?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The present work reports a simple, cost-effective, and ecofriendly method for the synthesis of silver nanoparticles (AgNPs) using Chrysanthemum indicum and its antibacterial and cytotoxic effects. The formation of AgNPs was confirmed by color change, and it was further characterized by ultraviolet–visible spectroscopy (435 nm). The phytochemical screening of C. indicum revealed the presence of flavonoids, terpenoids, and glycosides, suggesting that these compounds act as reducing and stabilizing agents. The crystalline nature of the synthesized particles was confirmed by X-ray diffraction, as they exhibited face-centered cubic symmetry. The size and morphology of the particles were characterized by transmission electron microscopy, which showed spherical shapes and sizes that ranged between 37.71–71.99 nm. Energy-dispersive X-ray spectroscopy documented the presence of silver. The antimicrobial effect of the synthesized AgNPs revealed a significant effect against the bacteria Klebsiella pneumonia, Escherichia coli, and Pseudomonas aeruginosa. Additionally, cytotoxic assays showed no toxicity of AgNPs toward 3T3 mouse embryo fibroblast cells (25 μg/mL); hence, these particles were safe to use.
Introduction
The advancement in the nanoparticle (NP) system has had an impact in scientific areas. The nature and unique properties of nanomaterials have a wide range of applications in biosensors, tissue engineering, deoxyribonucleic acid (DNA) modification, drug delivery systems, cosmetics, and medical devices.Citation1–Citation5 Silver NPs (AgNPs) are well known biocidal substances that can be incorporated as antimicrobial agents in pharmacology, veterinary medicine, implants, wound dressings, and topical ointments.Citation6–Citation9 AgNPs were also found to exhibit antibacterial and antiplatelet activities.Citation10,Citation11 This biological activity is due to the extremely small size of the NPs (1–100 nm), which enhances their physical, chemical, magnetic, and optical properties.Citation12–Citation14 The synthesis of AgNPs, through physical and chemical methods, are well known;Citation15–Citation19 however, due to their environmental impact, energy consumption, and use of toxic chemicals, the synthesis of AgNPs that uses a green approach is most preferable, as this type of approach is found to be ecofriendly and nontoxic.Citation20,Citation21 This ecofriendly approach might pave the path for researchers across the globe to explore the potential of different herbs in the synthesis of NPs. AgNPs have been reported to be green synthesized from various parts of plants such as the bark of cinnamon,Citation22 neem leaves,Citation23 and various plant leaves.Citation24 Metal NPs have received great attention for their use in developing materials for medical applications.Citation25 In recent times, several groups have reportedly achieved success in the synthesis of Au, Ag, and Pd NPs obtained from the extracts of plant parts (such as leaves);Citation26 biologically active molecules were used for the green synthesis of AgNPs of desired size and morphology using medicinal plants, bacteria, and fungi.Citation27,Citation28 These active molecules act as reducing and capping agents for the synthesis of NPs, which makes these NPs suitable for biomedical applications. Flowers are an area of interest in the synthesis of AgNPs due to their attractive carotenoid pigments. The essential oils from Chrysanthemum indicum flowers showed significant antimicrobial activity;Citation29 it is a well-known herbal tea in the east Asian countries and China. The whole plant is beneficial to humans, but the famous part of the plant is the flower used in chrysanthemum tea.Citation30C. indicum is used in the traditional treatment of several infectious diseases such as pneumonia, colitis, stomatitis, cancer, fever, sores, and it is also used to treat vertigo, pertussis, and hypertensive symptoms.Citation31 The active molecules in C. indicum are glycosides and flavonoids; the plant has the ability to act as antibiotic to many species of bacterial pathogens.Citation30C. indicum is an herb with single, small-head, yellow daisies, which belongs to the family of Sapotaceae. The flowers have been consumed as tea in Chinese medicine to prevent sore throats and fevers. In traditional medicine, the flowers have been used for the treatment of dizziness, ocular inflammation, and skin boils. The flowers possess antimicrobial, anti-inflammatory, and free radical scavenging activity.Citation32,Citation33 Therefore, this work is focused on C. indicum. Only a few works have been carried out using the flowers Lonicera japonica, Nelumbo nucifera, and Carthamus tinctorius.Citation34–Citation36
In this study, the novel, one-step biosynthesis of AgNPs using the flower extract of C. indicum L. at room temperature has been reported. The objective of this study is to synthesize AgNPs using a green synthesis method, and to characterize the NPs using ultraviolet–visible spectroscopy (UV–Vis), X-ray diffraction (XRD), transmission electron microscopy (TEM), and energy-dispersive X-ray spectroscopy (EDX), and to evaluate the NPs’ antimicrobial effects against bacteria, as well as their in vitro cytotoxic effects on mouse embryonic fibroblast cells (3T3).
