?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Iron oxide magnetic nanoparticles (MNPs) were synthesized by the coprecipitation of iron salts in sodium hydroxide followed by coating separately with chitosan (CS) and polyethylene glycol (PEG) to form CS-MNPs and PEG-MNPs nanoparticles, respectively. They were then loaded with kojic acid (KA), a pharmacologically bioactive natural compound, to form KA-CS-MNPs and KA-PEG-MNPs nanocomposites, respectively. The MNPs and their nanocomposites were characterized using powder X-ray diffraction, Fourier transform infrared spectroscopy, thermogravimetric analysis, vibrating sample magnetometry, and scanning electron microscopy. The powder X-ray diffraction data suggest that all formulations consisted of highly crystalline, pure magnetite Fe3O4. The Fourier transform infrared spectroscopy and thermogravimetric analysis confirmed the presence of both polymers and KA in the nanocomposites. Magnetization curves showed that both nanocomposites (KA-CS-MNPs and KA-PEG-MNPs) were superparamagnetic with saturation magnetizations of 8.1 emu/g and 26.4 emu/g, respectively. The KA drug loading was estimated using ultraviolet–visible spectroscopy, which gave a loading of 12.2% and 8.3% for the KA-CS-MNPs and KA-PEG-MNPs nanocomposites, respectively. The release profile of the KA from the nanocomposites followed a pseudo second-order kinetic model. The agar diffusion test was performed to evaluate the antimicrobial activity for both KA-CS-MNPs and KA-PEG-MNPs nanocomposites against a number of microorganisms using two Gram-positive (methicillin-resistant Staphylococcus aureus and Bacillus subtilis) and one Gram-negative (Salmonella enterica) species, and showed some antibacterial activity, which could be enhanced in future studies by optimizing drug loading. This study provided evidence for the promise for the further investigation of the possible beneficial biological activities of KA and both KA-CS-MNPs and KA-PEG-MNPs nanocomposites in nanopharmaceutical applications.
Introduction
Chemotherapy is the treatment of cancer with cytotoxic antineoplastic drugs. It is given to patients for treatment, or it may aim to prolong lifetimes or to palliate cancer symptoms. Traditional chemotherapy has limitations such as immunosuppression,Citation1 mucositis,Citation2 and alopecia.Citation3 These limitations are due to the nonselectivity of antineoplastic drugs, thus affecting normal cells as well as cancerous cells. This has encouraged pioneering therapeutic strategies where the drug is delivered at a targeted cancer site.Citation4,Citation5 Targeted drug delivery is the best way to accumulate active drug molecules in pathological areas and minimize the side effects of antineoplastic drugs.Citation6 Due to their ability to penetrate cells and possess prolonged circulation times, nanoparticles have extensive potential for chemotherapeutical applications. In this regard, superparamagnetic iron oxide magnetic nanoparticles (MNPs) and other nanoparticles coated with polymers have received considerable attention in the biomedical field.Citation7,Citation8 The application of magnetic nanoparticles in drug delivery systems depends on the use of an external magnetic field to avoid problems associated with traditional chemotherapy.Citation9–Citation11 Coated magnetic nanoparticles loaded with anticancer drugs can then be injected into the patient’s body via the circulatory system to develop a highly desirable on-demand drug delivery system. By using an external magnetic field, the nanoparticles, and thus drug, can localize only to tumor cells, and then the drug can be released locally from the nanocarriers to enter the cells. It has been shown that such magnetic nanoparticles caused a complete reduction in tumor cells without any negative side effects.Citation10,Citation12
Such work in cancer has forged a pathway to use magnetic nanoparticles for other medical applications. For example, several studies on model drug-loaded MNPs have been reported, including doxorubicin,Citation13–Citation16 5-aminosalicylic acid,Citation17 insulin,Citation18 10-hydroxy camptothecin,Citation19 gallic acid,Citation20 folic acid,Citation21 diclofenac sodium,Citation22 and dexamethasone.Citation23 It has been found that superparamagnetic MNPs are predisposed to aggregate due to strong dipole–dipole attractions. So, the modification of the surface of the superparamagnetic MNPs with biocompatible and biodegradable polymers can minimize their agglomeration while serving as a drug carrier. These polymers include chitosan (CS) and its derivatives, polysaccharides, and polyethylene glycol (PEG).
Along these lines, kojic acid (KA) (5-hydroxy-2-hydroxymethyl-4-pyranone), a secondary metabolite, is one of the oldest identified (dating back to 1903) natural antibiotics produced by filamentous fungi and bacteria of many species of Aspergillus, and Penicillium, and Aceto-bacter.Citation24–Citation27 KA is a natural compound of interest due to its low side effects and its polyfunctional structure that allows the development of a variety of biologically active compounds and pharmaceuticals.Citation28,Citation29 KACitation30 and its derivatives have been used in a variety of medical, food, agricultural, and industrial applications, such as an antibrowning agent (antispeck) and antioxidant;Citation31–Citation33 for skin care, antiacne, and cosmetic applications due to its potent skin lightening/depigmenting properties,Citation34–Citation37 and used for herbicide, insecticide, pesticide,Citation38–Citation41 antidiabetic,Citation42 antitumor,Citation43 antiproliferative,Citation44 anticonvulsant,Citation31 and antimicrobial (including both antibacterial and antifungal) activities.Citation29,Citation45–Citation48 In addition, it has also been shown that KA significantly enhances neutrophil phagocytosis and lymphocyte proliferation.49,Citation50
In this study, we have incorporated CS and PEG on the surface of MNPs as a first attempt to use them in antibacterial applications. Due to the importance of KA (and its derivatives) in antibacterial applications, as shown from the previous pharmacological studies, KA was chosen as the model active substance in this study. Thus, the aim of the study was to synthesize and characterize novel magnetic nanocomposites containing KA and study their release profile as well as to investigate the antimicrobial activity of these novel magnetic nanocomposites.
