Abstract
Thermosensitive liposomes are a promising tool for external targeting of drugs to solid tumors when used in combination with local hyperthermia or high intensity focused ultrasound. In vivo results have demonstrated strong evidence that external targeting is superior over passive targeting achieved by highly stable long-circulating drug formulations like PEGylated liposomal doxorubicin. Up to March 2014, the Web of Science listed 371 original papers in this field, with 45 in 2013 alone. Several formulations have been developed since 1978, with lysolipid-containing, low temperature-sensitive liposomes currently under clinical investigation. This review summarizes the historical development and effects of particular phospholipids and surfactants on the biophysical properties and in vivo efficacy of thermosensitive liposome formulations. Further, treatment strategies for solid tumors are discussed. Here we focus on temperature-triggered intravascular and interstitial drug release. Drug delivery guided by magnetic resonance imaging further adds the possibility of performing online monitoring of a heating focus to calculate locally released drug concentrations and to externally control drug release by steering the heating volume and power. The combination of external targeting with thermosensitive liposomes and magnetic resonance-guided drug delivery will be the unique characteristic of this nanotechnology approach in medicine.
Thermosensitive liposomes and their historical development
Liposomes are spherical vesicles formed by a membrane bilayer usually composed by phospholipids (). The membrane encloses an aqueous core that can be used to encapsulate hydrophilic drugs, whereas lipophilic drugs can be incorporated into the membrane. Several methods are available for preparation of liposomal formulations, ranging from laboratory scale to Good Manufacturing Practice production for clinical batches.Citation1 Loading of drugs can be achieved by active () or passive () loading methods. Stable encapsulation of a drug inside a liposomal formulation increases its half-life in the circulation after intravenous administration by avoiding rapid metabolism. Moreover, unwanted distribution in different compartments of the body is avoided, so the risk of drug-related side effects is reduced. The versatility of liposomal drug delivery systems reflects the fact that their biophysical characteristics, eg, vesicle size, lamellarity, surface charge, membrane fluidity, and surface, can be modified by the lipid composition and/or preparation method used. Since naturally occurring molecules like (phospho)lipids and cholesterol are used as the main components, liposomes are in general classified as biocompatible.
Figure 1 Structure of liposomes and examples for membrane components.
Notes: (A) Schematic representation of a liposome. The vesicle is formed by a membrane bilayer of phospholipids enclosing an aqueous internal core that can be loaded with hydrophilic molecules (1). The vesicle surface is often shielded by a polymer coating, eg, polyethylene glycol (2). Lipophilic molecules can be incorporated into the membrane bilayer (3). To destabilize the membrane for facilitating drug release, surfactants (eg, lysolipids) are incorporated into the membrane (4). Surface modification with antibodies, antibody fragments, or ligands yields formulations for active targeting towards the desired epitope (5). Incorporation of cationic lipids like DPTAP yields vesicles for endosomal targeting (6). Cholesterol is incorporated to stabilize the formulations (7). (B) Examples of amphiphilic molecules forming lamellar structures.
Abbreviations: DPPC, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine; DPPGn, 1,2-dipalmitoyl-sn-glycero-3-phosphodiglycerol; DPTAP, 1,2-dipalmitoyl-3-trimethylammonium-propane.
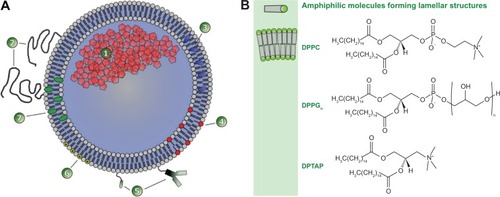
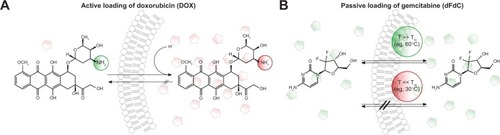
Figure 2 Different methods of drug loading into pre-formed liposomes.
Notes: (A) Active drug loading of weak base molecules (eg, doxorubicin) into preformed liposomes with a pH gradient. In the basic extraliposomal buffer, the drug is uncharged and able to transfer across the membrane bilayer. Inside, the drug is protonated due to the acidic buffer and trapped. The loading method reaches an encapsulation efficacy of up to 98%. (B) Passive drug loading (eg, gemcitabine) is achieved by incubating the drug and vesicles at temperatures where the membrane is in the liquid-disordered phase state and therefore permeable for the drug. After cooling, the membrane is in the solid gel phase state, and its permeability is negligible. Because the passive loading is an equilibrium process depending on the ratio between intraliposomal and extraliposomal volume, the encapsulation efficacy is low and the formulation has to be purified from the nonencapsulated drug.
Abbreviation: dFdC, gemcitabine; DOX, doxorubicin.
In 1965, Bangham et al described the spontaneous formation of liquid crystals after dispersing lecithin in aqueous medium.Citation2 Although the in vivo results were initially promising,Citation3,Citation4 the development of liposomes nearly came to an end in 1982 when doubts arose about their ability to target drugs to cells in tissues beyond the endothelial barrier.Citation5 Nevertheless, in the years since, due to discovery of steric stabilization of vesicles with polyethylene glycol (PEG),Citation6,Citation7 liposomes have been successfully developed as a carrier for drugs, and several liposomal drugs (eg, Doxil®/Caelyx® [Johnson & Johnson, New Brunswick, NJ, USA] and Ambisome® [Gilead, Foster City, CA, USA]) have been approved and entered the clinic.Citation8
In 1978, Yatvin et al described the first temperature-sensitive formulation (thermosensitive liposome, TSL) that was able to release a hydrophilic drug when the temperature was increased a few degrees above physiological temperature.Citation9 The original formulation based on 1,2-dipalmitoyl-sn-glyce-ro-3-phosphocholine (DPPC) and 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) 3:1 (mol/mol) has been modified frequently during recent decades to overcome several limitations. At the beginning of the 21st century, the first TSL formulation developed by Needham et al entered human clinical trials.Citation10 This was a breakthrough in the field, visible by approximately 300 citations of the original paperCitation11 since its publication. Heat-triggered drug release from liposomes can also be achieved by adding thermosensitive polymers to the formulation.Citation12 However, in the present review, we focus on formulations where thermosensitivity is achieved by the biophysical properties of the membrane-forming phospholipids and highlight the influence of lipid composition on the in vitro and in vivo behavior of the TSL formulations currently under investigation. This is in contrast with previously published reviews, which have concentrated on particular TSL formulationsCitation10,Citation13,Citation14 or image-guided drug delivery.Citation15,Citation16
Novel paradigm of drug targeting: intravascular temperature-triggered drug release by external targeting
Classical PEGylated long-circulating doxorubicin formulations like Doxil/Caelyx have been designed to exploit the enhanced permeability and retention effect (),Citation17 and passively accumulate inside tumor tissue because of the leaky tumor vasculature. Nevertheless, passive drug targeting has failed to achieve increased clinical efficacy in humans when compared with the free drug as a result of several shortcomings. Accumulation depends on the specific structure of the tumor vasculature and might be increased by heating the tumor tissue.Citation18,Citation19 However, extravasation of vesicles is the rate-limiting step, and nanoparticles have to circulate for days to accumulate in sufficiently high concentrationsCitation17 because accumulation in tumor tissue competes with uptake in the liver and spleen, and less than 10% of the injected dose accumulates in the tumor.Citation20 Doxil/Caelyx achieves these required long-circulating properties due to its high stability and ability to escape rapid recognition via the reticuloendothelial system. However, the bioavailability of doxorubicin is low.Citation21 Seynhaeve et al demonstrated cellular uptake of Doxil/Caelyx via endocytosis and transfer of the intact vesicles to the lysosomal compartment, which markedly impaired delivery of doxorubicin to the nucleus.Citation22
Figure 3 Schematic illustration of targeting concepts with liposomes.
