Abstract
To date, various nanodrug systems have been developed for different routes of administration, which include dendrimers, nanocrystals, emulsions, liposomes, solid lipid nanoparticles, micelles, and polymeric nanoparticles. Nanodrug systems have been employed to improve the efficacy, safety, physicochemical properties, and pharmacokinetic/pharmacodynamic profile of pharmaceutical substances. In particular, functionalized nanodrug systems can offer enhanced bioavailability of orally taken drugs, prolonged half-life of injected drugs (by reducing immunogenicity), and targeted delivery to specific tissues. Thus, nanodrug systems might lower the frequency of administration while providing maximized pharmacological effects and minimized systemic side effects, possibly leading to better therapeutic compliance and clinical outcomes. In spite of these attractive pharmacokinetic advantages, recent attention has been drawn to the toxic potential of nanodrugs since they often exhibit in vitro and in vivo cytotoxicity, oxidative stress, inflammation, and genotoxicity. A better understanding of the pharmacokinetic and safety characteristics of nanodrugs and the limitations of each delivery option is necessary for the further development of efficacious nanodrugs with high therapeutic potential and a wide safety margin. This review highlights the recent progress in nanodrug system development, with a focus on the pharmacokinetic advantages and safety challenges.
Introduction
Recently, considerable attention has been directed toward nanoscience, and a number of efforts have been made for the development and commercial applications of new nanotechnology in both academic and industrial institutions.Citation1–Citation3 As defined by the Royal Society and Royal Academy of Engineering, “nanoscience” is the study of phenomena and manipulation of materials at atomic, molecular, and macromolecular scales, where the properties differ significantly from those at a larger scale; and “nanotechnologies” are the design, characterization, production, and application of structures, devices, and systems by controlling shape and size at the nanometer scale.Citation4 The growing fields of nanoscience and nanotechnology have transformed many sectors of industry, with breakthrough applications in the areas of biotechnology, electronic, cosmetics, food sciences, and pharmaceutics. In particular, strategic application of nanotechnologies to pharmaceutical research and development has led to the successful development of nanodrugs, described as drug delivery systems developed to operate at the nanometer size range with novel engineered properties that provide medical benefits in the clinical treatment of several diseases.Citation1
Research into the rational delivery and targeting of pharmaceutical, therapeutic, and diagnostic agents is at the forefront of projects in nanodrugs. The early efforts in nanodrugs were focused on improving the molecular properties of already available therapeutic and diagnostic agents, but more recently, nanotechnology proponents have attempted to apply new therapeutic and diagnostic modalities for improving the developability. The major targets in the development of nanodrugs are 1) specific drug targeting and delivery; 2) greater safety and biocompatibility; 3) faster development of new medicine with a wide safety margin; and 4) improved pharmacokinetic behavior.Citation5 Theoretically, nanodrugs can easily pass through the fine capillary blood vessels and the lymphatic endothelium, and they might have longer circulation times in the blood and/or higher binding capability and accumulation at some target sites.Citation6 In particular, nanotechnologies have been used to develop site-specific drug targeting, for the treatment of brain diseases.Citation7 Nanodrugs might also produce less inflammatory and immune response in tissues compared with drugs of larger size.Citation8 In spite of these attractive characteristics, nanodrugs sometimes induce oxidative stress, genetic damage, and the inhibition of cell division and cell death, depending on their physicochemical characteristics (such as particle surface, size, and chemical composition).Citation9 Nanotoxicology is emerging as an important subdiscipline of nanoscience and nanotechnology because of the finding of increasing toxic effects of nanodrugs and nanomaterials on living organisms. However, the toxicology of nanoparticles is poorly understood as there are no sufficient methods to assess their safety. A better understanding of nanotoxicity and its mechanisms would be of great help to develop new nanodrugs with a wide safety margin. In this article, we review recent advances in nanodrug development and the pharmacokinetic/safety characteristics of nanodrugs.
General pharmacokinetic characteristics of nanodrugs
A number of efforts have been made in the development of nanodrugs over the past few decades, and the concept of nanodrugs has evolved considerably. There are various types of nanodrug systems, most designed with the drug encapsulated in a carrier (eg, dendrimers, liposomes, micelles, and polymeric nanoparticles). A nanodrug system can offer several pharmacokinetic advantages, such as specific drug delivery, high metabolic stability, high membrane permeability, improved bioavailability, and long duration of action (). Therefore, by altering the biopharmaceutic and pharmacokinetic properties of new drug candidates, nanodrug systems could be a promising approach to obtain the drug properties. The physicochemical properties of nanodrugs, such as size, surface charge, and hydrophobicity, affect their mucosal absorption characteristics, and smaller nanodrugs show higher transcellular uptake via follicle-associated epithelia than do larger ones.Citation3,Citation10–Citation12 Nanoparticles can enter cells via endocytotic processes, including caveolar-and clathrin-mediated endocytosis, potocytosis, pinocytosis, and patocytosis.Citation13 In contrast, larger particles can be quickly opsonized and removed from the bloodstream via the macrophages of the reticuloendothelial system (RES). In the formulation design of nanoparticles, it is necessary to minimize opsonization and prolong the circulation of nanoparticles in clinical use. This can be achieved by the surface coating of nanoparticles with hydrophilic polymers/surfactants, and/or the formulation of nanoparticles with biodegradable copolymers with hydrophilic segments, such as polyethylene glycol (PEG), polyethylene oxide, poloxamer, poloxamine, and polysorbate 80 (Tween 80).Citation14 Nanodrugs with a positive surface charge can interact with the negative charges of mucin owing to abundant sulfate sialic acid and sugar moieties, resulting in enhanced transportation across mucus and increased internalization by epithelial cells.Citation10 Strategic functionalization with some membrane permeation enhancers or ligands for receptors expressed on the cellular membrane may also promote the transcellular transport of entrapped drugs.Citation11,Citation12,Citation15 In addition to transcellular transport, nanodrugs equipped with bioadhesive polymers or chelators could enhance the paracellular transport of entrapped drugs, via the regulation of tight junctions.Citation16, Citation17 Surface modification of nanodrugs, with specific proteins, antibodies, and other biomolecules, can be used to design drugs that act selectively on particular tissues,Citation18 and this approach has been employed to provide improved therapeutic potential and reduced side effects of some anticancer drugs. In general, drug delivery systems employing nanotechnologies are designed to be administered via injection, transdermally, or orally, although recent studies have demonstrated the promising outcomes with pulmonary administration of nanodrug systems.Citation19,Citation20 Inhaled particles undergo pulmonary clearance, such as mucociliary clearance and macrophage clearance, leading to a limited duration of action. However, nanoparticles have been praised for their advantageous drug delivery properties to the lung, including their avoidance of mucociliary and macrophage clearance and long residence times before degradation or translocation by epithelial cells takes place.Citation21,Citation22 Thus, the nanodrug approach should enhance the therapeutic potential of entrapped drugs and contribute to the acceleration of pharmaceutical development.
