Abstract
Here, we investigated in diabetic mice the therapeutic effect of monocyte chemoattractant protein-1 (MCP-1), locally delivered by an electrospun scaffold, on transplanted islets. This therapeutic scheme is expected to exert a synergistic effect to ameliorate hyperglycemia and its associated nephrotic disorders. The cumulative amount of MCP-1 released from the scaffold in vitro within a 3-week window was 267.77±32.18 ng, without a compromise in bioactivity. After 8 weeks following the transplantation, the islet population stimulated by MCP-1 was 35.14%±7.23% larger than the non-stimulated islet population. Moreover, MCP-1 increased concentrations of blood insulin and C-peptide 2 by 49.83%±5.29% and 43.49%±9.21%, respectively. Consequently, the blood glucose concentration in the MCP-1 group was significantly lower than that in the control group at week 2 post-surgery. MCP-1 also enhanced the tolerance of sudden oral glucose challenge. The rapid decrease of blood creatinine, urine creatinine, and blood urea nitrogen suggested that the recovery of renal functions compromised by hyperglycemia could also be attributed to MCP-1. Our study shed new light on a synergistic strategy to alleviate hyperglycemia and nephrotic disorders in diabetic patients.
Introduction
Despite decades of scientific endeavor, diabetes has remained a leading disease in the US that inflicts millions of patients.Citation1 Moreover, chronic diabetes is believed to be the culprit for several other illnesses.Citation2 For example, diabetes frequently compromises renal function, prompting a need for kidney transplantation.Citation3 To that end, a comprehensive strategy to combat diabetes as well as its complications is in urgent need. In type I diabetes, pancreatic β-cells are destructed by abnormal autoimmunity, leading to the loss of insulin secretion and consequently to hyperglycemia.Citation4 To clinically recoup the lost capability of insulin production, islet transplantation has been widely used but suffers mixed outcomes of therapeutic effectiveness.Citation5 In conventional transplantation surgery, harvested islets are freely injected into the intrarenal capsule and start to secrete insulin thereafter. Clinical observations have revealed that a number of challenges have been associated with this means of islet transplantation.Citation6 For example, despite limited availability of islets, the poor survival rate mandates that a large population of islets must be pooled from multiple donors.Citation7 A number of factors could account for the poor survival rate after islet transplantation, such as local inflammation.Citation8,Citation9
Tissue engineering scientists have made tremendous progress on rebuilding a great variety of functional tissues using a wide range of biomaterials and nanofabrication technology.Citation10–Citation15 In light of previous research, we successfully developed an electrospun scaffold-assisted delivery of islets to enhance the control of hyperglycemia and associated nephrotic disorders in diabetic mice.Citation16 The study demonstrated that the islet population in the scaffold-assisted delivery group outgrew its peer in the freely injected delivery group as early as week 4 after surgery, and sustained this advantage through week 12. Consequently, functional readouts such as insulin secretion saw an unequivocal improvement, which effectively drove down the blood glucose level in diabetic mice. In addition, boosted functional outputs of transplanted islets arrested the spiral deterioration of renal functions, as evidenced by a suppressed level of creatinine in both blood and urine samples. Monocyte chemoattractant protein-1 (MCP-1), a prominent immunomodulatory protein, has garnered a growing interest in the tissue engineering community, in that it plays a regulatory role in a variety of tissue regenerations.Citation17–Citation21 Previous research suggests that MCP-1 sees a temporary spike in local tissues after islet transplantation and that it helps to recoup compromised renal functions lost to hyperglycemia.Citation16,Citation22 In particular, recent findings suggest that local MCP-1 in islets can engage the immune system by targeting dendritic cells and, potentially, T-cells to inhibit diabetes.Citation23 Therefore, these findings prompted us to hypothesize that local stimulation of islets and renal tissues by MCP-1 released from a scaffold would exert a synergistic effect on alleviating hyperglycemia and associated nephrotic disorders in diabetic mice. In this study, we engineered a drug-eluting electrospun scaffold composed of polycaprolactone (PCL) and poliglecaprone (PGC) to locally deliver MCP-1.
