?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Nanostructured lipid carriers (NLCs) were employed to formulate a lipophilic drug into hydrophilic polymeric microneedles (MNs). Hyaluronic acid (HA) was selected as a hydrophilic and bioerodible polymer to fabricate MNs, and nile red (NR) was used as a model lipophilic molecule. NR-loaded NLCs were consolidated into the HA-based MNs to prepare NLC-loaded MNs (NLC-MNs). A dispersion of NLCs was prepared by high-pressure homogenization after dissolving NR in Labrafil and mixing with melted Compritol, resulting in 268 nm NLCs with a polydispersity index of 0.273. The NLC dispersion showed a controlled release of NR over 24 hours, following Hixson–Crowell’s cube root law. After mixing the NLC dispersion with the HA solution, the drawing lithography method was used to fabricate NLC-MNs. The length, base diameter, and tip diameter of the NLC-MNs were approximately 350, 380, and 30 μm, respectively. Fluorescence microscopic imaging of the NLC-MNs helped confirm that the NR-loaded NLCs were distributed evenly throughout the MNs. In a skin permeation study performed using a Franz diffusion cell with minipig dorsal skin, approximately 70% of NR was localized in the skin after 24-hour application of NLC-MNs. Confocal laser scanning microscopy (z-series) of the skin at different depths showed strong fluorescence intensity in the epidermal layer, which appeared to spread out radially with the passage of time. This study indicated that incorporation of drug-loaded NLCs into MNs could represent a promising strategy for controlled dermal delivery of lipophilic drugs.
Introduction
The stratum corneum (SC) is the outermost layer of the skin. This layer acts as a significant barrier to the penetration of most drugs.Citation1 Microneedles (MNs) can penetrate the SC without stimulating nerve fibers in the dermal layer and have been efficiently used to deliver active pharmaceutical ingredients (APIs) into the skin, with negligible pain.Citation2 MN technology has been developed as a means to deliver drugs efficiently to target tissues. Studies conducted using calcein were the first to demonstrate that MNs could increase skin permeability.Citation3 Subsequent research has focused on MN fabrication and their use in drug delivery. MNs vary in length, ranging from 25 μm to 1,000 μm, and can be made from silicon, metal, glass, or polymers. The use of silicon or metal to fabricate MNs carries a risk of brittleness. A very small amount of silicon, metal, or glass deposited in the skin could also elicit inflammatory responses. In contrast, MNs made with biocompatible and biodegradable polymers can be safely applied to the skin.Citation4–Citation6 Moreover, polymers are generally cost-effective and suitable for mass production. Various polymeric materials such as poly-L-lactic acid, poly-glycolic acid, poly-carbonate, poly-lactic-co-glycolic acid (PLGA), poly-dimethylsiloxane, a copolymer of methyl vinyl ether and maleic anhydride, carboxymethyl cellulose, maltose, dextrin, and galactose have been used to fabricate MNs.Citation7–Citation11
Recently, a PLGA-based MN system was investigated to replace injection using a hypodermic needle.Citation12 This minimally invasive system is suitable for self-administration and does not cause pain. However, since PLGA is degraded over a period of months, if it remains stuck to the skin, the recovery of skin barrier function at the injection site may be delayed. To overcome these limitations, hyaluronic acid (HA)-based MNs have been introduced. HA, which is a widely used ingredient in skin care products, is a biocompatible and biodegradable polymer that dissolves rapidly in the biological milieu.Citation13 An additional advantage is that fabrication of HA-based MNs does not require the use of organic solvents, because of HA’s solubility in water.Citation14 However, it is difficult to incorporate many lipophilic APIs, including retinoids, corticosteroids, arildone, resveratrol, and cyclosporine, into these hydrophilic polymeric MNs. Although the research literature on this type of drug loading is very limited, one successful study described encapsulation of a hydrophobic drug molecule into PLGA nanoparticles, which were then dispersed homogenously in water-soluble polymeric MNs.Citation15
Maintenance of appropriate drug concentrations in the skin is necessary in many dermatological conditions such as pigmentation, vitiligo, topical infections, and psoriasis. Although MNs greatly enhance drug permeation rates, the permeated drugs are rapidly absorbed into the blood stream, leaving only a small quantity of drugs localized on the skin.Citation16 For controlled dermal delivery, incorporation of a particulate drug carrier within MNs has been investigated.Citation12,Citation15,Citation17 Recently, a particle-based MN system was introduced to develop a sustained skin immunization-based therapeutic strategy. This system entraps the antigen within a polymeric nanocarrier to prolong antigen release to the skin-resident dendritic cells.Citation17 Once the nanocarrier is liberated from the MN and is exposed to the dermal layer, it can control the release of the entrapped drug. Lipid-based particulate carriers, including solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs), have been introduced for formulation of lipophilic drugs into water-soluble polymeric matrices, since these drug-loaded nanoparticles can be dispersed homogeneously in an aqueous phase. NLCs represent the next generation of SLNs, consisting of liquid oil and solid lipid. Lipophilic APIs are easily dissolved or dispersed in the lipid phase, resulting in a high payload capacity.Citation18 Because the lipid phase of NLCs is biocompatible, there is no immune rejection.Citation19 These formulations are particularly useful for topical application, because their nanosize ensures close contact with the skin cells and helps them to remain on skin tissue as a thin occlusive film, thereby enabling controlled drug release.Citation18 Studies of lipid nanoparticles have recently been extended for the development of efficient loading methods for therapeutic proteins, confirmation of structural assembly, and surface modification to target specific sites.Citation20–Citation22
In the present study, NLCs were employed to formulate a lipophilic drug into hydrophilic polymeric MNs. HA was selected as a hydrophilic and bioerodible polymer to fabricate the MNs, and nile red (NR) was used as a model probe representing a lipophilic API. NR-loaded NLCs were consolidated into the HA-based MNs to prepare NLC-MNs. NR entrapment in NLCs, the physical characteristics of NLC-MNs in terms of homogeneity of NR distribution and MN array specifications, and the release of NR from either NLCs or NLC-MNs were evaluated. Finally, the skin distribution behavior of NR was examined by both quantitative determination and microscopic image analyses.