Materials and methods
Media and chemicals
Analytical-grade silver nitrate (AgNO3) was obtained from Sigma-Aldrich (St Louis, MO, USA). All of the media components and analytical reagents were purchased from Hi-Media Laboratories Pvt Ltd (Mumbai, India).
Microorganisms and cell line
Bacillus subtilis (Microbial Type Culture Collection and Gene Bank [MTCC] 121), Staphylococcus aureus (MTCC 96), S. epidermidis (MTCC 435), Escherichia coli (MTCC 433), Klebsiella pneumoniae (MTCC 109), and Pseudomonas aeruginosa (MTCC 1934) were used for this experiment, and they were obtained from the Microbial Type Culture Collection (MTCC), Chandigarh, India, and the mouse embryonic fibroblast cell line (3T3) was obtained from the National Center for Cell Science (Pune, India).
Preparation of flower extract and the synthesis of AgNPs
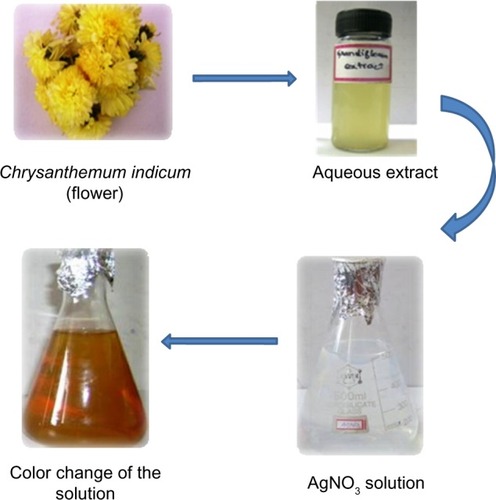
Fresh, healthy, and disease-free C. indicum flowers were selected and washed repeatedly with Milli-Q deionized water (EMD Millipore, Billerica, MA, USA) to remove surface contaminants. A total of 20 g of flowers were sliced into fine pieces, and 500 mL of Milli-Q deionized water was added and then boiled for 5 minutes before decantation. The crude extract was thus obtained, and it was filtered using Whatman No 1 filter paper (Whatman plc, Kent, UK). Then, 5 mL of flower extract was assorted with 500 mL of a silver nitrate solution (1 mmol). The reaction mixture was kept undisturbed until the colorless solution converted into a reddish-brown color, which indicated the formation of AgNPs. This process was carried out at room temperature. The particles were then purified by centrifugation. To remove excess silver ions, the silver colloids were washed at least three times with deionized water. It was then lyophilized and stored in screw-capped vials under ambient conditions for further characterization and application.
Phytochemical analysis
The aqueous extract obtained from the flower of C. indicum was tested for the presence of the phytochemicals – tannins, saponins, flavonoids, terpenoids, steroids, alkaloids, and glycosides – according to the method described by Trease and Evans.Citation37
Characterization of NPs
UV–Vis spectroscopy
The reduction of silver ions was monitored by measuring the UV–Vis spectrum of the reaction mixture after 2 minutes by using a UV–Vis spectrophotometer (UV100; Cyberlab® Inc, Miami, FL, USA) in the wavelength ranging from 300–650 nm.
X-ray diffraction
The crystalline structures of the synthesized AgNPs were investigated by XRD. Lyophilized and powdered samples were used, and the diffraction patterns were recorded in the scanning mode on an X’pert Pro diffractometer (PANalytical, Almelo, the Netherlands) operated at 40 kV and with a current of 30 mA, with Cu/kα radiation (λ=1.5418 Å) in the range of 20°–80° in 2θ angles. The average particle size of the synthesized silver AgNPs was calculated, using the Debye–Scherrer equation:
Transmission electron microscope and energy dispersive X-ray analysis
TEM and EDX were performed with a JEM 1101 transmission electron microscope (JEOL, Tokyo, Japan) to confirm the size, shape, and elemental composition of the AgNPs. The sample was dispersed in ethanol on a carbon-coated copper TEM grid, and the images were obtained by operating at an accelerating voltage of 120 kV.