Table 1 Targeted delivery of nanoparticles
General safety characteristics of nanodrugs
Despite attractive functions and a bright future outlook for nanodrugs, there is increasing concern over their safety. Knowledge of the toxic effects of nanodrugs is limited but is rapidly growing. Nanoparticles are expected to be able to diminish the toxicity of chemotherapy drugs or other drugs with a narrow therapeutic index; however, a number of in vitro and in vivo studies have shown that some nanoparticles demonstrated toxicity in biological systems, causing cytotoxicity, an allergic response, or inflammation.Citation23 Nanoparticles tend to produce reactive oxygen species (ROS) and free radicals, resulting in oxidative stress, inflammatory events, deoxyribonucleic acid (DNA) damage, multinucleus formation, and fibrosis.Citation13 Another toxicity concern associated with nanodrugs is their accumulation within cells, particularly with continuous exposure or long-term use. The upper size limit for the toxicity of nanoparticles is not fully clarified; however, it has been thought to lie between 65 nm and 200 nm.Citation24 Nanoparticle toxicity is extremely complex and multifactorial and depends on physicochemical properties, such as size, shape, and surface properties (charge, area, and reactivity). The size effect is likely to be more important for nanoparticle toxicity than the actual composition of the nanodrugs. The particle surface area can also be a better predictor of the toxic and pathological responses to nanoparticles than the particle mass dose. Although some nanodrugs were functionalized with cationic polymers, cationic formulations have been described to affect cell proliferation, differentiation, and proapoptotic genes, in human epithelial cells.Citation13 The polycationic nature of these formulations sometimes induces both necrosis and apoptosis; therefore, in the design of drug carriers, issues of safety, toxicity, and availability have to be taken into account. The surface reactivity of nanoparticles can cause chemical damage to surrounding tissues. When inhaled, micron-sized particles tend to deposit in the central airways; however, inhaled nanoparticles can deposit in the lung periphery, causing much greater inflammation.Citation25 In particular, needle-shaped carbon nanotubes, nanowires, and nanofibers might cause fibrotic lung disease and rare tumors, such as mesothelioma.Citation26 The interaction of nanoparticles with skin also has received significant attention recently because of the increasing use of nanoparticles in stain-resistant clothing, cosmetics, and sunscreens. The dermal penetration of nanoparticles can be dependent on the physicochemical properties of the nanoparticles and skin condition. Limited in vivo studies have been conducted to address the issue of cutaneous nanotoxicity,Citation25 and they demonstrated minimal irritancy potential and no evidence of irritation or allergic response for some nanoparticles. In contrast to these benign findings, many nanoparticles have been found to be cytotoxic and proinflammatory to dermal cell lines in vitro;Citation23 therefore, additional in vivo studies of chronic dosing of nanodrugs would be needed for further clarification of nanotoxicity. Few studies have examined the systemic toxicity of nanoparticles, and most of these have been acute-toxicity studies investigating the 50% lethal dose (LD50) values of tested nanoparticles. Since the nanoparticles are commonly taken up by the RES, many of the target organs have been thought to be members of RES, such as the liver and spleen.Citation27 However, nanoparticles used in biomedical applications are commonly coated with biocompatible materials to reduce opsonization and avoid the RES uptake, so the target organs for biomedical nanoparticles might be shifting away from the RES. The toxic potential of nanodrugs would be variable depending on administration routes, so an improved understanding of the risk factors related to nanodrugs and nanomaterials in the human body will aid the further development and exploitation of a variety of nanodrugs.
Dendrimers
Dendrimers, highly branched macromolecules with a specific size and shape, are a class of carriers of nanodrugs, in which the hydrophobic core and hydrophilic periphery exhibit micelle-like performance along with drug loading properties, in solution.Citation28 Drug payloads can be either entrapped within the dendrimer scaffold via the generation of noncovalent complexes or linked to the dendrimer surface via covalent conjugation, so dendrimers can incorporate a lower amount of drugs than other carriers.Citation29 Covalently constructed dendritic macromolecules have the advantage of more specific control over drug release and may be designed to limit drug release in the systemic circulation and to trigger release under tumor-specific conditions ().Citation30 Dendritic polymers have recently been shown to improve the delivery of doxorubicin and other cytotoxic drugs to solid tumors and to reduce their accumulation in noncancerous tissues.Citation31 Dendrimers have also been well proven as a tool in the solubilization of poorly soluble drugs, and poly(amidoamine) dendrimers and other polymeric dendrimers have been applied to flurbiprofen,Citation32 methotrexate,Citation33,Citation34 and piroxicamCitation35 for solubilization and targeted delivery. In particular, lactoferrin-conjugated dendritic nanocomposite exhibited an enhanced residence time in the systemic circulation and high lung delivery, possibly leading to reduced dosing frequency as well as nominal dose.Citation18 Other target selectivity has also been demonstrated via the conjugation of targeting ligands, such as folate, arginylglycylaspartic acid (RGD) peptides, epidermal growth factor, vascular endothelial growth factor (VEGF), and monoclonal antibodies, to the dendrimer surface ().Citation36 In spite of their attractive functions, most dendrimers demonstrate toxic and hemolytic activity because of their positively charged surface.Citation37 However, anionic dendrimers and modified dendrimers with masking of the peripheral cationic group can exhibit diminished hemolytic activity. Thus, surface engineering of dendrimers should lead to improvement of their pharmacokinetic and safety properties, in the context of biomedical applications.
Table 2 Nanodrugs and their biopharmaceutical characteristics
Table 3 Biopharmaceutical and safety characteristics of nanodrugs
Engineered nanoparticles (nanosized particles)
A particle size reduction approach is widely used to increase the dissolution rate, since the dissolution rate of a drug proportionally increases with increasing surface area of drug particles.Citation38 As defined in the Prandtl boundary layer equation, the decrease of diffusion layer thickness brought by reducing particle size, particularly down to <5 μm, would result in accelerated dissolution. Based on this, drug nanocrystal technology has been the highlight in the pharmaceutical field, and the approaches developed to produce drug nanosuspensions mainly include the so-called “bottom-up” (controlled precipitation) and “top-down” types (wet-milling with beads, and high-pressure homogenization).Citation39,Citation40 In both bottom-up and top-down approaches, hydrophilic polymer and/or surfactant are typically used to stabilize a nanosuspension. The nanoparticles of drug are dispersed into inert carriers after a drying process, such as spray drying or lyophilization, and the resulting solidified nanocrystal formulations can be defined as nanocrystalline solid dispersions.