Materials and methods
Materials
NR and Tween 80 were purchased from Sigma-Aldrich (St Louis, MO, USA). Compritol® 888 ATO (glyceryl behenate) and Labrafil M 1944 CS (oleyl macrogol glycerides) were kindly gifted by Gattefossé (Saint-Priest, France). HA sodium salt (29 kDa) was purchased from Soliance (Pomacle, France). Methanol was purchased from JT Baker (Avantor Performance Materials, Center Valley, PA, USA). Double-distilled water was used for all experiments. Minipig skin was purchased from Medi Kinetics Co., Ltd. (Pyeongtaek, Gyeonggi-do, Korea).
NR assay
The quantitative determination of NR was performed by highperformance liquid chromatography (HPLC). The system consisted of a pump (L-7100), an autosampler (L-7200), a fluorescence detector (L-7485), and a data station (LaChrom Elite; Hitachi Ltd., Tokyo, Japan). NR was separated using a Capcell pak C18, 0.5 μm, 4.6 × 150 mm (Shiseido Company, Limited, Tokyo, Japan) with methanol:water (93:7) as the mobile phase, at a flow rate of 1.0 mL/minute. The injection volume was 50 μL and NR was detected at 599 nm. The intraday and interday precision and accuracy of the NR assay were estimated in triplicate at three different concentrations: 10, 60, and 100 ng/mL. The calibration curve was based on seven different standard concentrations. Calibration curves were used to determine the limit of detection and limit of quantitation.
Preparation of NLCs and NLC-MNs
NLCs were prepared using the high-pressure homogenization (HPH) method. NR (0.5 mg) was dissolved in Labrafil (0.2 mL) and mixed with melted Compritol (0.3 g). Nine milliliters of an aqueous phase containing Tween 80 (2.5%) was heated to 85°C and mixed with the lipid phase. The mixture was sonicated for 60 seconds (Model 2210; Branson Ultrasonics Co, Danbury, CT, USA). The hot pre-emulsion was further processed by HPH, applying 10 cycles at 500 bar (M-110S; Microfluidics, Westwood, MA, USA) to obtain an oil/water nanosuspension. This was gradually cooled down to room temperature and stored in a refrigerator.
To fabricate NLC-MNs, the NLC dispersion (10 mL) was mixed with HA sodium salt (0.2 g). The mixture was dropped onto a 1-cm2 carboxymethyl cellulose base plate in a 7 × 7 arrangement using solution dispenser (ML-5000X; Musashi Engineering, Inc., Tokyo, Japan) and automated X-, Y-, and Z-stage (SHOT mini 100-s; Musashi Engineering, Inc.). The droplets were then transformed into MNs by the drawing lithography method ().Citation23 After the pillar contacted the 7 × 7 pattern of mixture droplets, drawing resulted in the appearance of a conical bridge between the plate and pillar. The desired liquid bridge was dried to generate a rigid structure. Isolation drawing was performed to separate the MNs from the pillar.