Methods
The fabrication and morphological characterizations of electrospun scaffolds
The scaffold was electrospun as previously described.Citation16 Briefly, PGC (Advanced Inventory Management, Mokena, IL, USA) and PCL (Absorbable Polymers, Birmingham, AL, USA) were used as received. Both polymers were dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol (Sigma-Aldrich, St Louis, MO, USA) to achieve a total concentration of 12% (weight/volume [w/v]) (weight ratio of 1:3). For drug-eluting scaffolds (DESs), recombinant MCP-1 (Peprotech, Rocky Hill, NJ, USA) was added to the dissolved polymer solution (2 μg/mL). Then, 0.5 mL of the solution was electrospun at a feeding rate of 3 mL/hour to a collection board distanced at 25 cm at a voltage of 30 kV. Retrieved scaffolds were desiccated in vacuum for 24 hours. For non-eluting scaffolds (NESs), the solution absent of MCP-1 was electrospun under the same conditions. Scaffold samples (1 cm × 1 cm) were sputter-coated by gold and studied using a scanning electron microscope.
In vitro pharmacokinetic study of MCP-1 release
To study the release profile of MCP-1 from the scaffold, DESs (diameter =1.6 cm) were incubated in Prigrow II medium supplemented with heat-inactivated fetal bovine serum to a final concentration of 10% and penicillin/streptomycin (Applied Biological Materials Inc., Richmond, BC, Canada) at 37°C and 5% CO2 for up to 3 weeks. The supernatant was collected every week for enzyme-linked immunosorbent assay (ELISA) assay (R&D Systems, Minneapolis, MN, USA) per manufacturer’s protocol. NESs were incubated under the same conditions as a control.
To assess the bioactivity of released MCP-1, a chemotaxis assay was performed (EMD Millipore, Billerica, MA, USA) per manufacturer’s protocol. Human CD14+ monocytes (Applied Biological Materials) were seeded into each migration chamber (1 × 105 cells/chamber). The supernatant from the medium in which DESs or NESs had been incubated for 3 weeks was used for samples. Medium containing MCP-1 (2 μg/mL) was used as positive control, and medium without MCP-1, as negative control.
The creation of mouse insulin I promoter–luciferase (MIP-luc) transgenic mice and the islet transplantation
Shanghai Changzheng Hospital approved the animal study protocol, and ensured the animal welfare throughout the study. The MIP-luc transgenic mice (in a C57BL/6 background) whose transgene contained an MIP promoter fragment leading to the expression of the firefly luciferase (MIP-luc) were generated as reported before.Citation24,Citation25 Previous research demonstrated that β-cells from MIP-luc mice can be visualized using bioluminescent imaging, and their mass is correlated with the bioluminescent signal.Citation26 Each hemizygous MIP-luc transgenic mouse received one dose of streptozotocin (50 mg/kg; Sigma Aldrich, St Louis, MO, USA) by intraperitoneal injection every 2 days for a total of five doses. The dosage was based on previous studyCitation16 to induce diabetes and a gradual renal deterioration before the islet transplantation. Mice were considered diabetic if the blood glucose concentration exceeded 400 mg/dL for more than two consecutive days without fasting. DESs and NESs (1 mm × 1 mm) were sterilized in 70% ethanol followed by an extensive rinse in sterile phosphate buffered saline. Harvested islets were seeded on respective scaffolds (100 islet equivalents/scaffold) and incubated for 3 hours at 37°C with 5% CO2 prior to the surgery. All mice were anesthetized by vaporized 2.5% isoflurane via inhalation. Islets on respective scaffolds were surgically placed under the kidney capsule. All mice were sacrificed 8 weeks after the surgery.
The in vivo growth of transplanted islets
Xenogen IVIS 200 imaging system (Xenogen Corporation, Alameda, CA, USA) was used to capture bioluminescent optical images as previously described.Citation24 Briefly, after a 4-hour fast, MIP-luc mice were anesthetized with vaporized isoflurane. Mice were positioned on their sides on the imaging stage to capture an initial image. Each mouse then received 15 mg/mL D-luciferin in sterile phosphate buffered saline (150 mg/kg) via intraperitoneal injection, and a bioluminescent image was captured (exposure time =1 min) at 14 minutes post-injection. Image processing and quantification of bioluminescence were carried out using Living Image® (Xenogen Corporation) software, version 2.05.
