Abstract
Mammalian metallothioneins (MTs) are low molecular weight (6–7 kDa) cysteine-rich proteins that are specifically induced by metal nanoparticles (NPs). MT induction in cell therapy may provide better protection by serving as antioxidant, anti-inflammatory, antiapoptotic agents, and by augmenting zinc-mediated transcriptional regulation of genes involved in cell proliferation and differentiation. Liposome-encapsulated MT-1 promoter has been used extensively to induce growth hormone or other genes in culture and gene-manipulated animals. MTs are induced as a defensive mechanism in chronic inflammatory conditions including neurodegenerative diseases, cardiovascular diseases, cancer, and infections, hence can serve as early and sensitive biomarkers of environmental safety and effectiveness of newly developed NPs for clinical applications. Microarray analysis has indicated that MTs are significantly induced in drug resistant cancers and during radiation treatment. Nutritional stress and environmental toxins (eg, kainic acid and domoic acid) induce MTs and aggregation of multilamellar electron-dense membrane stacks (Charnoly body) due to mitochondrial degeneration. MTs enhance mitochondrial bioenergetics of reduced nicotinamide adenine dinucleotide–ubiquinone oxidoreductase (complex-1), a rate-limiting enzyme complex involved in the oxidative phosphorylation. Monoamine oxidase-B inhibitors (eg, selegiline) inhibit α-synuclein nitration, implicated in Lewy body formation, and inhibit 1-methyl 4-phenylpyridinium and 3-morpholinosydnonimine-induced apoptosis in cultured human dopaminergic neurons and mesencephalic fetal stem cells. MTs as free radical scavengers inhibit Charnoly body formation and neurodegenerative α-synucleinopathies, hence Charnoly body formation and α-synuclein index may be used as early and sensitive biomarkers to assess NP effectiveness and toxicity to discover better drug delivery and surgical interventions. Furthermore, pharmacological interventions augmenting MTs may facilitate the theranostic potential of NP-labeled cells and other therapeutic agents. These unique characteristics of MTs might be helpful in the synthesis, characterization, and functionalization of emerging NPs for theranostic applications. This report highlights the clinical significance of MTs and their versatility as early, sensitive biomarkers in cell-based therapy and nanomedicine.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Mammalian metallothioneins (MT-1–4) are ubiquitous, low molecular weight (6–7 kDa), cysteine-rich, metal-binding proteins existing in all prokaryotes to eukaryotes. MTs were discovered for the first time by Marghoshes and Vallee in horse kidneys,Citation1 and in the rodent brain by the authors’ group.Citation2 Several researchers have elucidated the detailed molecular structure of MTs by performing nuclear magnetic resonance spectroscopic analysis.Citation3–Citation7 Zangger et al have determined the three-dimensional structure of mouse MT-1 by nuclear magnetic resonance analysis.Citation8 Briefly, these polypeptides possess 60–67 amino acid residues and 20 cysteine molecules which provide antioxidant properties to these metal detoxifying proteins. MTs possess two metal–thiolate clusters (C-terminal α-domain and N-terminal β-domain), including 20 cysteine residues and sulfur atoms, which bind to divalent or monovalent cations.Citation9 These domains are linked by a short peptide containing amino acid residue 30–32 in mammalian MTs. In the β-domain, three divalent or six monovalent metal ions are coordinated, while in the α-domain, four divalent or six monovalent cations remain bound. Zinc (Zn) is one of the most abundant divalent metal ions, and its concentration is greater than copper and manganese in the brain. Since free Zn is a potent inhibitor of sulfhydryl (–SH) enzymes, Zn in the brain is predominantly MT bound. However, there are several other proteins with which Zn can bind in the central nervous system (CNS) beyond the scope of this article. Unlike the hepatic or renal Zn thioneins, the Zn-binding proteins in the brain are not inducible following administration of Zn as it does not cross the blood–brain barrier efficiently. During oxidative stress, glutathione is significantly reduced.Citation10 Nitric oxide-mediated metal release is modulated from MTs by the redox state of glutathione in vitro.Citation11 MT induction attenuated carmustine-induced hippocampal toxicity, prevented glutathione reductase inhibition and glutathione depletion, and reduced tumor necrosis factor-α, malondialdehyde, and caspase-3 activity with preservation of cognition in rats.Citation12 These findings suggest the therapeutic potential of MTs in neurodegenerative disease and other disorders. Ferric nitrilotriacetate, which produces reactive oxygen species, induced MTs in the liver and kidney. Cadmium (Cd) released after an injection of ferric nitrilotriacetate induced MTs. Thus, MTs may act as antioxidants to compensate for glutathione depletion. MTs are more potent antioxidants compared to glutathione.Citation13 Thus, glutathione and MT synthesis is induced as an attempt to combat iron-induced oxidative stress.Citation14 Previous studies have shown that MTs are low molecular weight, heat-stable cytosolic proteins with a high content of cysteinyl sulfur that bind heavy metals like Cd, Zn, and copper.Citation15 As these proteins are induced following exposure to heavy metals, it is now well established that they have a detoxifying role during heavy metal toxicity. The primary function of MTs is in the homeostasis of the essential metals Zn and copper. In addition, a role MT plays in selenium (Se) metabolism in primates has been established. Furthermore, MT has gained significance in the clinical disorders related to trace metal metabolism. This report highlights some of the authors’ recent work on MTs and NPs. It also provides a brief update of this emerging field by highlighting the work from other laboratories.