Characterization of NLCs
The NLC dispersion was diluted 1,000 times with water, and the particle size and zeta potential were measured using photon correlation spectroscopy (Zetasizer Nano-ZS; Malvern Instruments, Malvern, UK) with a 50 mV laser at a scattering angle of 90°. The drug encapsulation efficiency (EE) (%) of NR in the NLC dispersion was determined by an ultrafiltration centrifugation method.Citation24 The amount of unencapsulated drug was determined indirectly by ultrafiltration using centrifugal filter tubes (Amicon Ultra-4; EMD Merck Millipore, Billerica, MA, USA) with a molecular weight cut-off of 30 kDa. The amount of NR encapsulated was calculated by the difference between the total amount used to prepare the systems and the amount of NR that remained in the aqueous phase after isolation of the systems. Free and total NR concentrations were measured using HPLC, and EE (%) was calculated using the following formula: [(D − C)/D] × 100, where D is the total amount of drug added and C is the amount of drug detected in the water phase.Citation25
Meanwhile, to determine NR loading into the NLCs, 10 μL of NLC dispersion was diluted with methanol and sonicated in a bath-type sonicator at 40°C for 10 minutes to dissolve the NR from the lipid carriers.Citation26 Drug loading (mg NR/g lipid) was calculated using the following formula: (R − C)/L, where R is the total amount of NR remaining in the NLC dispersion, C is the amount of drug detected in the water phase, and L is the total amount of lipid added. All measurements were carried out under ambient conditions and in triplicate.
In vitro release of NR
The release of NR from NLCs was determined in vitro using Franz diffusion cells with donor and receptor compartments, separated by a membrane filter. Cellulose nitrate membrane filters with a pore size of 0.1 μm (7181-004; GE Healthcare Europe GmbH, Freiburg, Germany) were mounted in the diffusion cells. The receptor phase was filled with distilled water containing 5% Tween 80 as an acceptor medium to achieve sink conditions during the experiment, and water-jacketed at 37°C. NLC dispersion (100 μL) was applied to the donor compartment, which had an available diffusion area of 1.76 cm2. Aliquots (300 μL) were withdrawn from the receptor compartment (11 mL) at predetermined time intervals for 48 hours and diluted 2.5-fold with water. Separately, an NR-containing micellar solution was prepared with Tween 80 (5% v/v) as a control, and the release of NR from micelles was observed using the procedure described above. After HPLC determination, the cumulative amount of NR in the receptor phase was plotted as a function of time.
Characterization of NLC-MNs
The length, base diameter, and tip diameter of seven arrays of NLC-MNs were determined in triplicate by microscopic observation (Motic AE31; Speed Fair Co, Ltd, Hong Kong). The aspect ratio was calculated by the ratio of length to base diameter. The amount of NR loaded in MNs was analyzed by HPLC. An array of NLC-MNs was dissolved in 7.0 mL of 2% Tween 80 in distilled water for 1 hour under moderate stirring (300 rpm). The solution was diluted with methanol and sonicated for 5 minutes to dissolve the NR from the NLC-MNs. The distribution behavior of NR in NLC-MNs was determined by fluorescence microscopy and confocal laser scanning microscopy (CLSM) as described below. A z-series was rotated (x: 72.5°, y: 316.7°, z: 287.7°) and stacked perpendicularly.
Determination of skin penetration and distribution
Distribution of NR in the skin was determined using a Franz diffusion cell, as described above, with the membrane filter replaced by excised back skin from a 15-month-old minipig. The skin was sectioned in 600- to 700-μm slices using a microdermatome (B Braun Melsungen AG, Melsungen, Germany) and cut into 3 × 3 cm pieces. The skins were wrapped in aluminum foil and stored at −20°C. Prior to the experiment, the frozen skin was pre-equilibrated in phosphate buffer saline to thaw completely. Hydrated skin was then placed at the flat bottom and wiped with a tissue to remove liquid. The MN array was inserted into the center of the skin (1.76 cm2) with a force of 20.0 N for 1 minute using a weight device. The skin with MNs was then carefully mounted in the Franz diffusion cell. Other processes were carried out as described above.
After the 24-hour MN array application period NR in the receptor compartment was detected using HPLC, and the skin sample was removed and separated from the MN array, which was then assayed for NR by HPLC. The specimen was positioned parallel to the bottom of the mold surrounded with Tissue-Tek® optimum cutting temperature compound 4583 (Sakura Finetek USA, Inc., Torrance, CA, USA), and flash frozen in liquid nitrogen. The frozen tissue was sectioned from the SC to the bottom using a cryotome (Ultracut UCT; Leica Microsystems, Wetzlar, Germany) at −25°C. Slice thickness was set at 50 μm and four consecutive slices were taken and placed into the same glass vial. This procedure was repeated until the entire section was sliced.Citation15 To each vial, 2.5 mL of methanol was added and sonicated for 1 hour at 40°C. This step was repeated twice. The extracted NR was quantified by HPLC assay.
Fluorescence microscopy
An inverted fluorescence microscope (Motic AE31; Speed Fair Co, Ltd) with 480-nm excitation and 535-nm emission filters (Motic MHG-100B; Speed Fair Co, Ltd) was used to investigate the entrapment of NR in NLC-MNs and the distribution of NR in minipig skin. Digital images were captured using a camera (Moticam Pro 825A; Speed Fair Co, Ltd) controlled with Motic Image Advanced 3.2 software.
