Abstract
Metal-organic frameworks (MOFs) are coordination polymers that comprise metal ions/clusters and organic ligands. MOFs have been extensively employed in different fields (eg, gas adsorption, energy storage, chemical separation, catalysis, and sensing) for their versatility, high porosity, and adjustable geometry. To be specific, Fe2+/Fe3+ exhibits unique redox chemistry, photochemical and electrical properties, as well as catalytic activity. Fe-based MOFs have been widely investigated in numerous biomedical fields over the past few years. In this study, the key index requirements of Fe-MOF materials in the biomedical field are summarized, and a conclusion is drawn in terms of the latest application progress, development prospects, and future challenges of Fe-based MOFs as drug delivery systems, antibacterial therapeutics, biocatalysts, imaging agents, and biosensors in the biomedical field.
Introduction
Metal-organic frameworks (MOFs) refer to porous coordination polymers (PCPs) formed through the self-assembly of metal ions (eg, Zn, Cu, Fe, Cr, Co, and Ni) and organic ligands (eg, phosphonates, carboxylates, sulphonates, and imidazolates).Citation1,Citation2 Due to the different reaction conditions (eg, the type of solvent, reaction temperature, reaction time, and the ratio of ligand to metal), MOFs are capable of self-assembling to form one-dimensional (1D),Citation3 two-dimensional (2D),Citation4,Citation5 or three-dimensional (3D)Citation6 skeleton topologies. Almost unlimited reasonable combinations are generated with the continuous expansion of metal clusters, organic ligands, and topological structures.Citation7 In this context, MOF materials exhibit a wide range of properties, including high porosity (from ultra-micropores to mesopores), high surface area, large pore volume for packaging, chemical and thermal stability, and so forth.Citation8,Citation9 Because of the above-described characteristics, MOFs have been extensively employed in a wide variety of research fields (eg, gas storage, photochemistry, catalysis, separation process, diagnosis, and drug delivery).Citation10–13 Besides, they are considered the most promising materials in biomedicine and nanomedicine.Citation9,Citation14
In the past few years, nanoscale MOFs (NMOFs) has played a crucial role in biomedical engineering. (i) NMOFs are the most fascinating nanocarriers in drug delivery, thus becoming better candidates for carrying anticancer drugs. NMOFs are more flexible and have better biodegradability compared to other delivery systems based on nanoparticles (eg, nano-silica).Citation14 (ii) The selection of internal components of NMOFs (eg, encapsulated fluorescent agents or organic dyes) can be adopted in the fields of biosensors and biological imaging.Citation10 (iii) The customized components of NMOFs can also be employed as therapeutic agents for a wide variety of treatments (eg, using drugs as organic ligands of MOFs, or using active metals as central metal ions of MOFs), which can be applied in the fields (eg, biocatalysis, antibacterial, and bacteriostatic).Citation13
Fe is one of the most abundant transition metal ions that are identified in the human body and other animals, and it serves as a vital component of hemoglobin. It serves as an excellent metal ion candidate for building coordination polymers because of its clear biological function and biological safety.Citation15 Accordingly, Fe-MOFs (eg, MIL-100,Citation16 MIL-88,Citation17 MIL-53,Citation18 MOF-n,Citation19 and PCNCitation20,Citation21) have aroused extensive attention in different biomedical applications. Its advantages are presented as follows: (i) It exhibits an open structural framework and a large specific surface area, such that the reduction of the framework can be induced to form a coordinated unsaturated iron site; it is selected for application in several fields (eg, biocatalysis and antibacterial). The open metal sites exhibit antibacterial activity.Citation16,Citation19 (ii) For its excellent chemical stability and high porosity, it can be combined with other organic and inorganic materials, and it is selected for several fields (eg, sensing and fluorescence detection).Citation21 (iii) With good biocompatibility and biodegradability, it can load drugs and metal particles in the frame, and it is selected for drug transportation, diagnosis, and treatment, and other fields.Citation17 (iv) It is easy to synthesize, and it can be produced through green synthesis and optimal scale.Citation15,Citation16 In general, Fe-MOFs become a powerful platform for achieving different biomedical goals for the above-mentioned biological characteristics of iron.
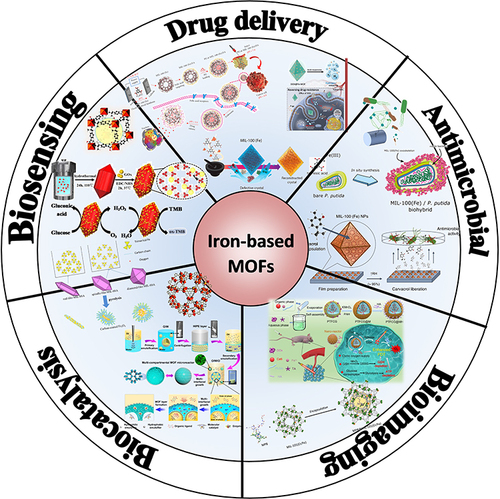
To date, there have been rare comprehensive summaries of Fe-MOFs and their biomedical progress despite a considerable number of comments on MOFs and their biomedical applications. Iron-based MOFs, as a subclass of the huge MOFs family, have made great progress in intelligent design and application strategies of various nano iron-based MOFs materials over the past 5 years. Hence, Fe MOFs and their biomedical progress are comprehensively summarized and systematically analyzed. In this study, the key index requirements for Fe-MOF materials in the biomedical field are first analyzed. Subsequently, the latest progress of Fe-MOFs in biomedical applications is systematically presented (eg, drug delivery systems, biosensors, biocatalysts, image guidance, and antibacterial), as shown in . Lastly, the development prospects and future challenges of Fe-MOFs in biomedical applications are summarized.
Key Indicators of Fe-MOFs Materials in Biomedical Applications
In general, Fe-MOF materials show the biomedical potential for their high porosity, strong adsorption capacity, good biocompatibility, as well as extensive fictionalizations advantages.Citation1 Notably, for the design of Fe-MOFs in biomedical applications, no matter the routes (host MOF or BIOMOF) or application (drug delivery, imaging, or combination of both), several vital indicators should be followed.Citation22
Accurate Particle Size Control
Fe-MOF materials employed in the biomedical field must have accurate particle size control and size optimization.Citation23 shows the application of MOF materials in biomedicine and particle size dependence relationships. As indicated by the results, (i) the reduction of particle size will affect its specific surface volume and increase its reactivity. (ii) The penetration ability of particles is enhanced with the reduction of size. (iii) Larger particles can be retained in the cell tissue, and they can lead to the increased stability of buffer solution and the reduced aggregation of particles. (iv) The different mechanisms of endocytosis (eg, phagocytosis, megacytosis, fossil-mediated endocytosis, or reticulin-mediated endocytosis) are particle size-dependent.Citation1 Thus, the size, charge, shape, and density of MOFs will significantly affect the cell uptake, circulation and retention time, biological distribution, and internalization mechanism.Citation24
Figure 2 Application of Fe-MOF nanomaterials in biomedicine and particle size dependence relationship.
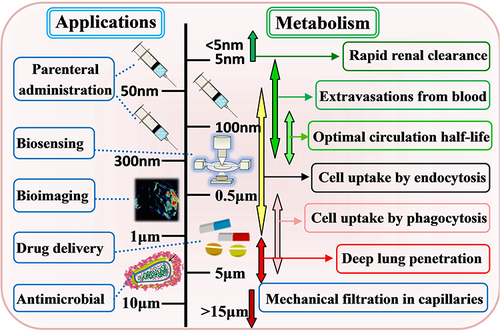
In general, MOFs materials with micron or even millimeter size are usually not suitable for biological applications.Citation25,Citation26 For instance, in the case of injection, the particle size should not exceed 200 nm; the size range between 50 and 300 nm can provide the best-circulating half-life for parenteral administration (). This is also why the synthesis of nanoscale drug delivery systems has become such a vital strategy.Citation27 Many literatures have adopted different strategies to control the particle size of MOF. For instance, crystal growth kinetics can be controlled by adjusting reaction parameters (eg, the type and quantity of solvent, reagent concentration, temperature, and pH) or introducing auxiliary additives (coordination modifiers, surfactant-mediated synthesis, etc.).Citation23–27 Accordingly, the study of a simple synthetic process for accurately controlling the particle size, morphology and crystallinity of Fe-MOFs has become a vital research direction in the application of this material in the biomedical field.Citation27
Simple Synthesis Process Route
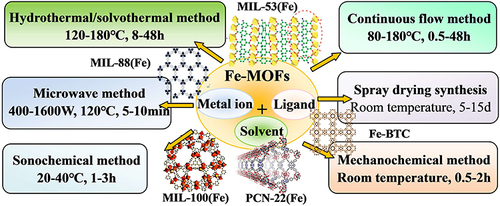
Different synthesis processes can affect the particle size, morphology, and crystallinity of Fe-MOFs for their advantages and disadvantages. A comprehensive schematic diagram of important synthesis technologies of Fe-MOFs materials is presented in . Hydrothermal or solvothermal methods are the most common methods to synthesize Fe-MOFs materials.Citation28–32 The above method usually forms porous metal-organic framework crystals through self-assembly nucleation and crystal growth in a sealed autoclave at a temperature higher than or close to the boiling point of the solvent, as well as through a long time of heating. The above method requires high time and energy consumption, which limits the feasibility of its large-scale synthesis and practical applications. The microwave radiation-based synthesis method is a highly promising synthesis technology of metal-organic skeleton for its several advantages (eg, rapid reaction and environmental friendliness).Citation33 Compared with conventional hydrothermal synthesis, microwaves can directly interact with mobile charge in solvent and solid, such that it can save energy and reaction time.
Ultrasonic-assisted synthesis refers to a type of energy-saving method for chemical reactions with high product selectivity and high speed at ambient temperature.Citation34,Citation35 The above method is to promote the chemical reaction in the solution containing the precursor through the interaction with the liquid through the cyclic mechanical vibration of the ultrasonic wave. The power, type, and ultrasonic time of the ultrasonic generator significantly affect the nucleation rate and crystal growth, and it is easy to form an amorphous MOF structure. Mechanochemical synthesis shows the potential of industrial scale for the advantages of zero waste generation and high output.Citation36,Citation37 In this synthetic route, mechanical energy causes chemical bond breaking and chemical reactions rapidly. Despite the improvements in reaction kinetics and rapid nucleation rate and the capability of rapidly synthesizing nano-sized particles, the above method is easy to form defective MOFs crystal structures.
The spray drying method has the advantages of simplicity, rapidity, less waste generation, and high solvent recovery.Citation38 In this method, through simple evaporation steps, the combined effect of evaporation of atomized droplets and local heating may lead to new chemical reactions. The advantage of the above method is that it can form dry spherical powder continuously and rapidly in a short time with minimum manufacturing cost, thus boosting the industrial production and large-scale application of MOFs. Flow chemistry refers to a mature technology for large-scale synthesis of functional nano-MOF materials.Citation39 The above method is to rapidly form MOF microcrystals through the continuous conversion of mixed reagents in a flow reactor at the reaction temperature. It has the advantages of high reproducibility and precise control of reaction parameters and can realize continuous production by adding reactant solution. Compared with the structure formed based on the conventional batch method, the obtained MOFs structure exhibits better crystallinity and porosity.
The existing synthesis methods have their advantages and disadvantages, and their practical applications are still limited (). It can be predicted that a great deal of research work in the future will be devoted to designing accurate, controllable, simple, clean, and sustainable synthesis routes for Fe-MOF materials while reducing the impact of their preparation on the environment.
