2018.09.00130184465
- Gnananath K, Sri Nataraj K, Ganga Rao B. Phospholipid Complex Technique for Superior Bioavailability of Phytoconstituents. Adv Pharm Bull. 2017;7(1):35–42.28507935
- Li M, Du C, Guo N, et al. Composition design and medical application of liposomes. Eur J Med Chem. 2019;164:640–653. doi:10.1016/j.ejmech.2019.01.00730640028
- Yucel Ç, Karatoprak GŞ, Aktas Y. Nanoliposomal Resveratrol as a Novel Approach to Treatment of Diabetes Mellitus. J Nanosci Nanotechnol. 2018;18(6):3856–3864. doi:10.1166/jnn.2018.1524729442719
- Asprea M, Tatini F, Piazzini V, Rossi F, Bergonzi MC, Bilia AR. Stable, Monodisperse, and Highly Cell-Permeating Nanocochleates from Natural Soy Lecithin Liposomes. Pharmaceutics. 2019;11(1):1. doi:10.3390/pharmaceutics11010034
- Zhong X, Chen B, Yang Z. Nanocochleates as the Potential Delivery Systems for Oral Antitumor of Hydroxycamptothecin. J Biomed Nanotechnol. 2018;14(7):1339–1346. doi:10.1166/jbn.2018.257229944107
- Bhosale RR, Ghodake PP, Mane AN, Ghadge AA. Nanocochleates: A novel carrier for drug transfer. J surg. 2013;2(5):964–969.
- Bothiraja C, Yojana BD, Pawar AP, Shaikh KS, Thorat UH. Fisetin-loaded nanocochleates: formulation, characterisation, in anticancer testing, bioavailability and biodistribution study. Expert Opin Drug Deliv. 2014;11(1):17–29. doi:10.1517/17425247.2013.86013124294925
- Shende P, Khair R, Gaud RS. Nanostructured cochleates: a multi-layered platform for cellular transportation of therapeutics. Drug Dev Ind Pharm. 2019;45(6):869–881. doi:10.1080/03639045.2019.158375730767577
- Ç YÜCEL, GŞ KARATOPRAK, Atmar A. Novel Resveratrol-Loaded Nanocochleates and Effectiveness in the Treatment of Diabetes. Fabad Journal of Pharmaceutical Sciences. 2018;43(2):35–44.
- Bozo T, Wacha A, Mihaly J, Bota A, Kellermayer MSZ. Dispersion and stabilization of cochleate nanoparticles. Eur J Pharm Biopharm. 2017;117:270–275. doi:10.1016/j.ejpb.2017.04.03028461084
- Mahale NB, Thakkar PD, Mali RG, Walunj DR, Chaudhari SR. Niosomes: novel sustained release nonionic stable vesicular systems — an overview. Adv Colloid Interface Sci. 2012;183-184:46–54. doi:10.1016/j.cis.2012.08.00222947187
- Pk S, P PS. Novel Encapsulation of Lycopene in Niosomes and Assessment of its Anticancer Activity. Journal of Bioequivalence & Bioavailability. 2016;8(5):5. doi:10.4172/jbb.1000300
- Ghanbarzadeh B, Babazadeh A, Hamishehkar H. Nano-phytosome as a potential food-grade delivery system. Food Bioscience. 2016;15:126–135. doi:10.1016/j.fbio.2016.07.006
- Lu M, Qiu Q, Luo X, et al. Phyto-phospholipid complexes (phytosomes): A novel strategy to improve the bioavailability of active constituents. Asian J Pharm Sci. 2019;14(3):265–274. doi:10.1016/j.ajps.2018.05.01132104457
- Mirzaei H, Shakeri A, Rashidi B, Jalili A, Banikazemi Z, Sahebkar A. Phytosomal curcumin: A review of pharmacokinetic, experimental and clinical studies. Biomed Pharmacother. 2017;85:102–112. doi:10.1016/j.biopha.2016.11.09827930973
- Babazadeh A, Zeinali M, Hamishehkar HH. Nano-Phytosome: A Developing Platform for Herbal Anti-Cancer Agents in Cancer Therapy. Curr Drug Targets. 2018;19(2):170–180. doi:10.2174/138945011866617050809525028482783