CLSM
CLSM (LSM 510; Carl Zeiss Meditec AG, Jena, Germany) was performed to scan optical sections at different z-positions along the optical axis (z-sectioning). NR was excited at 543 nm and its emission was detected with a long-pass filter (LP 560; Carl Zeiss). The NLC-MNs were placed on a microscope slide and scanned by CLSM to visualize the distribution of fluorescent NR-containing nanocarriers. Images were acquired in a series of the xy-sections (parallel to the base plate of MNs) as successive focal planes along the z-axis. Scanning was conducted at an interval of 56 μm from the tip of the MN through the z-axis, vertical to the xy-plane.
Visualization of skin distribution behavior
The skin sample was acquired as described above. A scalpel was used to excise the tissue exposed to the MN array. This tissue was positioned perpendicularly to the bottom of the mold and frozen with optimum cutting temperature compound in liquid nitrogen. The frozen skin was sectioned vertically using a cryotome and the 30-μm sections were investigated using light and fluorescence microscopy as described above. The images from the two techniques were merged to assess the distribution of NR in the different skin layers.
To observe the distribution pattern of NR in a time-dependent manner, skins treated with NLC-MNs were removed from Franz diffusion cells at time points of 1, 4, 8, 12, and 24 hours. The skins were separated from MN arrays and observed using CLSM.
Scanning was conducted at intervals of 60 μm from the SC. Images were taken automatically until a red channel response was detected. All other CLSM measurement settings (except scanning interval) were the same as those described above. For further comparison of CLSM images, pixel analyses were performed using the Adobe Photoshop® histogram feature (Adobe Systems Incorporated, San Jose, CA, USA). According to the intensity of redness, ranging from 0 (black) to 255 (red), four regions were categorized: invisible (less than 64); mild (64 to 127); moderate (128 to 191); and strong (more than 192). The number of pixels representing redness in every region were counted and expressed as the redness occupancy of the total 4,624 microphotograph pixels.
Statistical analysis
All reported data are mean ± standard deviation. The differences between groups were analyzed by Student’s t-test and considered to be statistically significant where P<0.05.
Results
HPLC NR assay validation
The seven-point calibration curve was linear in the concentration range of 5–100 ng/mL NR (y =6680.984 x + 1615.498, r2=0.9999). The calibration standards for intraday and interday accuracy and precision are listed in . Intraday and interday precision ranged from 1.31% to 3.74% and from 2.10% to 2.56%, respectively. The maximum values for intraday and interday variation at 10 ng/mL were 1.779 and 4.815, respectively.
Table 1 Intraday and interday accuracy and precision of the nile red assay
Characteristics of NLCs
NR-loaded NLCs were successfully prepared using the HPH technique and the size, polydispersity index, zeta potential, and entrapment of NR are listed in . The particle size was in the range of 250–295 nm with polydispersity index values of approximately 0.273. Zeta potential was approximately −20 mV Entrapment of NR into NLCs was evaluated in terms of drug loading and EE. A high load of NR was entrapped into NLCs, with the EE being greater than 99%.
Table 2 Physicochemical properties of NR-loaded NLCs
In vitro release profile of NR from NLCs
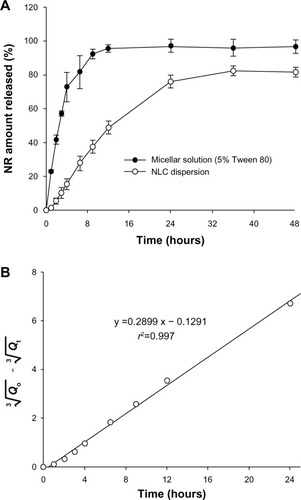
The in vitro release of NR from NLCs is shown in . The NLC dispersion showed a controlled release of NR for 24 hours, and the release reached plateau at around 36 hours. Over 75% of the NR in the NLC dispersion was released within 24 hours. In comparison, a micellar solution composed of 5% Tween 80 released NR more rapidly than the NLC dispersion and reached a plateau at around 9 hours, which indicated that 96% of the NR was released.
Characterization of NLC-MNs
The specifications of the MNs were as follows: length, 350 μm; base diameter, 380 μm; and dip diameter, 30 μm. Standard deviations were below 10% of each value, except those of tip diameter. The aspect ratio, defined as the ratio of length to base diameter, was approximately 0.9 (). This value was lower than those of other common conical-shaped polymeric MNs, which generally ranged from 1.2 to 5, suggesting that these MNs were short in length.