Notes: (A) Passive targeting of drug encapsulated in liposomes is achieved by extravasation of the vesicles into the tumor tissue due to the leaky tumor vasculature (1). This enhanced permeability and retention effect is the rate-limiting step in vesicle accumulation and requires highly stable liposomes with a long circulation half-life in the bloodstream. Even liposomes with targeting moieties attached to their surface (eg, immunoliposomes) have to exploit the enhanced permeability and retention effect to interact with their targets (2). An alternative approach is endothelial targeting with cationic liposomes (3). The drug delivered by stable liposomes is not fully bioavailable and does not reach the tumor cells in sufficient amounts (4). Even after endocytosis of the vesicles, drug delivery is limited, since the drug fails to escape from the endosomal compartment (5) and is subsequently degraded in the lysosome. (B) Intravascular drug release is achievable by thermosensitive liposomes. Release is externally steerable by changing the focus of local heating. After entering the heated tissue, the drug is released into the bloodstream (6), generating a high local drug concentration. The concentration gradient increases the penetration depth of the drug inside tumors (7). The heat-triggered drug release inside the target area overcomes the limitation of traditional targeting concepts, because the drug is fully bioavailable and able to enter its site of action inside the cell (8).
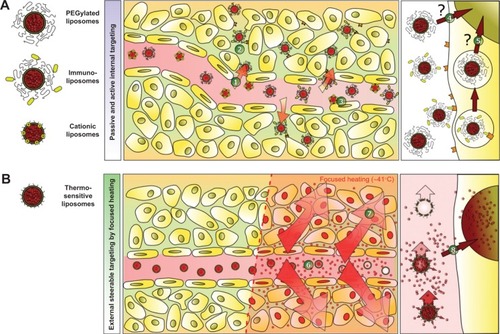
Surface modification of Doxil/Caelyx with the anticancer monoclonal antibody 2C5 resulted in enhanced vesicle accumulation in mouse tumors.Citation23 However, active targeting also requires extravasation of liposomes to reach deep-seated tumor cells and suffers from comparable shortcomings known for vesicles for passive targeting (). In recent years, the feasibility of passive drug targeting via the enhanced permeability and retention effect for improving therapeutic efficacy has been controversially discussed.Citation24 Another alternative is active targeting of tumor vessels that overexpress negatively charged macromolecules with cationic liposomes ().Citation25 However, cationic liposomes are characterized by an increased toxicity profile and rapid clearance from the blood after intravenous application.Citation25
A promising alternative, reported by Manzoor et al is external targeting achieved by temperature-triggered, localized intravascular drug release from TSL with focused heating ().Citation26 After reaching the heated tumor tissue, doxorubicin was released directly into the bloodstream, generating a high intravascular drug concentration.Citation26,Citation27 This led to a significantly increased penetration depth of bioavailable doxorubicin into the tumor tissue when compared with that in animals treated with free doxorubicin or Doxil/Caelyx.Citation26 Using this approach, poorly perfused tumor areas, which are known to be more difficult to treat due to a hypoxia-mediated resistance mechanism, could also be reached. The concept of intravascular drug release was then extended to targeting of more hydrophilic drugs, such as gemcitabine.Citation28 In contrast with doxorubicin, gemcitabine requires active cellular uptake and enzymatic intracellular activation to gemcitabine triphosphate. Continuous drug release should generate a consistently high intravascular concentration of gemcitabine during application of hyperthermia in the target tissue, reducing the risk of saturation of the gemcitabine-activating enzymes. A clinical pharmacokinetic study demonstrated the superiority of extending gemcitabine exposure by a prolonged infusion time, resulting in increased concentrations of gemcitabine triphosphate inside peripheral blood mononuclear cells.Citation29
Additional approaches for temperature-triggered drug delivery have been reported. For long-circulating TSL, it seems reasonable to include a pre-hyperthermia treatment to open up the tumor vasculature for passive accumulation of TSL,Citation18,Citation19 followed by a second heat trigger for interstitial drug release.Citation30 Such formulations might be further surface-modified for active targeting of the tumor vasculatureCitation31,Citation32 or tumor cells.Citation33,Citation34 For further information about these concepts, the reader is referred to a recent review by Dicheva and Koning.Citation35
Temperature-triggered drug targeting by TSL has the advantage of being able to externally control drug release spatially and temporally by steering the heating focus and heating power. Applicators for regional or localized heating of even deep-seated tumor tissue are well established in clinical practice, and are used to heat tumor tissue to temperatures of 42°C (mild hyperthermia).Citation36 Therefore, commonly used TSL are designed to release the encapsulated drug between 39°C and 42°C. In the following sections, these formulations are summarized and evaluated to their suitability regarding the above-mentioned targeting principles.
Influence of lipid composition on drug release
Encapsulated hydrophilic drugs are released from TSL at the melting phase transition temperature (Tm) of the lipid bilayer. At Tm, the structure of the lipid bilayer changes as transfer from a solid gel phase (Lβ) to a liquid-crystalline phase (Lα) occurs (). The membrane is more permeable to water and hydrophilic drugs in the liquid-crystalline phase than when in the gel phase.Citation15,Citation37 The permeability of hydrophilic drugs is highest at temperatures around the Tm because of the coexistence of membrane areas in both phases forming grain boundaries.Citation38,Citation39 DPPC is used as a major component in most TSL formulations because its Tm (41.4°C) is above body temperature.Citation40–Citation42 Unwanted drug leakage at body temperature can be reduced by mixing DPPC with small amounts of other phospholipids, such as DSPC (Tm =54.9°C).Citation15,Citation42–Citation45 The composition of miscible phospholipids determines the Tm of the formulation ().Citation45,Citation46 Basic requirements for TSL are stable drug retention at body temperature in the presence of blood components and a long in vivo half-life, combined with a fast drug release rate around Tm. Lipid-grafted PEG (eg, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-methoxy(PEG)-2000, DSPE-PEG2000, ) is commonly used in liposomes to create a steric barrier for inhibition of uptake by the reticuloendothelial system and increased blood circulation time,Citation47–Citation49 but also potentially affects vesicle stability ().Citation50 In addition to phospholipid composition, the manifestation of heat-triggered drug release depends to some degree on the drug molecule encapsulated (),Citation28,Citation51,Citation52 vesicle size,Citation51 and the presence of serum components.Citation45,Citation52
Figure 4 Factors affecting drug release from thermosensitive liposomes.