Materials and methods
Materials
All chemicals used in this study were reagent grade and were used as such without purification. Ferrous chloride tetrahydrate (FeCl2 · 4H2O) (>99%), ferric chloride hexahydrate (FeCl3 · 6H2O) (99%), and streptomycin sulfate were obtained from Merck KGaA (Darmstadt, Germany). Low molecular weight CS (deacetylation 75%–85%), PEG, KA (>98% purity; molecular weight 142.11 g/mol), and ampicillin were purchased from Sigma-Aldrich (Saint Louis, MO, USA) and were used as such without any pretreatment. Acetic acid (99.8%) was purchased from Hamburg Industries, Inc (Hamburg, Germany). Deionized water was used for all the experiments.
Preparation of MNPs
The MNPs were prepared by a coprecipitation method as previously described.Citation32,Citation33 The MNPs were prepared by mixing solutions of Fe2+ (0.15 mol) and Fe3+ (0.3 mol) in 40 mL of deionized water. The Fe ion solutions were added to a solution of NaOH (2 mol) and the pH value of the solution was kept above 10. The MNPs precipitates were produced according to the reaction,
The solution was sonicated for 60 minutes at room temperature. Finally, the precipitates were collected by centrifugal separation and were separately washed three times with deionized water.
Preparations of CS-MNPs and PEG-MNPs
MNPs surfaces were modified using CS and PEG polymers and were prepared as follows: the CS solution was prepared by dissolving 0.5 g of CS powder in a 1% acetic acid solution. Similarly, the PEG solution was prepared by dissolving 0.5 g of the polymer into 100 mL of water. CS coated magnetic nanoparticles (CS-MNPs) and PEG coated magnetic nano-particles (PEG-MNPs) were prepared by mixing CS and PEG solutions (40 mL), respectively, with a 10 mL suspension of Fe3O4 nanoparticles (50 mg/mL). The mixtures were stirred for 18 hours. Coated particles were separated by a permanent magnet and dried at 70°C for 2 hours.
Preparation of KA-CS-MNPs and KA-PEG-MNPs nanocomposites
KA-CS-MNPs (kojic acid-loaded chitosan coated magnetic nanoparticles) and KA-PEG-MNPs (kojic acid-loaded PEG coated magnetic nanoparticles) nanocomposites were prepared by mixing 5 mg/mL of KA with a known weight of each CS-MNPs and PEG-MNPs nanoparticles (40 mg/mL). The solutions were magnetically stirred at room temperature for 18 hours to facilitate the uptake of KA. Next, the products were separated using a permanent magnet.
Loading and release amounts of KA from KA-CS-MNPs and KA-PEG-MNPs nanocomposites
The percentage of KA loaded in the nanocomposite was measured spectrophotometrically. A measure of 5 mg of the nanocomposite was dissolved in concentrate HCl/HNO3, then the amount of the KA in the sample was measured using UV-VIS spectroscopy. KA release profiles from the nanocomposites were determined at room temperature using a phosphate-buffered saline solution (PBS) at a concentration of 0.01 M at pH 7.4. About 85 mg of the nanocomposites were added to 500 mL of the PBS media. The cumulative amount of KA released into the solution was measured at preset time intervals at λmax =218 nm using a UV-Vis spectrophotometer, model Lambda 35 (Perkin Elmer, Waltham, MA, USA). To compare the release rate of KA from KA-CS-MNPs and KA-PEG-MNPs nanocomposites with the physical mixture which contained KA with polymers and MNPs, approximately 1.0 mg of the physical mixture was obtained by mixing 0.14 mg KA with the 0.05 mg CS and 0.81 mg MNPs. In addition, 0.11 mg of KA was mixed with 0.17 mg PEG and 0.73 mg MNPs, which was used to compare with the release from the KA-PEG-MNPs. The release of the active KA was determined as described above.
Antimicrobial activity of KA nanocomposites
Two Gram-positive bacteria (methicillin-resistant Staphylococcus aureus and Bacillus subtilis) and one Gram-negative species (Salmonella enterica) were used to test the ability of the synthesized nanocomposites to inhibit bacteria and fungi. The microbial cultures were obtained from the microbial culture collection unit (UNiCC, Institute of Bioscience, Universiti Putra Malaysia). Bacterial cultures were maintained on Luria–Bertani (LB) agar (Sigma-Aldrich). Prior to incubation with the novel nanoparticles, bacteria were cultured overnight in 5 mL of LB broth (Sigma-Aldrich) in a Certomat BS-T incubation shaker (Sartorius, Goettingen, Germany) at 37°C, set to 150 rpm until the culture reached an optical density at a wavelength of 600 nm of 1.0 (Spekol UV VIS 3.02; AnalytikJena, Jena, Germany), corresponding to 108 CFU/mL.
The antimicrobial activities of the synthesized nanocomposites were evaluated against the aforementioned bacteria using the agar diffusion (cup diffusion) method according to the Clinical and Laboratory Standards Institute guidelines.Citation51 Briefly, a volume of 20 mL of liquid Mueller Hinton agar (pH 7.3±0.2 at 25°C) was poured onto disposable sterilized Petri dishes and allowed to solidify. The surfaces of the solidified agar plates were allowed to dry in the incubator prior to streaking of microorganisms onto the surface of the agar plates. Next, 100 μL of the microbial culture suspensions in LB broth was streaked over the dried surface of the agar plate and spread uniformly using sterilized glass rods and allowed to dry before use. The nanocomposites were suspended in sterilized distilled water. Next, wells were made at equal distances in the agar plates using a sterilized cork borer and were filled with the corresponding nanocomposite suspensions (150 μL), and the same concentration (100 μg/mL) of KA and control antibiotics (ampicillin for Gram-negative and streptomycin for Gram-positive) was added to a separate well. To test the antimicrobial effect of the nanocomposites after release, the nanocomposites were suspended in PBS, and the test was repeated as described above. The experiment was carried out in triplicate, and the diameters of the zones of inhibition were measured to the nearest millimeter after 24 hours of incubation at 37°C.