Table 3 Physicochemical properties of the NLC-MNs
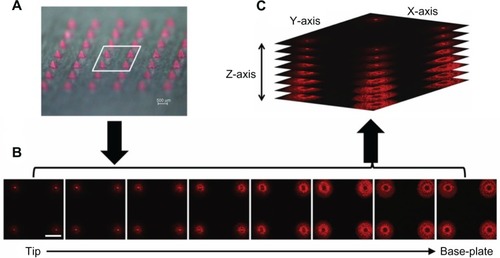
Fluorescence microscopic imaging of NLC-MNs proved that NLCs were distributed evenly throughout the MNs, as shown in . This finding was also supported by CLSM analysis (). Even distribution of the nanocarrier was found in fluorescence images in the xy-plane (horizontal view) and z-series (perpendicular direction). In the xy-plane micrographs arranged in z-series tiers, fluorescence was well distributed, but there were gaps in the center of the bottom portion (approximately 224–392 μm from the tip). For further visualization, the micrograph images were rotated and stacked together to analyze the distribution of fluorescence and the shape of the NLC-MNs.
Figure 3 Microscopic images of NLC-MNs under normal (A) and fluorescent (B) light sources.
Abbreviation: NLC-MNs, nanostructured-lipid-carrier-loaded microneedles.
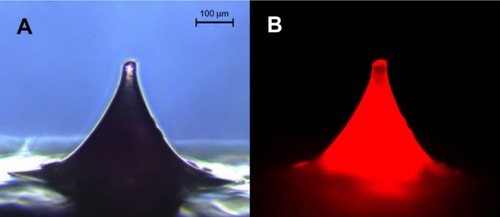
Figure 4 CLSM images of NR-NLC distribution in the MNs revealing even distribution of the nanocarrier throughout the needle.
Notes: (A) Microscopic image of NLC-MN array. (B) Confocal images of the xy-plane in z-series (sequential xy sections, interval =56 μm). (C) z-series rotated (x: 72.5°, y: 316.7°, z: 287.7°) and stacked perpendicularly. The white scale bar represents 500 μm.
Abbreviations: CLSM, confocal laser scanning microscopy; NLC-MN, nanostructured-lipid-carrier-loaded microneedle; NR, nile red; MNs, microneedles.
Quantitative analysis of skin localization of NR
This experiment measured relative NR distribution (). Approximately 70% of the NR dosage was distributed in the skin, of which 40% was localized in the upper layer of the skin (0–200 μm). The residual amount of NR in the NLC-MNs was limited to 10%, and the cumulative amount of NR that permeated into the receptor fluid was approximately 5%. There was a loss less than 15% of the NR in mass balance.
Table 4 NR localization after 24-hour NLC-MN array application on minipig skin in a Franz diffusion cell
Visualization of skin distribution behavior
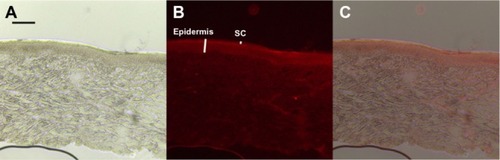
depicts fluorescent and light microscopy images of vertical skin sections following application of NLC-MNs for 24 hours. Strong fluorescent intensity was observed in the epidermis, while it was more diffuse throughout the dermal layer.
Figure 5 Microscopic images of minipig skin after NLC-MN application for 24 hours (A) under normal light, (B) under fluorescent light, and (C) a merged image of (A) and (B).
Notes: The red color in (B) and (C) represents NR. The black scale bar represents 100 μm.
Abbreviations: NLC-MN, nanostructured-lipid-carrier-loaded microneedle; NR, nile red; SC, stratum corneum.
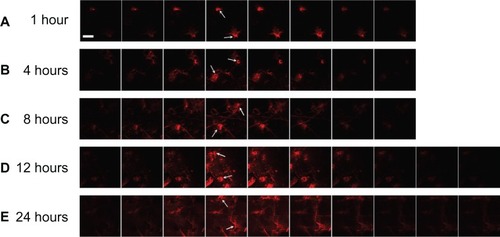
Images of the skin scanned by CLSM at different depths (z-series) showed that the fluorescence appeared to spread out radially with increased intensity as time passed (). After the application of NLC-MNs for 1 hour, fluorescence was detected in the spots pierced by MNs. Between 4 hours and 8 hours, the fluorescence permeated into the vicinity of the pierced spots. After 12 hours, CLSM detected fluorescence in deeper skin layers, throughout the deep dermal tissues.
Figure 6 CLSM images of a z-series (sequential xy sections, interval =60 μm) after application of NLC-MNs to minipig skin for (A) 1 hour, (B) 4 hours, (C) 8 hours, (D) 12 hours, and (E) 24 hours.
Notes: The arrows indicate the spot pierced with the MNs. The white scale bar represents 500 μm.
Abbreviations: CLSM, confocal laser scanning microscopy; NLC-MNs, nanostructured-lipid-carrier-loaded microneedles; MNs, microneedles.