Molecular biology of MTs
There are primarily four major isoforms of MTs. MT-1 and MT-2 are present ubiquitously; MT-3 is localized predominantly in the hippocampus and its depletion is implicated in the etiopathogenesis of Alzheimer’s disease (AD).Citation16 Recent studies have shown that MT3 expression is downregulated in the esophageal squamous cell carcinoma by DNA methylation.Citation17 MT-4 is localized in the buccal mucosa and pseudostratified squamous epithelial lining of the uterine cavity and is involved in desquamation during menstruation. The exact biological significance of MT-4 is yet to be established. MTs are capable of preventing oxidative stress and apoptotic cell death in the CNS. MTs promote neuronal survival and regeneration in vivo and are protective against metal ion toxicity, oxidative stress, and cytokine injury due to cerebral ischemia or infection, hence could be considered as early and sensitive biomarkers of redox signaling in neurodegenerative disorders such as Parkinson’s disease (PD), AD, multiple system atrophy, stroke, and epilepsy. However, the exact molecular mechanism of MT-mediated neuroprotection in these and other neurodegenerative disorders remains elusive. By using MT gene-manipulated mice and aging mitochondrial genome knockout (RhOmgko) cybrids as experimental models of PD and aging and micro-positron emission tomography neuroimaging with fluorine-18-L-dihydroxyphenylalanine and 18F fluorodeoxyglucose, it has been established that MTs may provide dopaminergic neuroprotection by (1) augmenting mitochondrial coenzyme Q10 synthesis, (2) attenuating α-synuclein nitration, (3) preserving mitochondrial glutathione, (4) enhancing neuromelanin synthesis, (5) preserving ferritin, (6) preventing metal ion accumulation, (7) acting as free radical scavengers, (8) attenuating peroxynitrite (ONOO−) ion neurotoxicity, (9) maintaining intracellular redox balance, (10) or through all of these mechanisms.Citation18 Whether augmentation of coenzyme Q10, glutathione, ferritin, melatonin, and neuromelanin synthesis in MT transgenic mice CNS occurs independently, is dependent on each other, or occurs synergistically, remains unknown.Citation19 Although it has been discovered that 3-morpholinosydnonimine (or SIN-1: a potent peroxynitrite donor) and 1-methyl, 4-phenyl, 1,2,3,6-tetrahydropyridine-induced α-synuclein nitration is attenuated in MT transgenic mice striatum, knowledge is very limited regarding the exact functional significance of these findings. In a recent study, the neuroprotective role of MTs in 3-morpholinosydnonimine and 1-methyl, 4-phenyl, 1,2,3,6-tetrahydropyridine-induced oxidative and nitrative stress was investigated, with a primary objective to explain the basic molecular mechanism of MT-mediated neuroprotection in PD and other neurodegenerative disorders. Based on these findings, it was reported that MTs are capable of inhibiting α-synuclein index and broadly classified neurodegenerative α-synucleinopathies.Citation20–Citation23 Cd/Se NP-labeled bone marrow-derived stem cells have also been used to determine their biodistribution, pharmacokinetics, and therapeutic potential in an experimental model of acute ischemic stroke (AIS) and have suggested that the therapeutic potential of stem cells can be augmented by MT induction.Citation24,Citation25 Furthermore, it was proposed that MT transgenic striatal fetal stem cells can be implanted in the striatal region of the homozygous weaver mutant (wv/wv) mice exhibiting progressive neurodegeneration and typical symptoms of PD, AD, and drug addiction to evaluate the therapeutic potential of MTs.Citation26
MTs and Zn homeostasis
Zn dyshomeostasis has been recognized as an important mechanism of cell death in acute brain injury. An increase in the level of free or histochemically reactive Zn in astrocytes and neurons is considered one of the major causes of cell death in ischemia and trauma. Although Zn dyshomeostasis can lead to cell death via diverse routes, the major pathway appears to involve oxidative stress. Recently, it has been discovered that a rise of Zn in autophagic vacuoles, including autolysosomes, is a prerequisite for lysosomal membrane permeabilization and cell death in cultured neurons exposed to oxidative stress. The source of Zn in this process is redox-sensitive Zn-binding protein MTs, which release Zn during oxidative stress. Of the MTs, MT-3 is particularly enriched in the CNS, but its exact biological significance is yet to be established. Like other MTs, MT-3 may function as a metal detoxicant, but may also inhibit neurite outgrowth and promote neuronal death by serving as a source of toxic Zn release. In addition, MT-3 regulates lysosomal functions. In the absence of MT-3, there are changes in lysosome-associated membrane protein-1 and -2 and reductions in lysosomal enzymes that result in decreased autophagic flux, which may have dual effects on cell survival. In acute oxidative injury, Zn dyshomeostasis and lysosomal membrane permeabilization are diminished in MT-3 knockout cells, resulting in reduced cell death. But during the chronic phase, diminished lysosomal function may lead to the accumulation of abnormal proteins and cytotoxicity. The role of Zn and MT-3 in autophagy and/or lysosomal function is now being explored. In light of evidence that autophagy and lysosomes may play significant roles in the pathogenesis of various neurological diseases, further insight into the contribution of Zn dynamics and MT-3 function may provide avenues to effectively regulate these processes in the CNS.Citation27 It has been discovered that the incidence of lysosomes and Charnoly body (CB) formation is significantly increased during nutritional stress and during environmental insult including kainic acid and domoic acid neurotoxicity, which may enhance MTs expression as a defensive mechanism (). CBs are electron-dense, multilamellar stacks of degenerated mitochondrial membranes that are generated as a consequence of free radical overproduction during oxidative and nitrative stress. MTs inhibit CB formation by acting as free radical scavengers to provide mitochondrial protection. Unfortunately, increased lysosomal activity as a consequence of severe nutritional stress or environmental neurotoxicity may structurally degrade induced MTs as well, thus compromising the intracellular defensive mechanisms which may lead to early morbidity and mortality in progressive neurodegenerative disorders including PD, AD, and drug addiction. Similarly, toxic NPs may induce autophagy as a result of intracellular acidosis which may trigger CB formation and its degradation by lysosomal activation during nutritional stress and/or environmental toxicity (). Recently, a peptide termed EmtinB has been designed, which is modeled after the β-domain of MT-2 and mimics the biological effects of MT-1/2 in vitro.Citation28 The neuroprotective effect of EmtinB in the in vitro and in vivo models of kainic acid-induced neurotoxicity has been investigated. EmtinB can pass through blood–brain barrier and is detectable in the plasma for up to 24 hours. Indeed, treatment with EmtinB attenuated seizures in C57BL/6J mice exposed to moderate (20 mg/kg) and high (30 mg/kg) kainic acid doses and decreased mortality. Furthermore, EmtinB treatment reduced kainic acid-induced neurodegeneration in the CA1 region, suggesting its role as a target for therapeutic development.
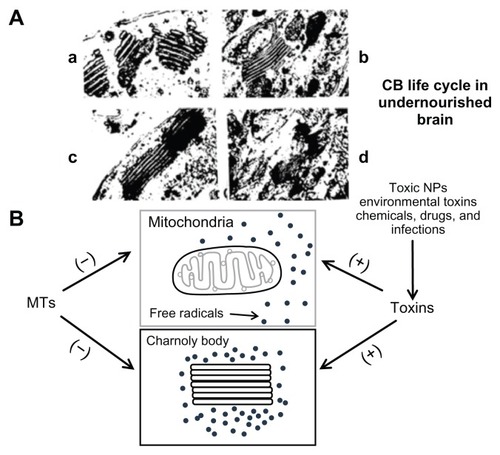
Figure 1 (A) Transmission electron microscope (magnification 50,000×) image illustrating CB formation in the developing undernourished Purkinje neurons as a consequence of degeneration of mitochondrial membranes (a). These membranes are transformed in to multilamellar (usually pentalamellar or heptalamellar) electron-dense structures (b) to form mature CBs (c). The structural degradation of CBs occurs eventually due to increased lysosomal activity (d). Based on the transmission electron microscopy and electrophysiological studies, it is proposed that CBs are involved in desmosomal repair, autophagy, and increased afterhyperpolarization duration due to increased intracellular calcium ions (Ca2+) in the 15-day undernourished rat cerebellar Purkinje neurons. (B) Free radicals (indicated by dots) are generated as a byproduct of mitochondrial oxidative phosphorylation.
Notes: Toxic NPs, environmental toxins, chemicals, drugs, nutritional stress, and infections augment free radical overproduction, which may trigger CB formation. MTs serve as free radical scavengers to protect mitochondrial structural and functional integrity by inhibiting CB formation. CB formation is a transitory state between apoptosis and cell death and is a reversible process. (+) indicates activation; (−) indicates inhibition.
Abbreviations: CB, Charnoly body; MT, metallothionein; NP, nanoparticle.