Instrumentation
Powder X-ray diffraction (XRD) patterns were used to determine the crystal structure of the samples in the range of 20–70 degrees on an XRD-6000 diffractometer (Shimadzu, Tokyo, Japan) using CuKα radiation (λ 1.5406 Å) at 30 kV and 30 mA. Fourier transform infrared spectroscopy (FTIR) spectra of the materials were recorded over the range of 400–4,000 cm−1 on a Thermo Nicolet Nexus, Smart Orbit spectrometer using the potassium bromide disc method. Thermogravimetric analysis (TGA) was carried out using a Metter-Toledo 851e instrument (Greifensee, Switzerland) with a heating rate of 10°C/minute in 150 μL alumina crucibles and in the range of 30°C–900°C. Scanning electron microscopy (SEM) was used to observe the surface morphology of the samples using a NOVA™ NanoSEM 230 (FEI, Hillsboro, OR, USA) scanning electron microscope. Magnetic properties were evaluated by a Lakeshore 7404 vibrating sample magnetometer (Westerville, OH, USA). An ultraviolet-visible spectrophotometer (Perkin Elmer) was used for the controlled release study.
Results and discussion
XRD
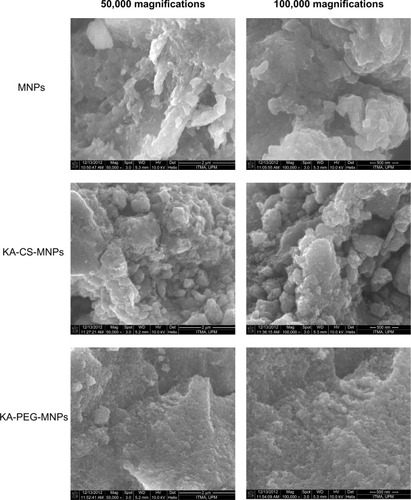
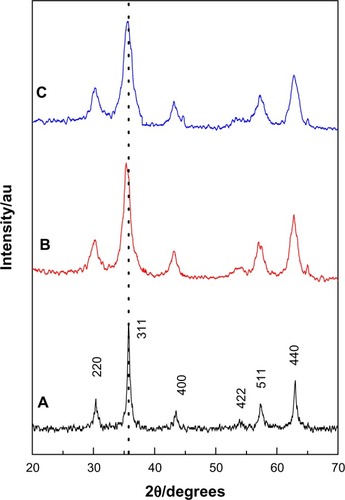
The XRD patterns of the prepared MNPs as well as KA-CS-MNPs and KA-PEG-MNPs nanocomposites are shown in –, respectively. shows six characteristic peaks for MNPs marked by their indices 220, 311, 400, 422, 511, and 440 which appeared at 2θ =30.1°, 35.4°, 43.2°, 53.4°, 57.0°, and 62.6°, respectively, which suggests the formation of spinel structure of iron oxide (magnetite-Fe3O4).Citation1,Citation25 These were observed also for both nanocomposite samples in , which indicates that the coating of both polymers and drug loading process did not result in any phase change for the MNPs.
Figure 1 Powder X-ray diffraction patterns of (A) MNPs, (B) KA-CS-MNPs, and (C) KA-PEG-MNPs nanocomposites.
Abbreviations: KA-CS-MNPs, kojic acid-chitosan-iron oxide nanoparticles; KA-PEG-MNPs, kojic acid-polyethylene glycol-iron oxide nanoparticles; MNPs, iron oxide magnetic nanoparticles.
The mean grain size was calculated using the Debye– Scherrer formula as shown in the equation,
where D is average grain size, k is Scherrer constant (0.89), λ is the X-ray diffraction wavelength (0.15418 nm), β is the peak width of half maximum intensity, and θ is the Bragg diffraction angle. The estimation of the average crystal size for the KA-CS-MNPs and KA-PEG-MNPs nanocomposites were calculated to be 19.2 nm and 16.7 nm, respectively.
FTIR
FTIR spectra for MNPs, KA-CS-MNPs, KA-PEG-MNPs, and free KA drug are shown in . The MNPs in showed an absorption band at 565 cm−1, which is due to the stretching of Fe-O in Fe3O4. However, this characteristic band of Fe-O was shifted to 579 cm−1 and 578 cm−1 for KA-CS-MNPs and KA-PEG-MNPs, respectively. This result confirmed the presence of magnetite nanoparticles.Citation52
Figure 2 FTIR spectra of (A) MNPs, (B) KA-CS-MNPs, (C) KA-PEG-MNPs, and (D) free KA.
Abbreviations: FTIR, Fourier transform infrared spectroscopy; KA, kojic acid; KA-CS-MNPs, kojic acid-chitosan-iron oxide nanoparticles; KA-PEG-MNPs, kojic acid-polyethylene glycol-iron oxide nanoparticles; MNPs, iron oxide magnetic nanoparticles.