Table 1 Summarizes Achievements Related to Fe-MOFs, Solvents Used in Their Preparation, and Their Applications
Biocompatibility and Non-Toxicity
Biocompatibility and non-toxicity are the necessary conditions for the application of Fe-MOF materials in biomedicine.Citation40 Thus, a complete toxicity evaluation (eg, cytotoxicity and in vivo research) takes on great significance, determining the applicability of MOF materials in vivo, and it is also one of the serious obstacles limiting the application of a wide variety of MOFs materials in biomedicine.Citation41 In general, biocompatibility research includes blood reaction, immune reaction, and tissue reaction. The evaluation of biological toxicity mainly comprises (i) the initial toxicity evaluation of the cations and ligands that make up the MOFs materials using LD50 (half lethal dose parameter),Citation42 (ii) the test of the cytotoxicity of MOFs to gain insights into the cytotoxicity at the cell level,Citation43 (iii) toxicity research in vivo, which is the critical test since it presents the most direct and valuable information.Citation44
Non-toxic metal cations should be employed to control the toxicity of MOFs, and the toxicity of cations is also significantly correlated with their ability to accumulate in vivo. Iron refers to a metal ion used by almost all living things, and is employed in biological processes (eg, DNA biosynthesis, oxygen transport, and cell energy generation).Citation41 Although Fe(III) is neutral to tissues and can be safely transported in the non-active state of oxidation and reduction, its high reactivity enhances its destructive power.Citation45 presents the key players in iron metabolism. When iron is excessive, it is also termed hemochromatosis, which can result in cell damage, cell death, and organ failure, mainly affecting the liver, heart, pancreas, thyroid, and central nervous system. Accordingly, Fe(III) should be adopted to synthesize Fe-MOF materials that can be employed in biomedicine, and the intake, distribution, and utilization of iron should be strictly controlled.Citation45
Figure 4 Biocompatibility evaluation of Fe-MOFs nanomaterials: (A) Key players in iron metabolism. Reprinted from Vogt A-CS, Arsiwala T, Mohsen M, et al. On Iron Metabolism and Its Regulation. Int J Mol Sci. 2021;22:4591. Creative Commons.Citation45 (B) In vitro and in vivo models employed to assess nano materials' toxicity. Reprinted from Loret T, Rogerieux F, Trouiller B, et al. Predicting the in vivo pulmonary toxicity induced by acute exposure to poorly soluble nanomaterials by using advanced in vitro methods. Part Fibre Toxicol. 2018;15:25. Creative Commons.Citation46 (C) Evaluation of MIL-89(Fe) toxicity on embryonic zebrafish development. Reprinted from Al-Ansari DE, Al-Badr M, Zakaria ZZ, et al. Evaluation of Metal‐Organic Framework MIL-89 nanoparticles toxicity on embryonic zebrafish development. Toxicology Reports. 2022;9:951–960. Creative Common.Citation44 (D) Effects of MIL-100(Fe) on human normal liver cells (HL-7702) cells’ viability and morphology. Reprinted from Chen G, Leng X, Luo J, et al. In Vitro Toxicity Study of a Porous Iron(III) Metal‒Organic Framework. Molecules. 2019;24(7):1211. Creative Commons.Citation47
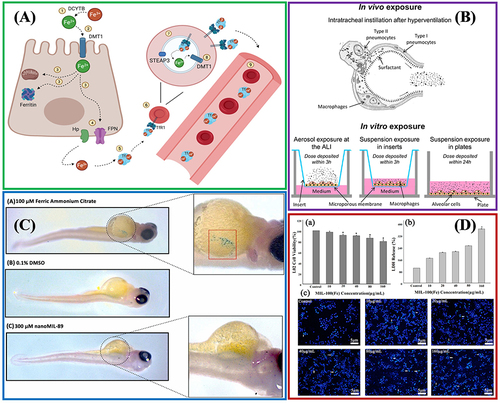
The toxicity of MOF materials also depends on the type of organic ligand.Citation48 presents the in vitro and in vivo models employed to assess MOFs’ toxicity. Both exogenous and endogenous substances can serve as organic ligands. There are many types of exogenous ligands, and functional substituents can be added to change the ADME (eg, absorption, distribution, metabolism, and excretion) process, and affect the drug–carrier interaction, making the delivery process more controllable. On the other hand, endogenous ligands (eg, fumaric acid) can be metabolized by organisms after MOF degradation. However, only a few MOFs based on endogenous ligands show appropriate porosity and structural stability.Citation47,Citation49,Citation50 also lists some LD50 example data of typical organic ligand toxicity, suggesting that their toxicity is at an acceptable level for biological applications.
Given the complete toxicity evaluation of Fe-MOF, Al-Ansari et al and Chen et al studied the toxicity of MIL-89 and MIL-100(Fe) in vivo ( and ).Citation44,Citation47 As indicated by all the parameters studied (eg, serum, enzymology, and histology), MIL-89 had no acute or subacute toxicity symptoms, and its degradation and excretion mechanism was believed to be that nano-Fe-MOFs particles were rapidly isolated by liver and spleen, and then further biodegradable and directly eliminated in urine or stool, without metabolic and substantive toxicity.Citation44 Within a safe dose of 80 μg/mL, MIL-100(Fe) particles had excellent biocompatibility with human normal liver cells (HL-7702) and hepatocellular carcinoma cells (HepG2), low cytotoxicity, and allowed a high cell survival rate.Citation47
The existing Fe-MOF materials still lack systematic biocompatibility and biological toxicity research, which is also one of the possible reasons for the delayed clinical application of Fe-MOF materials.Citation51 Metal ions and organic connectors significantly affect the biocompatibility and toxicity evaluation of MOF, while the solvent used in the synthesis of MOF and the crystal size of MOF will be the key research direction of the biocompatibility of Fe-MOF materials.
Chemical Stability and Controlled Degradation
The stability, reusability and controllable degradation of materials are also important conditions for the application of Fe-MOF materials in biomedicine.Citation40,Citation52 The stability of evaluation materials mainly includes mechanical stability, chemical stability, thermal stability, stability under acid and alkali conditions, and stability under physiological conditions. Different application environments may have very different requirements for the stability of materials. Industrial applications usually require materials that are very stable in thermal, chemical, and mechanical aspects. During drug transportation, it is necessary to be very stable in water, heat, pressure, and harsh chemical environment. Thus, moderately stable and reusable Fe-MOF materials can be recognized as an advantage in biomedical applications.
The stability of Fe-MOFs particles is primarily dependent on several parameters (eg, its components,Citation52 ligands, and metal ions, medium, temperature,Citation29 pH value,Citation32,Citation53 and physiological conditionsCitation54). To be specific, it is the potential representing the degree of electrostatic repulsion between adjacent particles in dispersion ζ. Moreover, it is one of the vital parameters to characterize the stability of components. In general, the greater the potential ζ, the greater the charge repulsion force between particles will be, and the less likely the particles will be to agglomerate. The thermal stability of materials can be monitored through thermo-gravimetric analysis (TGA) and differential scanning calorimetry (DSC). When the temperature reaches over 400 °C, the MOF structure collapses and decomposes to form Fe2O3, suggesting that Fe-MOFs show great thermal invariance in the physiological environment.Citation54,Citation55
The stability of numerous MOF structures, when exposed to water, is a vital issue. Accordingly, the systematic determination of the stability of MOF under water and acid–base conditions is crucial for its application in biomedicine. Bezverkhyy et al studied the stability of MIL-101 and MIL-53 in liquid water.Citation55 They found that: (i) dispersion of Fe-MOFs solid in water will lead to pH reduction, (ii) Due to the function of solid acid catalysts, MIL-101 can extend the effective pH range up to 10.2, and (iii) MIL-101 exhibited good reusability and stability in liquid water. However, there has been scarce research evaluating the stability of MOF in acidic, neutral, and alkaline solutions.
Besides, the stability of Fe-MOF in the biological medium is a crucial parameter.Citation46 If the material is non-biodegradable, the toxicity may also increase due to accumulation in the body.Citation56–58 Iron serves as a major participant in biological metabolism (eg, DNA biosynthesis, oxygen transport, and cell energy production). Under physiological conditions, iron ions are absorbed by intestinal cells in the duodenum and then loaded onto transferrin, such that they can be transported throughout the body to the places where high iron requires (eg, bone marrow, where red blood cells are produced). Aged red blood cells are recognized and swallowed by macrophages and degraded in cells.Citation45 In general, MOF materials are used in the form of nano-particles. When the particle size is within the range of 20–30nm, they mainly undergo kidney elimination, while larger particles (30–300nm) can be rapidly absorbed by mononuclear phagocytic system cells, and mainly exist in the liver, spleen, and bone marrow. However, there have been few studies on the stability of Fe-MOF materials under physiological conditions and the in vivo controlled degradation thus far, and more in vivo research should be conducted.
Overall analysis can suggest that the above-described key indicators of Fe-MOF materials in biomedical applications have interrelated characteristics.Citation59,Citation60 On the one hand, the organic connector in MOFs, the solvent employed in synthesis and the crystal size significantly affect the biocompatibility and toxicity evaluation. On the other hand, the structural stability and degradation tendency of MOF are dependent on the type of metal and ligand, the diameter of nanoparticles, as well as physiological conditions. Thus, to meet the clinical application needs of Fe-MOF materials, basic research on material synthesis, biocompatibility, and controllable degradation performance continues to be its main direction, covering the development of more organic ligands, nanocomposites, etc.
Application Status of Fe-MOFs Materials in Medical Treatment
Fe-MOF materials have become one of the most favorable candidates in biomedical applications for their high surface area and porosity, strong adsorption capacity and extensive functionalization advantages.Citation7,Citation61 The latest research of Fe-based MOFs in the fields of drug delivery, biosensors, biological imaging, biocatalysis, antibacterial and bacteriostasis has made great progress over the past few years, and its development prospects and future challenges should be discussed comprehensively.Citation62
Drug Delivery Systems
The components of numerous active drugs (API) are almost insoluble or unstable in the organism, and they are easy to rapidly and widely metabolize, and their distribution in the organism is usually non-selective.Citation63 This will lead to the generation of low therapeutic effect and may cause damage to healthy cells and tissues and serious adverse side effects. Conventional drug delivery systems (DDS) materials (inorganic materials, including silica,Citation64 porous carbon,Citation65 or zeoliteCitation66) are prone to cause the so-called “explosive effect” arising from insufficient adsorption capacity and no way to delay or control the release of drugs. The interaction between organic ligands and metals in MOF materials contributes to controlling the gradual release and degradation of API, including release under specific conditions (eg, pH, temperature, osmotic pressure, or through enzyme activity); it can also target specific cells through recognizing ligands (eg, folate and peptide), or using the magnetic field to reach specific target or position, and so forth.Citation67–72 Accordingly, the Fe-MOFs drug packaging and delivery system has been proposed and widely investigated for its high porosity, regular structure, excellent adsorption capacity, and high biocompatibility, as well as its wide range of functional advantages.Citation72–83 shows the current research status of the application of Fe MOF in the transportation of some typical drugs.
Table 2 Summary of the Drug, the MOF Type, Loading Capacity, and In Vitro Release Studies in This Review
In general, there are four common ways to synthesize Fe-based MOFs drug delivery systems, as shown in and , which are elucidated as follows: (i) Adsorption synthesis strategy. First, Fe-MOFs with specific properties are synthesized and activated, and drug molecules are added to the outer surface of the MOF carrier through chemical adsorption (covalent bonding with structure).Citation69 (ii) Indirect packaging strategy. MOF materials are synthesized first, and then bioactive compounds are blended into the channels, pores, or cavities of MOF through physical action (non-covalent bond) by impregnation or high pressure.Citation36,Citation67 (iii) Direct encapsulation strategy. Metal ions, organic ligands, and drug molecules are co-dissolved, and drug molecules are directly encapsulated into the MOFs carrier during the formation of the MOFs structure.Citation73,Citation75 (iv) Direct assembly strategy. A wide variety of biological molecules (eg, amino acids,Citation79 peptides,Citation80 and nitrogen-containing bases) or drug molecules are employed as ligands, and the biological metal-organic framework (BioMOF) is directly assembled and synthesized through the coordination bond between them and the metal Fe node.Citation41,Citation81–83
Figure 5 Different strategies for incorporating biomedical related reagents into Fe-MOFs nanomaterials.