- Kim S-M, Jung J-I, Chai C, Imm J-Y. Characteristics and Glucose Uptake Promoting Effect of Chrysin-Loaded Phytosomes Prepared with Different Phospholipid Matrices. Nutrients. 2019;11(10):10. doi:10.3390/nu11102549
- Torchilin VP, Lukyanov AN, Gao Z, Papahadjopoulos-Sternberg B. Immunomicelles: targeted pharmaceutical carriers for poorly soluble drugs. Proc Natl Acad Sci U S A. 2003;100(10):6039–6044. doi:10.1073/pnas.093142810012716967
- Simoes SM, Figueiras AR, Veiga F, Concheiro A, Alvarez-Lorenzo C. Polymeric micelles for oral drug administration enabling locoregional and systemic treatments. Expert Opin Drug Deliv. 2015;12(2):297–318. doi:10.1517/17425247.2015.96084125227130
- Reddy BP, Yadav HKS, Nagesha DK, Raizaday A, Karim A. Polymeric Micelles as Novel Carriers for Poorly Soluble Drugs— review. J Nanosci Nanotechnol. 2015;15(6):4009–4018. doi:10.1166/jnn.2015.971326369007
- Cho H, Lai TC, Tomoda K, Kwon GS. Polymeric micelles for multi-drug delivery in cancer. AAPS PharmSciTech. 2015;16(1):10–20. doi:10.1208/s12249-014-0251-325501872
- Han J, Oh J, Ihm S-H S-H, Lee M. Peptide micelle-mediated curcumin delivery for protection of islet β-cells under hypoxia. Journal of Drug Targeting. 2016;24(7):618–623. doi:10.3109/1061186X.2015.113222026768151
- Tousif Ayyub K, Moravkar K, Maniruzzaman M, Amin P. Effect of melt extrudability and melt binding efficiency of polyvinyl caprolactam polyvinyl acetate polyethylene glycol graft copolymer (Soluplus®) on release pattern of hydrophilic and high dose drugs. Mater Sci Eng C Mater Biol Appl. 2019;99:563–574. doi:10.1016/j.msec.2019.01.12630889730
- Li Z-L, Peng S-F, Chen X, et al. Pluronics modified liposomes for curcumin encapsulation: sustained release, stability and bioaccessibility. Food Res Int. 2018;108:246–253. doi:10.1016/j.foodres.2018.03.04829735054
- Batrakova EV, Kabanov AV. Pluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J Control Release. 2008;130(2):98–106. doi:10.1016/j.jconrel.2008.04.01318534704
- Lu Y, Yue Z, Xie J, et al. Micelles with ultralow critical micelle concentration as carriers for drug delivery. Nat Biomed Eng. 2018;2(5):318–325. doi:10.1038/s41551-018-0234-x30936455
- Fernandez-Pineiro I, Badiola I, Sanchez A. Nanocarriers for microRNA delivery in cancer medicine. Biotechnol Adv. 2017;35(3):350–360. doi:10.1016/j.biotechadv.2017.03.00228286148
- Sabio RM, Meneguin AB, Ribeiro TC, Silva RR, Chorilli M. New insights towards mesoporous silica nanoparticles as a technological platform for chemotherapeutic drugs delivery. Int J Pharm. 2019;564:379–409. doi:10.1016/j.ijpharm.2019.04.06731028801
- Ahmed HH, Abd El-Maksoud MD, Abdel Moneim AE, Aglan HA. Pre-Clinical Study for the Antidiabetic Potential of Selenium Nanoparticles. Biol Trace Elem Res. 2017;177(2):267–280. doi:10.1007/s12011-016-0876-z27785741
- Al-Quraishy S, Dkhil MA, Abdel Moneim AE. Anti-hyperglycemic activity of selenium nanoparticles in streptozotocin-induced diabetic rats. Int J Nanomedicine. 2015;10:6741–6756.26604749