Notes: (A) The encapsulated drug is released by passive transfer driven by a concentration gradient (1). At body temperature, the phospholipids are in the solid gel (Lβ) phase state characterized by low permeability to hydrophilic compounds (2). By increasing the temperature above the phase transition temperature (Tm), the phospholipids are in a liquid-crystalline (Lα) phase state with higher permeability to hydrophilic compounds, because of the higher disorder and movement in the phospholipid packing (3). Around Tm, permeability is the highest because of coexistence of membrane areas in both phases (4). Permeability is further mediated by disturbances in lipid packing induced by lipid incorporation (5), lipid loss (7), and interaction with blood components (8–10). As a specific mechanism of ultrafast drug release from lysolipid-containing TSL, the formation of membrane pores (6) by lysolipids around Tm was shown. (B) Tm of DPPC/DSPC/DPPG2 50:20:30 (mol/mol) (DPPG2-TSL) measured by dynamic scanning calorimetry. (C) Temperature-dependent release of hydrophilic compounds from DPPG2-TSL measured in fetal calf serum.
Abbreviations: CF, carboxyfluorescein; dFdC, gemcitabine; DOX, doxorubicin; DPPC, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine; DSPC, 1,2-distearoyl-sn-glycero-3-phosphocholine; DPPG2, 1,2-dipalmitoyl-sn-glycero-3-phosphodiglycerol; T, temperature; Tm, phase transition temperature; TSL, thermosensitive liposomes.
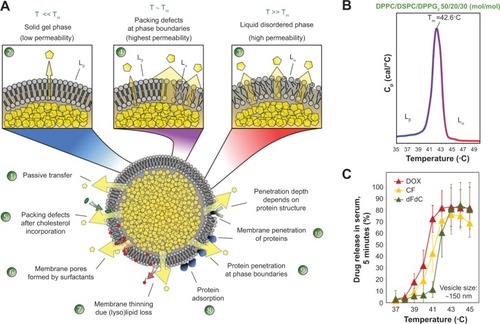
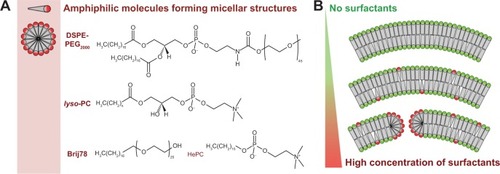
Figure 5 Micellar-forming amphiphilic molecules as membrane component in liposomes.
Notes: (A) Example of micellar-forming amphiphilic molecules (surfactants) used in liposomal formulations. DSPE-PEG2000 is used to increase the circulation half-life of liposomes, and lyso-PC, Brij78, and HePC can be used in thermosensitive formulations to facilitate drug release. (B) Surfactants are incorporated into the membrane bilayers at a low molecular content, but induce formation of usually unwanted structures (eg, membrane pores, dissolution of liposomes) at high concentrations.
Abbreviations: DSPE-PEG2000, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-methoxy(PEG)-2000; lyso-PC, acyl-sn-glycero-3-phosphocholine; Brij78, polyoxyethylene (20) stearyl ether; HePC, hexadecylphosphocholine.
In vitro and in vivo behavior of selected formulations
Until now distinct liposomal formulations have been described, which will be discussed in detail in this section ().
Table 1 Overview of thermosensitive liposomes
Traditional temperature-sensitive liposomes
In 1995, Gaber et al reported the effect of cholesterol and PEG-phosphatidylethanolamine with regard to stabilizing TSL formulations in vitro.Citation45 Incorporation of 30 mol% cholesterol into TSL formulations eliminated Tm by changing the phase state of the membrane to a liquid-ordered phase. Vesicles composed of DPPC/HSPC/cholesterol/DPPE-PEG 50:25:15:3 (mol/mol) released 60% of their doxorubicin content during 30 minutes of incubation at 42°C in human plasma.Citation45 In vivo fluorescence video microscopy in rats revealed liposome extravasation.Citation53 The doxorubicin content in the interstitial space was negligible at 34°C, but increased by 38-fold when the tumor was heated to 42°C for one hour.Citation53 A traditional temperature-sensitive liposome (TTSL) formulation with coencapsulated doxorubicin and a gadolinium-based contrast agent for MRI-guided delivery of doxorubicin is currently under investigation.Citation54–Citation57 The TTSL formulation has been used in these studies because of its higher stability when compared with lysolipid-containing low temperature-sensitive liposome (LTSL) formulations.Citation54
Lysolipid-containing low temperature-sensitive liposomes
The breakthrough in development of clinically usable TSL formulations was the incorporation of lysolipids into the membrane bilayer, as described by Needham et al in 2000.Citation11 The LTSL formulation was originally composed of DPPC/lyso-PC/DSPE-PEG2000 90:10:4 (mol/mol; ), but was modified slightly in recent years.Citation10 The surfactant, lyso-PC (), mediates drug release around Tm by formation of lysolipid-stabilized membrane pores.Citation10,Citation50,Citation58 The release rate of doxorubicin from LTSL at 41.3°C was 80% in 20 seconds.Citation14 In comparison, TTSL released only 40% of its doxorubicin content in 30 minutes on heating to 42°C.Citation11,Citation59
Complete regression of tumors was achieved in a preclinical xenograft mouse model using doxorubicin encapsulated in LTSL.Citation60 In an orthotopic murine mammary model, reduction of blood flow and microvascular density occurred after local application of hyperthermia.Citation61 Four xenograft models have been studied, and show that LTSL had improved efficacy in comparison with TTSL.Citation62 In 2006, Hauck et al published a Phase I study of LTSL performed in dogs with spontaneously grown solid tumors.Citation63 The tumor response achieved supported further evaluation of this formulation, but the maximum tolerated dose of 0.93 mg/kg was slightly lower than the published dose for free doxorubicin in dogs.Citation63 The LTSL formulation with encapsulated doxorubicin (Thermodox®) was licensed to Celsion Corporation (Columbia, MD, USA) and is currently under clinical investigation.Citation10,Citation64 Woo et al encapsulated cisplatin into LTSL, but until now only in vitro tests have been published.Citation65 Clinical development of the LTSL formulation was recently reviewed in detail, so interested readers are referred to this publication.Citation10
LTSL was the first TSL formulation suitable for use in the intravascular drug release approach. This formulation is characterized by ultrafast drug release upon heating, although incorporation of surfactants in the formulation decreases vesicle stability around Tm.Citation50 Moreover, approximately 70% of lysolipid was found to dissociate from the formulation within one hour post injection.Citation66
DPPG2-thermosensitive liposome