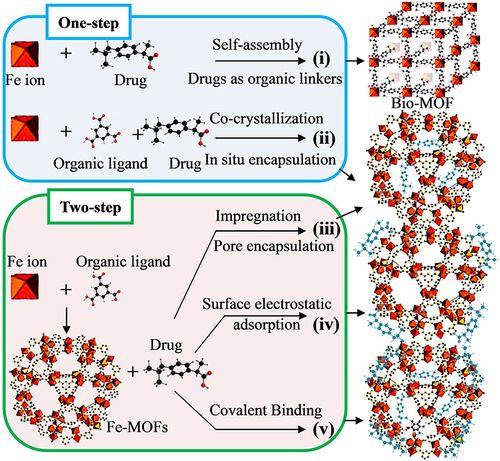
Figure 6 The practical application of Fe-MOFs in DDS: (A) Application of MIL-100 (Fe) in Guest Encapsulation. Reprinted from Souza BE, Möslein AF, Titov K, et al. Green reconstruction of MIL-100 (Fe) in water for high crystallinity and enhanced guest encapsulation. ACS Sustainable Chem Eng. 2020;8(22):8247–8255. Creative Commons.Citation36 (B) The combined formulation of the NH2-MIL-101(Fe)/d-pen and NH2-MIL-101(Fe)/CPT-11 and the anticancer mechanism. Reprinted from Ji HB, Kim CR, Min CH, et al. Fe-containing metal-organic framework with D-penicillamine for cancer-specific hydrogen peroxide generation and enhanced chemodynamic therapy. Bioeng Transl Med. 2023;8:e10477. Creative Commons.Citation77 (C) Green hydrothermal synthesis of iron based MOFs drug carriers,Citation74 (D) MOF-53 (Fe) nanoparticle embeds vancomycin drug. Lin S, Liu X, Tan L, et al. Porous Iron-Carboxylate Metal–Organic Framework: a Novel Bioplatform with Sustained Antibacterial Efficacy and Nontoxicity. ACS Appl Mater Interfaces. 2017;9(22):19248–19257. Copyright (2017), American Chemical Society.Citation82 (E) DOX@Fe-MOF nanocrystals for overcoming cancer resistance/metastasis. Reprinted from Yao XX, Chen DY, Zhao B, et al. Acid-Degradable Hydrogen-Generating Metal-Organic Framework for Overcoming Cancer Resistance/Metastasis and Off-Target Side Effects. Adv Sci. 2022;9:2101965. Creative Commons.Citation80
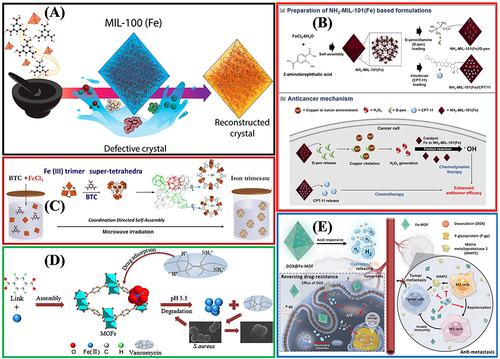
The basic methods of drug packaging exhibit special characteristics and unique defects. The major disadvantage of adsorption synthesis and indirect packaging methods, ie, the most used synthesis strategy for drug packaging systems thus far, refers to the heterogeneous distribution of active drugs in the MOFs structure. In this heterogeneous distribution, drug molecules generally tend to concentrate on the outer surface of MOFs, which makes delivery dynamics difficult to control while reducing drug utilization and efficacy (). The direct packaging strategy can help to improve the heterogeneous distribution. However, it is very important to evaluate the affinity of solvents and drugs to obtain better packaging efficiency, as shown in . Thus, the selectivity of metal ions, organic ligands, drug molecules, and solvents should be high, as shown in . The strategy of direct assembly and synthesis of BioMOFs employs biomolecules as organic ligands, which can make drugs uniformly distributed while showing the advantages of low toxicity and high biocompatibility.Citation84 However, the low symmetry of biological ligands may cause an unpredictable self-assembly process, and the chemical stability and thermal stability of BioMOFs cannot conform to the requirements of conventional biological materials.
In brief, the conventional adsorption synthesis and indirect packaging strategies remain the focus of future research for their simple methods and diverse structures. The direct packaging method has been scarcely investigated, whereas it has broad application prospects. BioMOFs materials will serve as an excellent drug delivery platform and a superstar in the field of smart materials for their beautiful structure, rich supramolecular chemistry, and unique bionic characteristics.
Biosensor
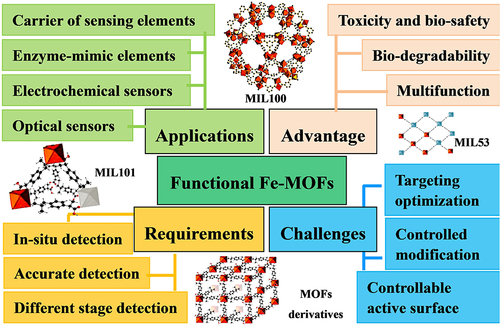
Biosensors are devices that accurately detect biochemical reactions with simple biological molecules, amino acids, proteins, enzymes, tissues, nucleic acids, cells, or microorganisms involved.Citation85 In the field of health care and medicine, accurate and rapid diagnosis and monitoring using developing biosensors that are capable of recording physical, chemical or biological changes and converting them into measurable signals takes on critical significance.Citation86 The development of biosensors primarily aims to develop efficient sensing materials. Fe-MOFs are also considered promising candidates for biosensors for their high porosity, functional sites, and high mass transfer capacity. Moreover, Fe-MOFs, as a vital material for biosensors, have numerous advantages (eg, versatility, toxicity, biosafety, and biodegradability).Citation87
From and , we can see that the core role of Fe-MOFs in biosensors mainly includes:
Carrier of sensing elements, porous Fe-MOFs exhibit strong surface area and adsorption capacity, thus making Fe-MOFs easy to be combined with various functional materials (eg, DNA molecules,Citation88 enzymes,Citation89 antibodies,Citation90 nanoions,Citation91 and dyesCitation92), and they have been extensively employed as carriers of sensitive elements in a wide variety of biosensors, as shown in .
Enzyme-mimic elements, Fe-MOFs show catalytic activity (also known as nanoenzymes). The above-mentioned nano-enzymes have the same or even stronger catalytic performance as natural biological enzymes, and can maintain stability under various biological unfriendly conditions.Citation106
Electrochemical signal transduction and electrochemical sensors. Fe-MOFs as electrochemical sensors also arouse wide attention for their strong redox activity and many catalytic active sites.Citation107 However, the original characteristics of MOF (eg, slow diffusion and low conductivity) significantly hinder its efficient electrochemical application, as shown in . The modification of electroactive ligands, other electroactive materials, or metal nanoparticles is a vital research direction to increase the electrochemical activity of sensors.Citation93,Citation101,Citation102,Citation108
Optical sensors, Fe-MOF have good oxidase-like catalytic performance, can be applied to colorimetric detection of color detection, and have the advantages of simple, fast, cheap, and direct visual readout.Citation94,Citation97–99,Citation104,Citation109 It has been widely used in the detection of enzymes,Citation98,Citation106 DNA,Citation94 hydrogen peroxide,Citation95,Citation100,Citation109 glucose,Citation89,Citation96,Citation103 and so on. Besides, using the specific coordination of hydroxyl or amino groups in Fe-MOF with heavy metals, based on colorimetric and PL chemosensory, can provide highly sensitive and selective detection of arsenicCitation93 or mercury.Citation105
Figure 8 The practical application of Fe-MOFs in Biosensors: (A) Integrated with Glucose oxidase as a biomimetic Glucose biosensor. Reprinted from Weiqing X, Jiao L, Yan H, et al. Glucose oxidase-integrated metal–organic framework hybrids as biomimetic cascade nanozymes for ultrasensitive glucose biosensing. ACS Appl Mater Interfaces. 2019;11:25, 22096–22101. Copyright (2019) American Chemical Society.Citation89 (B) Efficient Biocatalytic System for Biosensing by Combining MIL-53(Fe) MOF-Based Nanozymes and G-Quadruplex (G4)-DNAzymes. Reprinted from Mao XX, He FN, Qiu D, et al. Efficient Biocatalytic System for Biosensing by Combining Metal- Organic Framework (MOF)-Based Nanozymes and G-Quadruplex (G4)-DNAzymes. Anal Chem. 2022;94(20):7295–7302. Copyright (2022) American Chemical Society.Citation90 (C) PCN-224(Fe) hybridized gold nanoparticles as a bifunctional nanozyme for glucose sensing. Tong PH, Wang JJ, Hu X-L, et al. Metal-organic framework (MOF) hybridized gold nanoparticles as a bifunctional nanozyme for glucose sensing. Chem Sci. 2023;14:7762–7769. Creative Commons.Citation91 (D) Fe-MOF-525 Enables Benchmark Electrochemical Biosensing. Reprinted from Zhou ZY, Wang J, Hou S, et al. Room Temperature Synthesis Mediated Porphyrinic NanoMOF Enables Benchmark Electrochemical Biosensing. Small. 2023. 2301933. Creative Commons.Citation93 (E) MIL-88A-Derived Fe3O4@C Hierarchical Nanocomposites for Electrochemical Sensing. Wang L, Zhang Y, Li X, et al. The MIL-88A-Derived Fe3O4-Carbon Hierarchical Nanocomposites for Electrochemical Sensing. Sci Rep. 2015;5:14341. Creative Commons.Citation104
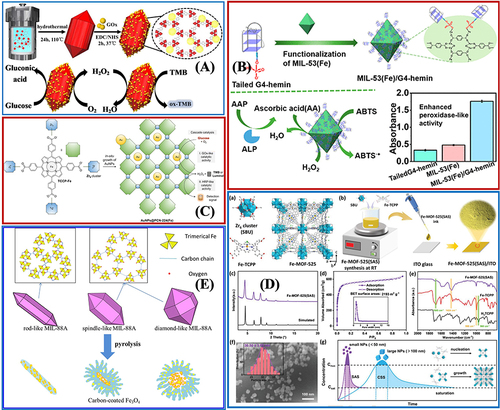
Table 3 Summary of Biosensing Applications of Fe-MOFs in This Review
As depicted in , the vital problems of clinical application of Fe-MOFs in biosensors mainly include:
In situ application or direct injection of Fe-MOFs biosensor is the vital factor for clinical application. Notably, in-situ detection with real-time diagnostic analysis takes on critical significance in detecting factors for toxicity and biosafety.Citation87
At different stages of the sensor based on MOF, the comparative study of selectivity and repeatability detection takes on crucial significance. Most of the published MOF-based biosensors use low-selectivity physical adsorption or chemical processes to identify target analytes, which are not suitable for analysis and detection in complex matrices.Citation108 Accordingly, selective detection of biomarkers at different stages of various diseases is helpful to develop rapid diagnostic clinical therapies; Reusable MOF materials are also very important in large-scale applications.Citation109
Rapid, accurate, and sensitive detection of MOFs biosensors is also recognized as a critical condition for clinical application accurate detection. If the specific recognition of biological probes (eg, antibodies or aptamers) can be directly coupled with those highly stable MOFs, the above bottleneck problem can be effectively solved. At present, although many technologies and methods have been developed to synthesize Fe-MOFs and their derivatives, the target optimization and controllable modification required for synthesis and detection, as well as Fe-based MOF materials with controllable active surfaces, is still a huge challenge.Citation110
Fe-MOFs show great application prospects in the field of biosensors. The synthesis and preparation place a major focus on biocompatible materials with specific pore microenvironments, stable structures and morphology, as well as controllable surface activity. Performance research focuses on high sensitivity in situ detection and wearable detection combining conventional biological insight with digital instruments; the unique advantages of Fe-MOFs in versatility, biosafety and biodegradability are bound to become the focus of research in the field of biosensors.
Biocatalysis
Biocatalysis refers to the process of using enzymes as catalysts to simulate complex biological transformation and then chemical transformation.Citation111 Enzymes, ie, the most efficient catalyst, exhibit unique characteristics (eg, high substrate affinity and specificity). However, the practical application of natural enzymes is hindered by high cost, highly restricted catalytic conditions, and fragility for their complex and fine structure, and natural enzymes should be protected by carrier materials.Citation112,Citation113 To solve the above-described problems in the practical application of enzymes, enzyme immobilization technology and the synthesis of efficient biomimetic catalysts have become the research focus in the field of biocatalysis.Citation114 Metal-organic frameworks (MOFs) have excellent tunability, good biocompatibility and significant surface modification in structural design.Citation113,Citation115 As candidates in the field of biocatalysis, they have become increasingly popular.