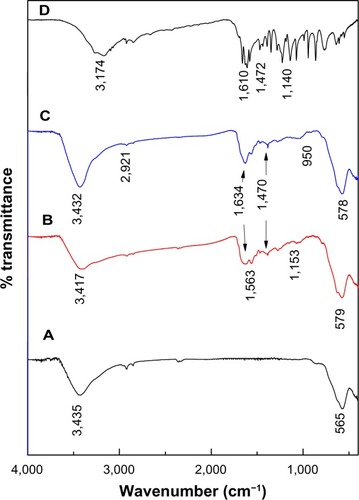
The FTIR spectra for KA-CS-MNPs and KA-PEG-MNPs nanocomposites showed the characteristic bands for CS and PEG, respectively, proving that magnetite nanoparticles were successfully coated with CS and PEG. For example in the KA-CS-MNPs nanocomposite, a band around 1,563 cm−1 was assigned to NH3+.Citation53 The bands at 1,153 cm−1 are due the C-N stretch in CS.Citation52 The FTIR spectra of the KA-PEG-MNPs nanocomposite are shown in . The bands around 2,921 cm−1 and 950 cm−1 were due to −CH2 stretching vibrations and −CH out-of-plane bending vibrations, respectively.Citation54 The −CH2 and −CH bands are strong evidence that PEG covered the nanoparticle surface.
The appearance of KA bands in KA-CS-MNPs and KA-PEG-MNPs nanocomposites confirmed the loading of KA on the CS-MNPs and PEG-MNPs nanoparticle surface; for example, there is a band at 1,634 cm−1. In addition, the band at 1,610 cm−1, which relates to the C=O of free KA was shifted to 1,563 cm−1 and 1,568 cm−1 in KA-CS-MNPs and KA-PEG-MNPs nanocomposites, respectively. This shifting was due to the loading of KA and binding with polymers by hydrogen bonds.
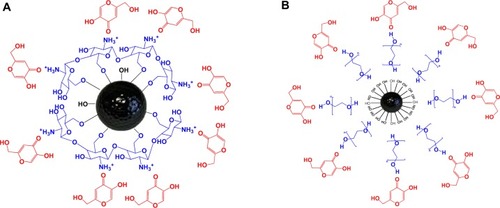
shows the interaction between the MNPs and the polymers, and it is clear that the MNPs interacted with CS by the glycosidic bonds,Citation19 whereas it interacted with PEG by hydrogen bonds. In addition, the KA drugs interacted with both polymers by hydrogen bonds.
Figure 3 Schematic representation of the interaction between KA, CS, and MNPs in the KA-CS-MNPs nanocomposite (A) and between KA, PEG, and MNPs in the KA-PEG-MNPs nanocomposite (B).
Abbreviations: CS, chitosan; KA, kojic acid; KA-CS-MNPs, kojic acid-chitosan-iron oxide nanoparticles; KA-PEG-MNPs, kojic acid-polyethylene glycol-iron oxide nanoparticles; MNPs, iron oxide magnetic nanoparticles; PEG, polyethylene glycol.
TGA
The thermal behavior of the MNPs before and after polymer coating and KA loading was further examined using TGA. The thermal analyses of MNPs, CS-MNPs, and KA-CS-MNPs are shown in , and the thermal analyses of MNPs, PEG-MNPs, and KA-PEG-MNPs are shown in . For MNPs, two main thermal events were clearly observed. The first event, which occurred in the region of 30°C–220°C, was attributed to the removal of surface hydroxyl groups and/or adsorbed water with a 3.9% weight loss.Citation12 This was followed by the second stage at 220°C–850°C with a 1.9% weight loss. The thermal analyses for CS-MNPs and PEG-MNPs showed a slight weight loss up to 260°C, which probably was due to the adsorbed water. Significant weight loss for CS-MNPs and PEG-MNPs was noticed between 260°C and 620°C, with 7.2% and 9.4% weight loss, respectively. These significant weight losses were attributed to the decomposition of CS and PEG polymers which absorbed on the surface of MNPs (see inset curve for ). By comparing the weight loss curves of CS-MNPs and PEG-MNPs nanocomposites with MNPs, the data suggested that the amount of CS and PEG in CS-MNPs and PEG-MNPs was estimated at 5.3% and 7.5%, respectively.
Figure 4 TGA curves for MNPs, CS-MNPs, and KA-CS-MNPs (A) and MNPs, PEG-MNPs, and KA-PEG-MNPs (B), with insets showing the TGA curves of pure CS and PEG, respectively.
Abbreviations: CS, chitosan; CS-MNPs, chitosan-iron oxide nanoparticles; KA-CS-MNPs, kojic acid-chitosan-iron oxide nanoparticles; KA-PEG-MNPs, kojic acid-polyethylene glycol-iron oxide nanoparticles; MNPs, iron oxide magnetic nanoparticles; PEG, polyethylene glycol; PEG-MNPs, polyethylene glycol-iron oxide nanoparticles; TGA, thermogravimetry analysis.
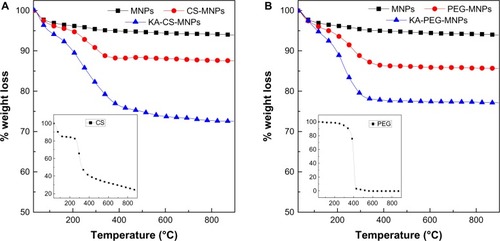
TGA thermal analysis can be used to also confirm the loading of KA at the surface of CS-MNPs and PEG-MNPs. A significant weight loss was noticed between 260°C and 600°C, with 20.7% and 19.9% total weight loss for KA-CS-MNPs and KA-PEG-MNPs, respectively. This weight loss is attributed to the decomposition of polymers and KA drugs.
The ultraviolet-visible spectrometry results, which were used to calculate the loading of KA in the final nanocomposites, gave 12.2% and 8.3% for the KA-CS-MNPs and KA-PEG-MNPs nanocomposites, respectively (data not shown).