MTs attenuate domoic acid toxicity
Domoic acid is an excitatory amino acid and a rigid structural analog to glutamate and kainic acid which acts through glutamate receptors and elicits a rapid and potent neurotoxic response. Domoic acid continues to pose a global risk to the health and safety of humans and wildlife. It is a marine biotoxin associated with harmful algal blooms and is the causative agent of amnesic shellfish poisoning in marine animals and humans. The hippocampus is a specific target site having increased sensitivity to domoic acid toxicity. It has been discovered that even intrauterine exposure to domoic acid can induce damage to hippocampal CA3 and dentate nucleus in the progeny as noticed in AD patients with no overt clinical seizure activity.Citation29 Histopathological evidence indicates that in addition to neurons, the astrocytes are also injured as consequence of domoic acid toxicity. Furthermore, the effect of domoic acid has been confirmed in cultured primary astrocytes from the hippocampus and the brain stem. The biomarker analysis for the early response genes including c-Fos, c-Jun, c-Myc, Hsp-72, specific marker for the astrocytes – glial fibrillary acidic protein – and the glutamate receptors including glutamate receptor-2, N-methyl-D-aspartic acid receptor-1, -2A, and -2B has been performed by microarray analysis.Citation30 Although, the astrocyte glial fibrillary acidic protein and c-Fos were not affected, c-Jun and glutamate receptor-2 were downregulated. The chemokines/cytokines, tyrosine kinases (Trk), and apoptotic genes were also altered. The cytokines that were upregulated included interleukin-1α (IL-1α), IL-1β, IL-6, the small inducible cytokine, interferon protein 10P-10, CXC chemokine LIX, and insulin-like growth factor-binding proteins, whereas Bax, Bcl-2, Trk-A, and Trk-B were downregulated. Only the hippocampal astrocytes were affected, suggesting that astrocytes may be used as a pharmacological target for the prevention and treatment of domoic acid poisoning and for other CNS pathologies involving excitotoxicity. A comprehensive review of domoic acid-induced brain pathology including ultrastructural changes associated to subchronic oral exposure, its molecular mechanism of aggregation in NPs, cell/tissue injury, food safety, and human health issues is now available.Citation31 Recently, field studies in the Pacific Ocean and laboratory studies have detected increased domoic acid production under conditions of iron limitation.Citation32
MTs and stem cells in experimental autoimmune encephalomyelitis (EAE)
MTs as anti-inflammatory and neuroprotective macromolecules are induced during EAE and multiple sclerosis.Citation33 EAE and multiple sclerosis are characterized by significant inflammation, demyelination, neuroglial damage, and cell death. EAE is characterized by demyelination, inflammation, and neurodegeneration of CNS, in which free radicals play a significant role. Exogenous administration of Zn-bound MT-2 to Lewis rats with EAE reduced clinical symptoms and the inflammatory response, oxidative stress, and apoptosis of the CNS areas. Zn-bound MT-2 treatment prevented demyelination and axonal damage and transection, and stimulated oligodendroglial regeneration, as well as the expression of the basic fibroblast growth factor, transforming growth factor-β, neurotrophin-3, neurotrophin-4/5, and nerve growth factor. These beneficial effects of Zn-bound MT-2 could not be attributable to its Zn content, suggesting potential application of Zn-bound MT-2 as a safe and successful therapy for multiple sclerosis. Recently, the efficacy of murine mesenchymal stem cells (MSCs) as treatment of EAE induced in mice by the encephalitogenic peptide myelin oligodendrocyte glycoprotein (35–55) has been demonstrated.Citation34 Various biomarkers of oxidative stress, inflammation/degeneration, and apoptosis such as MTs, antioxidant enzymes (superoxide dismutase, catalase, and glutathione-S-transferase), poly(adenosine diphosphate ribose) polymerase-1, and p53 during EAE progression and following MSC treatment have been analyzed. MT expression was significantly increased in EAE mice compared with healthy controls, but while expression of MT-1 and MT-3 increased along EAE course, MT-2 was upregulated at the onset, but returned to control levels during chronic phase. The changes in the transcription and activity of the antioxidant enzymes and poly(adenosine diphosphate ribose) polymerase-1 and p53 expressions exhibited similar kinetics for MT-1 and MT-3. MSCs reduced the EAE-induced increases in activities of all these proteins supporting an antioxidant and neuroprotective role of MSCs that was further confirmed in vitro in neuroblastoma cells exposed to oxidative stress.Citation35
MTs and liposome-encapsulated stem cells
MSC transplantation is now a promising method which is being actively explored in regenerative medicine. Thus, gene-modified MSCs (such as MTs) may possess superior characteristics of specific tissue differentiation, resistance to apoptosis, and directional migration. However, viral vectors have the disadvantages of potential immunogenicity, carcinogenicity, and complicated synthetic procedures. Polyethylene glycol (PEG)-grafted polyethyleneimine holds promise in gene delivery because of easy preparation and targeting modification. Coaxial electrospinning enables the incorporation of liposomes into nanofibers, and polyvinyl alcohol core/poly-ɛ-caprolactone shell nanofibers with embedded liposomes can preserve the enzymatic activity of encapsulated horseradish peroxidase. The clinical potential of this system has been demonstrated by the enhancement of MSC proliferation, suggesting that intact liposomes incorporated into nanofibers by coaxial electrospinning may serve as promising drug delivery vectors.Citation36
Recently, investigators have synthesized a PEG 8000-grafted polyethylenimine 25,000 graft copolymer and used agarose gel retardation assay and dynamic light scattering to determine the properties of NPs.Citation37 A multifunctional envelope-type nano device (MEND) has been developed as a novel nonviral gene delivery system.Citation38 Furthermore, a study has been performed to determine the effect of systemic delivery of prednisolone phosphate encapsulated within “stealth” liposomes on bone erosion and osteoclast activity during experimental antigen-induced arthritis.Citation39 As preclinical evaluation in animals does not necessarily portray human responses, liposome-encapsulated hemoglobin, an artificial oxygen carrier, has been tested in immunodeficient mice reconstituted with human hematopoietic stem cells (cord blood-transfused NOD/SCID/IL-2Rγnull) mice.Citation40 In a further study, investigators have attempted to improve the specificity and efficiency of gene transfection and make the liposome a better gene transfer vector to the brain by using monoclonal antibody (anti-Lex/stage-specific embryonic antigen-1)-mediated targeting of liposomes.Citation41 To determine the effect on erythropoiesis of liposome-encapsulated dichloromethylene diphosphonate-induced changes in bone marrow macrophages, red blood cell parameters and the formation of erythroid burst-forming unit-derived colonies in vitro have been evaluated.Citation42 It is known that the human multidrug resistance (MDR1) gene encodes a 170 kD glycoprotein (P-glycoprotein), which is an adenosine triphosphate-dependent transmembrane efflux pump for many different cytotoxic drugs. Therefore, an efficient expression of the human MDR1 gene in mouse bone marrow cells after transfection with a liposomal delivery system has been developed.Citation43 In another study, investigators compared the toxic effects of actinomycin-D encapsulated either in the aqueous phase or in the lipid phase of liposomes and the nonencapsulated actinomycin-D on the blood forming system on cell proliferation in the intestine and on antibody production by spleen lymphocytes and have confirmed that actinomycin-D is less toxic to mice than nonencapsulated actinomycin-D, but retains its tumoricidal activity.Citation44 Similarly, nerve growth factor has also been encapsulated into liposomes in order to protect it from the enzyme degradation in vivo and promote permeability across the blood–brain barrier.Citation45 Furthermore, MEND has been developed as a novel nonviral gene delivery system.Citation46 The host response to systemically administered lipid NPs encapsulating plasmid DNA has been studied in the spleen using a DNA microarray and MEND.Citation47 These authors have established that PEGylation is a useful method for achieving a longer circulation time for delivery of MEND to a tumor via the enhanced permeability and retention effect. However, PEGylation inhibits cellular uptake and endosomal escape, which results in significant loss of activity for the delivery system. Therefore, for successful gene delivery, particularly for cancer treatment, the crucial issue associated with the use of PEG – the “PEG dilemma” – has to be addressed. As