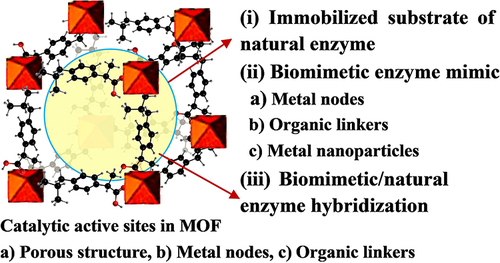
It can be seen from and that the core applications of Fe-MOFs in the field of biocatalysis mainly include:
Figure 10 The practical application of Fe-MOFs in biocatalysis: (A) Cu/Au/Pt TNP-modified TCPP-(Fe) nanozyme. Reprinted from Wu P, Gong F, Feng X, et al. Multimetallic nanoparticles decorated metal-organic framework for boosting peroxidase-like catalytic activity and its application in point-of-care testing. J Nanobiotechnol. 2023;21:185. Creative Commons.Citation112 (B) MIL-101(Cr/Fe) used as a platform for MP-8 enzyme immobilization. Reprinted from Kesse X, Sicard C, Steunou N, et al. Encapsulation of Microperoxidase-8 into MIL-101(Cr/Fe) Nanoparticles: a New Biocatalyst for the Epoxidation of Styrene. Eur J Inorg Chem. 2023;26:e202300040. Creative Commons.Citation115 (C) Multi-compartmental MOF microreactors for chemo-enzymatic cascade catalysis. Reprinted from Tian D, Hao R, Zhang X, et al. Multi-compartmental MOF microreactors derived from Pickering double emulsions for chemo-enzymatic cascade catalysis. Nat Commun. 2023;14:3226. Creative Commons.Citation116 (D) Fe-MOF based bio-/enzyme-mimics used for cancer treatment and anti-tumor principle. Reprinted from Xiang X, Pang H, Ma T, et al. Ultrasound targeted microbubble destruction combined with Fe-MOF based bio-/enzyme-mimics nanoparticles for treating of cancer. J Nanobiotechnol. 2021;19:92. Creative Commons.Citation117
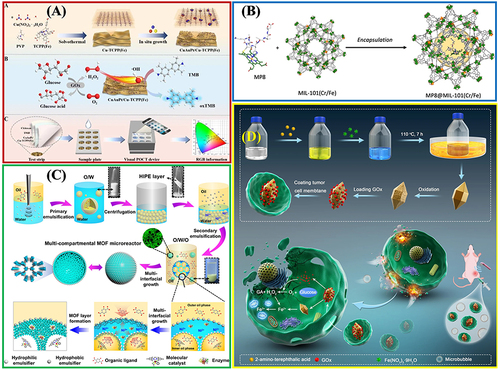
Natural enzyme immobilization matrix: Immobilizing the host enzyme on the porous organic framework carrier can protect the enzyme from the effect of harsh industrial conditions while promoting the selective diffusion of guest molecules through the carrier, and facilitating its recovery and recycling together with the carrier. MOF, a natural enzyme immobilization matrix, primarily comprises surface adhesion, covalent bond, pore interception and coprecipitation.Citation54 This requires the preparation of MOF materials with adjustable porosity, adjustable morphology and large surface area, and the immobilization of enzymes to stable MOFs with enzyme activity retention through appropriate strategies, as shown in and . This is the critical and challenging research direction in this field.Citation115,Citation118–120
Biomimetic enzyme mimics: To address the problems in the practical application of natural enzymes and inspired by the catalytic mechanism of natural enzymes, bionic schemes that mimic some key characteristics of enzymes have become effective in developing efficient biomimetic catalysts.Citation121 Biomimetic Fe-MOFs catalysts can satisfy some enzymatic characteristics (eg, the structural characteristics of enzymes, active sites, microenvironment, co-catalytic sites, and transmission channels) while showing the advantages of wider application conditions and stronger stability (). Besides, they will exhibit catalytic properties that natural enzymes do not exhibit, which are elucidated as follows: (1) homogeneous separation of biomimetic MOF catalytic sites to avoid self-accumulation and deactivation; (2) appropriate hydrophilic and hydrophobic pore properties to promote the recognition and accumulation of reactant molecules; (3) synergetic microenvironment to improve the reactivity between active sites and substrates; (4) restricted pore size, shape and function to improve chemistry, regionally, stereology and selectivity; (5) auxiliary active sites to improve catalytic efficiency and reaction rate; (6) simple reuse and high stability. Compared with enzymes, the applicable pH range, reaction temperature and solvent of biomimetic MOFs are significantly broadened.Citation89,Citation96,Citation116,Citation122–124 With the significant progress in the latest characterization technology to reveal the structure and catalytic mechanism of enzymes, the important synergy of microenvironments and co-catalytic sites has been recognized progressively, such that considerable scientific and technological efforts have been attracted for the development of efficient biomimetic MOFs catalysts.
Biomimetic enzyme/natural enzyme hybridization: Due to the comprehensive advantages of the selectivity of natural enzymes and the controllable catalytic activity of nanoenzyme, nanoenzyme/natural enzyme hybridization takes on critical significance in biosensor, treatments, and catalysis, as shown in . Extensive research should be conducted to properly address the interaction mechanism and biocompatibility of biomimetic enzyme/natural enzyme.Citation117,Citation125
In general, Fe-MOFs improve the stability and reusability of encapsulated biocatalysts while expanding their application scope in the field of biocatalysis.Citation126 The research focus will be placed on the development of simple and mild enzyme immobilization technology, the design and synthesis of novel MOF with a mesoporous structure to facilitate the transport of enzyme materials, and gaining insights into more detailed interaction mechanism between enzyme and Fe-MOF to more effectively boost industrialization. The integration of biocatalysts and Fe-MOFs is highly promising for industrial biocatalysis in the future.
Bioimaging and Phototherapy
The current cancer treatment methods mainly include surgery, chemotherapy, radiotherapy, endocrine therapy, and targeted therapy. To increase the therapeutic effect of chemotherapy, the image-guided chemical-photothermal combined therapy strategy combining chemotherapy and phototherapy has aroused wide interest.Citation127,Citation128 The above-mentioned method primarily refers to a treatment method that employs targeted recognition imaging or development technology to transform light energy into heat energy in the vicinity of tumor tissue by injecting materials with high light and heat conversion efficiency, and kill cancer cells locally. The above-described technology makes surgical treatment less invasive and more accurate, and it is capable of shortening hospital stays and reducing repeated operations.Citation129 At present, common imaging and photothermal agents comprise metal nanostructures, carbon-based materials, metal oxides and chalcogenides, metal-organic frameworks (MOFs), Mxenes, and so forth. To be specific, chemically and thermally stable Fe-MOFs are employed in drug delivery, biosensors, biocatalysts, and other fields, and they exhibit excellent optical and photothermal properties. Besides, they have been commonly adopted as imaging agents for magnetic resonance imaging (MRI)Citation130 and photoacoustic imaging (PAI).Citation131 Furthermore, they serve as a photothermal agent and carrier for photothermal therapy (PTT)Citation132 and photodynamic therapy (PDT).Citation133
(i) Imaging agent, developer, and carrier: Magnetic resonance imaging (MRI), a non-invasive method for imaging and tissue characterization of living organisms, has been the most widely applied and effective imaging technology.Citation134 Because Fe3+ is a paramagnetic metal ion, Fe-MOF has become an excellent candidate for MRI imaging, as shown in .Citation130 The existence of numerous paramagnetic metal centers makes Fe-MOFs tend to exhibit higher relaxation than small molecular contrast agents (CA). In general, a higher relaxation value will help to improve the sensitivity and specificity of contrast media. However, most of the single Fe-based MOF materials have only moderate relaxation (relaxation), which leads to the lack of sensitivity of MRI imaging.Citation135–137
Figure 11 (A) Schematic illustration of imaging-guided therapy. (B) Photodynamic tumor therapy (PDT) by reversing multiple resistances. Reprinted from Liu P, Zhou Y, Shi X, et al. A cyclic nano-reactor achieving enhanced photodynamic tumor therapy by reversing multiple resistances. J Nanobiotechnol. 2021;19:149. Creative Commons.Citation138 (C) MRI imaging of orthotopic pancreatic murine tumors. Reprinted from Rojas JD, Joiner JB, Velasco B, et al. Validation of a combined ultrasound and bioluminescence imaging system with magnetic resonance imaging in orthotopic pancreatic murine tumors. Sci Rep. 2022;12:102. Creative Commons.Citation130 (D) PAI images and (E) Photothermal photographs of doxorubicin@MIL-100 compound. Reprinted from Zhiming H, Caina X, Liang Y, et al. Multifunctional drug delivery nanoparticles based on MIL-100 (Fe) for photoacoustic imaging-guided synergistic chemodynamic/chemo/photothermal breast cancer therapy. Mater Design. 2022;223:111132. Creative Commons..Citation131
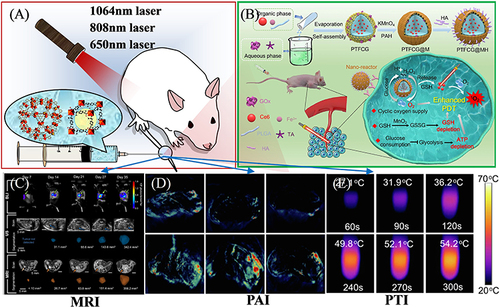
Photoacoustic imaging (PAI) and photothermal imaging (PTI) are novel non-invasive soft tissues medical imaging modes under photoacoustic (PA) and photothermal effects.Citation139 They refer to the generation of sound waves by local tissues irradiated by pulsed laser and the reception of local thermal expansion signals by ultrasonic detectors. Both PAI and PTI have the characteristics of high resolution, rich optical contrast, and tissue penetration depth, and show broad application prospects in visualizing tissue structure and function. Fe-MOF materials have less research in the application of PAI, which may be related to the weak optical absorption of the above-mentioned materials.Citation140,Citation141
(ii) Therapeutic agent and carrier for photothermal therapy and photodynamic therapy: Photothermal therapy (PTT) refers to a treatment method that uses materials with high photothermal conversion efficiency to kill cancer cells locally by converting light energy into heat energy.Citation132,Citation142 Photodynamic therapy (PDT) has been confirmed as a novel technology that exploits the photodynamic effect to diagnose and treat diseases.Citation133,Citation138,Citation143 Its mechanism of action is to induce photosensitizer to produce reactive oxygen species (ROS) by laser of specific wavelength, and produce cytotoxicity by oxidizing reaction with adjacent biological macromolecules, triggering tumor cell damage and even death. PTT and PDT are characterized by minimally invasive, long-term, high-precision, and controllable treatment process in the treatment of malignant tumors, and they have aroused wide attention by scientific researchers. In terms of PTT or PDT, non-toxic or low-toxic and biodegradable iron (III) based MOF serves as an ideal carrier of photosensitized Fe-soc-MOF nanoparticles combined with ICG.Citation144 It will be its main research direction in facilitating the near-infrared (NIR) absorption of Fe-MOFs and increasing the light stability, especially in the multi-functional therapeutic agent combining MOFs with other functional materials.Citation145–149
Fe-MOFs exhibit high research value and broad application prospects in image-guided chemical-photothermal therapy strategy. First, given the accumulation, retention, and metabolism of residues in the body, the degradation mechanism and biological stability of existing Fe-MOFs should be investigated in depth. Second, it is important to synthesize Fe-MOF materials with highly repeatable physical properties and systematically evaluate the toxicity in vivo. Besides, in PAI and PTT technology, the use of nanoparticles (NPs) with unique magnetism and excellent biocompatibility combined with appropriate Fe-MOFs to synthesize nanocomposites that can provide effective treatment and diagnosis will be its main research direction.
Antimicrobial MOFs
With the increasing resistance of pathogens to antibiotics (AMR) over the past few years, infections arising from microorganisms (eg, bacteria, fungi, viruses, and parasites) pose increasingly serious harm to public health.Citation150,Citation151 Thus, the development of antibacterial agents with high bactericidal activity (eg, antibacterial peptides, bacteriophages, and antibodies) has attracted great attention.Citation152 Although the conventional phytochemical antibacterial agents have high biocompatibility, their effect on treating pathogenic microorganisms is poor, and their stability and bioavailability in the water environment have also been limiting factors.Citation153,Citation154 The scientific progress of nanotechnology and material science has endowed nanoparticles (NPs) with higher antibacterial activity, including metals and their compounds (eg, metal oxides, metal salts, and metal hydroxides), inorganic-organic hybrid nanoparticles (NPs) and polymers, promising alternative methods for the treatment of AMR-associated infections.Citation155–159 However, their application in clinical research has triggered several problems (eg, cytotoxicity and non-targeted effects).
MOF materials and MOF matrix composites are considered a type of potential antibacterial materials, though their antibacterial effect research remains in its infancy.Citation160,Citation161 Fe-MOFs show several advantages (eg, low-cost composition, easy preparation, water solubility, and long-term stability), and they significantly apply to medical sterilization and bacterial eradication, as well as the growth inhibition of antibacterial agents.Citation162–165 presents the active site and main applications of antibacterial Fe-MOFs and their composites. The main research directions of Fe-MOFs in antimicrobials are presented as follows.
Figure 12 Active site and main applications of antibacterial Fe-MOFs nanomaterials and Their Composites.
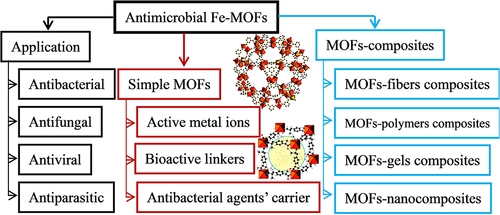
MOFs as metal ions reserve: Fe-MOFs can serve as sites of active metal ions, ie, original antibacterial. For instance, existing research has suggested that MIL-101 (Fe) exhibits high-free radical scavenging activity, and the MIC values of Escherichia coli (ATCC 512), Enterobacter planus (ATCC 256), Lactobacillus pneumophilus subspecies (ATCC 4), Bacillus cereus, Staphylococcus aureus (ATCC 256), Pseudomonas aeruginosa (ATCC 32), and Candida albicans reach 16, 256, 10536, 10541, 33152, 6538, and 9027, respectively.Citation43 As indicated by the above analysis, Fe-MOFs molecule exhibit high antibacterial activity against microorganisms, fungus, and yeast, as shown in and .Citation162,Citation163
MOFs containing bioactive links: For instance, it is obtained through the self-assembly of Fe3+ ion and doxorubicin hydrochloride (DOX) molecule Fe-DOX@Gd-MOF, showing higher stability and controllable DOX release, as shown in .Citation139 Nevertheless, there has been scarce research in this category.