Measurement of magnetic properties
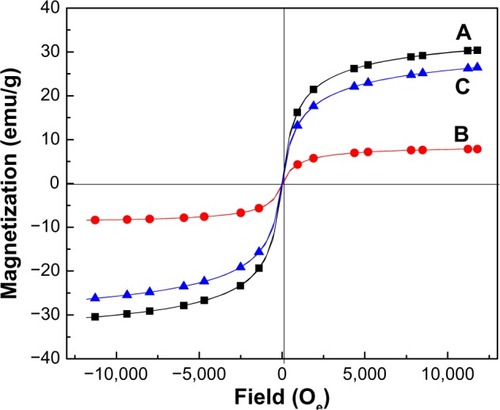
The results in show a typical magnetization (Ms) versus the applied magnetic field plot at room temperature for MNPs, KA-CS-MNPs, and KA-PEG-MNPs nanocomposites. The Ms curves of MNPs showed superparamagnetic properties (ie, no remanence effect) and saturation Ms of about 30.5 emu/g. As can be seen from , similar to magnetite particles, KA-CS-MNPs and KA-PEG-MNPs nanocomposites also indicated a zero remanence Ms and coercivity, thus suggesting that the superparamagnetic behavior is still retained with the nanocomposite materials. The saturation Ms for KA-CS-MNPs and KA-PEG-MNPs nanocomposites were approximately 8.1 emu/g and 26.4 emu/g, respectively. It can be seen from that the Ms of the MNPs decreased in the final products. The decrease in Ms value is only due to the exchange of electrons between the surface of Fe atoms with the CS and PEG polymers,Citation55–Citation57 providing further supporting evidence that the coating of CS and PEG did occur on MNPs.
Figure 5 Magnetization curves of (A) MNPs, (B) KA-CS-MNPs, and (C) KA-PEG-MNPs nanocomposites recorded at room temperature.
Abbreviations: KA-CS-MNPs, kojic acid-chitosan-iron oxide nanoparticles; KA-PEG-MNPs, kojic acid-polyethylene glycol-iron oxide nanoparticles; MNPs, iron oxide magnetic nanoparticles, Oe, applied magnetic field.
SEM analysis
The SEM images of the pure MNPs and the KA-CS-MNPs and KA-PEG-MNPs nanocomposites are shown in . The agglomeration of the synthesized MNPs was very strong due to the van der Waals forces between the particles.Citation58 After the surface coating, the agglomeration of the KA-CS-MNPs and KA-PEG-MNPs nanocomposites was reduced. This result indicated that the coating of the MNPs with CS and PEG decreased van der Waals forces between the MNPs.Citation59 The agglomeration observed in also provides insights into the antibacterial properties presented in the discussion below, as those particles with greater agglomeration decreased bacteria interactions and surface area exposure.
In vitro release study of KA from the nanocomposites
The release profiles of KA from the KA-CS-MNPs and KA-PEG-MNPs nanocomposites and the physical mixture of KA with polymers and MNPs are shown in . It can be seen from that the physical mixture of KA with polymers and MNPs exposed to a pH of 7.4 released KA quickly; the release was completed within 10 minutes. The release rate of KA from the KA-CS-MNPs and KA-PEG-MNPs nanocomposites are obviously slower than that from the physical mixture as shown in the , indicating that both the nanocomposites are potential drug controlled release systems which can be further tailored in future studies for specific applications. The slower release may be attributed to the hydrogen bond interactions between the polymers and KA as well as the aggregations that occurred in these nanocomposites.
Figure 7 (A) Release profiles of KA from the physical mixture of KA+MNPs+CS or KA+MNPs+PEG at pH 7.4, and (B) release profiles of KA from the KA-CS-MNPs (I) and KA-PEG-MNPs (II) nanocomposites at pH 7.4. Inset in (B) shows the release profiles of KA from KA-PEG-MNPs.
Abbreviations: KA, kojic acid; KA+CS+MNPs, physical mixture of kojic acid, chitosan, and iron oxide nanoparticles; KA-CS-MNPs, kojic acid-chitosan-iron oxide nanoparticles; KA+PEG+MNPs, physical mixture of kojic acid, polyethylene glycol, and iron oxide nanoparticles; KA-PEG-MNPs, kojic acid-polyethylene glycol-iron oxide nanoparticles.
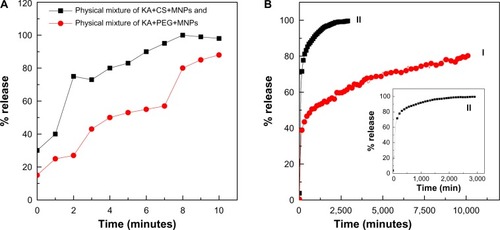
The percentage release of KA from the KA-CS-MNPs nanocomposite reached about 81.0% within about 8,000 minutes () compared to about 99.8% within about 2,000 minutes (). The differences in the release rate of KA from KA-CS-MNPs and KA-PEG-MNPs nanocomposites may be due to the difference in number of hydrogen bonds which formed between the KA and polymers. In , we can see the number of possible hydrogen bonds formed between the drug and CS was more than KA and PEG.
Release kinetics of KA from KA-CS-MNPs and KA-PEG-MNPs
The release behavior of KA from the KA-CS-MNPs and KA-PEG-MNPs nanocomposites can be described by different kinetics models, pseudo first-order (EquationEquation 2(2) ),Citation60 pseudo second-order (EquationEquation 3
(3) ),Citation10 Higuchi model (EquationEquation 4
(4) ),Citation61 Hixson–Crowell model (EquationEquation 5
(5) ),Citation61 and the Korsmeyer–Peppas model (EquationEquation 6
(6) );Citation61
where qe and qt are the equilibrium release amount and the release amount at any time (t), respectively, and Mo and Mt are the initial amount and the amount of the drug in the nanocomposite at time t, respectively.