Discussion
The advantages of using MNs for delivering various active molecules to deeper dermal tissues and/or the systemic circulation have been described previously.Citation3,Citation11,Citation27,Citation28 Puncturing the SC allows efficient dermal/transdermal delivery of not only small compounds such as calcein, vitamin C, and ketoprofen, but also of large molecules including vaccines, gene vectors, and antibodies. In the case of lipophilic drugs, permeation is not a matter of concern for dermal delivery, because of their easier partitioning into the SC than that of other drugs. Many lipophilic drugs are therefore commercially available as creams and/or ointments.Citation29–Citation31 Nevertheless, controlled delivery and localization of the drug to dermal layers are still necessary. MNs can deliver the drug with high efficiency in a controlled or sustained release pattern.
The selection of polymers, the matrix body architecture, and the means of drug loading are principal factors for MN technology. In addition, microparticulate carriers encapsulating a drug could be combined with MNs for successful drug delivery. The release of a hydrophilic probe by using microparticle-loaded MNs has been investigated, indicating that the release rate of calcein could be controlled by manipulation of the properties of the hydrogel particles.Citation32 PLGA nanoparticle-loaded polymeric MNs have also been studied as vehicles to enhance the dermal delivery and localization of a hydrophobic molecule. This PLGA-MN system exhibited improved delivery of NR to the skin, with 3.59% of the total NR in the MNs being localized to the skin, compared to 0.13% from a control patch.Citation15 Another PLGA-MN system facilitated controlled antigen delivery to skin dendritic cells. A single immunization with this system was sufficient to induce an antiviral adaptive immune response and to acquire protective immunity against a respiratory virus.Citation17 Puncture of skin is crucial for drugs with narrow therapeutic windows to avoid over- or under-dosing, and incorporation of the drug into a carrier can maintain the level within a therapeutic range by controlling drug release.Citation12
In the present study, NLCs were designed as a lipid-based nanocarrier for controlled delivery of NR, a hydrophobic model probe, and engrafted into a hydrophilic HA-based MN. Since NLCs are composed of liquid oil and solid lipid, oil-soluble drugs are easily entrapped with high EE. Our results showed perfect encapsulation of NR () because of its very poor solubility in aqueous media.Citation33 The kinetics of NR release from the NLC dispersion for the period of 24 hours were calculated using the Hixson–Crowell equation as follows:
, where Qo was the initial amount of drug in the NLC, Qt was the remaining amount of drug in the NLC at time t, and KHC was a constant incorporating the surface–volume relationship. As shown in , this model fitted the release of NR in the present study (r2=0.997). It described the NR release by dissolution and bioerosion, accompanied by changes in surface area and diameter of the NLC, suggesting complete biodegradation of the nanocarrier after releasing its contents. Meanwhile, the release profile of NR from the nanoemulsion was similar to that from the NLC (data not shown). This showed that sustained release of NR from the NLC was most strongly influenced by the oil component, rather than the solid lipid phase, of the carrier.
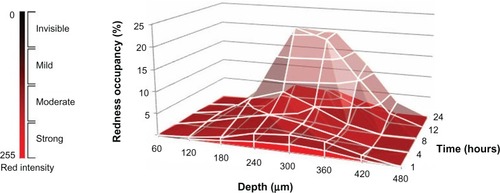
Nanocarriers, including NLCs, do not easily reach the deeper layers of the skin.Citation34 This means that delivery of low amounts of drug would not allow deeper tissues to be targeted.Citation35 However, micropuncture of the skin using MNs enables drug delivery to deep dermal layers.Citation15 The aspect ratio, expressed as the relative value of length to base diameter, is one of the crucial parameters determining the insertion capacity of MNs through the skin. The NLC-MNs used in this study were rather short, since the aspect ratio was much lower than those of other common conical polymeric MNs. The lower the aspect ratio of the MN, the harder it is for it to pierce the skin.Citation11 Nevertheless, as shown in , the delivery of NR was successfully achieved with our NLC-MN application. Strong fluorescence of NR in the dermal layer indicated the efficient localization effect of the system. This finding was corroborated by quantitation, demonstrating that approximately 70% of the NR was localized in the skin. Moreover, the fluorescence spread out radially to a larger area of the deep dermis as time passed. This time-dependent distribution behavior was further visualized by regional pixel analyses based on the intensity of redness. illustrates the three-dimensional cumulative distribution behavior of the NR by the variables of both time of application and depth of dermal layer. Moderate and strong redness regions peaked at 12 to 24 hours and in the 240 to 360 μm range. When the mild redness region was included, the redness occupancy at the same range was tripled. These results were consistent with previous literature describing the usefulness of nanoparticles with MNs for increasing skin penetration of drug and its deposition in the upper layer of the skin.Citation15,Citation16
Figure 7 Three-dimensional cumulative distributional behavior of NR, based on pixel analyses of CLSM images after application of NLC-MNs to minipig skin.