- Uma Suganya KS, Govindaraju K, Veena Vani C, Premanathan M, Ganesh Kumar VK. In vitro biological evaluation of anti-diabetic activity of organic–inorganic hybrid gold nanoparticles. IET Nanobiotechnol. 2019;13(2):226–229. doi:10.1049/iet-nbt.2018.513931051455
- Hussein JS, Rasheed W, Ramzy T, et al. Synthesis of docosahexaenoic acid–loaded silver nanoparticles for improving endothelial dysfunctions in experimental diabetes. Hum Exp Toxicol. 2019;38(8):962–973. doi:10.1177/096032711984358631018711
- Hussein J, El-Naggar ME, Latif YA, et al. Solvent-free and one-pot synthesis of silver and zinc oxide nanoparticles: activity toward cell membrane component and insulin signaling pathway in experimental diabetes. Colloids and Surfaces B: Biointerfaces. 2018;170:76–84. doi:10.1016/j.colsurfb.2018.05.05829883845
- Alkaladi A, Abdelazim AM, Afifi M. Antidiabetic activity of zinc oxide and silver nanoparticles on streptozotocin-induced diabetic rats. Int J Mol Sci. 2014;15(2):2015–2023. doi:10.3390/ijms1502201524477262
- Lushchak O, Zayachkivska A, Vaiserman A. Metallic Nanoantioxidants as Potential Therapeutics for Type 2 Diabetes: A Hypothetical Background and Translational Perspectives. Oxid Med Cell Longev. 2018;2018:3407375. doi:10.1155/2018/340737530050652
- Hanan NA, Chiu HI, Ramachandran MR, et al. Cytotoxicity of Plant-Mediated Synthesis of Metallic Nanoparticles: A Systematic Review. Int J Mol Sci. 2018;19(6):6. doi:10.3390/ijms19061725
- Anand K, Tiloke C, Naidoo P, Chuturgoon AA. Phytonanotherapy for management of diabetes using green synthesis nanoparticles. J Photochem Photobiol B. 2017;173:626–639. doi:10.1016/j.jphotobiol.2017.06.02828709077
- Rajarajeshwari T, Shivashri C, Rajasekar P. Synthesis and characterization of biocompatible gymnemic acid–gold nanoparticles: a study on glucose uptake stimulatory effect in 3T3-L1 adipocytes. RSC Adv. 2014;4(108):63285–63295. doi:10.1039/C4RA07087A
- Chockalingam S, Thada R, Dhandapani RK, Panchamoorthy R. Biogenesis, characterization, and the effect of vicenin-gold nanoparticles on glucose utilization in 3T3-L1 adipocytes: a bioinformatic approach to illuminate its interaction with PTP 1B and AMPK. Biotechnology Progress. 2015;31(4):1096–1106. doi:10.1002/btpr.211226014104
- Shamprasad BR, Keerthana S, Megarajan S, Lotha R, Aravind S, Veerappan A. Photosynthesized escin stabilized gold nanoparticles exhibit antidiabetic activity in L6 rat skeletal muscle cells. Materials Letters. 2019;241:198–201. doi:10.1016/j.matlet.2019.01.086
- Khaleel Basha S, Govindaraju K, Manikandan R, Ahn JS, Bae EY, Singaravelu G. Phytochemical mediated gold nanoparticles and their PTP 1B inhibitory activity. Colloids Surf B Biointerfaces. 2010;75(2):405–409.19815393
- Ranjous Y, Regdon G, Pintye-Hodi K, Sovany T. Standpoint on the priority of TNTs and CNTs as targeted drug delivery systems. Drug Discov Today. 2019;24(9):1704–1709. doi:10.1016/j.drudis.2019.05.01931158513
- Wong BS, Yoong SL, Jagusiak A, et al. Carbon nanotubes for delivery of small molecule drugs. Adv Drug Deliv Rev. 2013;65(15):1964–2015.23954402