Measurements of blood insulin, C-peptide 2, blood glucose, and oral glucose tolerance test
Each mouse underwent a blood withdrawal from the tail vein immediately before the surgery (week 0) and then on weeks 2, 4, and 8 post-surgery before the euthanasia. No fasting was imposed before blood withdrawals. A rat insulin ELISA kit with mouse insulin standards (Crystal Chem Inc., Chicago, IL, USA) and serum C-peptide 2 assayed with a rat/mouse C-peptide 2 ELISA kit (EMD Millipore) were used to measure the insulin and C-peptide 2 levels as per manufacturers’ protocols. Blood glucose was measured by OneTouch® Ultra® (LifeScan/Johnson and Johnson, Milpitas, CA, USA) glucometer. The oral glucose tolerance test (OGTT) was carried out 8 weeks after the surgery. After a 16-hour fast, blood samples were collected from the tail vein as the baseline glucose level (0 minutes). Thereafter, the glucose solution in sterile water (100 mg/mL) (Sigma-Aldrich) was administered to each mouse by oral gavage (2 g/kg). Blood glucose levels were measured in blood samples collected at 30, 60, and 120 minutes after the glucose challenge, as described above.
The evaluation of renal function following the islet transplantation
Blood and urine samples were collected immediately before the implantation (week 0) and on weeks 2, 4, and 8 after the surgery. Blood creatinine (Abcam, Cambridge, MA, USA), blood urea nitrogen (Bio Scientific Corp, Austin, TX, USA), urine creatinine (Abcam), and urine albumin (Abnova, Walnut, CA, USA) were assayed following manufacturers’ protocols.
Data analysis
Student’s t-test and analysis of variance were used where applicable (α=0.05). Images were processed with ImageJ (National Institutes of Health, Bethesda, MD, USA) software.
Results
Physical characterizations of scaffolds
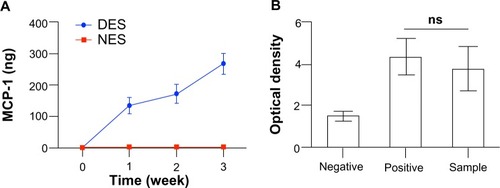
Electrospun scaffolds composed of various polymers and/or proteins have been extensively explored as drug-delivery vehicles for tissue engineering purposes.Citation27–Citation29 In light of previous research, we here successfully constructed DESs carrying MCP-1 for local delivery. Both DESs and NESs demonstrated a highly porous microstructure composed of randomly distributed fibers on the microscale (). No morphological difference was observed between the two scaffolds, suggesting that the incorporation of MCP-1 did not lead to a structural change. This lack of morphological change would rule out the possibility that the difference of biological readouts came from changes of environmental cues conferred on transplanted islets. The MCP-1 from DESs witnessed a rapid release within the first week and a reduced momentum in the next 2 weeks (). By the end of week 3, the DES had released a total of 267.77±32.18 ng MCP-1 into the medium, while no MCP-1 was detected in the NES group. Also, MCP-1 released from the scaffold retained its bioactivity (). This rapid release in the first week would be beneficial for the overall success of islet transplantation because it would quickly elevate the local MCP-1 concentrations for a maximal therapeutic effect. The sustained release through week 3 ensured that local tissues would consistently be stimulated by MCP-1 from the DES scaffold. Thereafter, local tissues are expected to compensate the exhausted MCP-1 from DESs as an immunological consequence of the islet transplantation.Citation22
Figure 1 The morphological characterization of non-eluting scaffolds (A) and MCP-1 drug-eluting scaffolds (B) by scanning electron microscope. The introduction of MCP-1 into the scaffold did not cause a morphological change of electrospun fibers.
Abbreviation: MCP-1, monocyte chemoattractant protein-1.
Figure 2 (A) The in vitro cumulative release of MCP-1 from DES. (B) The bioactivity of released MCP-1. The DES saw a rapid unleash of MCP-1 in the first week and then a steadfast release through week 3. No MCP-1 was detected in the NES group. MCP-1 retained its bioactivity throughout the delivery.
Notes: Negative = cell medium without MCP-1; positive = cell medium with MCP-1 (2 μg/mL); sample = supernatant containing released MCP-1 at week 3 (n=3).
Abbreviations: DES, drug-eluting scaffold; MCP-1, monocyte chemoattractant protein-1; NES, non-eluting scaffold; ns, not significant.