many as 1581 differentially expressed genes have been identified by PEG-unmodified NPs. PEGylation of NPs caused reduction in the expression of most of the genes. However, type I interferon expression was significantly increased. Based on these studies, it has been hypothesized that PEGylation inhibits the endosomal escape of NP and extends the interaction of toll-like receptor-9 with CpG DNA accompanied by the production of type I interferon. This hypothesis has been tested by introducing a pH-sensitive fusogenic peptide, GALA, which enhances the endosomal escape of PEGylated NP. As expected, type I interferon was reduced and IL-6 remained unchanged, suggesting that microarray analysis and the manipulation of intracellular trafficking constitute a rational strategy for reducing the host immune response to NPs. Furthermore, investigators have developed MEND for efficient delivery of nucleic acids. For tumor delivery of MEND, PEGylation has been considered a useful method, which confers a longer systemic circulation and tumor accumulation via the enhanced permeability and retention effect.Citation48 As preclinical evaluation in animals does not necessarily portray human responses, liposome-encapsulated hemoglobin (an artificial oxygen carrier) has been tested in immunodeficient (cord blood-transfused NOD/SCID/IL-2Rγnull) mice reconstituted with human hematopoietic stem cells.Citation49 However, PEGylation inhibits cellular uptake and subsequent endosomal escape. To overcome this, investigators developed a PEG-peptide-1,2-dioleoyl-sn-glycero-3-phosphoethanolamine that is cleaved in a matrix metalloproteinase-rich environment. Furthermore, the systemic delivery of small interfering ribonucleic acid to tumors has been examined by employing a MEND that is modified with PEG-peptide-1,2-dioleoyl-sn-glycero-3-phosphoethanolamine. PEG-peptide-1,2-dioleoyl-sn-glycero-3-phosphoethanolamine modification accelerated both cellular uptake and endosomal escape, compared to a conventional PEG-modified MEND. It is known that cancers often overexpress epidermal growth factor (EGF) and other growth factors to promote cell replication and migration. Liposomes bearing EGF receptors covalently crosslinked to p-Toluic acid or methyl-PEG 4-N-hydroxysuccinimide ester (ie, modified EGF receptor-bearing liposomes) exhibit an increased rate of release of encapsulated drug compounds when EGF is present in solution.Citation50 In all the liposomal encapsulation studies described above, the precise role of MTs has not been explored as yet. Studies in this direction will not only further elucidate the therapeutic potential of liposome-encapsulated genes or drugs but also their safety aspects of NPs for clinical applications.
MTs and NPs
It is now well established that the emission spectra of surfactant-coated semiconductor NPs depend on the size and the coating material.Citation51–Citation53 The blue series represents different sizes of Cd/Se NPs with diameters of 2.1, 2.4, 3.1, 3.6, and 4.6 nm. The green series is of indium phosphide NPs with diameters of 3.0, 3.5, and 4.6 nm. The red series is of indium arsenide NPs with diameters of 2.8, 3.6, 4.6, and 6.0 nm. A true-color image of a series of silica-coated NPs probes with a Cd/Se core and a Zn sulfide or Cd sulfide shell in aqueous buffer have also been developed. These NPs can be illuminated with ultraviolet light and used effectively to induce MTs in cell-based therapies as discussed in this report. Recently, Cd/Se/tellurium (Te)-based quantum dots (QD-705) have been developed with potential for biomedical applications. Although the biological fate of QD-705 is established, its chemical fate in the biological system still remains uncertain. One study investigated the chemical fate of QD-705 in mice kidneys. The molar ratio of Cd and Te (increased Cd/Te ratio signifies increased Cd release from QD-705) and the induction of tissue MTs are markers for elevated free Cd in tissues. Almost 100% of QD-705 was retained in the body even 16 weeks after exposure, with significant pharmacokinetics in the kidneys. Elevations in the Cd/Te ratio and MT-1 expression in the kidneys suggested that free Cd is released from QD-705. Thus, QD-705 is not stable or biologically inert as free Cd released from QD-705 may increase the risk of nephrotoxicity because MTs bind these metal NPs and are specifically induced to prevent nephrotoxicity.Citation54
MTs as molecular magnets
Recently, novel methods have been developed for preparing molecular magnets and patterning of the molecules on a semiconductor surface.Citation55 A magnetically aligned MT containing manganese and Cd is first synthesized, and the molecules are then placed into nanoporous silicon surfaces using electron beam lithography and reactive ion-etching techniques. The self-assembled growth of the MT molecules on the patterned silicon surface grows into rod- or ring-type three-dimensional nanostructures depending on the patterned surface nanostructures. Scanning electron microscopy, atomic force microscopy, and magnetic force microscopic analyses has revealed that these nanostructures exhibit molecular magnetization and are compatible with conventional semiconductors, which makes manganese and Cd-containing MT-2 NPs candidates for clinical applications and sensing nanodevices.
MTs as early and sensitive biomarkers of NPs toxicity
There is now evidence that MTs are induced in response to various semiconductor NPs. In particular, silver (Ag) NPs, Cd sulfide, Cd/Te NPs, and carbon nanotubes are known to induce MTs as a defensive mechanism to prevent toxicity. Uncoated Cd/Te QD in freshwater leads to the formation of aggregates and a dissolved component of Cd, where the latter explains the contribution of the accumulation pattern in mussel tissues and induced MT levels in mussels.Citation56–Citation58 Recently, Neupane has developed MT-capped Cd/Se NPs, in which up to eight MTs could be attached as detected by an increase in the fluorescence intensity.Citation59 Cd/Se QD NP-labeled bone marrow-derived mononuclear stem cells (MNCs) have been used to determine their biodistribution, pharmacokinetics, and therapeutic potential in experimental model of AIS.Citation60 By employing digital fluorescence microscopy and confocal microscopic analysis, it was discovered that MNCs exhibit preferential chemotaxis and are exponentially eliminated from the peri-infarct region as a function of time. These findings led to determining the therapeutic window of MNC-mediated recovery following AIS.Citation61 A detailed description of cell-based therapy in AIS has also been provided.Citation62 Furthermore, it was discovered for the first time that bone marrow-derived MNCs protect cortical neurons by modulating microglia in a cell culture model of AIS; additionally, the basic molecular mechanism of neuroprotection afforded by MNCs was elucidated.Citation63 Treatment of MNCs in AIS rats significantly increased brain regional IL-10, hence it is proposed that cortical neurons are protected directly by the MNC-mediated paracrine release of IL-10. Indeed, IL-10 can directly bind to its specific IL-10 receptor to execute anti-inflammatory response by activating upstream phosphatidylinositol 3-kinase and downstream signal transducer and activator of transcription-3-mediated signal transduction cascade.Citation64 To further confirm these findings, the effect of transcatheter injections on the viability and cytokine release of mononuclear cells has been examined.Citation65 These findings have indicated that MNCs are highly primitive, fragile, and can release anti-inflammatory cytokines such as IL-10 and other neurotrophic factors readily to exert their therapeutic effect in AIS and other neurodegenerative diseases. There could be several other molecular mechanisms in addition to MT-mediated MNC neuroprotection which remain unexplored yet. However, in vivo molecular imaging studies from the authors’ laboratories employing 18F fluorodeoxyglucose and fluorine-18-L-dihydroxyphenylalanine as positron emission tomography biomarkers strongly support MTs as potent antioxidant neuroprotective factors in the progressive neurodegenerative disorders such as PD, AD, and drug addiction.Citation66 Based on these findings, there will be further investigation into the detailed pharmacological properties of NPs using MTs and CB formation as sensitive biomarkers (). There is now evidence that Ag NP-induced changes in the action potential are associated with inhibitory effects on voltage-gated sodium currents of hippocampal CA1 neurons.Citation67 Furthermore, in vitro studies have indicated that Ag NPs can induce oxidative stress and acute calcium responses in the primary mixed neural cell cultures.Citation68 MTs can attenuate these deleterious events by acting as potent antioxidants and anti-inflammatory agents, as previously discussed.Citation25,Citation26 Further studies in this direction will not only enhance the theranostic potential but also the safety measure of NPs for future biomedical applications.