There have been numerous studies demonstrating the enhanced oral bioavailability and pharmacological effects of pharmaceuticals and neutraceuticals obtained via nanotechnologies ().Citation41–Citation52 In the nanosized formulation approach, maximum concentration (Cmax) and bioavailability were increased up to dozens of folds compared with conventional formulations with micrometer particle size. Interestingly, the neutral or acidic compounds, such as danazol,Citation44 cilostazol,Citation42 tranilast,Citation49 and curcuminCitation43 have shown better improvements in the pharmacokinetic parameters than have the basic compounds, via nanocrystal technologies. Recently, a lower-dose diclofenac submicron particle capsule was developed with the use of SoluMatrix™ technology (iCeutica, Philadelphia, PA, USA), and in 2013, the US Food and Drug Administration (FDA) approved this nanodrug for treatment of mild to moderate acute pain in adults.Citation51 In the Phase I study, the oral nanoformulated diclofenac (35 mg) demonstrated faster absorption and similar Cmax compared with diclofenac at higher dose (50 mg), in healthy subjects, and it also provided effective analgesia in the Phase III clinical study, in patients with acute pain.Citation53 The SoluMatrix™ technology was also applied to indomethacin, and a Phase I study demonstrated that the oral nanoformulated indomethacin exhibited a more rapid time to maximal concentration (Tmax) (1.1 hours) compared with indomethacin (2.0 hours), possibly leading to more rapid onset of action.Citation52 The Cmax for nanoformulated indomethacin (40 mg) was found to be slightly higher compared with standard oral indomethacin (50 mg) in healthy subjects (3,115 ng/mL vs 2,759 ng/mL, respectively). Thus, nanoformulated systems can lower the dose of drugs, thus improving their safety and tolerability, while maintaining their effectiveness. In general, neutral and acidic drugs would be poorly soluble in gastric fluid, and the improved dissolution behavior of these chemicals under acidic conditions via nanotechnologies could lead to marked enhancement in their oral bioavailability. Nanoparticles generally exhibit mucoadhesion to biological mucosa, and the mucoadhesion effect also plays an important role in the enhancement of oral bioavailability. The transcellular transport of nanosized particles through the endothelial cells of the small intestine via endocytosis has also been believed to be one of the major mechanisms for the improved oral absorption, and the transcellular uptake could be influenced by several factors, such as particle size and surface charge ().Citation16 The mechanisms for enhanced oral absorption can be mainly summarized as follows: 1) improved dissolution behavior; 2) bioadhesion to the intestinal wall; and 3) transcellular uptake. In addition to the enhanced dissolution and bioavailability, nanocrystals have provided further pharmaceutical benefits, including reproducibility of oral absorption, improved dose-bioavailability, proportionality, and increased patient compliance as a result of the reduction of the number of oral units to be taken.Citation54 Theoretically, as well as for oral dosing, nanocrystal formulations can be employed for dermal, ocular, and pulmonary drug delivery. An inhalable nanocrystalline solid dispersion of tranilast was previously prepared by wet-milling technology and exhibited high inhalation performance and improved anti-inflammatory effects, in a rat model of airway inflammation, compared with crystalline tranilast with a larger particle size.Citation50
Recently, there has been increasing concern about the potential nanotoxicity of nanosized particles, and the particles of major toxicological concern are those below 100 nm.Citation54 Although larger nanometer particles (>200 nm) can only be internalized by macrophages, causing effects inside those cells, smaller nanoparticles (with a diameter of 150 nm or much smaller) can be internalized by any cell via pinocytosis. In this context, small nanoparticles can access any cell of the body, possibly resulting in a higher cytotoxic potential. Orally taken nanocrystals could cause pharmacokinetic transition, with higher Cmax and shorter Tmax, and the higher and more rapid systemic exposure of drugs might lead to some adverse effects. In contrast, it was shown that the gastric irritancy of oral nonsteroidal anti-inflammatory drugs (NSAIDs) could be decreased via nanocrystal technologies because the nanocrystals achieved distribution uniformity in the gastrointestinal fluid without high and prolonged local concentration.Citation39
Lipid nanosystems
Lipid nanosystems, including emulsions, liposomes, and solid lipid nanoparticles, have been studied intensively to improve the biopharmaceutical properties and/or therapeutic index of drugs.Citation1 With respect to the safety concerns over lipid nanosystems, lipid-based colloidal carriers are believed to be well tolerated in living systems since they are usually made of physiological compounds and, therefore, metabolism should decrease the risk of acute and chronic toxicity.Citation55 Nevertheless, for the development of solid lipid nanoparticle emulsions with a wide safety margin, careful consideration should be taken of the potential toxicity of the emulsifiers.