Antibacterial activity of synthesized AgNPs
The antibacterial efficacy was assayed by the standard Kirby–Bauer disc diffusion methodCitation39 against Gram-positive and Gram-negative pathogenic bacteria. The bacterial suspension (108 colony-forming units/mL) was swabbed on the Mueller Hinton Agar plates using sterile cotton swabs. The sterile disc (Hi-Media Laboratories Pvt Ltd), which was 6 mm in diameter, was impregnated with the following four different components, including an aqueous extract of C. indicum, AgNO3, and synthesized AgNPs at the concentrations of 25 μg/disc. Streptomycin was used as a control. The discs were gently pressed and incubated at 37°C for 24 hours. The zone of inhibition in the diameter of each disc was measured in millimeters using a Hi-Media Laboratories Pvt Ltd. zone scale. The minimum inhibitory concentration (MIC) was determined according to the standard broth microdilution method.Citation40 The experiments were performed in triplicate.
Toxicity study of AgNPs
MTT assay on 3T3 cell lines
The mouse embryonic fibroblast cell line (3T3) was obtained from the National Center for Cell Science (Pune, India). The cells were seeded in a 75 cm2 flask containing Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% fetal bovine serum, 1.5 g/L of sodium bicarbonate, 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 0.25 μg/mL of amphotericin B. The cell line was grown in six-well plates at a density of 5 × 104 cells/mL in DMEM, and it was incubated to 75% confluency after 24 hours in 5% CO2 at 37°C. The cells were then treated with synthesized AgNPs at the concentrations of 25 μg/mL and 50 μg/mL of growth medium. The cells were then washed with phosphate buffered saline solution thrice and stained with 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoium bromide (MTT) dye.Citation41 The MTT solution (100 μL) was added to each well, followed by incubation at 37°C and 5% CO2 for an additional 4 hours. The insoluble formazan product formed within the living cells was solubilized by adding dimethyl sulfoxide:glycine buffer (125 μL). The intensity of the solutions was measured by a microplate reader (Bio-Rad 680; Bio-Rad Laboratories, Hercules, CA, USA) with relative cell viabilities being determined as a proportion of the control. After this treatment, the cells were added to the plate and incubated for 4 hours in 5% CO2, at 37°C. The viability of the cells was evaluated using a microplate reader (Bio-Rad 680) at 545 nm absorbance. The absorbance is directly proportional to the number of living cells in the culture. The relative cell viability (%) related to the control wells that contained the cell culture medium without NPs as a vehicle was calculated by
where (A) test is the absorbance of the test sample and (A) control is the absorbance of the control sample. Nontreated cells were used as the control, and the samples were imaged using an inverted photomicroscope (Nikon TE2000-E; Nikon Instruments, Melville, NY, USA).
Lactate dehydrogenase (LDH) leakage assay
The cytoplasmic enzyme release (lactate dehydrogenase [LDH]) and cell membrane instability were performed by the method of Borna et al,Citation42 with minor modifications. Various concentrations (25 μg/mL and 50 μg/mL) of the synthesized AgNPs were added to a 1 mL cuvette containing 0.9 mL of a reaction mixture to yield a final concentration of 1 mmol of pyruvate, 0.15 mmol of nicotinamide adenine dinucleotide (NADH) and 10 mmol of disodium hydrogen phosphate. After mixing thoroughly, the absorbance of the solution was measured at 340 nm. LDH activity was expressed as moles of NADH used in a minute per well.
Statistical analysis
All experiments were done in triplicate, and then the values were expressed as the mean ± standard deviation. Statistical significance was evaluated by one-way analysis of variance followed by Student’s t-test (P<0.05), using the Statistical Package for the Social Sciences (SPSS version 11; IBM Corporation, Armonk, NY, USA).
Results and discussion
In the present study, AgNPs were synthesized from the aqueous floral extract of C. indicum which acts as a reducing agent. It was observed that the reduction of silver ions into AgNPs was confirmed by the change of the silver nitrate solution to a reddish-brown color within 2 minutes at room temperature (), whereas the control AgNO3 solution (without the leaf extract) showed no change in color. This characteristic difference in color is due to the excitation of the surface plasmon resonance in the metal NPs.Citation43
Figure 1 Schematic illustration of the green synthesis of silver nanoparticles using an aqueous extract of the Chrysanthemum indicum (flower).