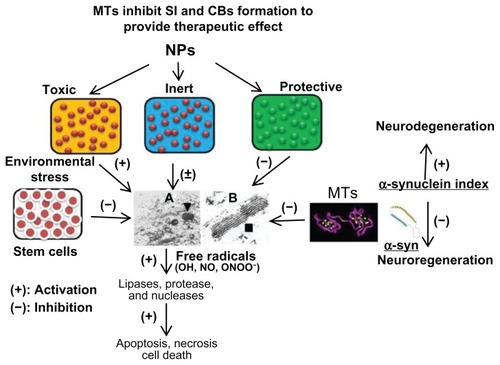
Figure 2 Three types of NPs have been proposed (1) toxic, (2) protective, and (3) inert. NPs augmenting the zinc ion homeostasis could be neuroprotective, those inducing dyshomeostasis could be toxic, and those producing no significant change could be neutral or inert. Thus, metal ion speciation of MTs following NP exposure employing sensitive procedure such as inductively coupled plasma mass spectroscopy may provide a better understanding of the therapeutic potential of MTs in nanomedicine. At the ultrastructural level, protective NPs may prevent CB formation and toxic NPs will induce CB formation, whereas inert NPs will remain ineffective. Stem cells provide MT-mediated mitochondrial protection and inhibit CB formation to facilitate regenerative process. Any physical, physiological, and/or pharmacological intervention involving MT induction will inhibit CB formation and provide neuroprotection. Thus, MT induction and CB formation may be utilized as early and sensitive biomarkers of NPs safety, effectiveness, and toxicity for theranostic applications. (The specificity of these biomarkers may be explored in the future). Furthermore, toxic NPs and environmental stress may induce overproduction of free radicals (OH, NO) to cause lysosomal membrane destabilization and CB formation. Free radicals may induce lipases, proteases, and nucleases to cause membranolysis, proteolysis, and nuclear DNA depolymerization leading to apoptosis, necrosis, and even cell death. MTs may serve as free radical scavengers to inhibit CB formation and provide therapeutic effect in neurodegenerative disease and other disorders. (A) Transmission electron microscope picture (magnification 20,000×) demonstrating the cytoplasmic organization of Purkinje neuron from the undernourished rat cerebellar cortex demonstrating increased incidence of lysosomes (▼). (B) Multilamellar electron-dense membrane stacks (CB ■) in the 15-day undernourished rat Purkinje neurons (magnification 50,000×).
Notes: (+) indicates activation; (−) indicates inhibition.
Abbreviations: CB, Charnoly body; MT, metallothionein; NO, nitric oxide; NP, nanoparticle; OH, hydroxyl; ONOO, peroxynitrite; SI, α-synuclein index.
MTs for future NPs development
Oxidative stress and secondary damage can occur during carcinogenesis and neurodegenerative disorders, as has been reported recently.Citation25 MTs as reactive oxygen species scavengers inhibit oxidative stress due to their metal ion release and inhibit apoptosis by attenuating mitochondrial cytochrome c leakage and caspase-3 activation. It is well established that MTs are also significantly induced by metal NPs and by environmental neurotoxins. To test the prolonged consequences of a short transient exposure of astrocytes to Ag NPs, cultured primary astrocytes were incubated for 4 hours in the presence of Ag NPs, and the cell viability and metabolic parameters were investigated.Citation69 Ag NPs caused concentration-dependent increase in Ag up to 46 nmol/mg protein, but did not compromise cell viability. During subsequent incubation in Ag NP-free medium, the Ag content remained constant for up to 7 days. The presence of Ag NPs neither induced any delayed cellular toxicity nor glucose metabolism, lactate production, or the ratio of glutathione to glutathione disulfide. However Ag NP-treated astrocytes upregulated MT expression suggesting that MT induction may prevent Ag NP-mediated Ag ion toxicity.
It is now realized that gold (Au) NPs may have theranostic applications in humans. Since Au NPs have significant clinical applications, their release in the environment may induce toxicological effects. In a recent study, the marine bivalve Scrobicularia plana was exposed to 5 nm, 15 nm, and 40 nm Au NPs during a 16-day laboratory exposure at 100 μg L−1 Au.Citation70 After exposure to Au NPs forming aggregates (>700 nm), the Au was accumulated in the soft tissues. Au NP-induced MTs (5 nm and 40 nm) increased activities of catalase and superoxide dismutase and of glutathione S-transferase, indicating induction of the defense mechanisms against NP-induced oxidative stress. Exposure to Au NPs also impaired burrowing behavior. However, these effects were observed at higher doses than expected in the environment. Recently, an extended fish embryo test was utilized to bridge the gap between cell culture and small animal models to investigate the toxicity of Au NPs in wild type and transgenic zebrafish.Citation71 The fish embryo test confirmed all findings of a study in HeLa cells and added new information on teratogenicity and hepatotoxicity that could not be obtained from cultured cells. Recently, mesoporous silicon nanochips have been developed for diagnostic applications.Citation72 Biocompatible and biodegradable silicon NPs, mesoporous silicon nanochips, and silicon dioxide nanocoating microdevices can be used for the sensitive and early detection of disease biomarkers from serum using mass spectrometry. Silicon is converted to silicic acid and excreted through the urine. However, if inhaled, it can cause silicosis similar to asbestosis, mesothelioma, and lung cancer.Citation73 Although silicon nullifies aluminum toxicity, in the absence of ascorbic acid it may facilitate iron accumulation to induce further toxicity.