For antimicrobial control delivery carriers: For instance, silver ion was loaded into Fe-MOFs as a typical antibacterial agent to form an antibacterial system, showing excellent antibacterial behavior against Staphylococcus aureus and Escherichia coli.Citation167,Citation168 Gentamicin (GM) can also be encapsulated with Fe-MIL-100 through a simple permeation procedure. Antibacterial tests on Staphylococcus aureus, Streptococcus epidermidis and Pseudomonas aeruginosa also confirmed that the released transgenic antibiotic activity was retained.Citation83 Fe-MOF-112 prepared in the form of nanoparticles has been used as a nano-carrier for the precise transport of 3-azido-d-alanine (d-AzAla).Citation160 After intravenous injection, 112 NPs have been found to accumulate in the infected tissue and release d-AzAla in the inflammatory environment with high H2O2, which is selectively incorporated into the cell wall of MRSA bacteria.Citation160 and shows the antibacterial mechanism of La3+, Gd3+ metal ion dopingCitation165 and Carvacrol encapsulated in Fe-based MOFs.Citation166 On the other hand, Fe-MOFs can also appear in the form of various composites, including fiber, polymer, gel or nanocomposite.Citation169–172
Figure 13 (A) Surface-Anchored MOFs-Cotton Material with antibacterial properties. Reprinted from Rubin HN, Neufeld BH, Reynolds MM. Surface-anchored metal–organic framework–cotton material for tunable antibacterial copper delivery. ACS Appl Mater Interfaces. 2018;10(17):15189–15199. Copyright (2018) American Chemical Society.Citation162 (B) The antibacterial mechanisms of Fe(III)-MOF towards different types of microorganisms, fungus, and yeast. Reprinted from Sheta SM, Salem SR, El‑Sheikh SM. A novel Iron (III)‑based MOF: synthesis, characterization, biological, and antimicrobial activity study. J Mater Res. 2022;37(14):2357–2367. Creative Commons.Citation163 (C) Synthesis of a mesoporous MIL-100(Fe) bacteria exoskeleton Reprinted from Permyakova A, Kakar A, Bachir J, et al. In Situ Synthesis of a Mesoporous MIL-100(Fe) Bacteria Exoskeleton. ACS Materials Lett. 2023;5(1):79–84. Copyright (2023) American Chemical Society.Citation139 (D) Smart MIL-88B(Fe) coating as a host matrix for the antibiofilm compound. Reprinted from Claes B, Boudewijns T, Muchez L, et al. Smart metal–organic framework coatings: triggered antibiofilm compound release. ACS Appl Mater Interfaces. 2017;9(5):4440–4449. Copyright (2017) American Chemical Society.Citation165 (E) Antibacterial properties of Carvacrol encapsulated in MIL-100 (Fe) nanoparticles. Reprinted from Caamaño K, Heras-Mozos R, Calbo J, et al. Exploiting the Redox Activity of MIL-100(Fe) Carrier Enables Prolonged Carvacrol Antimicrobial Activity. ACS Appl Mater Interfaces. 2022;14:10758–10768. Creative Commons.Citation166
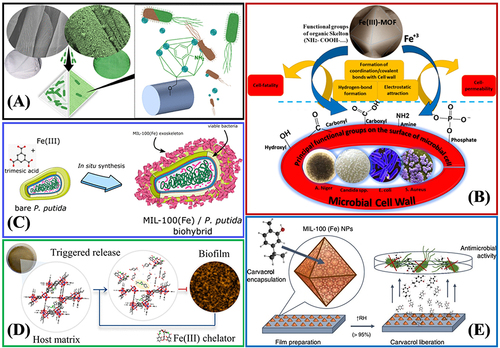
In general, biosafety Fe-MOF materials seem to be very suitable for medical sterilization, bacterial eradication or as carriers of antiviral agents, because they combine the advantages of two different fields, namely inorganic antibacterial agents with long sterilization time and broad-spectrum sterilization.Citation173,Citation174 However, at present, there are few research results on antibacterial Fe-MOFs. We can foresee that research in this field will increase significantly under the context of the recent COVID-19 pandemic and the rise of potential risks in the microbial world.
Discussion and Future Outlook
This study focuses on the key index requirements of Fe-MOFs materials in biomedical applications, as well as their extensive research in biomedical applications (eg, drug delivery, biosensors, photothermal therapy and antibacterial activity), suggesting the huge scientific and technological potential of the above-described materials. Fe-MOFs material serves as an ideal candidate in the biomedical field for its numerous advantages. For instance, its good biocompatibility and biodegradability can be applied to drug delivery; large specific surface area and high porosity provide a solid basis for the use of biosensors and biocatalysts; the open structural framework and active metal sites expand the ability of photothermal therapy and antibacterial; finally, its simple green synthesis process is also conducive to large-scale production. However, the research of Fe-MOF materials for biomedical applications is in the early stage of exploration, many long-term obstacles and several challenges remain.
The size optimization, shape control and structural stability of Fe-MOFs take on critical significance in drug delivery and biosensors. The uniform size and stable morphology are conducive to expediting the distribution and reuse of Fe-MOFs in the organism. However, it is difficult for the self-assembly synthesis process to apply to the synthesis of Bio-MOFs due to the low symmetry of many biological ligands. Moreover, the size, shape, and porosity of Fe-MOFs can hardly be accurately controlled. Thus, improving the structural stability, accurate particle size control, and simple synthetic process route are crucial in widening their biological application. This will serve as crucial content to guide the design and application of Fe-MOF materials in the future.
A considerable number of physiological mechanisms of Fe-MOFs (eg, biodegradation mechanism, pharmacokinetics, antibacterial and bacteriostasis mechanism) remain unclear. Although some Fe-MOFs materials or their nanocomposites have been extensively explored in vitro and in vivo, there is little research on the metabolic mechanism of Bio-MOF materials. Further exploring the biological logic and molecular level theoretical research of absorption, distribution, metabolism, and excretion (ADME) in vivo under complex substrate conditions (eg, the effect of pH, temperature, or other active molecules) is also a vital research direction of Fe-MOFs.
The application of single-structure Fe-MOF materials is also restricted by biocompatibility and cytological toxicity. Their biosafety and biodistribution in vitro and/or in vivo must approach the requirements of practical biomedical applications. Future research will focus on the development of mixed metal MOF materials based on highly biocompatible metal ions (eg, Ca and Zn), functional MOF materials combined with bioactive ligands, and graded MOF materials with surface modification, so as to significantly promote the expansion of the application range of Fe-MOF materials.
In brief, early reports on Fe-MOF materials in the biomedical field have revealed their extraordinary and unique properties, while some challenges should be explored in future research. With the latest development of MOF-based nanocomposites, their application in biomedicine provides exciting new avenues for the clinical development of nanotechnology. Hence, technologies based on Fe-MOF materials have brighter prospects in the biomedical field.
Disclosure
The authors declare no conflicts of interest in this work.
Acknowledgments
This work is supported by Innovatively Scientific Research Project for Young People of Sichuan Medical Association (Q20041 and Q21064), and Novel corona virus infection emergency research project of Mianyang Science and Technology Bureau (2020YJKY006).
References
- Velásquez-Hernández MD, Linares-Moreau M, Astria E, et al. Towards applications of bioentities@MOFs in biomedicine. Coordin Chem Rev. 2021;429:213651. doi:10.1016/j.ccr.2020.213651
- Cai H, Huang Y-L, Dan L. Biological metal–organic frameworks: structures, host–guest chemistry and bio-applications. Coordin Chem Rev. 2019;378:207–221. doi:10.1016/j.ccr.2017.12.003
- Liu W, Yin R, Xu X, et al. Structural engineering of low-dimensional metal–organic frameworks: synthesis, properties, and applications. Adv Sci. 2019;6(12):1802373. doi:10.1002/advs.201802373
- Pan Y, Abazari R, Yao J, Gao J. Recent progress in 2D metal-organic framework photocatalysts: synthesis, photocatalytic mechanism and applications. J Phys Energy. 2021;3:032010. doi:10.1088/2515-7655/abf721
- Ming X, Yang S-S, Zhi-Yuan G. Two-Dimensional Metal-Organic Framework Nanosheets: a Rapidly Growing Class of Versatile Nanomaterials for Gas Separation, MALDI-TOF Matrix and Biomimetic Applications. Chem Eur J. 2018;24:15131–15142. doi:10.1002/chem.201800556
- Xin Y, Zhou J, Xing YH, et al. A series of porous 3D inorganic–organic hybrid framework crystalline materials based on 5-aminoisophthalic acid for photocatalytic degradation of crystal violet. New J Chem. 2021;45:3432–3440. doi:10.1039/D0NJ05472K
- Yang J, Yang Y-W. Metal–Organic Frameworks for Biomedical Applications. Small. 2020;16:1906846. doi:10.1002/smll.201906846
- Xueying G, Wong R, Anisa A, et al. Recent development of metal-organic framework nanocomposites for biomedical applications. Biomaterials. 2022;281:121322. doi:10.1016/j.biomaterials.2021.121322
- Auer B, Telfer SG, Gross AJ. Metal Organic Frameworks for Bioelectrochemical Applications. Electroanalysis. 2023;35:2200145. doi:10.1002/elan.202200145
- Karimi-Maleh H, Lütfi Yola M, Atar N, et al. A novel detection method for organophosphorus insecticide fenamiphos: molecularly imprinted electrochemical sensor based on core-shell Co3O4@MOF-74 nanocomposite. J Colloid Interf Sci. 2021;592:174–185. doi:10.1016/j.jcis.2021.02.066
- Luna-Triguero A, Vicent-Luna JM, Madero-Castro RM, et al. Acetylene Storage and Separation Using Metal−Organic Frameworks with Open Metal Sites. ACS Appl Mater Interfaces. 2019;11:31499–31507. doi:10.1021/acsami.9b09010
- Dolgopolova EA, Rice AM, Martin CR, et al. Photochemistry and photophysics of MOFs: steps towards MOF-based sensing enhancements. Chem Soc Rev. 2018;47:4710–4728. doi:10.1039/C7CS00861A
- Qin H, Yangjie F, Ge X, et al. Facile fabrication of Fe-BDC/Fe-2MI heterojunction with boosted photocatalytic activity for Cr(VI) reduction. J Environ Chem Eng. 2021;9(5):105961. doi:10.1016/j.jece.2021.105961
- Ibrahim M, Sabouni R, Husseini GA, et al. Synthesis of Metal-Organic Framework from Iron Nitrate and 2,6-Naphthalenedicarboxylic Acid and Its Application as Drug Carrier. J Nanosci Nanotechnol. 2018;18(8):5266–5273. doi:10.1166/jnn.2018.15373
- Zhang S, Zhang Y, Baig F, et al. Synthesis and applications of stable iron-based metal−organic framework materials. Cryst Growth Des. 2021;21:3100–3122. doi:10.1021/acs.cgd.0c01500
- Fang Y, Yang Z, Li H, et al. MIL-100(Fe) and its derivatives: from synthesis to application for wastewater decontamination. Environ Sci Pollut Res. 2020;27:4703–4724. doi:10.1007/s11356-019-07318-w
- Kim S-N, Gwon Park C, Huh BK, et al. Metal-organic frameworks, NH2-MIL-88(Fe), as carriers for ophthalmic delivery of brimonidine. Acta Biomater. 2018;79:344–353. doi:10.1016/j.actbio.2018.08.023
- Liu N, Huang W, Tang M, et al. In-situ fabrication of needle-shaped MIL-53(Fe) with 1T-MoS2 and study on its enhanced photocatalytic mechanism of ibuprofen. Chem Eng J. 2019;359:254–264. doi:10.1016/j.cej.2018.11.143