Emulsions
In recent years, self-emulsifying drug delivery systems (SEDDSs) have been utilized to enhance the oral bioavailability of poorly water-soluble drugs, especially of highly lipophilic drugs.Citation56–Citation60 SEDDSs are isotropic mixtures of oil, surfactant, cosolvent, and solubilized drug, and the SEDDS approach requires fine dissolution and chemical stability of the drugs in the oil phase. These formulations can rapidly form oil in water (O/W) fine emulsions, when dispersed in the aqueous phase under mild agitation. SEDDSs are additionally classified as either self-microemulsification drug delivery systems (SMEDDSs) or self-nanoemulsification drug delivery systems (SNEDDSs), according to the size range of their oil droplets.Citation38 SMEDDSs form microemulsions ranging in droplet size from 100 to 250 nm, and finer nanoemulsions, with a diameter of less than 100 nm, can be obtained using SNEDDSs. The SEDDS approach has been thought to be suitable for the Biopharmaceutics Classification System (BCS)Citation38 class II drugs, the characteristics of which are low solubility and high permeability. Generally, the bioavailability of a BCS class II drug is rate-limited by its dissolution so that even a small increase in dissolution rate sometimes results in a large increase in bioavailability. Therefore, the rapid emulsification of these formulations in the gastrointestinal tract can provide both improved oral bioavailability and a reproducible plasma concentration profile (). The droplet size of the emulsion could influence the bioavailability of orally administered drugs. For instance, two SEDDSs formulations of cyclosporin A (Sandimmune® [Novartis Pharmaceuticals Corp, Basel, Switzerland], a coarse SMEDDS formulation, and Neoral® [Novartis], a fine SNEDDS formulation) are available on the market, and Neoral® is more rapidly and consistently absorbed than Sandimmune®, in humans. The strategic application of emulsion approaches to poorly soluble drugs could result in the rapid increase of systemic exposure, which might show unwanted side effects if the drugs have low therapeutic index (). To overcome this limitation, sustained-release SEDDS would be a viable dosage option to modulate high peak plasma concentrations of the administered drugs.Citation16 In addition to the oral dosage form, an inhalable dry emulsion of cyclosporin A was proposed for the treatment of asthma, chronic obstructive pulmonary disease (COPD), and allograft rejection after pulmonary transplantation, and the insufflated dry emulsions showed higher potency than did cyclosporin A particles, in a rat model of acute airway inflammation.Citation19
Liposomes
Liposomes, a type of microcapsule, enclose liquid compartments with a multilamellar structure consisting of lipid bilayers. A liposomal formulation can be prepared by the dehydration–rehydration method, the reverse-phase evaporation vesicle method, and by the proliposome method. Several pharmacokinetic challenges have been pointed out for conventional liposomes, including nonspecific uptake, within a few minutes to a few hours, by the RES; rapid clearance; and opsonization.Citation1 These pharmacokinetic properties of liposomes depend on their physicochemical characteristics, such as size, surface charge, membrane lipid packing, steric stabilization, dose, and route of administration. A number of efforts have been made to overcome these drawbacks, and recent studies demonstrated that liposomes coated or grafted with hydrophilic polymers were efficacious for attenuating the opsonization of the liposomes and rapid clearance. Considerable attention has been drawn to PEG-modified liposomes since they have exhibited an increased systemic half-life for the encapsulated drug, based on significant reduction in nonspecific RES uptake.Citation61 PEG-modified liposomes also have advantages in terms of passive targeting to tumors.Citation3 Tumor vasculature is well characterized by a chaotic network of thin-walled, leaky vessels, so liposomes have the ability to cross into the interstitial spaces in viable tumor areas, with limited washout. This process is referred to as the enhanced permeability and retention (EPR) effect, and small PEG-modified liposomes with a diameter of 100–200 nm can permeate through the tumor vasculature, eventually leading to their accumulation in tumor tissue (). As a result of basic research in both academia and industry, liposomes have been widely used as pharmaceutical carriers in the past decade because of their attractive biopharmaceutical properties: 1) high encapsulation efficiency for both hydrophilic and hydrophobic therapeutic agents; 2) protection of encapsulated drugs from undesired effects of external conditions; 3) functionalization upon conjugation with specific ligands, for the targeting specific cells, tissues, and organs of interest; 4) prolonged systemic circulation with the use of inert and biocompatible polymers; and 5) controllable size and surface charge ().Citation29,Citation61–Citation65 Currently, a number of liposomal formulations have obtained approval for the treatment of cancer, infections, and meningitis, including amphotericin B (Abelcet®; Sigma-Tau Pharmaceuticals, Inc., Gaithersburg, MD, USA) and doxorubicin (Doxil® [Janssen Pharmaceuticals, Inc., Titusville, NJ, USA] and Myocet® [Enzon Pharmaceuticals, Piscataway, NJ, USA]). Doxil® is the first FDA-approved nanodrug, pharmacokinetic characteristics of which are 1) prolonged drug circulation time and 2) avoidance of RES uptake, due to the use of PEGylated nanoliposomes.Citation66 In humans, the area under the concentration-time curve (AUC) of plasma doxorubicin after the intravenous (IV) administration of Doxil® (50 mg/m2) is ~300-fold greater than that with free drug at same dose, and clearance and volume of distribution are drastically reduced, by at least 250-and 60-fold, respectively.Citation66 CPX-351 (liposome-encapsulated cytarabine and daunorubicin) is currently under clinical development for the treatment of leukemia.Citation67 Following IV administration of CPX-351 in patients with advanced leukemia, the clearance of cytarabine and daunorubicin was found to be less than 120 mL/h/m2 across all the dose levels (24–134 units/m2), which was markedly less than the clearance rates for unencapsulated daunorubicin (38,600 mL/h/m2) and cytarabine (134,000 mL/h/m2). The very low rate of clearance might be attributed to the apparent lack of a distribution phase for the encapsulated drugs. In addition to the therapeutic agents, liposomal delivery has been applied to imaging techniques, such as magnetic resonance imaging (MRI), positron or single-photon emission (computed) tomography (PET/SPECT), and fluorescence, at the forefront of medical diagnostics in preclinical and clinical settings, for the assessment of treatment efficacy.
Solid lipid nanoparticles
Solid lipid nanoparticles are described as colloidal nanoparticles of highly purified triglycerides, complex glyceride mixtures, monoglycerides, hard fats, or waxes stabilized by a surfactant and fabricated via a high-pressure homogenization and nanoemulsion technique. Solid lipid nanoparticles have recently emerged as an alternative to liposomal formulations, owing to various advantages: 1) improved physical stability; 2) modulated release of the loaded drugs; 3) relative low cost compared with phospholipids used for liposomes; and 4) easy scale-up and manufacturing (). In contrast, solid lipid nanoparticles have some disadvantages: 1) drug expulsion after recrystallization; 2) limited loading capacity, depending on the solubility of drugs in the oil phase; and 3) relatively high water content of the dispersions.Citation16
A variety of pharmaceutical substances, such as small molecules, peptides, and proteins, can be applied to solid lipid nanoparticle systems with the aim of improving pharmacokinetic behavior, and many different routes are available for the administration of solid lipid nanoparticles, unlike for liposomes ().Citation68–Citation72 There are major challenges for the oral delivery of therapeutic peptides/proteins – to overcome the gastrointestinal barriers and protect the structure in the gastrointestinal tract; despite this, promising results in the oral delivery of insulin have been achieved with the use of the solid lipid nanoparticle approach.Citation71 Solid lipid nanoparticles might thus be a promising approach for the formulation of other therapeutic peptides and proteins. As observed with the liposomal formulations, IV-administered solid lipid nanoparticles of drug also exhibited longer systemic circulation, due to decreased clearanceCitation69 and higher accumulation in the tissues,Citation68 compared with that exhibited by the drug itself.