Notes: The redness occupancy is represented by two different panels: bottom panel for regions with strong and moderate redness; overlaid panel for the mild redness region.
Abbreviations: CLSM, confocal laser scanning microscopy; NLC-MNs, nanostructured-lipid-carrier-loaded microneedles; NR, nile red.
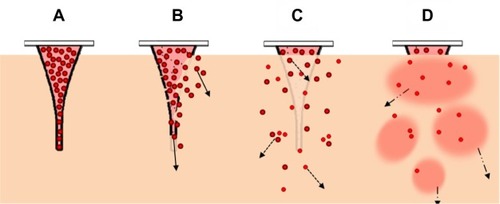
The enhanced delivery efficiency of the NLC-MNs could be explained in two stages, as illustrated by step-wise controlled dermal delivery of NR (). Firstly, MNs perforate the SC and liberate the NLCs as the polymeric matrix erodes. Since HA is a biocompatible and bioerodible polymer, the matrix body rapidly melts down and delivers the loaded nanoparticles. Numerous studies using bioerodible HA-based MNs have been carried out. Biodegradable HA-MNs fabricated with an appropriate geometry and sufficient strength for insertion into the skin dramatically increased transdermal transport of molecules.Citation14 HA-MNs rapidly dissolved in the skin’s extracellular fluid after application.Citation6,Citation14 Secondly, NR was released from the liberated NLCs in a controlled manner and finally reached the target site. During this stage, the NLCs diffused and/or partitioned into the specific dermal layer while releasing the drug slowly. As discussed earlier, NR release from the NLCs was controlled by both diffusion and surface erosion, according to Hixson–Crowell’s cube root law. The NLCs then decomposed to their elementary components, which would be digested by endogenous lipases, followed by biochemical dissipation of the resulting fatty acid molecules.
Figure 8 Step-wise illustration of the controlled dermal delivery of a lipophilic drug by NLC-MNs.
Notes: (A) Immediately after the application of the NLC-MNs to the skin. (B) Bioerosion of the MNs to release the NLCs carrying the drug. (C) Liberated NLCs diffuse and/or partition into the dermal layer while releasing the drug. (D) Drug is totally exposed and orientates to the target sites in dermal tissue. Solid black or grey lines represent the surface of the MNs as intact or eroded, respectively. Red colored circles represent the NLCs containing NR (as a model lipophilic molecule).
Abbreviations: NLC-MNs, nanostructured-lipid-carrier-loaded microneedles; NR, nile red; MNs, microneedles; NLCs, nanostructured-lipid-carriers.
In summary, the NLC-MNs developed in this study might provide a useful platform for developing a sophisticated dermal delivery system for lipophilic compounds. However, further studies on their optimization and practical application for therapeutic drug delivery are still necessary.
Conclusion
NLCs containing NR, a model lipophilic compound, were successfully incorporated into hydrophilic polymeric MNs. A skin permeation study using a Franz diffusion cell with minipig dorsal skin confirmed the efficient localization of NR to the upper layer of the skin. The present study also demonstrated that dermal delivery of NR could be controlled by NLC-MNs.
Acknowledgments
This study was supported by the Seoul R and BD program (SS100001).
Disclosure
The authors report no conflicts of interest in this work.
References
- WiechersJWThe barrier function of the skin in relation to percutaneous absorption of drugsPharm Weekbl Sci19891161851982694089
- GillHSDensonDDBurrisBAPrausnitzMREffect of microneedle design on pain in human volunteersClin J Pain200824758559418716497
- HenrySMcAllisterDVAllenMGPrausnitzMRMicrofabricated microneedles: a novel approach to transdermal drug deliveryJ Pharm Sci19988789229259687334
- DonnellyRFSinghTRWoolfsonADMicroneedle-based drug delivery systems: microfabrication, drug delivery, and safetyDrug Deliv201017418720720297904
- JinCYHanMHLeeSSChoiYHMass producible and biocompatible microneedle patch and functional verification of its usefulness for transdermal drug deliveryBiomed Microdevices20091161195120319609679
- KatsumiHLiuSTanakaYDevelopment of a novel self-dissolving microneedle array of alendronate, a nitrogen-containing bisphosphonate: evaluation of transdermal absorption, safety, and pharmacological effects after application in ratsJ Pharm Sci201210193230323822467424
- DonnellyRFMorrowDISinghTRProcessing difficulties and instability of carbohydrate microneedle arraysDrug Dev Ind Pharm200935101242125419555249
- GittardSDOvsianikovAMonteiro-RiviereNAFabrication of polymer microneedles using a two-photon polymerization and micromolding processJ Diabetes Sci Technol20093230431120144361