The in vivo growth of transplanted islets
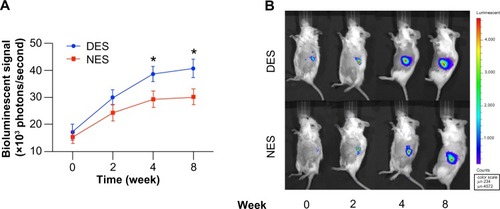
Prior research demonstrated that the strength of the bioluminescent signal is positively associated with the mass of transplanted islets, establishing that it was a quantitative and noninvasive way to measure the islet proliferation.Citation24 Previously, we demonstrated NESs provide a more favorable microenvironment that ultimately translates into an accelerated proliferation than freely injected islets.Citation16 To that end, in this study, we specifically focused on whether MCP-1 released from the DESs could further enhance the proliferation of islets than that released from NES. The islet population in the DES group started to outgrow its peer in the NES group as early as week four and maintained this proliferative advantage through week 8 (). At week 4, the bioluminescent signal strength of the islet population in the DES group was (38.73±2.82) × 103 photons/second, whereas the strength in the NES group was (29.35±3.01) × 103 photons/second. At week eight, the strength in the DES group grew to (40.89±3.35) × 103 photons/second, whereas that in the NES group was (30.26±2.99) × 103 photons/second. The islet population in the DES group was significantly larger at both week 4 (P=0.0078) and week 8 (P=0.0102). However, no difference was observed between the two groups at week 2. These results suggested that the local delivery of MCP-1 might not have translated into a marked increase of islet proliferation in the initial stage but might have contributed to the long-term proliferation.
Figure 3 (A) The quantification of bioluminescent signals. (B) Representative bioluminescent images. The islet population in the DES group outgrew its peer in the NES group at week 4. This proliferative advantage was further pronounced at week 8.
Note: *Statistical difference between the groups (n=7 in the DES group; n=6 in the NES group).
Abbreviations: DES, drug-eluting scaffold; NES, non-eluting scaffold.
The functional outputs of transplanted islets
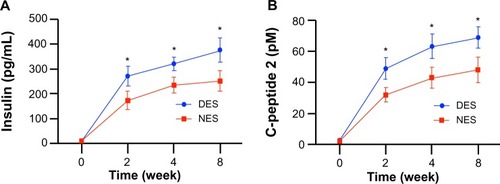
Both blood insulin and C-peptide 2 concentrations saw a sustained difference between the DES and NES groups from week 2 after the surgery (). The insulin concentrations in the DES and NES groups were 272.34±38.94 pg/mL and 173.45±37.21 pg/mL, respectively, at week 2. By week 8, the insulin concentrations were 377.91±49.34 pg/mL in the DES group and 252.22±40.11 pg/mL in the NES group. Correspondingly, the C-peptide 2 concentrations were 49.23±6.97 pM in the DES group and 32.11±4.56 pM in the NES group at week 2. By week 8, the C-peptide 2 concentrations were 69.29±6.82 pM in the DES group and 48.29±8.22 pM in the NES group.
Figure 4 Functional outputs of transplanted islets: (A) blood insulin concentrations; and (B) C-peptide 2 concentrations. The insulin level in the DES group outperformed the NES group as early as week 2 and maintained this advantage through week 8. A correlated advantage of C-peptide 2 was observed.
Note: *Statistical difference between the groups (n=7 in the DES group; n=6 in the NES group).
Abbreviations: DES, drug-eluting scaffold; NES, non-eluting scaffold.
At week 2, the blood glucose levels were 228±31 mg/dL in the DES group and 321±37 mg/dL in the NES group, showing a significant difference (P=0.0098) (). However, this difference disappeared by week 4, when the blood glucose levels in both groups fell into the physiological range. On the other hand, the OGTT result clearly showed that mice in the DES group possessed a stronger capability to tolerate glucose challenge (). At 30 minutes after the oral gavage, blood glucose levels in mice from the DES and NES groups were 352±35 mg/dL and 475±58 mg/dL, respectively, showing a significant difference (P=0.0012).