In 2004, a new liposomal formulation (DPPG2-TSL) composed of the phospholipids DPPC, DSPC, and 1,2-dipalmitoyl-sn-glycero-3-phosphodiglycerol (DPPG2), was reported by Lindner et al.Citation67 DPPG2 is a synthetic phospholipid with a molecular weight close to that of natural occurring 1,2-dipalmitoyl-sn-glycero-3-phosphoglycerol, because only one additional glycerol molecule is bound via an ether bond to the head group.Citation67 The molecular class of oligoglycerols (, DPPGn) was developed to increase the circulation half-life of vesicles in the same way as for PEGylated lipids. Lasic et al postulated that highly hydrated groups like PEG on the liposomal surface are capable of sterically inhibiting electrostatic and hydrophobic interactions with blood components.Citation68 Incorporation of DPPG2 led to a prolonged circulation time in non-thermosensitiveCitation69 and thermosensitiveCitation28,Citation67 formulations. The plasma half-life of carboxyfluorescein encapsulated into DPPG2-TSL was reported to be 9.6 hours in hamsters and 5 hours in rats.Citation67 Because of the significantly smaller head group modification of the phospholipid compared to DSPE-PEG2000 (74 Da versus approximately 2,000 Da), DPPGn forms lamellar structures and could be incorporated into TSL formulations with up to 70 mol%.Citation67 Incorporation of DSPE-PEG2000 instead is limited to concentrations below 10% mol, since it acts like a surfactant with a critical micelle concentration of 0.5–1.0 μM.Citation70 The Tm for DPPG2-TSL is around 42°C, with a narrow transition range ().
In contrast with the LTSL formulation, incorporation of surfactants into DPPG2-TSL was avoided. However, the release rates of carboxyfluorescein and doxorubicin from DPPG2-TSL were as fast as measured with the LTSL formulation,Citation46 but drug release from the DPPG2-TSL formulation started at approximately one degree higher temperature.Citation46 DPPG2-TSL showed improved in vitro stability in complete serum when compared with LTSL.Citation52 The presence of serum components at 37°C stabilized the formulation over time, whereas the opposite was found for LTSL. Interestingly, the lipid composition of a TSL formulation markedly influenced the effect of serum components on vesicle stability. DPPC/DSPC/DSPE-PEG2000 80:15:5 (mol/mol) (Stealth TSL) and LTSL were more susceptible towards destabilizing effects by cholesterol-containing vesicles,Citation52 whereas the presence of immunoglobulin type G stronger affected the stability of DPPG2-TSL.Citation52 Moreover, the stability of DPPG2-TSL was less affected by size changes in the range of 100–150 nm compared to surfactant containing LTSL.Citation51
Incorporation of 10% mol hexadecylphosphocholine (HePC; ) into the membrane of DPPG2-TSL further increased the release rate of the encapsulated drug, similar to lyso-PC.Citation71 HePC is structurally related to lyso-PC, but has better chemical and metabolic stability, and is approved as a lipophilic drug for the treatment of skin metastasis in breast cancer and for leishmaniasis. The in vitro cytotoxicity of HePC in DPPG2-TSL was heat-inducible and stronger than that induced by micellar HePC, which did not respond to heat.Citation71
Limmer et al passively loaded gemcitabine into DPPG2-TSL,Citation28 and their pharmacokinetic studies in rats using gemcitabine 6 mg/kg body weight showed an initial plasma half-life of 0.53 hours for gemcitabine encapsulated in DPPG2-TSL, with a size of 109 nm. The plasma half-life was increased to 2.59 hours when the vesicle size was increased to 129 nm. In a therapeutic study, significant delay of tumor growth was found for heat-triggered gemcitabine from DPPG2-TSL when compared with other gemcitabine formulations, including gemcitabine encapsulated in DPPG2-TSL without hyperthermia.Citation28
DPPG2-TSL is currently the only TSL formulation that fulfills all the criteria for heat-triggered intravascular drug release. Drug release upon activation from this formulation is comparable fast as observed with LTSL formulation. Moreover, the absence of surfactants yields a long-circulating formulation, with high plasma levels after intraveneous application for the duration of a typical hyperthermia treatment in the clinic.
Stealth TSL
A sterically stabilized TSL formulation (Stealth TSL; ) was developed from the original Yatvin formulation by adding DSPE-PEG2000 for improved stability and a better in vivo half-life when compared with the LTSL formulation,Citation46,Citation72 and enabled passive accumulation of TSL in tumor tissue.Citation19 Li et al compared Stealth TSL and LTSL, and found that the former had superior in vitro stability at 37°C in serum.Citation27 The maximum release of doxorubicin from Stealth TSL was at 42°C.Citation27 In comparison with LTSL, release of doxorubicin from Stealth TSL starts at higher temperatures (39°C versus 37°C).Citation27 Because of the absence of lyso-PC in Stealth TSL, the rate of release of doxorubicin at 42°C was slower (75%, one minute) when compared with LTSL (99%, one minute).Citation27 In a BFS-1 mouse model, Stealth TSL showed improved tumor growth control over LTSL when combined with mild hyperthermia.Citation27 Recently, Li et al published a two-step treatment approach.Citation30 After prehyperthermia treatment to open up the tumor vasculature,Citation18,Citation19 Stealth TSL passively accumulated in the tumor tissue, and a subsequent second hyperthermia treatment allowed interstitial drug release for precise intratumoral drug delivery.Citation30 Nevertheless, in tumor growth delay studies, this treatment was less effective than the intravascular temperature-triggered drug release approach, but could be a promising alternative for large and more deep-seated tumors.Citation30
Hyperthermia-activated cytotoxic formulation
Another TSL formulation with encapsulated doxorubicin currently under investigation is the hyperthermia-activated cytotoxic (HaT) liposome formulation described by Tagami et al ().Citation73 HaT is composed of DPPC and the non-ionic surfactant, polyoxyethylene (20) stearyl ether (Brij78; ). Brij78 consists of a PEGylated acyl chain, so it was hypothesized that Brij78 could replace the function of lyso-PC and DSPE-PEG2000 in the LTSL formulation.Citation73,Citation74 The HaT formulation showed 100% doxorubicin release within 3 minutes at a temperature of 40°C–42°C in buffer.Citation74 In comparison with LTSL, HaT showed enhanced drug release rates at 40°C, with similar blood pharmacokinetics.Citation73 For both formulations, a blood circulation half-life of approximately 0.5 hours was observed after injection.Citation73 A single intravenous treatment with HaT at a doxorubicin dose of 3 mg/kg body weight in combination with local hyperthermia showed enhanced tumor regression when compared with LTSL.Citation73
Gemcitabine and oxaliplatin have also been encapsulated into the HaT formulation.Citation75 In a pharmacokinetic study in mice, 40% of the injected dose was detectable 2 hours after intravenous administration of gemcitabine encapsulated in HaT.Citation75 For oxaliplatin, a three-fold reduction in clearance was observed in comparison with the free drug.Citation75 HaT showed a 25-fold improvement in delivery of gemcitabine to the heated tumor relative to free gemcitabine.Citation75 Unfortunately, superiority of external targeting was not shown in the therapeutic study, because there was no appropriate control group (ie, HaT without hyperthermia).