- ItoYYoshimitsuJShiroyamaKSugiokaNTakadaKSelf-dissolving microneedles for the percutaneous absorption of EPO in miceJ Drug Target200614525526116882545
- LeeKLeeCYJungHDissolving microneedles for transdermal drug administration prepared by stepwise controlled drawing of maltoseBiomaterials201132113134314021292317
- ParkJHAllenMGPrausnitzMRBiodegradable polymer microneedles: fabrication, mechanics and transdermal drug deliveryJ Control Release20051041516615866334
- ParkJHAllenMGPrausnitzMRPolymer microneedles for controlled-release drug deliveryPharm Res20062351008101916715391
- KoganGSoltésLSternRGemeinerPHyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applicationsBiotechnol Lett2007291172517091377
- LiuSJinMNQuanYSThe development and characteristics of novel microneedle arrays fabricated from hyaluronic acid and their application in the transdermal delivery of insulinJ Control Release2012161393394122634072
- DonnellyRFMorrowDIFayFMicroneedle-mediated intradermal nanoparticle delivery: Potential for enhanced local administration of hydrophobic pre-formed photosensitisersPhotodiagnosis Photodyn Ther20107422223121112544
- ZhangWGaoJZhuQPenetration and distribution of PLGA nanoparticles in the human skin treated with microneedlesInt J Pharm20104021–220521220932886
- ZaricMLyubomskaOTouzeletODendritic cell targeting via microneedle arrays laden with antigen-encapsulated poly-d,l-lactide-co-glycolide nanoparticles induces efficient antitumor and antiviral immune responsesACS Nano2013732042205523373658
- MüllerRHRadtkeMWissingSASolid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparationsAdv Drug Deliv Rev200254Suppl 1S131S15512460720
- PugliaCBlasiPRizzaLLipid nanoparticles for prolonged topical delivery: An in vitro and in vivo investigationInt J Pharm20083571–229530418343059
- AngelovBAngelovaAPapahadjopoulos-SternbergBHoffmannSVNicolasVLesieurSProtein-containing PEGylated cubosomic particles: Freeze-Fracture Electron Microscopy and Synchrotron Radiation Circular Dichroism StudyJ Phys Chem B2012116267676768622720820
- AngelovaAAngelovBDrechslerMGaramusVMLesieurSProtein entrapment in PEGylated lipid nanoparticlesInt J Pharm2013454262563223791734
- AngelovBAngelovaAFilippovSKDNA/fusogenic lipid nanocarrier assembly: millisecond structural dynamicsJ Phys Chem Lett201341119591964
- LeeKJungHDrawing lithography for microneedles: A review of fundamentals and biomedical applicationsBiomaterials201233307309732622831855
- ZhangXPanWGanLZhuCGanYNieSPreparation of a dispersible PEGylate nanostructured lipid carriers (NLC) loaded with 10-hydroxycamptothecin by spray-dryingChem Pharm Bull (Tokyo)200856121645165019043233
- LeeSGJeongJHKimSRTopical formulation of retinyl retinoate employing nanostructured lipid carriersJ Pharm Invest2012425243250
- TeeranachaideekulVMüllerRHJunvaprasertVBEncapsulation of ascorbyl palmitate in nanostructured lipid carriers (NLC) – Effects of formulation parameters on physicochemical stabilityInt J Pharm20073401–219820617482778
- OhJHParkHHDoKYInfluence of the delivery systems using a microneedle array on the permeation of a hydrophilic molecule, calceinEur J Pharm Biopharm20086931040104518411045
- NohYWKimTHBaekJSIn vitro characterization of the invasiveness of polymer microneedle against skinInt J Pharm20103971–220120520619328
- PardeikeJSchwabeKMüllerRHInfluence of nanostructured lipid carriers (NLC) on the physical properties of the Cutanova Nanorepair Q10 cream and the in vivo skin hydration effectInt J Pharm20103961–216617320541000
- MrowietzUWustlichSHoexterGGraeberMBräutigamMLugerTAn experimental ointment formulation of pimecrolimus is effective in psoriasis without occlusionActa Derm Venereol200383535135314609102
- GodfreyHRinventorGodfreyHRassigneeTopical zinc compositions and methods of use United States patent US 6558710562003
- KimMJungBParkJHHydrogel swelling as a trigger to release biodegradable polymer microneedles in skinBiomaterials201233266867822000788
- GreenspanPFowlerSDSpectrofluorometric studies of the lipid probe, nile redJ Lipid Res19852677817894031658
- Lombardi BorgiaSRegehlyMSivaramakrishnanRLipid nanoparticles for skin penetration enhancement-correlation to drug localization within the particle matrix as determined by fluorescence and parelectric spectroscopyJ Control Release2005110115116316297487
- CevcGVierlUNanotechnology and the transdermal route: A state of the art review and critical appraisalJ Control Release2010141327729919850095