Micelles
Polymeric micelles are spherical nanostructures formed by supermolecular assembly of amphiphilic copolymers in aqueous environments, normally as a consequence of ion pair or hydrophobic interaction. Micellar nanoparticles have received considerable attention in contemporary drug delivery research since micellar formulations can achieve the protection of internal drugs from degradation, solubility enhancement, and target-specific delivery.Citation1,Citation73–Citation77 Hydrophobic drugs tend to be entrapped in the semisolid core of micelles, and the core–shell structure can mimic the naturally occurring transport system. Therefore, micellar nanoparticles can improve the absorption and distribution of internal drugs and also, avoid opsonization and phagocytic clearance by RES uptake.Citation78
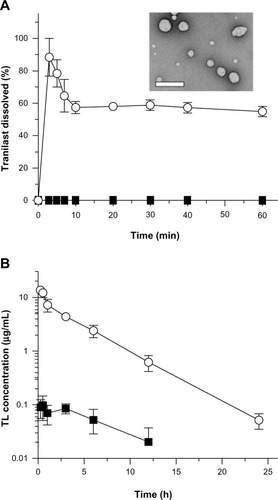
Micellar nanoparticles can be a viable alternative to liposomal formulations, in terms of passive and active targeting of the disease site in the body. After the IV administration of micelles, their tissue distribution and clearance has tended to be highly altered compared with those of free drugs, possibly resulting in better clinical outcomes ().Citation73–Citation75 The micellar nanoparticles can also be applied to liquid eye drops, to attenuate the rapid elimination of the drugs from the precorneal area, offering longer duration of action.Citation76 Recently, Onoue et al developed a self-micellizing solid dispersion of poorly water-soluble drugs, with the use of an amphiphilic block copolymer of 2-methacryloyloxyethyl phosphorylcholine (MPC) unit and a n-butyl methacrylate (BMA) unit ([poly(MPC-co-BMA)]).Citation77 The new solid dispersion system, tranilast, showed significant improvement in dissolution behavior and rapid formation of micelles with a diameter of 100–150 nm, and there appeared to be accelerated absorption of tranilast, with ~50-fold enhancement of oral bioavailability in rats (). Clinical development of several micellar nanoparticles is ongoing, with the aim of improving the pharmacokinetic behavior and reducing the potential side-effects of anticancer drugs.Citation79 NK105, a polymeric micellar nanoparticle formulation of paclitaxel, is currently under clinical trial in the patients with gastric cancer and breast cancer.Citation75 In a Phase I study, the AUC of NK105 at 150 mg/m2 (recommended Phase II dose) was ~15-fold larger than that of the conventional paclitaxel formulation at the dose of 210 mg/m2 (clinical dose for a 3-week regimen in Japanese patients). The volume of distribution and the clearance of NK105 were significantly lower than those of the conventional formulation, while the hematological and nonhematological toxicities of NK105 were mild and well manageable. Although the micellar nanoparticles have been thought to be a safe delivery system, there are some safety concerns, including possible side effects after rapid elevation of systemic exposure, and toxicity of the surfactant used. In particular, prior to clinical use, the safety of newly developed micellizing agents has to be checked carefully with respect to chronic dosing.
Figure 1 Biopharmaceutical characteristics of self-micellizing solid dispersions.
Notes: (A) Dissolution profiles of tranilast formulations in acidic solution (pH 1.2). ■, crystalline tranilast; ○, self-micellizing solid dispersion. Data represent mean ± SE of three independent experiments. Transmission electron microscopic image (inset) shows the self-micellizing solid dispersion redispersed in distilled water. Bar represents 500 nm. (B) Systemic exposure of tranilast after oral administration of tranilast formulations in rats. ■, crystalline tranilast (10 mg/kg); ○, self-micellizing solid dispersion (10 mg-tranilast/kg). Data represent mean ± SE of four to six experiments. Reprinted from Onoue S, Kojo Y, Suzuki H, et al. Development of novel solid dispersion of tranilast using amphiphilic block copolymer for improved oral bioavailability. Int J Pharm. 452(1–2):220–226.77 © 2013 with permission from Elsevier.
Abbreviations: h, hours, min, minutes, SE, standard error; TL, tranilast.
Polymeric nanoparticles
Polymeric nanoparticles can be defined as solid particles with a size in the range of 10–1,000 nm; they allow encapsulation of the drugs inside a polymeric matrix, protecting them from enzymatic and hydrolytic degradation.Citation80 The polymeric nanoparticles can be prepared by several classical methods, including nanoprecipitation, emulsion–diffusion, double emulsification, emulsion–coacervation, and polymer-coating. Polymeric nanoparticles show some advantages with respect to other drug delivery systems for several types of pharmaceutical substance (small molecules, peptides, proteins and small interfering ribonucleic acid [siRNA]) (),Citation20,Citation81–Citation84 which include 1) high stability during storage; 2) controlled release; 3) multiple available routes of administration; and 4) prolonged duration of action. Once the polymeric nanoparticles reach the target tissues, the drug may be released by desorption, diffusion through the polymer matrix or polymer wall, or nanoparticle erosion. To obviate the need for surgical retrieval of the exhausted depot, clearance of the dosage from the injection site requires the use of biodegradable polymers. Of the available biomaterials, poly(lactic-co-glycolic acid) (PLGA) is the most commonly used FDA-approved polymer for biodegradable and biocompatible controlled-release devices, with high versatility provided by the suitable selection of the polymer molecular weight, copolymerization, and functionalization.Citation20,Citation85–Citation88 The number of commercial polymeric nanoparticles employing biodegradable carriers is growing and is expected to continue to do so, in line with the promise of further peptide-, protein-, and DNA/ribonucleic acid (RNA)-based drugs emerging from the biotechnology sector. A new polymeric nanoparticle of docetaxel, targeting the extracellular domain of prostate-specific membrane antigen, was developed for the treatment of patients with solid tumors.Citation84 In the Phase I study, the plasma levels of docetaxel (30 mg/m2, IV) administered as polymeric nanoparticles of docetaxel were at least twofold higher than those of an equivalent dose of docetaxel solution, and the high plasma concentrations of docetaxel persisted for at least 48 