In 2012, Tagami et al reported an improved method for active loading of doxorubicin into the HaT formulation based on a copper(II) gradient (HaT-II).Citation76 HaT-II showed improved in vitro stability at 37°C, together with a faster drug release rate at 41°C in the presence of serum when compared with LTSL.Citation76 In comparison with LTSL, HaT-II showed a 2.5-fold longer blood circulation time in mice and a 2.0-fold increase in drug delivery to the heated tumor.Citation76 This resulted in improved antitumor efficacy.Citation76
STL formulation
In 2013, Park et al reported another stabilized formulation composed of DPPC, DSPE-PEG2000, cholesterol, and fatty acid-conjugated elastin-like polypeptide 55:2:15:0.4125 (mol/mol) (STL) with encapsulated doxorubicin ().Citation77 Pharmacokinetic studies in mice showed plasma half-lives of 2.03 hours and 0.92 hours for doxorubicin encapsulated in STL and LTSL,Citation77 respectively. In combination with high intensity focused ultrasound, STL achieved significantly better tumor growth delay 7 days after injection when compared with LTSL.Citation77
Thermosensitive liposomes for MRI-guided drug delivery
MRI is the method of choice for image-guided drug delivery with TSL. Its abilities with regard to morphological and functional tumor characterization without exposure to ionizing radiation are well known, and it is a standard method in clinical use. Further, MRI thermometry is established for the control of thermotherapies, such as radiofrequency hyperthermia and high intensity focused ultrasound. Dedicated hybrid systems have already been introduced into clinical applications.Citation15,Citation78,Citation79 Localized drug release from TSL has been demonstrated in rodents,Citation55,Citation80 and nonrodents,Citation81–Citation83 using MRI for the control of hyperthermia. Beyond controlling the volume of heating, encapsulation of MRI contrast agents in TSL formulations allows additional characterization of the drug delivery only accessible in humans when using MRI.
Signal mechanism
Paramagnetic gadolinium chelatesCitation54,Citation82,Citation84–Citation86 and manganese ionsCitation87–Citation90 are typical MRI-active contrast agents for encapsulation in TSL formulations (). The nuclear magnetic resonance of water protons is the primary origin of MRI signal and not the contrast agent itself. MRI contrast agents are only visualized by their ability to accelerate the water proton relaxation in the vicinity of the contrast agent molecules. This indirect signal forming process is only effective if the contrast agent molecule is allowed to interact with a large number of water protons. For visualization of temperature-induced release, the contrast agent has to be encapsulated inside the TSL.Citation91–Citation93 Below the Tm, the contrast agent interacts mainly with the water present inside the TSL, because water exchange with the exterior of the TSL is limited. As a result, the visibility of the contrast agent is reduced when compared with free contrast agents. When approaching the Tm, the increase in water exchange results in a signal increase in T1-weighted images.Citation84 Around the Tm, the contrast agent is released and the signal change is maximal and comparable with the signal change achieved with free contrast agent.Citation84,Citation86,Citation91,Citation92 This makes it possible to strongly change an MRI signal by altering temperature.Citation92
The maximum achievable signal change depends on the type of contrast agent,Citation86 lipid composition,Citation86,Citation91 vesicle size,Citation51,Citation91 and concentration of the encapsulated contrast agent.Citation86,Citation91 The heating method might also play a role, eg, focused ultrasound adds a mechanical release component to the signal mechanism.Citation15 The signal mechanism described in the paragraph before is mainly related to the contrast agent induced change of the longitudinal (T1) relaxation time of the water protons. But signal formation in MRI is complex, often showing a weighted signal depending not only on the T1 relaxation time shortening effect but also on the type of pulse sequence, choice of sequence parameters, and effects related to T2 relaxation. Quantification strategies in MRI thus try to determine a single parameter, such as the T1 relaxation time, with the aim of being independent of variables such as system settings of an individual measurement or inhomogeneity of the receiver coil. T1 relaxation remains the parameter of choice because it is directly related to the membrane dynamics.
Applications
TSL can be applied with an encapsulated contrast agent to distinguish heated from unheated tissueCitation85,Citation94 or to quantify absolute temperatures complementing traditional MRI thermometry methods,Citation95,Citation96 thus serving as a tool for quality assurance in thermotherapy in patients.
It has been demonstrated that potentially quantitative estimation of drug release based on T1 relaxation time changes is possible if the contrast agent and drug are both encapsulated in a TSL formulation,Citation55,Citation87–Citation89,Citation97 thus allowing “drug dose painting”Citation89 or “chemodosimetry”.Citation88 Because the drug itself is not observed by MRI, a correlation between the contrast agent and drug release had to be established. For that purpose, LTSL were actively loaded with doxorubicin using a manganese(II) gradient.Citation87 Doxorubicin and manganese(II) form a stable complex,Citation98 with the paramagnetic manganese(II) serving as an MRI contrast agent. Thus the release kinetics are the same for the contrast agent and the drug, allowing for correlation between change in T1 relaxation time (determined by MRI) and amount of doxorubicin (determined by high-performance liquid chromatography).Citation88 Using this strategy, it was possible to show that release of doxorubicin was heterogeneously distributed in the tumor model, and that LTSL administered during hyperthermia had the greatest antitumor effect when compared with other administration strategies.
The major drawback of the above approach is the toxicity related to manganese(II).