- Huang W, Shao H, Song M, et al. Perylene diimides coated Fe-MOFs as acid-tolerant photo-Fenton catalyst for phenol removal. Appl Surf Sci. 2021;547:149222. doi:10.1016/j.apsusc.2021.149222
- Chen Y, Sun X, Biswas S, et al. Integrating polythiophene derivates to PCN-222(Fe) for electrocatalytic sensing of L-dopa. Biosens Bioelectron. 2019;141:111470. doi:10.1016/j.bios.2019.111470
- Zheng W, Liu J, Yi D, et al. Ficin encapsulated in mesoporous metal-organic frameworks with enhanced peroxidase-like activity and colorimetric detection of glucose. Spectrochimica Acta A. 2020;233:118195. doi:10.1016/j.saa.2020.118195
- Joseph J, Iftekhar S, Srivastava V, et al. Iron-based metal-organic framework: synthesis, structure and current technologies for water reclamation with deep insight into framework integrity. Chemosphere. 2021;284:131171. doi:10.1016/j.chemosphere.2021.131171
- Marshall CR, Staudhammer SA, Brozek CK. Size control over metal–organic framework porous nanocrystals. Chem Sci. 2019;10:9396–9408. doi:10.1039/C9SC03802G
- Iqbal B, Laybourn A, ul-Hamid A, et al. Size-controlled synthesis of spinel nickel ferrite nanorods by thermal decomposition of a bimetallic Fe/Ni-MOF. Ceram Int. 2021;47(9):12433–12441. doi:10.1016/j.ceramint.2021.01.100
- Kumar S, Jain S, Nehra M, et al. Green synthesis of metal–organic frameworks: a state-of-The-art review of potential environmental and medical applications. Coordin Chem Rev. 2020;420:213407. doi:10.1016/j.ccr.2020.213407
- Chen W, Wu C. Synthesis, functionalization, and applications of metal–organic frameworks in biomedicine. Dalton Trans. 2018;47:2114–2133. doi:10.1039/C7DT04116K
- Zhang Y, Yang L, Yan L, et al. Recent advances in the synthesis of spherical and nanoMOF-derived multifunctional porous carbon for nanomedicine applications. Coordin Chem Rev. 2019;391:69–89. doi:10.1016/j.ccr.2019.04.006
- Zhang L, Jiejun L, Wang C, et al. A novel kaempferol electrochemical sensor based on glass carbon electrode modified by poly (3, 4-ethylenedioxythiophene) decorated with green synthesized MIL-100(Fe)-multi- walled carbon nanotubes composites, Colloid. Surface A. 2022;649:129484. doi:10.1016/j.colsurfa.2022.129484
- Lin Y-S, Lin K-S. Characterization of the size and porous temperature sensitivity of Pluronic F127‒Coated MIL‒88B(Fe) for drug release. Micropor Mesopor Mat. 2021;328:111456. doi:10.1016/j.micromeso.2021.111456
- Dariush Taherzade S, Rojas S, Soleimannejad J, et al. Combined cutaneous therapy using biocompatible metal-organic frameworks. Nanomaterials. 2020;10(12):2296. doi:10.3390/nano10122296
- Bagherzadeh E, Mojtaba Zebarjad S, Madaah Hosseini HR, et al. Interplay between morphology and band gap energy in Fe-MIL-88A prepared via a high temperature surfactant-assisted solvothermal method. Mater Chem Phys. 2022;277:125536. doi:10.1016/j.matchemphys.2021.125536
- Jun Y, Xiong W, Li X, et al. Functionalized MIL-53(Fe) as efficient adsorbents for removal of tetracycline antibiotics from aqueous solution. Micropor Mesopor Mat. 2019;290:109642. doi:10.1016/j.micromeso.2019.109642
- Dong Y, Tianding H, Pudukudy M, et al. Influence of microwave-assisted synthesis on the structural and textural properties of mesoporous MIL-101(Fe) and NH2-MIL-101(Fe) for enhanced tetracycline adsorption. Mater Chem Phys. 2020;251:123060. doi:10.1016/j.matchemphys.2020.123060
- Vaitsis C, Sourkouni G, Argirusis C. Metal Organic Frameworks (MOFs) and ultrasound: a review. Ultrason Sonochem. 2019;52:106–119. doi:10.1016/j.ultsonch.2018.11.004
- Amaro-Gahete J, Klee R, Esquivel D, et al. Fast ultrasound-assisted synthesis of highly crystalline MIL-88A particles and their application as ethylene adsorbents. Ultrason Sonochem. 2019;50:59–66. doi:10.1016/j.ultsonch.2018.08.027
- Souza BE, Möslein AF, Titov K, et al. Green reconstruction of MIL-100 (Fe) in water for high crystallinity and enhanced guest encapsulation. ACS Sustainable Chem Eng. 2020;8(22):8247–8255. doi:10.1021/acssuschemeng.0c01471
- Jeong H, Lee J. 3D-superstructured networks comprising fe-mil-88a metalorganic frameworks under mechanochemical condition. Eur J Inorg Chem. 2019;2019:4597–4600. doi:10.1002/ejic.201900979
- Troyano J, Çamur C, Garzón-Tovar L, et al. Spray-Drying Synthesis of MOFs, COFs, and Related Composites. Acc Chem Res. 2020;53(6):1206–1217. doi:10.1021/acs.accounts.0c00133
- Rasmussen EG, Kramlich J, Novosselov IV. Scalable Continuous Flow Metal-Organic Framework (MOF) Synthesis Using Supercritical CO2. ACS Sustainable Chem Eng. 2020;8(26):9680–9689. doi:10.1021/acssuschemeng.0c01429
- Haider J, Shahzadi A, Akbar MU, et al. A review of synthesis, fabrication, and emerging biomedical applications of metal-organic frameworks. Biomaterials Adv. 2022;140:213049. doi:10.1016/j.bioadv.2022.213049
- Nikpour S, Ansari-Asl Z, Sedaghat T, et al. Curcumin-loaded Fe-MOF/PDMS porous scaffold: fabrication, characterization, and biocompatibility assessment. J Ind Eng Chem. 2022;110:188–197. doi:10.1016/j.jiec.2022.02.052
- Wang Q, Zhao Y, Shi Z, et al. Magnetic amino functionalized MOF(M = Fe, Ti, Zr)@COFs with superior biocompatibility: performance and mechanism on adsorption of azo dyes in soft drinks. Chem Eng J. 2021;420:129955. doi:10.1016/j.cej.2021.129955
- Gecgel C, Bulut Simsek U, Turabik M, et al. Synthesis of titanium doped iron based metal–organic frameworks and investigation of their biological activities. J Inorg Organomet P. 2020;30:749–757. doi:10.1007/s10904-019-01329-3
- Al-Ansari DE, Al-Badr M, Zakaria ZZ, et al. Evaluation of Metal‐Organic Framework MIL-89 nanoparticles toxicity on embryonic zebrafish development. Toxicology Reports. 2022;9:951–960. doi:10.1016/j.toxrep.2022.04.016
- Vogt A-CS, Arsiwala T, Mohsen M, et al. On Iron Metabolism and Its Regulation. Int J Mol Sci. 2021;22:4591. doi:10.3390/ijms22094591
- Loret T, Rogerieux F, Trouiller B, et al. Predicting the in vivo pulmonary toxicity induced by acute exposure to poorly soluble nanomaterials by using advanced in vitro methods. Part Fibre Toxicol. 2018;15:25. doi:10.1186/s12989-018-0260-6
- Chen G, Leng X, Luo J, et al. In Vitro Toxicity Study of a Porous Iron(III) Metal‒Organic Framework. Molecules. 2019;24(7):1211. doi:10.3390/molecules24071211
- Liu C-H, Chiu H-C, Sung H-L, et al. Acute oral toxicity and repeated dose 28-day oral toxicity studies of MIL-101 nanoparticles. Regul Toxicol Pharm. 2019;107:104426. doi:10.1016/j.yrtph.2019.104426
- Kumar P, Anand B, Tsang YF, et al. Regeneration, degradation, and toxicity effect of MOFs: opportunities and challenges. Environ Res. 2019;176:108488. doi:10.1016/j.envres.2019.05.019
- Ettlinger R, Lächelt U, Gref R, et al. Toxicity of metal–organic framework nanoparticles: from essential analyses to potential applications. Chem Soc Rev. 2022;51:464–484. doi:10.1039/D1CS00918D
- Yuyu Z, Weicong L, Rao C, et al. Recent Advances in Fe-MOF Compositions for Biomedical Applications. Curr Med Chem. 2021;28(30):6179–6198. doi:10.2174/0929867328666210511014129
- Ameta RK, Koshti RR, Vyas A, et al. Fe(CN)6]4−/[Fe(CN)6]3− based metal organic ionic frameworks and impact of Fe2+/Fe3+ on material-medicinal-properties. J Mol Liq. 2018;268:677–684. doi:10.1016/j.molliq.2018.07.057
- Du C, Zhang Y, Zhang Z, et al. Fe-based metal organic frameworks (Fe-MOFs) for organic pollutants removal via photo-Fenton: a review. Chem Eng J. 2022;431(2):133932. doi:10.1016/j.cej.2021.133932
- Nowroozi-Nejad Z, Bahramian B, Hosseinkhani S. Efficient immobilization of firefly luciferase in a metal organic framework: fe-MIL-88(NH2) as a mighty support for this purpose. Enzyme Microb Tech. 2019;121:59–67. doi:10.1016/j.enzmictec.2018.10.011
- Bezverkhyy I, Weber G, Bellat J-P. Degradation of fluoride-free MIL-100(Fe) and MIL-53(Fe) in water: effect of temperature and pH. Micropor Mesopor Mat. 2016;219:117–124. doi:10.1016/j.micromeso.2015.07.037
- Liu N, Wang J, Wu J, et al. Magnetic Fe3O4@MIL-53(Fe) nanocomposites derived from MIL-53(Fe) for the photocatalytic degradation of ibuprofen under visible light irradiation. Mater Res Bull. 2020;132:111000. doi:10.1016/j.materresbull.2020.111000
- Ur Rasheed H, Xiaomeng L, Zhang S, et al. Ternary MIL-100(Fe)@Fe3O4/CA magnetic nanophotocatalysts (MNPCs): magnetically separable and Fenton-like degradation of tetracycline hydrochloride. Adv Powder Technol. 2018;29(12):3305–3314. doi:10.1016/j.apt.2018.09.011
- Chen X, Chang R, Liu H, et al. Moving research direction in the field of metallic bioresorbable stents-A mini-review. Bioact Mater. 2023;24:20–25. doi:10.1016/j.bioactmat.2022.12.004
- Porras CA, Rouault TA. Iron Homeostasis in the CNS: an Overview of the Pathological Consequences of Iron Metabolism Disruption. Int J Mol Sci. 2022;23(9):4490. doi:10.3390/ijms23094490
- Rafael Quijia C, Lima C, Silva C, et al. Application of MIL-100(Fe) in drug delivery and biomedicine. J Drug Deliv Sci Tec. 2021;61:102217. doi:10.1016/j.jddst.2020.102217
- Sun Y, Zheng L, Yang Y, et al. Metal–organic framework nanocarriers for drug delivery in biomedical applications. Nano-Micro Lett. 2020;12:103. doi:10.1007/s40820-020-00423-3
- Xin M, Lepoitevin M, Serre C. Metal–organic frameworks towards bio-medical applications. Mater Chem Front. 2021;5(15):5573–5594. doi:10.1039/D1QM00784J
- Mallakpour S, Nikkhoo E, Mustansar Hussain C. Application of MOF materials as drug delivery systems for cancer therapy and dermal treatment. Coordin Chem Rev. 2022;451:214262. doi:10.1016/j.ccr.2021.214262
- Manzano M, Vallet-Regí M. Mesoporous Silica Nanoparticles for Drug Delivery. Adv Funct Mater. 2020;30:1902634. doi:10.1002/adfm.201902634
- Wang Z-X, Wang Z, Fu-Gen W. Carbon dots as drug delivery vehicles for antimicrobial applications: a minireview. Chem Med Chem. 2022;17:e202200003. doi:10.1002/cmdc.202200003
- Servatan M, Zarrintaj P, Mahmodi G, et al. Zeolites in drug delivery: progress, challenges and opportunities. Drug Discov Today. 2020;25(4):642–656. doi:10.1016/j.drudis.2020.02.005
- Siyu H, Wu L, Li X, et al. Metal-organic frameworks for advanced drug delivery. Acta Pharm Sin B. 2021;11(8):2362–2395. doi:10.1016/j.apsb.2021.03.019
- Cai W, Wang J, Chu C, et al. Metal–organic framework-based stimuli-responsive systems for drug delivery. Adv Sci. 2019;6:1801526. doi:10.1002/advs.201801526
- Darvishi S, Javanbakht S, Heydari A, et al. Ultrasound-assisted synthesis of MIL-88(Fe) coordinated to carboxymethyl cellulose fibers: a safe carrier for highly sustained release of tetracycline. Int J Biol Macromol. 2021;181:937–944. doi:10.1016/j.ijbiomac.2021.04.092
- Liu XB, Liang TT, Zhang RT, et al. Iron-Based Metal-Organic Frameworks in Drug Delivery and Biomedicine. ACS Appl Mater Interfaces. 2021;13 8 :9643–9655. doi:10.1021/acsami.0c21486