hours. Albumin nanoparticle technology is particularly well adapted for applications with lipophilic drugs, and albumin-bound paclitaxel (Abraxane®; Celgene Corp, Summit, NJ, USA) was approved by the FDA for the treatment of metastatic breast cancer (2005) and non–small cell lung cancer (2012).Citation79 Abraxane® is obtained by high-pressure homogenization of paclitaxel in the presence of serum human albumin, devoid of any solvent excipients. In humans, Abraxane® has exhibited good pharmacokinetics linearity over the various doses tested, up to 300 mg/m2, and inter-/intrapatient variability in the pharmacokinetic parameters was low. The volume of distribution for Abraxane® was found to be markedly higher than that of free paclitaxel solution, thus suggesting a greater extravascular distribution of Abraxane®. Hydrogel nanoparticles of insulin were also developed, comprising cross-linked materials with the ability to absorb a large amount of water without dissolving,Citation83 and this new technology should allow the oral delivery of insulin and high clinical compliance. In general, polymeric nanoparticles exhibit low immunogenicity and low toxicity (). Polymeric nanoparticles are commonly coated with nonionic surfactants, and the presence of surfactants on the particle surface markedly reduces immunological interactions, such as opsonization, and also, the interactions between the surface chemical group of nanodrugs via van der Waals forces, hydrophobic interaction, or hydrogen bonding.Citation89
Conclusion and future outlook
In pharmaceutical research and development, the important biopharmaceutical characteristics of drug candidates can be listed as 1) solubility; 2) membrane permeability; 3) metabolic stability; and 4) systemic pharmacokinetics and pharmacodynamics; these factors would have major impact on the drugability and developability of new pharmaceutical products. Nanodrug approaches might resolve the biopharmaceutical problems related to imprecise control of drug release, poor stability, limited pharmacokinetic behavior, and toxicity of the active ingredient. In recent years, as much as ~70% of new drug candidates have shown poor aqueous solubility, and ~40% of marketed drugs for oral use are identified to be practically insoluble in aqueous media (<100 μg/mL).Citation38 Some nanodrug approaches have been found to be efficacious in improving the dissolution behavior of drugs with limited solubility (BCS class II/IV drugs), and current nanotechnologies and ongoing research should bring clinically useful nanodrug systems with improved pharmacokinetic profiles. Therefore, interest in nanodrugs has increased significantly in the last two decades, and several nanodrugs with reduced drug toxicity or enhanced drug efficacy have been successfully developed. In contrast, nanodrugs for targeted delivery are still under development. The suitable selection and further development of highly qualified targeting ligands, such as antibodies, peptides, or aptamers, might accelerate the development of the next generation of nanodrugs with high therapeutic potential. According to the report by Uchegbu and Siew,Citation90 there were 6,242 entries on clinical trials for nanodrugs, and over half of these clinical trials originated from USA. The vast majority of clinical trials are studying cancer patients (~72%), and the other biomedical applications are in infectious diseases (~6%), imaging (~2%), and dental composites (~0.2%). Currently, the nanodrug systems are believed to be feasible and promising in cancer therapy since the nanodrug systems theoretically allow targeting of the particles to increase the concentration of the drug at the site of interest, while reducing the systemic side effects. However, in addition to cancer, there are many serious diseases (diabetes, COPD, dementia, etc) that need to be addressed, and these might eventually be treated effectively via nanotechnology, upon further maturation of the technology platform.
In addition to the nanoparticles presented in this review article, carbon nanotubes have recently emerged as a new option for possible use in methodologies for cancer treatment, bioengineering, and gene therapy. In spite of such attractive features, the toxicity of carbon nanotubes is a prime concern. A deeper understanding of the toxic mechanisms and related physicochemical properties is needed, and carbon nanotubes have to be further developed to optimize the drug payload and reduce their potential toxicity. Via such efforts in academia and the pharmaceutical industry, carbon nanotubes might be a viable and safe option as a nanodrug carrier in the future.
We might still be far from the ultimate goal of the nanodrug approach; however, further development and/or strategic use of suitable nanotechnologies should provide a bright future in the treatment of several diseases, upon successful improvement in the safety, efficacy, and the quality of drugs.
Disclosure
The authors report no conflicts of interest in this work.
References
- DevalapallyHChakilamAAmijiMMRole of nanotechnology in pharmaceutical product developmentJ Pharm Sci200796102547256517688284
- NaahidiSJafariMEdalatFRaymondKKhademhosseiniAChenPBiocompatibility of engineered nanoparticles for drug deliveryJ Control Release2013166218219423262199
- PetrosRADeSimoneJMStrategies in the design of nanoparticles for therapeutic applicationsNat Rev Drug Discov20109861562720616808
- YangWPetersJIWilliamsROInhaled nanoparticles – a current reviewInt J Pharm20083561–223924718358652
- De JongWHBormPJDrug delivery and nanoparticles: applications and hazardsInt J Nanomedicine20083213314918686775
- WinKYFengSSEffects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugsBiomaterials200526152713272215585275
- KreuterJNanoparticulate systems for brain delivery of drugsAdv Drug Deliv Rev2001471658111251246
- LiuWYangXLHoWSPreparation of uniform-sized multiple emulsions and micro/nano particulates for drug delivery by membrane emulsificationJ Pharm Sci20111001759320589949
- NelAXiaTMädlerLLiNToxic potential of materials at the nanolevelScience2006311576162262716456071
- RogerELagarceFGarcionEBenoitJPBiopharmaceutical parameters to consider in order to alter the fate of nanocarriers after oral deliveryNanomedicine (Lond)20105228730620148639
- JainAJainSKIn vitro and cell uptake studies for targeting of ligand anchored nanoparticles for colon tumorsEur J Pharm Sci200835540441618824095