- Martincic M, Tobias G. Filled carbon nanotubes in biomedical imaging and drug delivery. Expert Opin Drug Deliv. 2015;12(4):563–581.25430876
- Ilie I, Ilie R, Mocan T, Tabaran F, Iancu C, Mocan L. Nicotinamide-functionalized multiwalled carbon nanotubes increase insulin production in pancreatic beta cells via MIF pathway. Int J Nanomedicine. 2013;8:3345–3353.24039418
- Li Z, Zhang Y, Feng N. Mesoporous silica nanoparticles: synthesis, classification, drug loading, pharmacokinetics, biocompatibility, and application in drug delivery. Expert Opin Drug Deliv. 2019;16(3):219–237.30686075
- Yang G, Phua SZF, Bindra AK, Degradability ZY. Clearance of Inorganic Nanoparticles for Biomedical Applications. Adv Mater. 2019;31(10):e1805730.30614561
- Croissant JG, Fatieiev Y, Almalik A, Khashab NM, Silica M. Organosilica Nanoparticles: physical Chemistry, Biosafety, Delivery Strategies, and Biomedical Applications. Adv Healthc Mater. 2018;7:4.
- Mohammadpour R, Dobrovolskaia MA, Cheney DL, Greish KF, Ghandehari H. Subchronic and chronic toxicity evaluation of inorganic nanoparticles for delivery applications. Adv Drug Deliv Rev. 2019;144:112–132.31295521
- Lai F, Schlich M, Pireddu R, Fadda AM, Sinico C. Nanocrystals as Effective Delivery Systems of Poorly Water-soluble Natural Molecules. Curr Med Chem. 2019;26(24):4657–4680.30543163
- Liversidge GG, Cundy KC. Particle size reduction for improvement of oral bioavailability of hydrophobic drugs: I. Absolute oral bioavailability of nanocrystalline danazol in beagle dogs. Int J Pharm. 1995;125(1):91–97.
- Chen C, Fu X. Spheroidization on Fructus Mori polysaccharides to enhance bioavailability and bioactivity by anti-solvent precipitation method. Food Chem. 2019;300:125245.31352287
- Wang Y, Zheng Y, Zhang L, Wang Q, Zhang D. Stability of nanosuspensions in drug delivery. J Control Release. 2013;172(3):1126–1141.23954372
- Wang L, Du J, Zhou Y, Wang Y. Safety of nanosuspensions in drug delivery. Nanomedicine. 2017;13(2):455–469.27558350
- Gao L, Liu G, Ma J, Wang X, Zhou L, Li X. Drug nanocrystals: in vivo performances. Journal of Controlled Release. 2012;160(3):418–430.22465393
- Souto EB, Souto SB, Campos JR, et al. Nanoparticle Delivery Systems in the Treatment of Diabetes Complications. Molecules. 2019;24:23.
- Rho JG, Han HS, Han JH, et al. Self-assembled hyaluronic acid nanoparticles: implications as a nanomedicine for treatment of type 2 diabetes. J Control Release. 2018;279:89–98.29649530
- Rahimi HR, Mohammadpour AH, Dastani M, et al. The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: a randomized clinical trial. Avicenna Journal of Phytomedicine. 2016;6(5):567–577.27761427
- Asadi S, Gholami MS, Siassi F, Qorbani M, Khamoshian K, Sotoudeh G. Nano curcumin supplementation reduced the severity of diabetic sensorimotor polyneuropathy in patients with type 2 diabetes mellitus: A randomized double-blind placebo- controlled clinical trial. Complement Ther Med. 2019;43:253–260.30935539
- Veiseh O, Tang BC, Whitehead KA, Anderson DG, Langer R. Managing diabetes with nanomedicine: challenges and opportunities. Nat Rev Drug Discov. 2015;14(1):45–57.25430866