Indeed, MTs are protective when induced at the physiological and pharmacological level; however, their enhanced induction may reflect the toxicity of NPs. Particularly when the protective layer of Zn oxide is removed from QDs in vivo, as discussed above. Recently developed QDs are composed of Cd/Se, which provides excellent fluorescent properties and may be used for tumor localization to help the surgeon to identify normal cancer cells from cancer stem cells. However, when the protective Zn oxide layer is dislodged, these NPs could induce Cd nephrotoxicity. Therefore, the primary objective of coating the NPs with Zn oxide is to avoid direct toxicity and simultaneously induce MTs as protective antioxidants and free radical scavengers to provide mitochondrial protection. Indeed, a considerable amount of research is required in this direction to stabilize NPs in the biological system and enhance their theranostic potential by employing MTs as early and sensitive biomarkers, as emphasized in this report. At present, the exact pathophysiological significance of MT induction is not understood due to a lack of existing knowledge. Zn ions (Zn2+) act as physiological neuromodulators at glutamatergic synapses; however, in order to avoid neurotoxic damage, the intracellular Zn2+ must be controlled by: (1) Zn2+ transporters, (2) Zn2+ buffering MTs, and (3) mitochondrial sequestration systems. Particularly in physiological aging, if any of these systems is impaired and/or not adequately coordinated with the other two, the resulting rise of intracellular Zn2+ may inhibit the cellular energy and affect mitochondria as a primary target.Citation74 MTs could be directly or indirectly involved in the modulation of cellular senescence and might represent a potential therapeutic target against the aggregation of dysfunctional aging cells.Citation75 Therefore, it may be important to perform metal ion speciation of MTs employing atomic absorption spectrophotometry or preferably inductively coupled plasma mass spectrometry to assess their exact pathophysiological significance in neurodegenerative disease and other disorders. For instance, Zn-bound MTs may play a different role during cancer or aging, switching from a protective to a deleterious one in immune, endocrine, and cerebral activities. Intracellular Zn2+ homeostasis is affected by oxidative stress, which is a trigger for detrimental Zn2+ release from MTs. Moreover, Zn2+ at higher concentrations may induce oxidative stress by promoting mitochondrial and extramitochondrial production of reactive oxygen species in the aging brain. Thus, it will be extremely necessary to consider MTs as important biomarkers while developing biologically-safe metal NPs for theranostic applications in future.
Clinical significance of MTs in nanomedicine
Recent studies have established that bone marrow-derived stem cells are able to alleviate symptoms of lung silicosis.Citation9 Thus, MT semiconductor NPs are now being developed to encapsulate silicon as third-generation NPs for theranostic applications. Indeed, semiconductor NPs have potential applications in medicine, engineering, and biology. Size tunable emission, through the quantum confinement effect of semiconductor NPs, make them useful chromophores as fluorescence biomarkers, light emitting diodes, and laser materials. The NPs can be stabilized by thiol-containing capping molecules such as MTs. Although hydrophilic monothiols render the NPs water soluble, they are degraded by light exposure. Thus, peptides based on polycysteine-containing MTs may provide robust, water soluble, and biocompatible capping groups for developing semiconductor NPs. Up to eight MT peptides may be attached to each Cd/Se NPs to provide structural and functional stability and prevent cytotoxicity.Citation76,Citation77 Complementary DNA and protein microarray analysis have revealed that various stress-related genes including metallothioneins (MTs), heat shock proteins (HSPs), glutathione S-transferase (GST), p53, cytochrome p450-1A (Cyp-1A), and transferrin are induced following toxic insult.Citation78 MTs are specifically induced in metal toxicity, cancer, inflammation, and infections, hence can serve as early and sensitive biomarkers of environmental safety and effectiveness of newly developed NPs.Citation78,Citation79 Because of their unique physical, optical, and mechanical properties, NPs hold great promise in improving clinical diagnosis and effective treatment. Their use in research and consumer products is increasing rapidly, hence contamination of the environment with various NPs seems inevitable. Because surface waters receive pollutants and contaminants from many sources including NPs, and act as reservoirs and conduits for many environmental contaminants, understanding the impacts of NPs on the organisms within these environments is critical for evaluating their toxicity. Thus, MTs could be used as early and sensitive biomarkers to evaluate environmental toxicity. MTs can also serve as early and sensitive biomarkers of neurotoxicity as well as drug addiction.Citation25,Citation26 Moreover, frequent structural heterogeneity and mutations in bacterial MTs may provide genetic resistance to antibiotics and augment pathogenicity.Citation80 Local application of Zn ointments enhances MTs, which induces cell proliferation and wound healing.Citation81 Thus, material composition and design of fracture patties for amputees, wound clips, and dressings coated with Ag and Au NPs are being developed to prevent osteomyelitis, septicemia, inflammation, and allergic reactions.Citation82 In addition, drug (sirolimus)-eluting carbon nanocoated stents are being developed to prevent platelet activation/aggregation, 5-hydroxytryptamine release, vascular neointimal hyperplasia, restenosis, thrombosis, and persistent infections due to the formation of biofilms on these nanodevices.Citation83 By altering the surface topography of nanocoatings, the activation of platelets can be affected, while the carbon nanocoatings having higher surface roughness is less thrombogenic in terms of platelet adhesion for improving the stent coating fabrication.Citation84 MT-capped Cd/Se NPs are being further developed for their clinical application as theranostic agents.Citation59 Further studies in this direction will go a long way in the effective clinical management of patients.
Conclusion
MTs have a diversified and versatile therapeutic role as free radical scavengers in the CNS and other tissues. Through Zn-mediated transcriptional activation, these proteins can regulate cellular growth and development in health and disease. MTs induction in response to nutritional stress and environmental toxicity (particularly from glutamate analogs and NPs) is of considerable clinical interest. Hence it is important to verify NPs-mediated MTs induction before they could be used routinely as potential theranostic agents. As NPs aggregate and accumulate quite frequently in the biological system, it would of significant interest to study the contribution of MT induction and CB formation in these conditions. Furthermore, liposomal encapsulation and MT induction in stem cell therapeutics will have a promising future for developing safe and effective NPs for biomedical applications.
Acknowledgments
Moral support and encouragement by Dr Kallol Guha, Saint James School of Medicine, Bonaire is gratefully acknowledged.
Disclosure
The authors report no conflicts of interest in this work.