Phytochemical analysis
The phytochemical analysis of C. indicum revealed the presence of tannins, flavonoids, and glycosides (). These constituents may be responsible for the formation of AgNPs by reducing AgNO3 and acting as a capping agent to prevent agglomeration and to provide stability to the medium.Citation44 Flavonoids, tannins, proteins, and reducing sugars that are present in plants have been reported to act as bioreductants and capping agents.Citation45
Table 1 Phytochemical screening of the flower extract of Chrysanthemum indicum
UV–Vis spectroscopic analysis
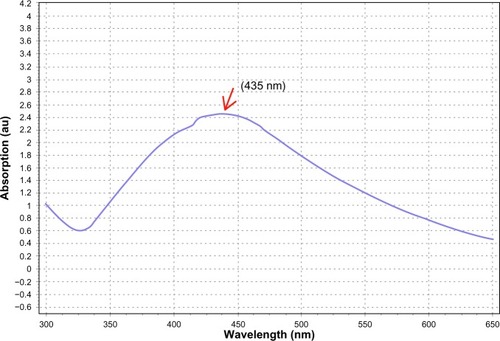
The UV–Vis spectrum is one of the imperative techniques used to find low-level concentrations of metal NPs in solution. In this study, the absorption spectra were noted at 435 nm (), and a broadening of the peak indicated that the particles were polydispersed. A similar report was observed by Loo et al.Citation46
XRD
The crystalline nature of the AgNPs that were synthesized using C. indicum was confirmed by XRD pattern analysis (). The XRD pattern indicates the five main characteristic peaks at 2θ values of 38°, 44°, 64°, 77° and 81° corresponding to 111, 200, 220, 311 and 222 planes for silver, suggesting that these NPs were crystalline in nature.Citation47 The mean particle diameter of the synthesized AgNPs was calculated from the XRD pattern using Scherrer’s equation:
Figure 3 X-ray diffraction pattern of synthesized silver nanoparticles from the floral extract of Chrysanthemum indicum.
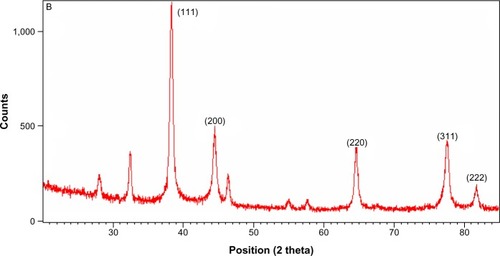
The equation uses the reference peak width at angle θ, where λ is the X-ray wavelength (1.5418 Å), β1/2 is the width of the XRD peak at half-height, and k is a shape factor. From this equation, the average crystalline size of the synthesized AgNPs was found to be 52 nm.
TEM and EDX analysis
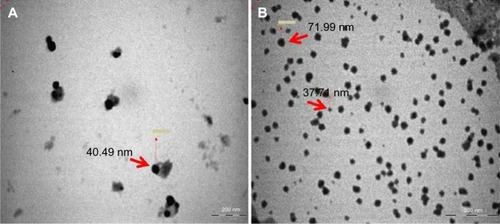
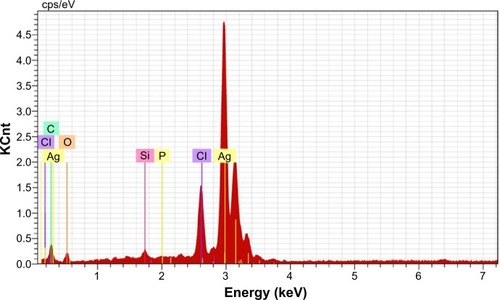
The size and morphology of the synthesized AgNPs were studied by TEM (). The size of the particles was found to be in the range of 37.71–71.99 nm, and they had a smooth surface and polydispersed particles. shows the clear elemental composition profile of the synthesized AgNPs. The intense signal at 3 keV strongly suggests that Ag was the major element of these NPs, as it has optical absorption in this range due to the surface plasmon resonance.Citation48 The other signals (carbon and oxygen) indicate the presence of plant extract, which corresponds to the biomolecules that were capping over the AgNPs.