To overcome this, other researchers are using clinically approved gadolinium-based contrast agents. Hossann et al investigated six of these contrast agents for encapsulation in DPPG2-TSL, and considered a nonionic contrast agent with a low contribution to osmolality to be optimal.Citation86 Two strategies of encapsulation are possible using gadolinium-based contrast agents, but the release kinetics and signal mechanisms for both the contrast agent and drug have to be considered. One strategy is to combine two subsets of TSL, with one encapsulating only the contrast agent and a second encapsulating only the drug.Citation99 This strategy allows a higher amount of contrast agent and drug to be encapsulated whilst avoiding osmotic effects.Citation86 The second strategy is to coencapsulate both drug and contrast agent in the same TSL,Citation54–Citation57,Citation82,Citation83,Citation97 which limits the amount of both components in each TSL. Nevertheless, for both strategies, it has to be ensured that the temperature-dependent drug release rate and MRI signal change are correlated.Citation82
An important risk associated with clinical application of a gadolinium-based contrast agent is nephrogenic systemic fibrosis, a rare side effect in patients. The pathophysiological mechanism involves a reduced glomerular filtration rate and a long retention time combined with transmetallation of gadolinium(III). Hijnen et al addressed this concern in a rat model, but could not detect dissociation of gadolinium-DTPA in high intensity focused ultrasound ablation therapy.Citation100 De Smet et al investigated the blood kinetics and biodistribution of 111 In-labeled TTSL with coencapsulated doxorubicin and gadolinium-(HPDO3A)(H2O) in Fisher rats,Citation57 and found significant clearance with ≤0.3% of the injected dose in all analyzed organs one month after injection. Nevertheless, before application in humans, further investigation of the risks associated with this strategy seems necessary.
Conclusion
TSL are a promising tool for external targeting of drugs to solid tumors in combination with local hyperthermia or high intensity focused ultrasound. Several formulations have been developed, with one currently under clinical investigation. In vivo results show strong evidence that external targeting is superior over passive targeting of highly stable long-circulating drug formulations. Moreover, MRI-guided drug delivery adds the possibilities of online monitoring of heating focus, calculating locally released drug concentrations, and externally controlling drug release by steering the heating focus and power. The combination of external targeting with TSL and MRI-guided drug delivery will be the unique characteristic of this nanotechnology approach in medicine.
Acknowledgments
BK was funded by the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreement 603028 (iPaCT project).
Disclosure
The authors report no conflicts of interest in this work.
References
- WagnerAVorauer-UhlKLiposome technology for industrial purposesJ Drug Deliv2011201159132521490754
- BanghamADStandishMMWatkinsJCDiffusion of univalent ions across the lamellae of swollen phospholipidsJ Mol Biol1965132382525859039
- GregoriadisGWillsEJSwainCPTavillASDrug-carrier potential of liposomes in cancer chemotherapyLancet19741131313164134296
- GregoriadisGDrug entrapment in liposomesFEBS Lett1973362922964763309
- PosteGBucanaCRazABugelskiPKirshRFidlerIJAnalysis of the fate of systemically administered liposomes and implications for their use in drug deliveryCancer Res198242141214227060015
- BlumeGCevcGMolecular mechanism of the lipid vesicle longevity in vivoBiochim Biophys Acta199311461571688452853
- AllenTMHansenCMartinFRedemannCYau-YoungALiposomes containing synthetic lipid derivatives of poly(ethylene glycol) show prolonged circulation half-lives in vivoBiochim Biophys Acta1991106629362065067
- WagnerVDullaartABockAKZweckAThe emerging nanomedicine landscapeNat Biotechnol2006241211121717033654
- YatvinMBWeinsteinJNDennisWHBlumenthalRDesign of liposomes for enhanced local release of drugs by hyperthermiaScience197820212901293364652
- LandonCDParkJYNeedhamDDewhirstMWNanoscale drug delivery and hyperthermia: the materials design and preclinical and clinical testing of low temperature-sensitive liposomes used in combination with mild hyperthermia in the treatment of local cancerOpen Nanomed J20113386423807899
- NeedhamDAnyarambhatlaGKongGDewhirstMWA new temperature-sensitive liposome for use with mild hyperthermia: characterization and testing in a human tumor xenograft modelCancer Res2000601197120110728674
- van ElkMDeckersROerlemansCTriggered release of doxorubicin from temperature-sensitive poly(N-(2-hydroxypropyl)-methacrylamide mono/dilactate) grafted liposomesBiomacromolecules2014151002100924476227
- MayJPLiSDHyperthermia-induced drug targetingExpert Opin Drug Deliv20131051152723289519
- TaTPorterTMThermosensitive liposomes for localized delivery and triggered release of chemotherapyJ Control Release201316911212523583706
- GrüllHLangereisSHyperthermia-triggered drug delivery from temperature-sensitive liposomes using MRI-guided high intensity focused ultrasoundJ Control Release201216131732722565055
- LangereisSGeelenTGrüllHStrijkersGJNicolayKParamagnetic liposomes for molecular MRI and MRI-guided drug deliveryNMR Biomed20132672874423703874
- MaedaHThe enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targetingAdv Enzyme Regul20014118920711384745
- KongGAnyarambhatlaGPetrosWPEfficacy of liposomes and hyperthermia in a human tumor xenograft model: importance of triggered drug releaseCancer Res2000606950695711156395
- LiLten HagenTLBolkesteinMImproved intratumoral nanoparticle extravasation and penetration by mild hyperthermiaJ Control Release201316713013723391444
- HarringtonKJMohammadtaghiSUsterPSEffective targeting of solid tumors in patients with locally advanced cancers by radiolabeled pegylated liposomesClin Cancer Res2001724325411234875
- LaginhaKMVerwoertSCharroisGJAllenTMDetermination of doxorubicin levels in whole tumor and tumor nuclei in murine breast cancer tumorsClin Cancer Res20051119 Pt 16944694916203786
- SeynhaeveALDichevaBMHovingSKoningGAten HagenTLIntact Doxil is taken up intracellularly and released doxorubicin sequesters in the lysosome: evaluated by in vitro/in vivo live cell imagingJ Control Release201317233034024012486
- ElBayoumiTATorchilinVPTumor-targeted nanomedicines: enhanced antitumor efficacy in vivo of doxorubicin-loaded, long-circulating liposomes modified with cancer-specific monoclonal antibodyClin Cancer Res2009151973198019276264