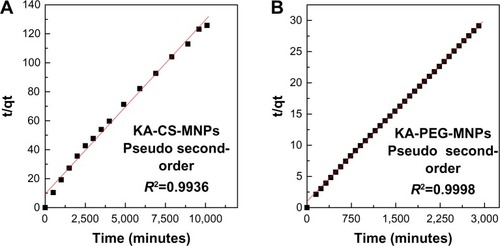
With the use of the five kinetic models as mentioned earlier in the release kinetic data, it was found that the pseudo second-order model is more satisfactory for describing the release kinetic processes of KA from the KA-CS-MNPs and KA-PEG-MNPs nanocomposites compared to the other models used in this work. shows the plots of t/qt against time for the pseudo second-order kinetic model, and the resulting correlation coefficient as well as the rate constant k-values are given in .
Table 1 Correlation coefficients (R2) obtained by fitting the KA release data from KA-CS-MNPs and KA-PEG-MNPs nanocomposites in phosphate-buffered saline solutions at pH 7.4
Figure 8 Fitting of data for KA release from (A) KA-CS-MNPs and (B) KA-PEG-MNPs nanocomposites for pseudo second-order kinetic model at pH 7.4.
Abbreviations: CS-MNPs, chitosan-iron oxide nanoparticles; KA, kojic acid; PEG-MNPs, polyethylene glycol-iron oxide nanoparticles; KA-CS-MNPs, kojic acid-chitosan-iron oxide nanoparticles; KA-PEG-MNPs, kojic acid-polyethylene glycol-iron oxide nanoparticles.
In vitro antimicrobial activity of the KA nanocomposites
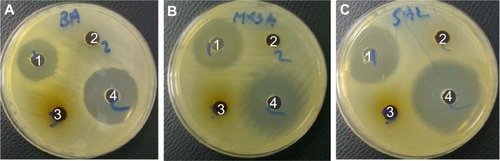
In the present study, the antimicrobial activity of the synthe-sized KA-CS-MNPs nanocomposite was determined using an agar-based method against three bacterial strains of clinical significance, which can cause a range of minor to life-threatening infections in the skin due to burns and respiratory, urinary tract, gastrointestinal, or blood infections (). The antimicrobial activity of the MNPs alone as well as the KA loaded nanocomposites were tested, and it was found that MNPs did not show any antimicrobial effect on the microorganisms tested (data not shown). In contrast, the KA-CS-MNPs and KA-PEG-MNPs nanocomposites showed minimal to no antimicrobial activity against the selected microorganisms under the test conditions using the agar diffusion method as compared to the free KA, which showed comparable to less antimicrobial activity, in agreement with previous studies. Further investigation into optimal drug loading, polymer degradation, etc will improve the antimicrobial activity of the KA incorporated novel nanocomposites. Specifically, for Bacillus subtilis, the zone of inhibition was one-half for KA alone and one-tenth for KA-CS-MNPs compared to controls. For methicillin-resistant Staphylococcus aureus, the zone of inhibition was two-thirds for KA alone and one-tenth for KA-CS-MNPs compared to controls. For Salmonella enterica, the zone of inhibition was one-half for KA alone and was one-fifteenth for the KA-CS-MNPs compared to controls. At the drug concentrations used and time point studied, no zone of inhibition was clearly observed for any of the bacteria and KA-PEG-MNPs. Such results may be expected due to the aforementioned decreased KA loading for KA-PEG-MNPs compared to KA-CS-MNPs.
Figure 9 Antimicrobial activity of 1) kojic acid, 2) KA-CS-MNPs, and 3) KA-PEG-MNPs nanocomposites using an agar diffusion method against different microorganisms.
Notes: (A) Bacillus subtilis, (B) methicillin-resistant Staphylococcus aureus, and (C) Salmonella choleraesuis showing the antimicrobial activity of 1), whereby 2) showed some and 3) lacked antimicrobial activity as compared to control antibiotics 4).
Abbreviations: KA-CS-MNPs, kojic acid-chitosan-iron oxide nanoparticles; KA-PEG-MNPs, kojic acid-chitosan-iron oxide nanoparticles.
The antimicrobial activity of KA and its derivatives against a number of microorganisms was reported previously;Citation29,Citation45,Citation48,Citation62,Citation63 there are no similar reports in the literature for KA nano-composites. Incorporating other derivatives of KA of higher antibacterial activity may also reveal additional information towards understanding the antimicrobial activity of KA-polymer-nanocomposites and their applications. Nonetheless, this study provided critical evidence of KA loading into composites as a new strategy into nanoantimicrobials to treat microbial infections that can be enhanced in further studies.
Conclusion
KA-CS-MNPs and KA-PEG-MNPs nanocomposites were prepared in this work. The attachment of the CS and PEG onto the MNPs surface was revealed by FTIR, similarly to the loaded KA. However, studies in the biological activities of KA nano-composites have been limited, and this study is the first to report a novel nanocomposite for biomedical applications. Whereby, this work only detected minimal antimicrobial activity for the KA-CS-MNPs nanocomposite against the tested microorganisms, further studies on the possible biological activities are underway. Thus, this study provided promising evidence for KA and KA-CS-MNPs in antibacterial applications, and their potential therapeutic role deserves further attention.
Acknowledgments
The authors would like to thank the Malaysian Ministry of Higher Education for supporting this research to be conducted in Universiti Putra Malaysia under grant ERGS/1/11/STG/UPM/01/18 (vote 5527050).
Disclosure
The authors report no conflicts of interest in this work.