Antimicrobial activity of AgNPs
The antimicrobial efficacy of the aqueous floral extract of C. indicum and of the AgNPs was examined against three Gram-positive and three Gram-negative bacteria such as B. subtilis (MTCC 121), S. aureus (MTCC 96), S. epidermidis (MTCC 435), E. coli (MTCC 433), K. pneumoniae (MTCC 109), and P. aeruginosa (MTCC 1934). Primary screening revealed that the biologically synthesized AgNPs showed significant activity, as compared to the flower extract. The synthesized AgNPs demonstrated prominent results against K. pneumoniae (19 mm) E. coli (14 mm), and they exhibited moderate activity against P. aeruginosa when compared to streptomycin ( and ).
Table 2 Antibacterial activity of AgNPs synthesized by the aqueous flower extract of Chrysanthemum indicum
Figure 6 The antimicrobial efficacy of the aqueous extracts of Chrysanthemum indicum, AgNO3, and AgNPs were examined against three selected Gram-positive bacterial strains (D, E, and F) and three selected Gram-negative bacterial strains (A, B, and C).
Notes: The Gram-positive bacterial strains included BS (MTCC 121), SE (MTCC 435), and SA (MTCC 96), and the three Gram-negative bacterial strains included KP (MTCC 109), EC (MTCC 433), and PA (MTCC 1934). The arrows indicate the zone of inhibition.
Abbreviations: PE, plant extract; C, control (streptomycin); KP, Klebsiella pneumoniae; AgNP, silver nanoparticle; EC, Escherichia coli; PA, Pseudomonas aeruginosa; BS, Bacillus subtilis; SE, Staphylococcus epidermidis; SA, Staphylococcus aureus; MTCC, Microbial Type Culture Collection and Gene Bank.
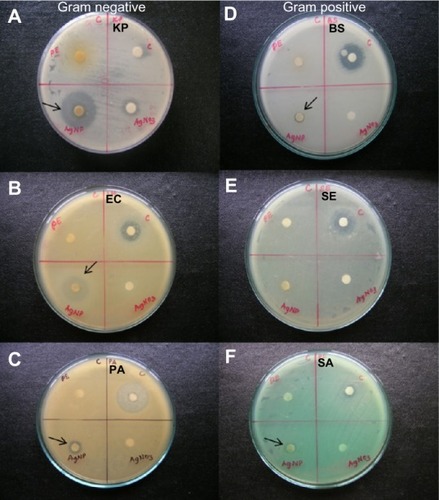
The antibacterial potential of the AgNPs in terms of their MIC are shown in . The AgNPs exhibited a MIC value of 25 μg mL−1 for the Gram-positive bacteria, S. epidermidis, and 6.25 μg mL−1 for the Gram-negative bacteria, K. pneumoniae. The MIC values of the AgNPs were slightly similar to or less than that of the standard broad spectrum antibiotic, streptomycin. This antibacterial activity exhibited by the AgNPs that were synthesized from C. indicum may be due to one or more of these following mechanisms. First, the effect may be due to the ultrafine size and larger surface area of the AgNPs, as their positively charged Ag+ ions bind to the negatively charged bacterial cell wall, deactivating the cellular enzymes, causing disruptions in membrane permeability.Citation49 Second, interactions with the thiol group of L-cysteine protein residues may lead to enzymatic dysfunction.Citation50 Finally, the AgNPs may facilitate the release of reactive oxygen species, which cause damage to proteins and DNA,Citation51 ultimately leading to cell death. This bactericidal effect of the synthesized AgNPs may also be facilitated by the shape of AgNPs. Pal et alCitation52 illustrated that the spherical and hexagonal shapes of AgNPs inhibited the Gram-negative bacterium, E. coli; similarly, our TEM micrographs showed spherical particles. It is noteworthy that the biologically synthesized AgNPs from C. indicum showed significant activity against Gram-negative bacteria when compared to Gram-positive bacteria. This difference was possibly due to the difference in the peptidoglycan layer. Gram-negative bacteria have a thin layer, whereas Gram-positive bacteria have a thick peptidoglycan layer (30–100 nm). As a result, the Gram-positive bacteria are defended from the bactericidal mechanisms, which are released by AgNPs.Citation53,Citation54
Table 3 Minimum inhibitory concentration of AgNPs synthesized by aqueous flower extract of Chrysanthemum indicum