- ParkKQuestions on the role of the EPR effect in tumor targetingJ Control Release201317239124113486
- Abu LilaASIshidaTKiwadaHTargeting anticancer drugs to tumor vasculature using cationic liposomesPharm Res2010271171118320333455
- ManzoorAALindnerLHLandonCDOvercoming limitations in nanoparticle drug delivery: triggered, intravascular release to improve drug penetration into tumorsCancer Res2012725566557522952218
- LiLten HagenTLHossannMMild hyperthermia triggered doxorubicin release from optimized stealth thermosensitive liposomes improves intratumoral drug delivery and efficacyJ Control Release201316814215023524188
- LimmerSHahnJSchmidtRGemcitabine treatment of rat soft tissue sarcoma with phosphatidyldiglycerol-based thermosensitive liposomesPharm Res Epub201436
- TemperoMPlunkettWRuiz Van HaperenVRandomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinomaJ Clin Oncol2003213402340812885837
- LiLTen HagenTLHaeriAA novel two-step mild hyperthermia for advanced liposomal chemotherapyJ Control Release201417420220824269966
- NegussieAHMillerJLReddyGDrakeSKWoodBJDreherMRSynthesis and in vitro evaluation of cyclic NGR peptide targeted thermally sensitive liposomeJ Control Release201014326527320067811
- DichevaBMten HagenTLLiLCationic thermosensitive liposomes: a novel dual targeted heat-triggered drug delivery approach for endothelial and tumor cellsNano Lett2013132324233122616659
- GaberMHModulation of doxorubicin resistance in multidrug-resistance cells by targeted liposomes combined with hyperthermiaJ Biochem Mol Biol Biophys2002630931412385965
- KullbergMMannKOwensJLA two-component drug delivery system using Her-2-targeting thermosensitive liposomesJ Drug Target2009179810719089689
- DichevaBMKoningGATargeted thermosensitive liposomes: an attractive novel approach for increased drug delivery to solid tumorsExpert Opin Drug Deliv2014118310024320104
- IsselsRDLindnerLHVerweijJNeo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre studyLancet Oncol20101156157020434400
- EvansENeedhamDPhysical properties of surfactant bilayer- membranes – thermal transitions, elasticity, rigidity, cohesion, and colloidal interactionsJ Phys Chem19879142194228
- MouritsenOGZuckermannMJModel of interfacial meltingPhys Rev Lett19875838939210034921
- KaasgaardTLeidyCCroweJHMouritsenOGJorgensenKTemperature-controlled structure and kinetics of ripple phases in one- and two-component supported lipid bilayersBiophys J20038535036012829489
- DemelRADe KruyffBThe function of sterols in membranesBiochim Biophys Acta1976457109132184844
- ChowdhryBZLipkaGDalzielAWSturtevantJMEffect of lanthanum ions on the phase-transitions of lecithin bilayersBiophys J1984456336356546889
- MabreySSturtevantJMInvestigation of phase-transitions of lipids and lipid mixtures by high sensitivity differential scanning calorimetryProc Natl Acad Sci U S A197673386238661069270
- BassettJBAndersonRUTackerJRUse of temperature-sensitive liposomes in the selective delivery of methotrexate and cisplatinum analogs to murine bladder-tumorJ Urol19861356126153944919
- MaruyamaKUnezakiSTakahashiNIwatsuruMEnhanced delivery of doxorubicin to tumor by long-circulating thermosensitive liposomes and local hyperthermiaBiochim Biophys Acta199311492092168323940
- GaberMHHongKHuangSKPapahadjopoulosDThermosensitive sterically stabilized liposomes: formulation and in vitro studies on mechanism of doxorubicin release by bovine serum and human plasmaPharm Res199512140714168584472
- HossannMWiggenhornMSchwerdtAIn vitro stability and content release properties of phosphatidylglyceroglycerol containing thermosensitive liposomesBiochim Biophys Acta200717682491249917618599
- PapahadjopoulosDGabizonALiposomes designed to avoid the reticuloendothelial systemProg Clin Biol Res199034385932198586
- AllenTMHansenCBLopes de MenezesDEPharmacokineics of long-circulating liposomesAdv Drug Deliv Rev199516267284
- NeedhamDMcIntoshTJLasicDDRepulsive interactions and mechanical stability of polymer-grafted lipid membranesBiochim Biophys Acta1992110840481643080
- IckensteinLMArfvidssonMCNeedhamDMayerLDEdwardsKDisc formation in cholesterol-free liposomes during phase transitionBiochim Biophys Acta2003161413513812896806
- HossannMWangTWiggenhornMSize of thermosensitive liposomes influences content releaseJ Control Release201014743644320727921
- HossannMSyunyaevaZSchmidtRProteins and cholesterol lipid vesicles are mediators of drug release from thermosensitive liposomesJ Control Release201216240040622759980
- GaberMHWuNZHongKHuangSKDewhirstMWPapahadjopoulosDThermosensitive liposomes: extravasation and release of contents in tumor microvascular networksInt J Radiat Oncol Biol Phys199636117711878985041
- de SmetMLangereisSvan den BoschSGrüllHTemperature-sensitive liposomes for doxorubicin delivery under MRI guidanceJ Control Release201014312012719969035
- de SmetMHeijmanELangereisSHijnenNMGrüllHMagnetic resonance imaging of high intensity focused ultrasound mediated drug delivery from temperature-sensitive liposomes: an in vivo proof-of-concept studyJ Control Release201115010211021059375
- de SmetMHijnenNMLangereisSMagnetic resonance guided high-intensity focused ultrasound mediated hyperthermia improves the intratumoral distribution of temperature-sensitive liposomal doxorubicinInvest Radiol20134839540523399809
- de SmetMLangereisSvan den BoschSSPECT/CT imaging of temperature-sensitive liposomes for MR-image guided drug delivery with high intensity focused ultrasoundJ Control Release2013169829023598044
- MillsJKNeedhamDLysolipid incorporation in dipalmitoylphosphatidylcholine bilayer membranes enhances the ion permeability and drug release rates at the membrane phase transitionBiochim Biophys Acta20051716779616216216
- GaberMHHongKLHuangSKPapahadjopoulosDThermosensitive sterically stabilized liposomes – formulation and in vitro studies on mechanism of doxorubicin release by bovine serum and human plasmaPharm Res199512140714168584472
- NeedhamDDewhirstMWThe development and testing of a new temperature-sensitive drug delivery system for the treatment of solid tumorsAdv Drug Deliv Rev20015328530511744173
- ChenQKrolAWrightANeedhamDDewhirstMWYuanFTumor microvascular permeability is a key determinant for antivascular effects of doxorubicin encapsulated in a temperature sensitive liposomeInt J Hyperthermia20082447548218608573
- YarmolenkoPSZhaoYLLandonCComparative effects of thermosensitive doxorubicin-containing liposomes and hyperthermia in human and murine tumoursInt J Hyperthermia20102648549820597627
- HauckMLLaRueSMPetrosWPPhase I trial of doxorubicin-containing low temperature sensitive liposomes in spontaneous canine tumorsClin Cancer Res2006124004401016818699