References
- BarrettAPA long-term prospective clinical study of oral complications during conventional chemotherapy for acute leukemiaOral Surg Med Path1987633313316
- BrandaRFNaudSJBrooksEMChenZMussHEffect of vitamin B12, folate, and dietary supplements on breast carcinoma chemotherapy-induced mucositis and neutropeniaCancer200410151058106415329916
- DanilenkoDMRingBDYanagiharaDKeratinocyte growth factor is an important endogenous mediator of hair follicle growth, development, and differentiation. Normalization of the nu/nu follicular differentiation defect and amelioration of chemotherapy-induced alopeciaAm J Pathol199514711451547604876
- AkbulutMD’AddioSMGindyMEPrud’hommeRKNovel methods of targeted drug delivery: the potential of multifunctional nanoparticlesExpert Rev Clin Pharmacol20092326528224410705
- ChariRVTargeted cancer therapy: conferring specificity to cytotoxic drugsAcc Chem Res20074119810717705444
- KimSKimJYHuhKMAcharyaGParkKHydrotropic polymer micelles containing acrylic acid moieties for oral delivery of paclitaxelJ Controlled Release20081323222229
- TranNWebsterTJMagnetic nanoparticles: biomedical applications and challengesJ Mater Chem2010204087608767
- MahmoudiMSantSWangBLaurentSSenTSuperparamag-netic iron oxide nanoparticles (SPIONs): development, surface modification and applications in chemotherapyAdv Drug Deliv Rev2011631–2244620685224
- GuptaAKGuptaMSynthesis and surface engineering of iron oxide nanoparticles for biomedical applicationsBiomaterials200526183995402115626447
- AlexiouCSchmidRJJurgonsRTargeting cancer cells: magnetic nanoparticles as drug carriersEur Biophys J200635544645016447039
- PankhurstQAConnollyJJonesSKDobsonJApplications of magnetic nanoparticles in biomedicineJ Phys D Appl Phys20033613R167
- AlexiouCArnoldWHulinPMagnetic mitoxantrone nanoparticle detection by histology, X-ray and MRI after magnetic tumor targetingJ Magn Magn Mater20012251–2187193
- JainTKMoralesMASahooSKLeslie-PeleckyDLLabhasetwarVIron oxide nanoparticles for sustained delivery of anticancer agentsMol Pharm20052319420515934780
- KaakiKHervé-AubertKChiperMMagnetic nanocarriers of doxorubicin coated with poly(ethylene glycol) and folic acid: relation between coating structure, surface properties, colloidal stability, and cancer cell targetingLangmuir20122821496150522172203
- KayalSRamanujanRVDoxorubicin loaded PVA coated iron oxide nanoparticles for targeted drug deliveryMater Sci Eng C2010303484490
- QuanQXieJGaoHHSA coated iron oxide nanoparticles as drug delivery vehicles for cancer therapyMol Pharm2011851669167621838321
- SaboktakinMRTabatabaieRMaharramovARamazanovMASynthesis and characterization of superparamagnetic chitosan-dextran sulfate hydrogels as nano carriers for colon-specific drug deliveryCarbohydr Polym2010812372376
- FinotelliPVDa SilvaDSola-PennaMMicrocapsules of alginate/chitosan containing magnetic nanoparticles for controlled release of insulinColloids Surf B Biointerfaces201081120621120688491
- QuJBShaoHHJingGLHuangFPEG-chitosan-coated iron oxide nanoparticles with high saturated magnetization as carriers of 10-hydroxycamptothecin: preparation, characterization and cytotoxicity studiesColloids Surf B Biointerfaces2013102374423000675
- DornianiDHusseinMZKuraAUFakuraziSShaariAHAhmadZPreparation of Fe3O4 magnetic nanoparticles coated with gallic acid for drug deliveryInt J Nanomedicine201275745575623166439
- SunCSzeRZhangMFolic acid-PEG conjugated superparamagnetic nanoparticles for targeted cellular uptake and detection by MRIJ Biomed Mater Res A200678355055716736484
- AriasJLLópez-ViotaMLópez-ViotaJDelgadoAVDevelopment of iron/ethylcellulose (core/shell) nanoparticles loaded with diclofenac sodium for arthritis treatmentInt J Pharm20093821–227027619712736
- ButoescuNJordanOBurdetPDexamethasone-containing biodegradable superparamagnetic microparticles for intra-articular administration: physicochemical and magnetic properties, in vitro and in vivo drug releaseEur J Pharm Biopharm200972352953819303928
- BeélikAKojic acidAdv Carbohydr Chem1956481114518313469630
- MortonHEKocholatyWJunowicz-KocholatyRKelnerAToxicity and antibiotic activity of kojic acid produced by Aspergillus luteo-virescensJ Bacteriol1945505579584
- PrescottSCDunnCGIndustrial MicrobiologyNew York, NYMcGraw-Hill1940
- YadutaTKojic acid, a new organic acid formed by Aspergillus oryzaeJ Chem Soc Japan19163711851233
- AytemirMDKarakayaGEkinciDKojic acid derivativesEkinciDenizMedicinal Chemistry and Drug Design2012126 Available from: http://www.intechopen.com/books/medicinal-chemistry-and-drug-design/kojic-acid-derivatives
- BrtkoJRondahlLFickováMHudecováDEyblVUherMKojic acid and its derivatives: history and present state of artCent Eur J Public Health200412SupplS16S1815141965
- MohamadRMohamedMSSuhailiNSallehMMAriffABKojic acid: applications and development of fermentation process for productionBiotechnol Mol Biol Rev2010522437
- AytemirMDSeptioğluECalişUSynthesis and anticonvulsant activity of new kojic acid derivativesArzneimittelforschung2010601222920184223