- FrancisMFCristeaMWinnikFMExploiting the vitamin B12 pathway to enhance oral drug delivery via polymeric micellesBiomacromolecules2005652462246716153081
- Vega-VillaKRTakemotoJKYáñezJARemsbergCMForrestMLDaviesNMClinical toxicities of nanocarrier systemsAdv Drug Deliv Rev200860892993818313790
- des RieuxAFievezVGarinotMSchneiderYJPréatVNanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approachJ Control Release2006116112717050027
- MoriSMatsuuraARama PrasadYVTakadaKStudies on the intestinal absorption of low molecular weight heparin using saturated fatty acids and their derivatives as an absorption enhancer in ratsBiol Pharm Bull200427341842114993814
- ShahbaziMASantosHAImproving oral absorption via drug-loaded nanocarriers: absorption mechanisms, intestinal models and rational fabricationCurr Drug Metab2013141285622497568
- ChuangEYLinKJSuFYCalcium depletion-mediated protease inhibition and apical-junctional-complex disassembly via an EGTA-conjugated carrier for oral insulin deliveryJ Control Release2013169329630523195534
- KozlowskaDForanPMacMahonPShellyMJEustaceSO’KennedyRMolecular and magnetic resonance imaging: The value of immunoliposomesAdv Drug Deliv Rev200961151402141119796661
- OnoueSSatoHOgawaKInhalable dry-emulsion formulation of cyclosporine A with improved anti-inflammatory effects in experimental asthma/COPD-model ratsEur J Pharm Biopharm2012801546022008148
- OnoueSMatsuiTKuriyamaKInhalable sustained-release formulation of long-acting vasoactive intestinal peptide derivative alleviates acute airway inflammationPeptides201235218218922484228
- MobleyCHochhausGMethods used to assess pulmonary deposition and absorption of drugsDrug Discov Today20016736737511267923
- BurMHenningAHeinSSchneiderMLehrCMInhalative nanomedicine – opportunities and challengesInhal Toxicol200921Suppl 1S137S143
- AiJBiazarEJafarpourMNanotoxicology and nanoparticle safety in biomedical designsInt J Nanomedicine201161117112721698080
- DrobneDNanotoxicology for safe and sustainable nanotechnologyArh Hig Rada Toksikol200758447147818063532
- ChoiHSFrangioniJVNanoparticles for biomedical imaging: fundamentals of clinical translationMol Imaging20109629131021084027
- WickPManserPLimbachLKThe degree and kind of agglomeration affect carbon nanotube cytotoxicityToxicol Lett20071682121131
- SternSTMcNeilSENanotechnology safety concerns revisitedToxicol Sci2008101142117602205
- LiuMFréchetJMJDesigning dendrimers for drug deliveryPharm Sci Technolo Today199921039340110498919
- HondaMAsaiTOkuNArakiYTanakaMEbiharaNLiposomes and nanotechnology in drug development: focus on ocular targetsInt J Nanomedicine2013849550323439842
- KaminskasLMMcLeodVMKellyBDA comparison of changes to doxorubicin pharmacokinetics, antitumor activity, and toxicity mediated by PEGylated dendrimer and PEGylated liposome drug delivery systemsNanomedicine20128110311121704192
- MaedaHSawaTKonnoTMechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCSJ Control Release2001741–3476111489482
- AsthanaAChauhanASDiwanPVJainNKPoly(amidoamine) (PAMAM) dendritic nanostructures for controlled site-specific delivery of acidic anti-inflammatory active ingredientAAPS PharmSciTech200563E536E54216354015
- KaminskasLMKellyBDMcLeodVMCapping methotrexate α-carboxyl groups enhances systemic exposure and retains the cytotoxicity of drug conjugated PEGylated polylysine dendrimersMol Pharm20118233834921171585
- KurmiBDGajbhiyeVKayatJJainNKLactoferrin-conjugated dendritic nanoconstructs for lung targeting of methotrexateJ Pharm Sci201110062311232021491447
- PrajapatiRNTekadeRKGuptaUGajbhiyeVJainNKDendimer-mediated solubilization, formulation development and in vitro-in vivo assessment of piroxicamMol Pharm20096394095019231841
- KaminskasLMPorterCJTargeting the lymphatics using dendritic polymers (dendrimers)Adv Drug Deliv Rev20116310–1189090021683746
- ZiembaBMatuszkoGBryszewskaMKlajnertBInfluence of dendrimers on red blood cellsCell Mol Biol Lett2012171213522086186
- KawabataYWadaKNakataniMYamadaSOnoueSFormulation design for poorly water-soluble drugs based on biopharmaceutics classification system: basic approaches and practical applicationsInt J Pharm2011420111021884771
- GaoLLiuGMaJWangXZhouLLiXDrug nanocrystals: In vivo performancesJ Control Release2012160341843022465393
- ChanHKKwokPCProduction methods for nanodrug particles using the bottom-up approachAdv Drug Deliv Rev201163640641621457742
- JiaLWongHWangYGarzaMWeitmanSDCarbendazim: disposition, cellular permeability, metabolite identification, and pharmacokinetic comparison with its nanoparticleJ Pharm Sci200392116117212486692
- JinnoJKamadaNMiyakeMEffect of particle size reduction on dissolution and oral absorption of a poorly water-soluble drug, cilostazol, in beagle dogsJ Control Release20061111–2566416410029
- OnoueSTakahashiHKawabataYFormulation design and photochemical studies on nanocrystal solid dispersion of curcumin with improved oral bioavailabilityJ Pharm Sci20109941871188119827133
- WuCYBenetLZPredicting drug disposition via application of BCS: transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification systemPharm Res2005221112315771225
- HanafyASpahn-LangguthHVergnaultGPharmacokinetic evaluation of oral fenofibrate nanosuspensions and SLN in comparison to conventional suspensions of micronized drugAdv Drug Deliv Rev200759641942617566595
- SylvestreJPTangMCFurtosALeclairGMeunierMLerouxJCNanonization of megestrol acetate by laser fragmentation in aqueous milieuJ Control Release2011149327328021047539
- XiaDCuiFPiaoHEffect of crystal size on the in vitro dissolution and oral absorption of nitrendipine in ratsPharm Res20102791965197620585842
- OnoueSNakamuraTUchidaAPhysicochemical and biopharmaceutical characterization of amorphous solid dispersion of nobiletin, a citrus polymethoxylated flavone, with improved hepatoprotective effectsEur J Pharm Sci201349445346023707470
- KawabataYYamamotoKDebariKOnoueSYamadaSNovel crystalline solid dispersion of tranilast with high photostability and improved oral bioavailabilityEur J Pharm Sci201039425626220038453
- OnoueSAokiYKawabataYDevelopment of inhalable nanocrystalline solid dispersion of tranilast for airway inflammatory diseasesJ Pharm Sci2011100262263320653048
- ManvelianGDanielsSGibofskyAThe pharmacokinetic parameters of a single dose of a novel nano-formulated, lower-dose oral diclofenacPostgrad Med2012124111712322314121