- Bui A, Guillen SG, Sua A, et al. Iron-containing metal-organic framework thin film as a drug delivery system. Colloid Surface A. 2022;650:129611. doi:10.1016/j.colsurfa.2022.129611
- Guillen SG, Parres-Gold J, Ruiz A, et al. pH-responsive metal-organic framework thin film for drug delivery. Langmuir. 2022;38(51):16014–16023. doi:10.1021/acs.langmuir.2c02497
- Nejadshafiee V, Naeimi H, Goliaei B, et al. Magnetic bio-metal–organic framework nanocomposites decorated with folic acid conjugated chitosan as a promising biocompatible targeted theranostic system for cancer treatment. Mat Sci Eng C. 2019;99:805–815. doi:10.1016/j.msec.2019.02.017
- Li X, Lachmanski L, Safi S, et al. New insights into the degradation mechanism of metal-organic frameworks drug carriers. Sci Rep. 2017;7:13142. doi:10.1038/s41598-017-13323-1
- Strzempek W, Menaszek E, Gil B. Fe-MIL-100 as drug delivery system for asthma and chronic obstructive pulmonary disease treatment and diagnosis. Micropor Mesopor Mat. 2019;280:264–270. doi:10.1016/j.micromeso.2019.02.018
- Latifi L, Sohrabnezhad S. Drug delivery by micro and meso metal-organic frameworks. Polyhedron. 2020;180:114321. doi:10.1016/j.poly.2019.114321
- Ji HB, Kim CR, Min CH, et al. Fe-containing metal-organic framework with D-penicillamine for cancer-specific hydrogen peroxide generation and enhanced chemodynamic therapy. Bioeng Transl Med. 2023;8:e10477. doi:10.1002/btm2.10477
- Lajevardi A, Hossaini Sadr M, Badiei A, et al. Synthesis and characterization of Fe3O4@SiO2@MIL-100(Fe) nanocomposite: a nanocarrier for loading and release of celecoxib. J Mol Liq. 2020;307:112996. doi:10.1016/j.molliq.2020.112996
- Wang Y, Zhang J, Zhang C, et al. Functional-protein-assisted fabrication of fe–gallic acid coordination polymer nanonetworks for localized photothermal therapy. ACS Sustainable Chem Eng. 2019;7(1):994–1005. doi:10.1021/acssuschemeng.8b04656
- Yao XX, Chen DY, Zhao B, et al. Acid-Degradable Hydrogen-Generating Metal-Organic Framework for Overcoming Cancer Resistance/Metastasis and Off-Target Side Effects. Adv Sci. 2022;9:2101965. doi:10.1002/advs.202101965
- Gisela Quintero-álvarez F, Karina Rojas-Mayorga C, et al. Physicochemical Modeling of the Adsorption of Pharmaceuticals on MIL-100-Fe and MIL-101-Fe MOFs. Adsorpt Sci Technol. 2022;4482263. doi:10.1155/2022/4482263
- Lin S, Liu X, Tan L, et al. Porous Iron-Carboxylate Metal–Organic Framework: a Novel Bioplatform with Sustained Antibacterial Efficacy and Nontoxicity. ACS Appl Mater Interfaces. 2017;9(22):19248–19257. doi:10.1021/acsami.7b04810
- Unamuno X, Imbuluzqueta E, Salles F, et al. Biocompatible porous metal-organic framework nanoparticles based on Fe or Zr for gentamicin vectorization. Eur J Pharm Biopharm. 2018;132:11–18. doi:10.1016/j.ejpb.2018.08.013
- Nur Hasan M, Bera A, Maji TK, et al. Sensitization of nontoxic MOF for their potential drug delivery application against microbial infection. Inorg Chim Acta. 2021;523:120381. doi:10.1016/j.ica.2021.120381
- Du L, Chen W, Zhu P, et al. Applications of functional metal-organic frameworks in biosensors. Biotechnol J. 2021;16:1900424. doi:10.1002/biot.201900424
- Bieniek A, Terzyk AP, Wiśniewski M, et al. MOF materials as therapeutic agents, drug carriers, imaging agents and biosensors in cancer biomedicine: recent advances and perspectives. Prog Mater Sci. 2021;117:100743. doi:10.1016/j.pmatsci.2020.100743
- Liao X, Haomin F, Yan T, et al. Electroactive metal–organic framework composites: design and biosensing application. Biosens Bioelectron. 2019;146:111743. doi:10.1016/j.bios.2019.111743
- Guo L, Zhaode M, Yan B, et al. A novel electrochemical biosensor for sensitive detection of non-small cell lung cancer ctDNA using NG-PEI-COFTAPB-TFPB as sensing platform and Fe-MOF for signal enhancement. Sensor Actuat B-Chem. 2022;350:130874. doi:10.1016/j.snb.2021.130874
- Xu WQ, Jiao L, Yan HY, et al. Glucose oxidase-integrated metal–organic framework hybrids as biomimetic cascade nanozymes for ultrasensitive glucose biosensing. ACS Appl Mater Interfaces. 2019;11 25 :22096–22101. doi:10.1021/acsami.9b03004
- Mao XX, He FN, Qiu D, et al. Efficient Biocatalytic System for Biosensing by Combining Metal- Organic Framework (MOF)-Based Nanozymes and G-Quadruplex (G4)-DNAzymes. Anal Chem. 2022;94(20):7295–7302. doi:10.1021/acs.analchem.2c00600
- Tong PH, Wang JJ, Hu X-L, et al. Metal-organic framework (MOF) hybridized gold nanoparticles as a bifunctional nanozyme for glucose sensing. Chem Sci. 2023;14:7762–7769. doi:10.1039/D3SC02598E
- Let S, Samanta P, Dutta S, et al. A Dye@MOF composite as luminescent sensory material for selective and sensitive recognition of Fe(III) ions in water. Inorg Chim Acta. 2020;500:119205. doi:10.1016/j.ica.2019.119205
- Zhou ZY, Wang J, Hou S, et al. Room Temperature Synthesis Mediated Porphyrinic NanoMOF Enables Benchmark Electrochemical Biosensing. Small;2023. 2301933. doi:10.1002/smll.202301933
- Ding Z, Lu Y, Wei Y, et al. DNA-Engineered iron-based metal-organic framework bio-interface for rapid visual determination of exosomes. J Colloid Interf Sci. 2022;612:424–433. doi:10.1016/j.jcis.2021.12.133
- Yan W, Zhou J, Jiang Y-S, et al. Silver Nanoparticles@Metal-Organic Framework as Peroxidase Mimics for Colorimetric Determination of Hydrogen Peroxide and Blood Glucose. Chinese J Anal Chem. 2022;50(12):100187. doi:10.1016/j.cjac.2022.100187
- Zhu N, Lantian G, Wang J, et al. Novel and Sensitive Chemiluminescence Sensors Based on 2D-MOF Nanosheets for One-Step Detection of Glucose in Human Urine. J Phys Chem C. 2019;123(14):9388–9393. doi:10.1021/acs.jpcc.9b00671
- Yao J, Xie Z, Zeng X, et al. Bimetallic Eu/Fe-MOFs ratiometric fluorescent nanoenzyme for selective cholesterol detection in biological serum: synthesis, characterization, mechanism and DFT calculations. Sensor Actuat B-Chem. 2022;354:130760. doi:10.1016/j.snb.2021.130760
- Zhang Y, Feng Y-S, Ren X-H, et al. Bimetallic molecularly imprinted nanozyme: dual-mode detection platform. Biosens Bioelectron. 2022;196:113718. doi:10.1016/j.bios.2021.113718
- Jiang Y, Yang Q-M, Xu Q-J, et al. Metal organic framework MIL-53(Fe) as an efficient artificial oxidase for colorimetric detection of cellular biothiols. Anal Biochem. 2019;577:82–88. doi:10.1016/j.ab.2019.04.020
- Jungyeon J, So Yeon K, Choi KM, et al. Hydrogen peroxide sensor using the biomimetic structure of peroxidase including a metal organic framework. Appl Surf Sci. 2021;554:148786. doi:10.1016/j.apsusc.2020.148786
- Ling P-H, Zang X-N, Qian C-H, et al. A metal–organic framework with multienzyme activity as a biosensing platform for real-time electrochemical detection of nitric oxide and hydrogen peroxide. Analyst. 2021;146:2609. doi:10.1039/D1AN00142F
- Tu RX, Wang YJ, Peng JY, et al. Integration of multiple redox centers into porous coordination networks for ratiometric sensing of dissolved oxygen. ACS Appl Mater Interfaces. 2021;13 34 :40847–40852. doi:10.1021/acsami.1c13601
- Hou C, Zhao D, Wang Y, et al. Preparation of magnetic Fe3O4/PPy@ZIF-8 nanocomposite for glucose oxidase immobilization and used as glucose electrochemical biosensor. J Electroanal Chem. 2018;822:50–56. doi:10.1016/j.jelechem.2018.04.067
- Wang L, Zhang Y, Li X, et al. The MIL-88A-Derived Fe3O4-Carbon Hierarchical Nanocomposites for Electrochemical Sensing. Sci Rep. 2015;5:14341. doi:10.1038/srep14341
- Basaleh AS, Sheta SM. Novel advanced nanomaterial based on ferrous metal–organic framework and its application as chemosensors for mercury in environmental and biological samples. Anal Bioanal Chem. 2020;412:3153–3165. doi:10.1007/s00216-020-02566-z
- Wang X-N, Zhao Y, Li J-L, et al. Biomimetic catalysts of iron-based metal–organic frameworks with high peroxidase-mimicking activity for colorimetric biosensing. Dalton Trans. 2021;50:3854–3861. doi:10.1039/D0DT02504F
- Daniel M, Mathew G, Anpo M, et al. MOF based electrochemical sensors for the detection of physiologically relevant biomolecules: an overview. Coordin Chem Rev. 2022;468:214627. doi:10.1016/j.ccr.2022.214627
- Mohan B, Kumar S, Xi H, et al. Fabricated Metal-Organic Frameworks (MOFs) as luminescent and electrochemical biosensors for cancer biomarkers detection. Biosens Bioelectron. 2022;197:113738. doi:10.1016/j.bios.2021.113738
- Liang A, Zhao Y, Huang X, et al. A facile and sensitive fluorescence assay for glucose via hydrogen peroxide based on MOF-Fe catalytic oxidation of TMB. Spectrochim Acta A. 2022;265:120376. doi:10.1016/j.saa.2021.120376
- Aguilar-Palma R, Malankowska M, Coronas J. Applications of metal-organic frameworks and zeolites to virus detection and control: biosensors, barriers, and biocomposites, Z. Anorg Allg Chem. 2021;647:1532–1541. doi:10.1002/zaac.202000453
- Chen K, Wu C-D. Designed fabrication of biomimetic metal–organic frameworks for catalytic applications. Coordin Chem Rev. 2019;378:445–465. doi:10.1016/j.ccr.2018.01.016
- Wu P, Gong F, Feng X, et al. Multimetallic nanoparticles decorated metal-organic framework for boosting peroxidase-like catalytic activity and its application in point-of-care testing. J Nanobiotechnol. 2023;21:185. doi:10.1186/s12951-023-01946-8
- Zhu G, Wang S, Yu Z, et al. Application of Fe‑MOFs in advanced oxidation processes. Res Chem Int. 2019;45:3777–3793. doi:10.1007/s11164-019-03820-5
- Tocco Cristina Carucci D, Todde D, Todde D, et al. Enzyme immobilization on metal organic frameworks: laccase from Aspergillus sp. is better adapted to ZIF-zni rather than Fe-BTC. Colloid Surface B. 2021;208:112147. doi:10.1016/j.colsurfb.2021.112147
- Kesse X, Sicard C, Steunou N, et al. Encapsulation of Microperoxidase-8 into MIL-101(Cr/Fe) Nanoparticles: a New Biocatalyst for the Epoxidation of Styrene. Eur J Inorg Chem. 2023;26:e202300040. doi:10.1002/ejic.202300040
- Tian D, Hao R, Zhang X, et al. Multi-compartmental MOF microreactors derived from Pickering double emulsions for chemo-enzymatic cascade catalysis. Nat Commun. 2023;14:3226. doi:10.1038/s41467-023-38949-w
- Xiang X, Pang H, Ma T, et al. Ultrasound targeted microbubble destruction combined with Fe-MOF based bio-/enzyme-mimics nanoparticles for treating of cancer. J Nanobiotechnol. 2021;19:92. doi:10.1186/s12951-021-00835-2
- Li SF, Chen Y, Wang Y-S, et al. Integration of enzyme immobilization and biomimetic catalysis in hierarchically porous metal-organic frameworks for multi-enzymatic cascade reactions. Sci China Chem. 2022;65:1122–1128. doi:10.1007/s11426-022-1254-5
- Qin J-S, Yuan S, Lollar C, et al. Stable metal–organic frameworks as a host platform for catalysis and biomimetics. Chem Commun. 2018;54:4231–4249. doi:10.1039/C7CC09173G
- Liu J, Liang J, Xue J, et al. Metal–Organic Frameworks as a Versatile Materials Platform for Unlocking New Potentials in Biocatalysis. Small. 2021;17:2100300. doi:10.1002/smll.202100300
- Fandzloch M, Maldonado CR, Navarro JAR, et al. Biomimetic 1‑Aminocyclopropane-1-Carboxylic Acid Oxidase Ethylene Production by MIL-100(Fe)-Based Materials. ACS Appl Mater Interfaces. 2019;11:34053–34058. doi:10.1021/acsami.9b13361