Figure 5 Blood glucose concentrations after the islet transplantation: blood glucose levels (A) and oral glucose tolerance test (B) at week 8. The blood glucose in the DES group returned to the physiological range as early as week 2, outpacing its peer from the NES group. Correspondingly, islet transplants in the DES group unleashed a greater resistance to sudden glucose challenge, evidencing the improved therapeutic effect of DESs.
Note: *Statistical difference between the groups (n=7 in the DES group; n=6 in the NES group).
Abbreviations: DES, drug-eluting scaffold; NES, non-eluting scaffold.
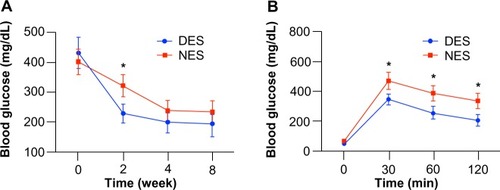
The blood glucose level in mice from the DES group was consistently lower than that in the NES group throughout the 120-minute period. These results substantiated that the increased insulin production due to the stimulation of MCP-1 from DESs successfully translated into an accelerated return of blood glucose levels into the physiological range and a greater capacity to withstand sudden glucose challenges.
Renal protein concentrations after the islet transplantation
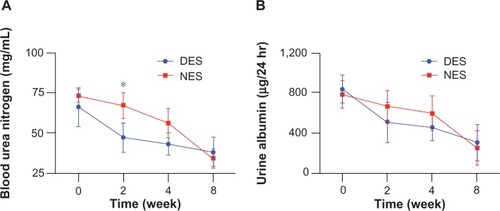
A significant difference of blood creatinine, urine creatinine, and blood urea nitrogen concentrations were observed between the DES and NES groups at week 2. Specifically, the blood creatinine concentrations were 0.94±0.15 mg/dL in the DES group and 1.56±0.15 mg/dL in the NES group (). The urine creatinine concentrations were 145±12 mg/dL in the DES group and 178±13 mg/dL in the NES group (). The blood urea nitrogen concentrations were 47±9 mg/dL in the DES group and 67±8 mg/dL in the NES group (). However, no difference in urine albumin was observed between the two groups (). These results suggest that MCP-1 accelerated the recovery of renal functions following the local delivery. The disappearance of a difference by week 4 could be attributed to the exhausted source of MCP-1 in the DESs and the increased MCP-1 secretion by local renal tissues in response to the islet transplantation.
Figure 6 Creatinine levels in blood (A) and urine (B). The concentrations of blood creatinine and urine creatinine in the DES group fell to the physiological range by week 2, while those from the NES group were still abnormally high.
Note: *Statistical difference between the groups (n=7 in the DES group; n=6 in the NES group).
Abbreviations: DES, drug-eluting scaffold; NES, non-eluting scaffold.
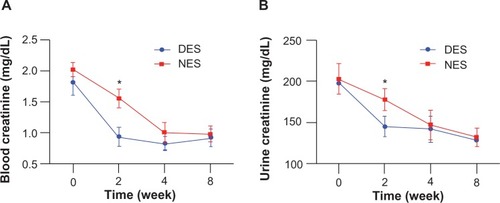
Figure 7 Blood urea nitrogen (A) and urine albumin (B) levels. The concentration of blood urea nitrogen in the DES group was reduced to the physiological range by week 2, while no difference of urine albumin concentrations between DES and NES groups was observed.
Note: *Statistical difference between the groups (n=7 in the DES group; n=6 in the NES group).
Abbreviations: DES, drug-eluting scaffold; NES, non-eluting scaffold.
Discussion
The tissue-engineered microenvironment could exert a profound influence on individual cells and their functions.Citation30–Citation32 In our research, this tissue-engineered scaffold was supposed to confer a favorable microenvironment for islet cells to adhere, proliferate, and ultimately produce insulin to rein in the hyperglycemia. In previous research, we demonstrated that the NES increased the islet proliferation and insulin production in vitro with no cytotoxic effects observed, suggesting that the degradation of scaffolding materials posed no threat to the population.Citation16 The lack of morphological change of fibers in DESs guaranteed that the optimal performance attained by NESs in previous research would not be compromised.