References
- MarghoshesMValleeBLA cadmium protein from equine kidney cortexJ Am Chem Soc19577948134814
- ItohMEbadiMSwansonSThe presence of zinc-binding proteins in brainJ Neurochem19834138238296875568
- RuppHWeserUConversion of metallothionein into Cu-thionein, the possible low molecular weight form of neonatal hepatic mitochondrocupreinFEBS Lett19744432932974472041
- VasakMGaldesAHillHAKagiJHBremnerIYoungBWInvestigation of the structure of metallothioneins by proton nuclear magnetic resonance spectroscopyBiochemistry19801934164257356935
- VasakMKagiJHHillHAZinc(II), cadmium(II), and mercury(II) thiolate transitions in metallothioneinBiochemistry19812010285228567248252
- BoulangerYGoodmanCMForteCPFesikSWArmitageIMModel for mammalian metallothionein structureProc Natl Acad Sci U S A1983806150115056572910
- BraunWVasakMRobbinsAHComparison of the NMR solution structure and the X-ray crystal structure of rat metallothionein-2Proc Natl Acad of Sci U S A1992892110124101281438200
- ZanggerKOzGOtvosJDArmitageIMThree-dimensional solution structure of mouse (Cd7)-metallothionein-1 by homonuclear and heteronuclear NMR spectroscopyProtein Sci19998122630263810631978
- NielsenAEBohrAPenkowaMThe balance between life and death of a cell: role of metallothioneinsBiomark Insights200719911119690641
- PastoreAPiemonteFS-glutathionylation signaling in cell biology: progress and prospectsEur J Pharm Sci201246527929222484331
- KhataiLGoesslerWLorencovaHZanggerKModulation of nitric oxide-mediated metal release from metallothionein by the redox state of glutathione in vitroEur J Biochem2004271122408241615182356
- HelalGKAleisaAMHelalOKMetallothionein induction reduces caspase-3 activity and TNFalpha levels with preservation of cognitive function and intact hippocampal neurons in carmustine-treated ratsOxid Med Cell Longev200921263520046642
- MinKSPhysiological significance of metallothionein in oxidative stressYakugaku Zasshi20071274695702 Japanese [with English abstract]17409699
- KooncumchooPSharmaSPorterJGovitrapongPEbadiMCoenzyme Q10 provides neuroprotection in iron-induced apoptosis in dopaminergic neuronsJ Mol Neurosci200628212514116679553
- NathRKambadurRGulatiSPaliwalVKSharmaMMolecular aspects, physiological function, and clinical significance of metallothioneinsCrit Rev Food Sci Nutr198827141853293923
- UchidaYTakioKTitaniKIharaYTomonagaMThe growth inhibitory factor that is deficient in the Alzheimer’s disease brain is a 68 amino acid metallothionein-like proteinNeuron1991723373471873033
- SmithEDrewPATianZQMetallothionien 3 expression is frequently down-regulated in oesophageal squamous cell carcinoma by DNA methylationMol Cancer200544216351731
- SharmaSKEbadiMMetallothionein attenuates 3-morpoholinosydnonimine (SIN-1)-induced oxidative stress in dopaminergic neuronsAntioxid Redox Signal20035325126412880480
- EbadiMSharmaSKPeroxynitrite and mitochondrial dysfunction in the pathogenesis of Parkinson’s diseaseAntioxid Redox Signal20035331933512880486
- EbadiMSharmaSMetallothioneins 1 and 2 attenuate peroxynitrite-induced oxidative stress in Parkinson’s diseaseExp Biol Med (Maywood)200623191576158317018883
- EbadiMBrown-BorgHEl RefaeyHMetallothionein-mediated neuroprotection in genetically engineered mouse models of Parkinson’s diseaseBrain Res Mol Brain Res20051341677515790531
- EbadiMSharmaSWanpenSShavaliSMetallothionein isoforms attenuate peroxynityrite-induced oxidative stress in Parkinson’s diseaseEbadiMPfeifferRFParkinson’s DiseaseBoca Raton, FLCRC Press2004739765
- EbadiMSharmaSKMuralikrishnanDMTs provides ubiquinone-mediated neuroprotection in Parkinson’s diseaseProc West Pharmacol Soc200245363812434520
- EbadiMSharmaSKGhafourifarPBrown-BorgHEl RefaeyHPeroxynitrite in the pathogenesis of Parkinson’s disease and the neuroprotective role of metallothioneinsMethods Enzymol200539627629816291239
- SharmaSEbadiMMetallothioneins as early and sensitive biomarkers of redox signaling in neurodegenerative disordersThe IIOAB Journal20112698106
- SharmaSEbadiMTherapeutic potential of metallothioneins as anti-inflammatory agents in polysubstance abuseThe IIOAB Journal2011265061
- LeeSJKohJYRoles of zinc and metallothionein-3 in oxidative stress-induced lysosomal dysfunction, cell death, and autophagy in neurons and astrocytesMol Brain2010313020974010
- SonnKPankratovaSKorshunovaIA metallothionein mimetic peptide protects neurons against kainic acid-induced excitotoxicityJ Neurosci Res20108851074108219937811
- DakshinamurtiKSharmaSKSundaramMWatanabeTHippocampal changes in developing postnatal mice following intrauterine exposure to domoic acidJ Neurosci19931310448644958105041
- GillSSHouYGhaneTPulidoOMRegional susceptibility to domoic acid in primary astrocyte cells cultured from the brain stem and hippocampusMar Drugs200861253818648670
- PulidoOMDomoic acid toxicologic pathology: a reviewMar Drugs20086218021918728725
- RueEBrulandKDomoic acid binds iron and copper: a possible role for the toxin produced by the marine diatom Pseudo-nitzschiaMarine Chemistry200176127134
- PedersenDSFredericiaPMPedersenMOMetallic gold slows disease progression, reduces cell death and induces astrogliosis while simultaneously increasing stem cell responses in an EAE rat model of multiple sclerosisHistochem Cell Biol2012138578780222820857
- ZappiaECasazzaSPedemonteEMesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergyBlood200510651755176115905186
- LanzaCMorandoSVociANeuroprotective mesenchymal stem cells are endowed with a potent antioxidant effect in vivoJ Neurochem200911051674168419619133
- MickovaABuzgoMBenadaOCore/shell nanofibers with embedded liposomes as a drug delivery systemBiomacromolecules201213495296222401557
- ChenXAZhangLJHeZJPlasmid-encapsulated polyethylene glycol-grafted polyethylenimine nanoparticles for gene delivery into rat mesenchymal stem cellsInt J Nanomedicine2011684385321589652
- ScottRCRosanoJMIvanovZTargeting VEGF-encapsulated immunoliposomes to MI heart improves vascularity and cardiac functionFASEB J200923103361336719535683
- HofkensWGreversLCWalgreenBIntravenously delivered glucocorticoid liposomes inhibit osteoclast activity and bone erosion in murine antigen-induced arthritisJ Control Release2011152336336921396411
- TangSLiuZZhaoLZouZDuMTransfection of pEGFP-C2 in brain mediated by targeting liposome P-MMA-DOSPERSheng Wu Yi Xue Gong Cheng Xue Za Zhi200825511701174 Chinese19024469
- GiulianiALWienerELeeMJBrownINBertiGWickramasingheSNChanges in murine bone marrow macrophages and erythroid burst-forming cells following the intravenous injection of liposome-encapsulated dichloromethylene diphosphonate (Cl2MDP)Eur J Haematol200166422122911380601
- AksentijevichIPastanILunardi-IskandarYGalloRCGottesmanMMThierryARIn vitro and in vivo liposome-mediated gene transfer leads to human MDR1 expression in mouse bone marrow progenitor cellsHum Gene Ther199679111111228773513