- ManvelianGDanielsSAltmanRA phase I study evaluating the pharmacokinetic profile of a novel, proprietary, nano-formulated, lower-dose oral indomethacinPostgrad Med2012124419720522913908
- GibofskyASilbersteinSArgoffCDanielsSJensenSYoungCLLower-dose diclofenac submicron particle capsules provide early and sustained acute patient pain relief in a phase 3 studyPostgrad Med2013125513013824113671
- ShegokarRMüllerRHNanocrystals: industrially feasible multifunctional formulation technology for poorly soluble activesInt J Pharm20103991–212913920674732
- MartinsSSarmentoBFerreiraDCSoutoEBLipid-based colloidal carriers for peptide and protein delivery – liposomes versus lipid nanoparticlesInt J Nanomedicine20072459560718203427
- LarsenATOhlssonAGPolentaruttiBOral bioavailability of cinnarizine in dogs: relation to SNEDDS droplet size, drug solubility and in vitro precipitationEur J Pharm Sci2013481–2339350
- OnoueSUchidaAKuriyamaKNovel solid self-emulsifying drug delivery system of coenzyme Q10 with improved photochemical and pharmacokinetic behaviorsEur J Pharm Sci201246549249922498005
- StrickleyRGSolubilizing excipients in oral and injectable formulationsPharm Res200421220123015032302
- ThomasNHolmRMüllertzARadesTIn vitro and in vivo performance of novel supersaturated self-nanoemulsifying drug delivery systems (super-SNEDDS)J Control Release20121601253222405903
- ThomasNHolmRGarmerMKarlssonJJMüllertzARadesTSupersaturated self-nanoemulsifying drug delivery systems (Super-SNEDDS) enhance the bioavailability of the poorly water-soluble drug simvastatin in dogsAAPS J201315121922723180162
- TeshimaMFumotoSNishidaKProlonged blood concentration of prednisolone after intravenous injection of liposomal palmitoyl prednisoloneJ Control Release20061123320328
- ZhangLGuFXChanJMWangAZLangerRSFarokhzadOCNanoparticles in medicine: therapeutic applications and developmentsClin Pharmacol Ther2008835761769
- TomiiYLipid formulation as a drug carrier for drug deliveryCurr Pharm Des20028646747412069383
- KawakamiSYamamuraKMukaiTSustained ocular delivery of tilisolol to rabbits after topical administration or intravitreal injection of lipophilic prodrug incorporated in liposomesJ Pharm Pharmacol20015381157116111518027
- FetterlyGJStraubingerRMPharmacokinetics of paclitaxel-containing liposomes in ratsAAPS PharmSci200354E3215198520
- BarenholzYDoxil® – the first FDA-approved nano-drug: lessons learnedJ Control Release2012160211713422484195
- FeldmanEJKolitzJETrangJMPharmacokinetics of CPX-351; a nano-scale liposomal fixed molar ratio formulation of cytarabine: daunorubicin, in patients with advanced leukemiaLeuk Res201236101283128922840315
- ReddyLHSharmaRKChuttaniKMishraAKMurthyRREtoposide-incorporated tripalmitin nanoparticles with different surface charge: formulation, characterization, radiolabeling, and biodistribution studiesAAPS J200463e2315760108
- ManjunathKVenkateswarluVPharmacokinetics, tissue distribution and bioavailability of clozapine solid lipid nanoparticles after intravenous and intraduodenal administrationJ Control Release2005107221522816014318
- PiaoHKamiyaNHirataAFujiiTGotoMA novel solid-in-oil nanosuspension for transdermal delivery of diclofenac sodiumPharm Res200825489690117896098
- ZhangNPingQHuangGXuWChengYHanXLectin-modified solid lipid nanoparticles as carriers for oral administration of insulinInt J Pharm20063271–215315916935443
- PathakPNagarsenkerMFormulation and evaluation of lidocaine lipid nanosystems for dermal deliveryAAPS PharmSciTech200910398599219641997
- WatanabeMKawanoKYokoyamaMOpanasopitPOkanoTMaitaniYPreparation of camptothecin-loaded polymeric micelles and evaluation of their incorporation and circulation stabilityInt J Pharm20063081–218318916324807
- MatsumuraYHamaguchiTUraTPhase I clinical trial and pharmacokinetic evaluation of NK911, a micelle-encapsulated doxorubicinBr J Cancer200491101775178115477860
- KatoKChinKYoshikawaTPhase II study of NK105, a paclitaxel-incorporating micellar nanoparticle, for previously treated advanced or recurrent gastric cancerInvest New Drugs20123041621162721728023
- PepićIJalsenjakNJalsenjakIMicellar solutions of triblock copoly-mer surfactants with pilocarpineInt J Pharm20042721–2576415019069
- OnoueSKojoYSuzukiHDevelopment of novel solid dispersion of tranilast using amphiphilic block copolymer for improved oral bioavailabilityInt J Pharm20134521–222022623694807
- KabanovAVAlakhovVYPluronic block copolymers in drug delivery: from micellar nanocontainers to biological response modifiersCrit Rev Ther Drug Carrier Syst200219117212046891
- FanciullinoRCiccoliniJMilanoGChallenges, expectations and limits for nanoparticles-based therapeutics in cancer: A focus on nano-albumin-bound drugsCrit Rev Oncol Hematol201388350451323871532
- Mora-HuertasCEFessiHElaissariAPolymer-based nanocapsules for drug deliveryInt J Pharm20103851–211314219825408
- MorgenMBloomCBeyerinckRPolymeric nanoparticles for increased oral bioavailability and rapid absorption using celecoxib as a model of a low-solubility, high-permeability drugPharm Res201229242744021863477
- ZhangJHeCTangCYinCTernary polymeric nanoparticles for oral siRNA deliveryPharm Res20133051228123923307349
- ChaturvediKGangulyKNadagoudaMNAminabhaviTMPolymeric hydrogels for oral insulin deliveryJ Control Release2013165212913823159827
- HrkachJVon HoffDMukkaram AliMPreclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profileSci Transl Med20124128128ra39
- PandeyRAhmadZSharmaSKhullerGKNano-encapsulation of azole antifungals: potential applications to improve oral drug deliveryInt J Pharm20053011–226827616023808
- ReddyLHMurthyRSPharmacokinetics and biodistribution studies of Doxorubicin loaded poly(butyl cyanoacrylate) nanoparticles synthesized by two different techniquesBiomed Pap Med Fac Univ Palacky Olomouc Czech Repub2004148216116615744366
- OnoueSKuriyamaKUchidaAMizumotoTYamadaSInhalable sustained-release formulation of glucagon: in vitro amyloidogenic and inhalation properties, and in vivo absorption and bioactivityPharm Res20112851157116621287249
- SharmaASharmaSKhullerGKLectin-functionalized poly (lactide-co-glycolide) nanoparticles as oral/aerosolized antitubercular drug carriers for treatment of tuberculosisJ Antimicrob Chemother200454476176615329364
- WilczewskaAZNiemirowiczKMarkiewiczKHCarHNanoparticles as drug delivery systemsPharmacol Rep20126451020103723238461
- UchegbuIFSiewANanomedicines and nanodiagnostics come of ageJ Pharm Sci2013102230531023175462