It should be noted that tissue engineered scaffolds are supposed to degrade over time to allow the growth of native tissues and the deposition of native extracellular matrix.Citation33–Citation35 Both PGC and PCL are widely used biodegradable suture materials for various tissue-engineering purposes, without cytotoxicity, and both demonstrated an excellent in vivo biocompatibility to promote the growth and functional output of transplanted islets.Citation10–Citation16
To further enhance the therapeutic effect of our electrospun scaffold, we focused an investigative spotlight on MCP-1 in this study. MCP-1 is an immunomodulating factor that mediates a wide range of biological events, ranging from the regeneration of vascular tissues to local inflammation in renal tissues in diabetic patients.Citation17,Citation19 Prior research has even proposed that the level of MCP-1 be used as a clinical indicator of renal inflammation caused by diabetes.Citation36–Citation38
Interestingly, a recent study discovered that local MCP-1 was able to inhibit diabetes, possibly by engaging dendritic cells and T-cells in islets, although the exact underlying mechanism remains elusive.Citation23 A rapid release of MCP-1 is believed to contribute to the acceleration of the therapeutic regeneration of diseased or injured tissues and angiogenesis.Citation39,Citation40 However, previous research suggests that a prolonged high level of inflammation is detrimental to the tissue regeneration and functional recovery.Citation37,Citation38 Therefore, a rapid yet temporary release of MCP-1 from the DES might be a desired scheme to kick start the functional recovery of local tissues without causing unnecessary stress. It should be noted that administered MCP-1 is rapidly distributed to various organs and injured tissues, and features a transient half-life in blood circulation.Citation40 Also, MCP-1 is produced by a variety of tissues under both physiological and pathological conditions, rendering it impossible to discriminate native MCP-1 from the administered one.Citation41,Citation42
A satisfactory recovery of insulin secretion is a critical benchmark for the success of this bioengineering strategy, because it would return the blood glucose concentration to a physiological level, thus relieving other organs from the biological stress caused by hyperglycemia. The injection of insulin into diabetic patients to control the blood glucose level has been a popular clinical therapy. But, unfortunately, it could cause a variety of complications, including hypoglycemia, hyperglycemia, and seizure. To that end, the reestablishment of an in vivo native-like biological insulin-production mechanism through islet transplantation was developed. In this therapeutic scheme, C-peptide, a byproduct with no known functions during the native production of insulin by the pancreas, is an important and convenient clinical indicator of the production of endogenous insulin. Consequently, a correlated presence of C-peptide evidences the therapeutic effectiveness of transplanted islets. The results that both insulin and C-peptide 2 levels in the DES group were higher than those in the NES group at week 2 suggests that DESs enhanced the functional output of transplanted islets and thus would potentially improve the overall therapeutic effectiveness of the islet transplantation.
Consistent hyperglycemia is the culprit for the various complications in diabetic patients, particularly the loss of renal functions.Citation43 So an effective management of blood glucose is critical to rescue failing kidneys in diabetic patients. In our previous research, we showed that the improved islet functions due to the use of scaffolds helped to rein in blood glucose level faster than the traditional means of islet transplantation.Citation16 In the present study, we demonstrated that the incorporation of MCP-1 into the scaffold could further pronounce this advantage in regulating blood glucose. The OGTT result spoke to the fact that transplanted islets possessed a stronger blood glucose regulatory capacity in the face of a sudden spike of blood glucose, evidencing the long-term benefits afforded by the DES.
According to American Diabetes Association, diabetic patients who suffer failing kidneys frequently undergo kidney transplantation to recoup critical renal functions. Unfortunately, kidneys are extremely limited at best, and out of sight at worst. For the cohort of diabetic patients, an alternative strategy to simultaneously restore insulin production and renal functions is of great significance. Our previous research showed that blood MCP-1 level spiked after islet transplantation, probably due to enhanced immune response to the transplanted islets.Citation16 In that study, the spike of the MCP-1 level correlated to improved functional outputs of transplanted islets and renal tissues, which prompted us to further investigate the therapeutic role of MCP-1 in renal functions in diabetes. We later discovered that MCP-1 via intraperitoneal injection would accelerate the recovery of renal functions compromised by diabetes. The half-life of injected MCP-1 in the blood circulation was extremely short because MCP-1 would rapidly accumulate into various organs, which might explain the late onset of therapeutic effect.Citation40 In addition, the fact that cells in various tissues could secrete MCP-1 further complicates the task to tease out the relationship between MCP-1 and renal function recovery in a diabetic context.Citation41,Citation43 These challenges prompted us to functionalize the scaffold to locally deliver MCP-1 in the hope of enhancing the therapeutic effect. We found that MCP-1 from DESs accelerated the recapitulation of renal functions, but this advantage would soon diminish. This phenomenon might be attributed to the activation of a native immunoregulatory mechanism, because an artificially increased level of MCP-1 would induce an overwhelming immune response that might be detrimental to a functional recovery. All of the four renal proteins returning to physiological range demonstrated that the DESs accelerated renal function recovery without introducing cytotoxic effects.