Cytotoxicity of silver nanoparticles
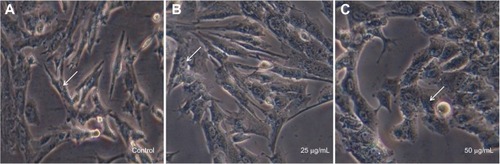
Recently, researchers have focused on the development of methods to reduce the toxicity of AgNPs. AgNPs are toxic to both microorganisms and human cells, but when exposed to adequate concentrations, different reactions may be observed.Citation55 In this study, the toxicity of AgNPs synthesized from C. indicum were evaluated in vitro against a 3T3 mouse embryo fibroblast cell line at 25 μg/mL and 50 μg/mL concentrations by MTT and LDH assays. In our study, the cells treated with 25 μg/mL of AgNPs did not seem to affect cell viability, which was observed to be 93.33% (). However, when the cells were treated with increased concentrations of AgNPs (50 μg/mL), they showed decreased cell viability (45.14%). The effect of AgNPs on LDH leakage in 3T3 cells are presented in . From the results, it is clear that there was no significant increase in LDH at 25 μg/mL (0.12 μmol/NADH) when compared to control, but there is an increase in LDH activity of 0.42 μmol/NADH at a concentration of 50 μg/mL. The toxicity of the synthesized AgNPs depends on their dose. Investigations of the morphological features of treated cells using an inverted microscope revealed that changes in the cell morphology were clearly reflected at 50 μg/mL when compared to the cells treated at concentrations of 25 μg/mL (). It can be clearly observed that the cells treated with AgNPs (25 μg/mL) have a well-developed nucleus, devoid of peripheral cellular distribution. Conversely, in the cells treated with a concentration of 50 μg/mL, systematic changes in the morphological features of the cells occurred. Further, with the LDH assay, a rise in LDH enzyme activity was observed at 50 μg/mL when compared to the control cells. LDH is a stable cytoplasmic enzyme that is present in most of the eukaryotic cells, and it is secreted into the culture medium during the damage or rupture of the cell’s cytoplasmic membrane. The elevated levels of LDH enzyme activity are directly proportional to the number of dead or damaged plasma membrane cells.Citation56 In this study, the synthesized AgNPs from the aqueous flower extract of C. indicum were found to exhibit highly antibacterial activity against K. pneumoniae and E. coli at concentrations of 25 μg/mL, and no cytotoxic effects on mouse embryonic fibroblast cells were observed. This result may provide a safe start for the use of AgNPs in pharmaceutical and other medical applications.
Table 4 Cell viability and LDH leakage in control- and AgNP-treated 3T3 cells after 24 hours of exposure
Conclusion
The present study reported on the synthesis of AgNPs by C. indium without using any harmful reducing or capping agents, as confirmed by UV–Vis spectrophotometer, XRD, TEM, and EDX techniques. Spherical, smooth surface, and poly-dispersed particles of sizes ranging from 37.71–71.99 nm with an average size of 52.9±4.6 nm were obtained. The lowest MIC of the AgNPs was exhibited against E. coli and K. pneumoniae (12.5 μg/mL and 6.25 μg/mL, respectively). The synthesized AgNPs act as excellent bactericidal agents, without exhibiting any toxic effects on mouse embryonic fibroblast cells.
Acknowledgments
The authors gratefully acknowledge the National Institute of Animal Science, Republic of Korea, for supporting this research. In addition, thanks to CERD, Loyola College, Chennai, India for providing the laboratory facilities.
Disclosure
The authors report no conflicts of interest in this work.