- Wang JN, Bao MY, Wei TX, et al. Bimetallic metal–organic framework for enzyme immobilization by biomimetic mineralization: constructing a mimic enzyme and simultaneously immobilizing natural enzymes. Anal Chim Acta. 2020;1098:148–154. doi:10.1016/j.aca.2019.11.039
- Liu X, Wei Q, Wang Y, et al. Rational Design of Mimic Multienzyme Systems in Hierarchically Porous Biomimetic Metal-Organic Frameworks. ACS Appl Mater Interfaces. 2018;10(39):33407–33415. doi:10.1021/acsami.8b09388
- Liang J, Johannessen B, Wu Z, et al. Regulating the Coordination Environment of Mesopore-Confined Single Atoms from Metalloprotein-MOFs for Highly Efficient Biocatalysis. Adv Mater. 2022;34:2205674. doi:10.1002/adma.202205674
- Yang J, Li J, Ng DHL, et al. Micromotor-Assisted Highly Efficient Fenton Catalysis by Laccase/Fe-BTC-NiFe2O4 Nanozyme Hybrid with 3D Hierarchical Structure. Environ Sci-Nano. 2020;7 9 :2573–2583. doi:10.1039/c9en01443h
- Yang Q, Chena D, Chu L, et al. Enhancement of ionizing radiation-induced catalytic degradation of antibiotics using Fe/C nanomaterials derived from Fe-based MOFs. J Hazard Mater. 2020;389:122148. doi:10.1016/j.jhazmat.2020.122148
- Zhao Y, Jiang X, Liu X, et al. Application of photo-responsive metal-organic framework in cancer therapy and bioimaging. Front Bioeng Biotechnol. 2022;10:1031986. doi:10.3389/fbioe.2022.1031986
- Zheng Q, Liu X, Zheng Y, et al. The recent progress on metal–organic frameworks for phototherapy. Chem Soc Rev. 2021;50:5086–5125. doi:10.1039/d1cs00056j
- Nikazar S, Barani M, Rahdar A, et al. Photo- and Magnetothermally Responsive Nanomaterials for Therapy, Controlled Drug Delivery and Imaging Applications. ChemistrySelect. 2020;5:12590–12609. doi:10.1002/slct.202002978
- Rojas JD, Joiner JB, Velasco B, et al. Validation of a combined ultrasound and bioluminescence imaging system with magnetic resonance imaging in orthotopic pancreatic murine tumors. Sci Rep. 2022;12:102. doi:10.1038/s41598-021-03684-z
- Zhiming H, Caina X, Liang Y, et al. Multifunctional drug delivery nanoparticles based on MIL-100 (Fe) for photoacoustic imaging-guided synergistic chemodynamic/chemo/photothermal breast cancer therapy. Mater Design. 2022;223:111132. doi:10.1016/j.matdes.2022.111132
- Cai X, Deng X, Xie Z, et al. Controllable synthesis of highly monodispersed nanoscale Fe-soc-MOF and the construction of Fe-soc-MOF@polypyrrole core-shell nanohybrids for cancer therapy. Chem Eng J. 2019;358:369–378. doi:10.1016/j.cej.2018.10.044
- Sui C, Tan R, Chen Y, et al. MOFs-Derived Fe–N Codoped Carbon Nanoparticles as O 2 -Evolving Reactor and ROS Generator for CDT/PDT/PTT Synergistic Treatment of Tumors. Bioconjugate Chem. 2021;32:318–327. doi:10.1021/acs.bioconjchem.0c00694
- Peller M, Böll K, Zimpel A, et al. Metal–organic framework nanoparticles for magnetic resonance imaging. Inorg Chem Front. 2018;5:1760–1779. doi:10.1039/C8QI00149A
- Böll K, Zimpel A, Dietrich O, et al. Clinically Approved MRI Contrast Agents as Imaging Labels for a Porous Iron-Based MOF Nanocarrier: a Systematic Investigation in a Clinical MRI Setting. Adv Therap. 2020;3:1900126. doi:10.1002/adtp.201900126
- Wang Z, Wanjian Y, Yu N, et al. Construction of CuS@Fe-MOF nanoplatforms for MRI-guided synergistic photothermal-chemo therapy of tumors. Chem Eng J. 2020;400:125877. doi:10.1016/j.cej.2020.125877
- Alanagh Hamideh R, Akbari B, Fathi P, et al. Biodegradable MRI Visible Drug Eluting Stent Reinforced by Metal Organic Frameworks. Adv Healthcare Mater. 2020;9:2000136. doi:10.1002/adhm.202000136
- Liu P, Zhou Y, Shi X, et al. A cyclic nano-reactor achieving enhanced photodynamic tumor therapy by reversing multiple resistances. J Nanobiotechnol. 2021;19:149. doi:10.1186/s12951-021-00893-6
- Permyakova A, Kakar A, Bachir J, et al. In Situ Synthesis of a Mesoporous MIL-100(Fe) Bacteria Exoskeleton. ACS Materials Lett. 2023;5(1):79–84. doi:10.1021/acsmaterialslett.2c00820
- Demir Duman F, Forgan RS. Applications of nanoscale metal–organic frameworks as imaging agents in biology and medicine. J Mater Chem B. 2021;9:3423–3449. doi:10.1039/D1TB00358E
- Zhang WM, Wang J, Su LC, et al. Activatable nanoscale metal-organic framework for ratiometric photoacoustic imaging of hydrogen sulfide and orthotopic colorectal cancer in vivo. Sci China Chem. 2020 63 9 ;1315–1322. doi:10.1007/s11426-020-9775-y
- Wang L, Qu XZ, Zhao YX, et al. Exploiting Single Atom Iron Centers in a Porphyrin-like MOF for Efficient Cancer Phototherapy. ACS Appl Mater Interfaces. 2019;11 38 :35228–35237. doi:10.1021/acsami.9b11238
- Zheng X, Zhong J, Dong M-Y, et al. Synthesis of porphyrin-based 2D ytterbium metal organic frameworks for efficient photodynamic therapy. RSC Adv. 2022;12:34318. doi:10.1039/D2RA06655F
- Cai X, Liu B, Pang M, et al. Interfacially synthesized Fe- soc -MOF nanoparticles combined with ICG for photothermal/photodynamic therapy. Dalton Trans. 2018;47:16329–16336. doi:10.1039/C8DT02941E
- Jingchao H, Ramachandraiah K, Huang T, et al. Core-shell structured hollow copper sulfide@metal-organic framework for magnetic resonance imaging guided photothermal therapy in second near-infrared biological window. Biochem Bioph Res Co. 2023;638:51–57. doi:10.1016/j.bbrc.2022.11.036
- Yao J, Liu Y, Wang J, et al. On-demand CO release for amplification of chemotherapy by MOF functionalized magnetic carbon nanoparticles with NIR irradiation. Biomaterials. 2019;195:51–62. doi:10.1016/j.biomaterials.2018.12.029
- Wang Z, Liu B, Sun QQ, et al. Upconverted Metal−Organic Framework Janus Architecture for Near-Infrared and Ultrasound Co-Enhanced High Performance Tumor Therapy. ACS Nano. 2021;15 7 :12342–12357. doi:10.1021/acsnano.1c04280
- Bao Z, Kexin L, Hou P, et al. Nanoscale metal-organic framework composites for phototherapy and synergistic therapy of cancer. Mater Chem Front. 2021;5:1632. doi:10.1039/D0QM00786B
- Huang X, Sun X, Wang W, et al. Nanoscale metal–organic frameworks for tumor phototherapy. J Mater Chem B. 2021;9:3756–3777. doi:10.1039/D1TB00349F
- Li R, Chen TT, Pan XL. Metal−Organic-Framework Based Materials for Antimicrobial Applications. ACS Nano. 2021;15 3 :3808–3848. doi:10.1021/acsnano.0c09617
- Joakim Larsson DG, Flach C-F. Antibiotic resistance in the environment. Nat Rev Microbiol. 2022;20(5):257–269. doi:10.1038/s41579-021-00649-x
- Ghosh C, Sarkar P, Issa R, et al. Alternatives to Conventional Antibioticsinthe Era of Antimicrobial Resistance. Trends Microbiol. 2019;27:4. doi:10.1016/j.tim.2018.12.010
- Gorniak I, Bartoszewski R, et al. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem Rev. 2019;18 1 :241–272. doi:10.1007/s11101-018-9591-z
- Ciulla MG, Gelain F. Structure–activity relationships of antibacterial peptides. Microb Biotechnol. 2023;16 4 :757–777. doi:10.1111/1751-7915.14213
- Fan XZ, Yahia L, Sacher, E. Antimicrobial Properties of the Ag, Cu Nanoparticle System. Biology-Basel. 2021;10 2 :137. doi:10.3390/biology10020137
- Gharpure S, Akash A, Ankamwar B. A Review on Antimicrobial Properties of Metal Nanoparticles. J Nanosci Nanotechnol. 2020 20 6 :3303–3339. doi:10.1166/jnn.2020.17677
- Vasantharaj S, Sathiyavimal S, Senthilkumar P, et al. Biosynthesis of iron oxide nanoparticles using leaf extract of Ruellia tuberosa: antimicrobial properties and their applications in photocatalytic degradation. J Photoch Photobio B. 2019;192:74–82. doi:10.1016/j.jphotobiol.2018.12.025
- Kumar Dey U, Sankar Maiti P, Koley T, et al. Development of nano–magnesium oxide modified hybrid resin system for antimicrobial coating. J Coat Technol Res. 2023;20(1):223–231. doi:10.1007/s11998-022-00650-w
- Razaviamri S, Wang K, Liu B, et al. Catechol-Based Antimicrobial Polymers. Molecules. 2021;26:559. doi:10.3390/molecules26030559
- Mao D, Fang H, Ji S, et al. Metal–Organic-Framework-Assisted In Vivo Bacterial Metabolic Labeling and Precise Antibacterial Therapy. Adv Mater. 2018;30:1706831. doi:10.1002/adma.201706831
- Pettinari C, Pettinari R, Di Nicola C, et al. Antimicrobial MOFs. Coordin Chem Rev. 2021;446:214121. doi:10.1016/j.ccr.2021.214121
- Rubin HN, Neufeld BH, Reynolds MM. Surface-anchored metal–organic framework–cotton material for tunable antibacterial copper delivery. ACS Appl Mater Interfaces. 2018;10(17):15189–15199. doi:10.1021/acsami.7b19455
- Sheta SM, Salem SR, El‑Sheikh SM. A novel Iron (III)‑based MOF: synthesis, characterization, biological, and antimicrobial activity study. J Mater Res. 2022;37(14):2357–2367. doi:10.1557/s43578-022-00644-9
- Golmohamadpour A, Bahramian B, Khoobi M, et al. Antimicrobial photodynamic therapy assessment of three indocyanine green-loaded metal-organic frameworks against Enterococcus faecalis. Photodiagn Photodyn Therapy. 2018;23:331–338. doi:10.1016/j.pdpdt.2018.08.004
- Claes B, Boudewijns T, Muchez L, et al. Smart metal–organic framework coatings: triggered antibiofilm compound release. ACS Appl Mater Interfaces. 2017;9(5):4440–4449. doi:10.1021/acsami.6b14152
- Caamaño K, Heras-Mozos R, Calbo J, et al. Exploiting the Redox Activity of MIL-100(Fe) Carrier Enables Prolonged Carvacrol Antimicrobial Activity. ACS Appl Mater Interfaces. 2022;14:10758–10768. doi:10.1021/acsami.1c21555
- Yang M, Zhang J, Wei Y, et al. Recent advances in metal-organic framework-based materials for anti-staphylococcus aureus infection. Nano Res. 2022;15:6220–6242. doi:10.1007/s12274-022-4302-x
- Huang X, Shijiang Y, Lin W, et al. A metal-organic framework MIL-53(Fe) containing sliver ions with antibacterial property. J Solid State Chem. 2021;302:122442. doi:10.1016/j.jssc.2021.122442
- Liu Y, Zhou L, Dong Y, et al. Recent developments on MOF-based platforms for antibacterial therapy. RSC Med Chem. 2021;12:915–928. doi:10.1039/D0MD00416B
- Reza Ramezani M, Ansari-Asl Z, Hoveizi E, et al. Polyacrylonitrile/Fe(III) metal-organic framework fibrous nanocomposites designed for tissue engineering applications. Mater Chem Phys. 2019;229:242–250. doi:10.1016/j.matchemphys.2019.03.031
- Zhao J, Wei F, Xu W, et al. Enhanced antibacterial performance of gelatin/chitosan film containing capsaicin loaded MOFs for food packaging. Appl Surf Sci. 2020;510:145418. doi:10.1016/j.apsusc.2020.145418
- Uthappa UT, Sriram G, Arvind OR, et al. Engineering MIL-100(Fe) on 3D porous natural diatoms as a versatile high performing platform for controlled isoniazid drug release, Fenton’s catalysis for malachite green dye degradation and environmental adsorbents for Pb2+ removal and dyes. Appl Surf Sci. 2020;528:146974. doi:10.1016/j.apsusc.2020.146974
- Zhang X, Peng F, Wang D. MOFs and MOF-derived materials for antibacterial application. J Funct Biomater. 2022;13:215. doi:10.3390/jfb13040215
- Nong WQ, Wu J, Ghiladi RA, et al. The structural appeal of metal–organic frameworks in antimicrobial applications. Coordin Chem Rev. 2021;442:214007. doi:10.1016/j.ccr.2021.214007