- IyengarRMcEvilyAJAnti-browning agents: alternatives to the use of sulfites in foodsTrends Food Sci Technol199236064
- UchinoKNagawaMTonosakiYOdaMFukuchiAKojic acid as an anti-speck agent (foof and nutrition)Agric Biol Chem1988521026092610
- BentleyRFrom miso, saké and shoyu to cosmetics: a century of science for kojic acidNat Prod Rep20062361046106217119644
- MishimaYHattaSOhyamaYInazuMInduction of melanogenesis suppression: cellular pharmacology and mode of differential actionPigment Cell Res1988163673743148920
- ChoiCMBersonDSCosmeceuticalsSeminars in Cutaneous Medicine and Surgery200625316316817055397
- CabanesJChazarraSGarcia-CarmonaFKojic acid, a cosmetic skin whitening agent, is a slow-binding inhibitor of catecholase activity of tyrosinaseJ Pharm Pharmacol199446129829857714722
- BeardRLWaltonGSKojic acid as an insecticidal mycotoxinJ Invertebr Pathol19691415359
- VeverkaMSynthesis of some biologically active derivatives of 2-hydroxymethyl-5-hydroxy-4H-pyran-4-one. 2. Synthesis and biological properties of S-substituted 2-thiomethyl-5-O-acyl derivativesChem Pap1992463206210
- AlversonJCohenACEffect of antifungal agents on biological fitness of Lygus hesperus (Heteroptera: Miridae)J Econ Entomol200295225626012019998
- HigaYKawabeMNabaeKKojic acid-absence of tumor-initiating activity in rat liver, and of carcinogenic and photo-genotoxic potential in mouse skinJ Toxicol Sci200732214315917538239
- XiongXPirrungMCModular synthesis of candidate indole-based insulin mimics by Claisen rearrangementOrg Lett20081061151115418303898
- YamatoMHashigakiKYasumotoYSynthesis and antitumor activity of tropolone derivatives. 6. Structure-activity relationships of antitumor-active tropolone and 8-hydroxyquinoline derivativesJ Med Chem19873010189719003116257
- FickovaMPravdovaERondhalLUherMBrtkoJIn vitro antiproliferative and cytotoxic activities of novel kojic acid derivatives: 5-benzyloxy-2-selenocyanatomethyl- and 5-methoxy-2-selenocyanatomethyl-4-pyranoneJ Appl Toxicol200828455455917879241
- HudecováDUherMBrtkoJHalogen derivatives of kojic acid with antifungal effectsBiologia199247483488
- NohynekGJKirklandDMarzinDToutainHLeclerc-RibaudCJinnaiHAn assessment of the genotoxicity and human health risk of topical use of kojic acid [5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one]Food Chem Toxicol20044219310514630133
- KimJHChangPKChanKLEnhancement of commercial antifungal agents by kojic AcidInt J Mol Sci20121311138671388023203038
- CheeHYLeeEHFungistatic activity of kojic acid against human pathogenic fungi and inhibition of melanin-production in Cryptococcus neoformansMycobiology2003314248250
- RodriguesAPCarvalhoASSantosASAlvesCNdo NascimentoJLSilvaEOKojic acid, a secondary metabolite from Aspergillus sp, acts as an inducer of macrophage activationCell Biol Int201135433534321044044
- National Committee for Clinical Laboratory StandardsMethods for Dilution of Antimicrobial Susceptibility Test for Bacteria that Grow Aerobically4th edApproved Standard NCCLS Document M7-A4172Wayne, PANCCLS1997
- UnsoyGYalcinSKhodadustRGunduzGGunduzUSynthesis optimization and characterization of chitosan-coated iron oxide nanoparticles produced for biomedical applicationsJ Nanopart Res2012141111322448125
- MarchessaultRHRavenelleFoZhuXXPolysaccharides for Drug Delivery and Pharmaceutical Applications934Washington, DCAmerican Chemical Society2006
- JayanthiSASukanyaDPragasamAJASagayarajPThe influence of PEG 20,000 concentration on the size control and magnetic properties of functionalized bio-compatible magnetic nanoparticlesDer Pharma Chemica20135190102
- KumarRInbarajBSChenBHSurface modification of superparamagnetic iron nanoparticles with calcium salt of poly(γ-glutamic acid) as coating materialMater Res Bull2010451116031607
- ShanZYangWSZhangXHuangQMYeHPreparation and characterization of carboxyl-group functionalized superparamagnetic nanoparticles and the potential for bio-applicationsJ Brazil Chem Soc200718713291335
- YuSChowGMCarboxyl group (–CO2H) functionalized ferrimagnetic iron oxide nanoparticles for potential bio-applicationsJ Mater Chem2004141827812786
- DungTTDanhTMDucNHChienDMPreparation and characterization of magnetic nanoparticles coated with polyethylene glycolJ Phys Conf Ser20091871012048
- Yue-JianCJuanTFeiXSynthesis, self-assembly, and characterization of PEG-coated iron oxide nanoparticles as potential MRI contrast agentDrug Dev Ind Pharm201036101235124420818962
- DongLYanLHouWGLiuSJSynthesis and release behavior of composites of camptothecin and layered double hydroxideJ Solid State Chem2010183818111816
- SakoreSChakrabortyBFormulation and evaluation of enalapril maleate sustained release matrix tabletsInt J Pharm Biomed Res2013412126
- BalázSUherMBrtkoJRelationship between antifungal activity and hydrophobicity of kojic acid derivativesFolia Microbiol (Praha)19933853873918262449
- AytemirMDÖzçelikBSynthesis and biological activities of new Mannich bases of chlorokojic acid derivativesMed Chem Res2011204443452