- RahmanYEHansonWRBharuchaJAinsworthEJJaroslowBNMechanisms of reduction of antitumor drug toxicity by liposome encapsulationAnn N Y Acad Sci1978308325342279296
- WangYZengBLiXExpression of human calcitonin by microencapsulated recombinant myoblastsBiotechnol Lett200628181453145816823598
- XieYYeLZhangXTransport of nerve growth factor encapsulated into liposomes across the blood–brain barrier: in vitro and in vivo studiesJ Control Release20051051–210611915893839
- HatakeyamaHItoEYamamotoMA DNA microarray-based analysis of the host response to a nonviral gene carrier: a strategy for improving the immune responseMol Ther20111981487149821386823
- HatakeyamaHAkitaHItoESystemic delivery of siRNA to tumors using a lipid nanoparticle containing a tumor-specific cleavable PEG-lipidBiomaterials201132184306431621429576
- HatakeyamaHAkitaHHarashimaHA multifunctional envelope type nano device (MEND) for gene delivery to tumours based on the EPR effect: a strategy for overcoming the PEG dilemmaAdv Drug Deliv Rev201163315216020840859
- KawaguchiATKametaniYKatoSFuruyaHTamaokiKHabuSEffects of liposome-encapsulated hemoglobin on human immune system: evaluation in immunodeficient mice reconstituted with human cord blood stem cellsArtif Organs200933216917619178463
- WongAModified epidermal growth factor receptor (EGFR)-bearing liposomes (MRBLs) are sensitive to EGF in solutionPLoS One2009410e739119816581
- XiaoQXiaoCPreparation and characterization of silica-coated magnetic-fluorescent bifunctional microspheresNanoscale Res Lett2009491078108420596403
- LeungKQuantum dot-prostate-specific membrane antigen antibody J591Molecular Imaging and Contrast Agent Database (MICAD)Bethesda, MDNational Center for Biotechnology Information2005 Available from: http://www.ncbi.nlm.nih.gov/books/NBK23039/pdf/QD-PMSA.pdfAccessed February 20, 2013
- ChoiYKimKHongSKimHKwonYJSongRIntracellular protein target detection by quantum dots optimized for live cell imagingBioconjug Chem20112281576158621718016
- LinCHChangLWChangHThe chemical fate of the Cd/Se/Te-based quantum dot 705 in the biological system: toxicity implicationsNanotechnology2009202121510119423922
- ChangCCSunKWLeeSFKanLSSelf-assembled molecular magnets on patterned silicon substrates: bridging bio-molecules with nanoelectronicsBiomaterials200728111941194717223191
- PeyrotCGagnonCGagneFWillkinsonKJTurcottePSauveSEffects of cadmium telluride quantum dots on cadmium bioaccumulation and metallothionein production to the freshwater mussel, Elliptio complanataComp Biochem Physiol C Toxicol Pharmacol200915024625119427919
- WeiLThakkarMChenYNtimSAMitraSZhangXCytotoxicity effects of water dispersible oxidized multiwalled carbon nanotubes on marine alga, Dunaliella tertiolectaAquat Toxicol2010100219420120673592
- MounicouSOuerdaneLL’AzouBIdentification of metallothionein subisoforms in HPLC using accurate mass and online sequencing by electrospray hybrid linear ion trap-orbital ion trap mass spectrometryAnal Chemistry2010821669476957
- NeupaneKPDeveloping Metallothionein Capped Cadmium Selenide Nanoparticles [doctoral thesis]Detroit, MIWayne State University2005
- BrennemanMSharmaSHartingMAutologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged ratsJ Cereb Blood Flow Metab201030114014919773802
- YangBStrongRSharmaSTherapeutic time window and dose response of autologous bone marrow mononuclear cells for ischemic strokeJ Neurosci Res201189683383921412816
- MisraVYangBSharmaSSavitzSCell-based therapy for strokeCoxCSProgenitor Cell Therapy for Neurological InjuryNew York, NYHumana Press2011143162
- SharmaSYangBStrongRBone marrow mononuclear cells protect neurons and modulate microglia in cell culture models of ischemic strokeJ Neurosci Res201088132869287620629187
- SharmaSYangBXiXGrottaJAronowskiJSavitzSIIL-10 directly protects cortical neurons by activating PI-3 kinase and STAT-3 pathwaysBrain Res2011137318919421138740
- El KhouryRMisraVSharmaSThe effect of transcatheter injections on viability and cytokine release of mononuclear cellsAJNR Am J Neuroradiol20103181488149220395386
- SharmaSEbadiMIn vivo molecular imaging in Parkinson’s diseasePfeifferRFWszolekZKEbadiMParkinson’s Disease2nd edBoca Raton, FLCRC Press2013787802
- LiuZRenGZhangTYangZAction potential changes associated with inhibitory effects on voltage-gated sodium currents of hippocampal CA1 neurons by silver nanoparticlesToxicology2009246317918419683029
- HaaseARottSMantionAEffects of silver nanoparticles on primary mixed neural cell cultures: uptake, oxidative stress and acute calcium responsesToxicol Sci2012126245746822240980
- LutherEMSchmidtMMDiendorfJEppleMDringenRUpregulation of metallothioneins after exposure of cultured primary astrocytes to silver nanoparticlesNeurochem Res20123781639164822476984
- PanJFBuffetPEPoirierLSize dependent bioaccumulation and ecotoxicity of gold nanoparticles in an endobenthic invertebrate: the Tellinid clam Scrobicularia planaEnviron Pollut2012168374322595760
- PanYLeifertAGrafMHigh-sensitivity real-time analysis of nanoparticle toxicity in green fluorescent protein-expressing zebrafishSmall Epub 2012 Nov 12
- FanJGallagherJWWuHJLow molecular weight protein enrichment on mesoporous silica thin films for biomarker discoveryJ Vis Exp201262387622546927
- SteenlandKStaynerLSilica, asbestos, man-made mineral fibers, and cancerCancer Causes Control1997834915039498906
- Bertoni-FreddariCFattorettiPCasoliTDi StefanoGGiorgettiBBaliettiMBrain aging: the zinc connectionExp Gerontol200843538939318078729
- MocchegianiECostarelliLGiacconiRPiacenzaFBassoAMalavoltaMMicronutrient (Zn, Cu, Fe)-gene interactions in ageing and inflammatory age-related diseases: implications for treatmentsAgeing Res Rev201211229731922322094
- DerfusAMChanWCWBhatiaSNProbing the cytotoxicity of semiconductor quantum dotsNano Lett2004411118
- LeighKBouldinJBuchananREffects of exposure to semiconductor nanoparticles on aquatic organismsJ Toxicol2012201239765722131989
- PenkowaMMetallothioneins are multipurpose neuroprotectants during brain pathologyFEBS J200627391857187016640552
- PenkowaMTioLGiraltMSpecificity and divergence in the neurobiologic effects of different metallothioneins after brain injuryJ Neurosci Res200683697498416493670
- StankovicRKChungRSPenkowaMMetallothioneins I and II: neuroprotective significance during CNS pathologyInt J Biochem Cell Biol200739348448917097331
- LansdownABMirastschijskiUStubbsNScanlonEAgrenMSZinc in wound healing: theoretical, experimental, and clinical aspectsWound Repair Regen200715121617244314
- WongKKCheungSOHuangLFurther evidence of the anti-inflammatory effects of silver nanoparticlesChem Med Chem2009471129113519405063
- RajenderGNarayananNGLiquid chromatography-tandem mass spectrometry method for determination of sirolimus coated drug eluting nano porous carbon stentsBiomed Chromatogr201024332933419662626
- KaragkiozakiVCLogothetidisSDKassavetisSNGiannoglouGDNanomedicine for the reduction of the thrombogenicity of stent coatingsInt J Nanomedicine2010523924820463940