References
- KotthausSGuntherBHHangRSchaferHStudy of isotropically conductive bondings filled with aggregates of nano-sited Ag-particlesIEEE Trans Compon Packaging Manuf Technol19972011520
- Klaus-JoergerTJoergerROlssonEGranqvistCBacteria as workers in the living factory: metal-accumulating bacteria and their potential for materials scienceTrends Biotechnol2001191152011146098
- GoldsmithBRMitalaJJJosueJBiomimetic chemical sensors using nanoelectronic readout of olfactory receptor proteinsACS Nano2011575408541621696137
- TolaymatTMEl BadawyAMGenaidyAScheckelKGLuxtonTPSuidanMAn evidence-based environmental perspective of manufactured silver nanoparticle in syntheses and applications: a systematic review and critical appraisal of peer-reviewed scientific papersSci Total Environ20104085999100619945151
- WeirAWesterhoffPFabriciusLHristovskiKvon GoetzNTitanium dioxide nanoparticles in food and personal care productsEnviron Sci Technol20124642242225022260395
- KimKJSungWSSuhBKAntifungal activity and mode of action of silver nano-particles on Candida albicansBiometals200922223524218769871
- NadwornyPLWangJTredgetEEBurrellREAnti-inflammatory activity of nanocrystalline silver-derived solutions in porcine contact dermatitisJ Inflamm (Lond)201071320170497
- LaraHHAyala-NuñezNVIxtepan-TurrentLRodriguez-PadillaCMode of antiviral action of silver nanoparticles against HIV-1J Nanobiotechnology20108120145735
- BeckerROSilver ions in the treatment of local infectionsMet Based Drugs199964–531131418475906
- XiuZMZhangQBPuppalaHLColvinVLAlvarezPJNegligible particle-specific antibacterial activity of silver nanoparticlesNano Lett20121284271427522765771
- ShrivastavaSBeraTSinghSKSinghGRamachandraraoPDashDCharacterization of antiplatelet properties of silver nanoparticlesACS Nano2009361357136419545167
- Bogunia-KubikKSugisakaMFrom molecular biology to nanotechnology and nanomedicineBiosystems2002652–312313812069723
- ZharovVPKimJWCurielDTEvertsMSelf-assembling nanoclusters in living systems: application for integrated photothermal nanodiagnostics and nanotherapyNanomedicine20051432634517292107
- DobrovolskaiaMAMcNeilSEImmunological properties of engineered nanomaterialsNat Nanotechnol20072846947818654343
- KruisFEFissanHRellinghausBSintering and evaporation characteristics of gas-phase synthesis of size-selected PbS nanoparticlesMaterials Science and Engineering: B20006970329334
- MafunéFKohnoJYTakedaYKondowTFormation and size control of silver nanoparticles by laser ablation in aqueous solutionJ Phys Chem B20001043991119117
- WileyBSunYMayersBXiaYShape-controlled synthesis of metal nanostructures: the case of silverChemistry200511245446315565727
- MergaGWilsonRLynnGMilosavljevicBHMeiselDRedox catalysis on “naked” silver nanoparticlesJ Phys Chem C Nanomater Interfaces2007111331222012226
- OliveiraMMUgarteDZanchetDZarbinAJInfluence of synthetic parameters on the size, structure, and stability of dodecanethiol-stabilized silver nanoparticlesJ Colloid Interface Sci2005292242943516055140
- AnkamwarBDamleCAhmadASastryMBiosynthesis of gold and silver nanoparticles using Emblica officinalis fruit extract, their phase transfer and transmetallation in an organic solutionJ Nanosci Nanotechnol20055101665167116245525
- GeethalakshmiRSaradaDVGold and silver nanoparticles from Trianthema decandra: synthesis, characterization, and antimicrobial propertiesInt J Nanomedicine201275375538423091381
- SathishkumarMSnehaKWonSWChoCWKimSYunYSCinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activityColloids Surf B Biointerfaces200973233233819576733
- TripathiAChandrasekaranNRaichurAMMukherjeeAAntibacterial applications of silver nanoparticles synthesized by aqueous extract of Azadirachta indica (Neem) leavesJ Biomed Nanotechnol200951939820055111
- SongJYKimBSRapid biological synthesis of silver nanoparticles using plant leaf extractsBioprocess Biosyst Eng2009321798418438688
- ShahverdiARFakhimiAShahverdiHRMinaianSSynthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coliNanomedicine20073216817117468052
- ShankarSSAhmadASastryMGeranium leaf assisted biosynthesis of silver nanoparticlesBiotechnol Prog20031961627163114656132
- GhoshSPatilSAhireMSynthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agentsInt J Nanomedicine2012748349622334779
- PrakashPGnanaprakasamPEmmanuelRArokiyarajSSaravananMGreen synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolatesColloids Surf B Biointerfaces201310825525923563291
- ShunyingZYangYHuaidongYYueYGuolinZChemical composition and antimicrobial activity of the essential oils of Chrysanthemum indicumJ Ethnopharmacol2005961–215115815588664