- WoodBJPoonRTLocklinJKPhase I study of heat-deployed liposomal doxorubicin during radiofrequency ablation for hepatic malignanciesJ Vasc Interv Radiol201223248255.e24722178041
- WooJChiuGNKarlssonGUse of a passive equilibration methodology to encapsulate cisplatin into preformed thermosensitive liposomesInt J Pharm2008349384617728083
- BannoBIckensteinLMChiuGNThe functional roles of poly(ethylene glycol)-lipid and lysolipid in the drug retention and release from lysolipid-containing thermosensitive liposomes in vitro and in vivoJ Pharm Sci2010992295230819902527
- LindnerLHEichhornMEEiblHNovel temperature-sensitive liposomes with prolonged circulation timeClin Cancer Res2004102168217815041738
- LasicDDMartinFJGabizonAHuangSKPapahadjopoulosDSterically stabilized liposomes: a hypothesis on the molecular origin of the extended circulation timesBiochim Biophys Acta199110701871921751525
- SchagonOLiposomen als potentielle Arzneistoffträger: Variation der bio-pharmazeutischen Eigenschaften durch 1,2-Dipalmitoyl-sn-glycero-oligo-glycerine. [Liposomes as drug carriers - variation of biopharmaceutical characteristics by incorporation of 1,2-dipalmitoyl-sn-glycero-3-phospho-oligoglycerols]Aachen, GermanyShaker Verlag1997 German
- AshokBArlethLHjelmRPRubinsteinIOnyukselHIn vitro characterization of PEGylated phospholipid micelles for improved drug solubilization: effects of PEG chain length and PC incorporationJ Pharm Sci2004932476248715349957
- LindnerLHHossannMVogeserMDual role of hexa decylphosphocholine (miltefosine) in thermosensitive liposomes: active ingredient and mediator of drug releaseJ Control Release200812511212018022271
- LiLten HagenTLSchipperDTriggered content release from optimized stealth thermosensitive liposomes using mild hyperthermiaJ Control Release201014327427920074595
- TagamiTErnstingMJLiSDEfficient tumor regression by a single and low dose treatment with a novel and enhanced formulation of thermosensitive liposomal doxorubicinJ Control Release201115230330921338635
- TagamiTErnstingMJLiSDOptimization of a novel and improved thermosensitive liposome formulated with DPPC and a Brij surfactant using a robust in vitro systemJ Control Release201115429029721640149
- MayJPErnstingMJUndzysELiS-DThermosensitive liposomes for the delivery of gemcitabine and oxaliplatin to tumorsMol Pharm2013104499450824152292
- TagamiTMayJPErnstingMJLiSDA thermosensitive liposome prepared with a Cu2+ gradient demonstrates improved pharmacokinetics, drug delivery and antitumor efficacyJ Control Release201216114214922504351
- ParkSMKimMSParkSJNovel temperature-triggered liposome with high stability: formulation, in vitro evaluation, and in vivo study combined with high-intensity focused ultrasound (HIFU)J Control Release201317037337923770213
- PellerMLofflerRBaurAMRI-controlled regional hyperthermiaRadiologe199939756763 German10525633
- GellermannJWlodarczykWHildebrandtBNoninvasive magnetic resonance thermography of recurrent rectal carcinoma in a 1.5 Tesla hybrid systemCancer Res2005655872588015994965
- HijnenNMHeijmanEKohlerMOTumour hyperthermia and ablation in rats using a clinical MR-HIFU system equipped with a dedicated small animal set-upInt J Hyperthermia20122814115522335228
- StaruchRChopraRHynynenKLocalised drug release using MRI-controlled focused ultrasound hyperthermiaInt J Hyperthermia20112715617121158487
- NegussieAHYarmolenkoPSPartanenAFormulation and characterisation of magnetic resonance imageable thermally sensitive liposomes for use with magnetic resonance-guided high intensity focused ultrasoundInt J Hyperthermia20112714015521314334
- RanjanAJacobsGCWoodsDLImage-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit Vx2 tumor modelJ Control Release201215848749422210162
- WangTTHossannMReinlHMIn vitro characterization of phosphatidylglyceroglycerol-based thermosensitive liposomes with encapsulated H-1 MR T-1-shortening gadodiamideContrast Media Mol Imaging20083192618330933
- PellerMSchwerdtAHossannMMR characterization of mild hyperthermia-induced gadodiamide release from thermosensitive liposomes in solid tumorsInvest Radiol20084387789219002060
- HossannMWangTSyunyaevaZNon-ionic Gd-based MRI contrast agents are optimal for encapsulation into phosphatidyldiglycerol-based thermosensitive liposomesJ Control Release2013166222923246469
- VigliantiBLAbrahamSAMichelichCRIn vivo monitoring of tissue pharmacokinetics of liposome/drug using MRI: illustration of targeted deliveryMagn Reson Med2004511153116215170835
- VigliantiBLPonceAMMichelichCRChemodosimetry of in vivo tumor liposomal drug concentration using MRIMagn Reson Med2006561011101817029236
- PonceAMVigliantiBLYuDMagnetic resonance imaging of temperature-sensitive liposome release: drug dose painting and antitumor effectsJ Natl Cancer Inst200799536317202113
- ReinlHMHossannMLindnerLHReiserMThermosensitive Mn2+ liposomes for MR-guided hyperthermia – solvent-dependent Mn2+ releaseIFMBE Proc2010252124
- FossheimSLFahlvikAKKlavenessJMullerRNParamagnetic liposomes as MRI contrast agents: influence of liposomal physicochemical properties on the in vitro relaxivityMagn Reson Imaging19991783899888401
- FossheimSLIl‘yasovKAHennigJBjornerudAThermosensitive paramagnetic liposomes for temperature control during MR imaging-guided hyperthermia: in vitro feasibility studiesAcad Radiol200071107111511131055
- ReinlHMLindnerLHSchneiderPNew thermosensitive liposomes for MR-guided hyperthermiaProc Intl Soc Mag Reson Med2003111209
- McDannoldNFossheimSLRasmussenHMartinHVykhodtsevaNHynynenKHeat-activated liposomal MR contrast agent: initial in vivo results in rabbit liver and kidneyRadiology200423074375214764890
- LindnerLHReinlHMSchlemmerMStahlRPellerMParamagnetic thermosensitive liposomes for MR-thermometryInt J Hyperthermia20052157558816147441
- HeySde SmetMStehningCSimultaneous T1 measurements and proton resonance frequency shift based thermometry using variable flip anglesMagn Reson Med20126745746322052363
- TagamiTFoltzWDErnstingMJMRI monitoring of intratumoral drug delivery and prediction of the therapeutic effect with a multifunctional thermosensitive liposomeBiomaterials2011326570657821641639
- ChiuGNAbrahamSAIckensteinLMEncapsulation of doxorubicin into thermosensitive liposomes via complexation with the transition metal manganeseJ Control Release200510427128815907579
- WangTHossannMPellerMDual phosphatidylglyceroglycerol-based thermosensitive liposomes for MR-guided chemothermotherapyIFMBE Proc201025259260
- HijnenNMEleveltAGrüllHStability and trapping of magnetic resonance imaging contrast agents during high-intensity focused ultrasound ablation therapyInvest Radiol20134851752423695082