- JungEKChemical composition and antimicrobial activity of the essential oil of Chrysanthemum indicum against oral bacteriaJournal of Bacteriology and Virology20093926169
- PitinidhipatNYasurinPAntibacterial activity of Chrysanthemum indicum, Cantella asiatica and Andrographis paniculata against Bacillus cereus and Listeria monocytogenes under osmotic stressAssumption University Journal of Technology2012154239245
- ChengWLiJYouTHuCAnti-inflammatory and immunomodulatory activities of the extracts from the inflorescence of Chrysanthemum indicum LinnéJ Ethnopharmacol20051011–333433716029939
- PongjitCNinsontiaCChaothamChanvorachotePProtective effect of Glycine max and Chrysanthemum indicum extracts against cisplatin-induced renal epithelial cell deathHuman Exp Toxicol2011301219311944
- NagajyothiPCLeeSEAnMLeeKDGreen synthesis of silver and gold nanoparticles using Lonicera japonica flower extractBull Korean Chem Soc201233826092612
- ArokiyarajSUdaya PrakashNKElakkyaVijayKamalaTBhuvaneswariSDinesh KumarVGreen synthesis of silver nano-particles using aqueous floral extract of Nelumbo NuciferaMaterials Science Forum2013756106111
- SreekanthTVMLeeKDGreen synthesis of silver nanoparticles from Carthamus tinctorius flower extract and evaluation of their antimicrobial and cytotoxic activitiesCurr Nanosci20117610461053
- TreaseGEEvansWCPharmacognosy11th edLondonBailliere Tindall19894550
- AminMAnwarFJanjuaMRIqbalMARashidUGreen synthesis of silver nanoparticles through reduction with Solanum xanthocarpum L. berry extract: characterization, antimicrobial and urease inhibitory activities against Helicobacter pyloriInt J Mol Sci20121389923994122949839
- BauerAWKirbyWMSherrisJCTurckMAntibiotic susceptibility testing by a standardized single disk methodAm J Clin Pathol19664544934965325707
- Clinical and Laboratory Standards InstituteMethods for Determining Bactericidal Activity of Antimicrobial Agents; Approved GuidelineWayne, PAClinical and Laboratory Standards Institute1999
- MosmannTRapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assaysJ Immunol Methods1983651–255636606682
- BornaSAbdollahiAMirzaeiFPredictive value of mid-trimester amniotic fluid high-sensitive C-reactive protein, ferritin, and lactate dehydrogenase for fetal growth restrictionIndian J Pathol Microbiol200952449850019805955
- KrishnarajCJaganEGRajasekarSSelvakumarPKalaichelvanPTMohanNSynthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogensColloids Surf B Biointerfaces2010761505619896347
- JhaAKPrasadKPrasadKKulkarniARPlant system: nature’s nanofactoryColloids Surf B Biointerfaces200973221922319539452
- LiSShenYXieAGreen synthesis of silver nanoparticles using Capsicum annuum L. extractGreen Chemistry20079852858
- LooYYChiengBWNishibuchiMRaduSSynthesis of silver nanoparticles by using tea leaf extract from Camellia sinensisInt J Nanomedicine201274263426722904632
- KhanMKhanMAdilSFGreen synthesis of silver nanoparticles mediated by Pulicaria glutinosa extractInt J Nanomedicine201381507151623620666
- NajithaBanu ABalasubramanianCMoorthiPVBiosynthesis of silver nanoparticles using Bacillus thuringiensis against dengue vector, Aedes aegypti (Diptera: Culicidae)Parasitol Res Epub20131031
- SuHLChouCCHungDJThe disruption of bacterial membrane integrity through ROS generation induced by nanohybrids of silver and clayBiomaterials200930305979598719656561
- GordonOVig SlentersTBrunettoPSSilver coordination polymers for prevention of implant infection: thiol interaction, impact on respiratory chain enzymes, and hydroxyl radical inductionAntimicrob Agents Chemother201054104208421820660682
- HossainZHuqFStudies on the interaction between Ag(+) and DNAJ Inorg Biochem200291239840412161309
- PalSTakYKSongJMDoes the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coliAppl Environ Microbiol20077361712172017261510
- SaravananMVemuAKBarikSKRapid biosynthesis of silver nanoparticles from Bacillus megaterium (NCIM 2326) and their antibacterial activity on multi drug resistant clinical pathogensColloids Surf B Biointerfaces201188132533121798729
- KimSHLeeHSRyuDSChoiSJLeeDSAntibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coliKorean Journal of Microbiology and Biotechnology20113917785
- YouCHanCWangXThe progress of silver nanoparticles in the antibacterial mechanism, clinical application and cytotoxicityMol Biol Rep20123999193920122722996
- SasakiTKawaiKSaijo-KuritaKOhnoTDetergent cytotoxicity: simplified assay of cytolysis by measuring LDH activityToxicol In Vitro19926545145720732144