Islet transplantation is currently the gold standard clinical solution to recoup lost insulin secretion in diabetic patients. We previously proved that the employment of an electrospun scaffold could promote the islet proliferation and functional output, in particular, the insulin secretion. In addition, we discovered an active role of MCP-1 in recouping renal functions lost to diabetes.Citation16 In this present study, we further explored a synergistic strategy to simultaneously enhance the functional output of transplanted islets as well as the recovery of renal functions. Our results confirmed that the local delivery of MCP-1 from a DES enhanced the insulin secretion of transplanted islets despite islet proliferation being only moderately increased. This increased insulin secretion successfully drove down the blood glucose to the physiological range within 2 weeks. Moreover, renal functions were swiftly recouped within 2 weeks. These increased functions could be attributed to the local stimulation of MCP-1. Our study offered a synergistic bioengineering strategy to simultaneously manage blood glucose and recoup compromised renal functions due to hyperglycemia.
Acknowledgments
The authors are grateful for the financial support from China’s National Natural Science Foundation (Grant Numbers: 30300357, 81270550 and 81270551).
Disclosure
The authors report no conflicts of interest in this work.
References
- GreggEWChengYJSaydahSTrends in death rates among US adults with and without diabetes between 1997 and 2006: findings from the National Health Interview SurveyDiabetes Care20123561252125722619288
- PorteDJrSchwartzMWDiabetes complications: why is glucose potentially toxic?Science199627252626997008614830
- GacklerDJakelSFrickeLReinschBFischerFDiabetes and kidneysDtsch Med Wochenschr201313818949955 German23613374
- MathisDVenceLBenoistCBeta-cell death during progression to diabetesNature2001414686579279811742411
- RyanEALakeyJRPatyBWSuccessful islet transplantation: continued insulin reserve provides long-term glycemic controlDiabetes20025172148215712086945
- RobertsonRPIslet transplantation as a treatment for diabetes – a work in progressN Engl J Med2004350769470514960745
- RicordiCStromTBClinical islet transplantation: advances and immunological challengesNat Rev Immunol20044425926815057784
- JaegerCBrendelMDHeringBJEckhardMBretzelRGProgressive islet graft failure occurs significantly earlier in autoantibody-positive than in autoantibody-negative IDDM recipients of intrahepatic islet allograftsDiabetes19974611190719109356046
- BarshesNRWyllieSGossJAInflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic graftsJ Leukoc Biol200577558759715728243
- ZhangXXuYThomasVBellisSLVohraYKEngineering an antiplatelet adhesion layer on an electrospun scaffold using porcine endothelial progenitor cellsJ Biomed Mater Res A201197214515121370444
- ZhangXThomasVXuYBellisSLVohraYKAn in vitro regenerated functional human endothelium on a nanofibrous electrospun scaffoldBiomaterials201031154376438120199808
- ZhangXThomasVVohraYKTwo ply tubular scaffolds comprised of proteins/poliglecaprone/polycaprolactone fibersJ Mater Sci Mater Med201021254154919902335
- ThomasVZhangXVohraYKA biomimetic tubular scaffold with spatially designed nanofibers of protein/PDS bio-blendsBiotechnol Bioeng200910451025103319575442
- ZhangXThomasVVohraYKIn vitro biodegradation of designed tubular scaffolds of electrospun protein/polyglyconate blend fibersJ Biomed Mater Res B Appl Biomater200989113514718780360
- ThomasVZhangXCatledgeSAVohraYKFunctionally graded electrospun scaffolds with tunable mechanical properties for vascular tissue regenerationBiomed Mater20072422423218458479
- YinHGaoMLeoniLHanHZhangXFuZThe therapeutic role of monocyte chemoattractant protein-1 in a renal tissue engineering strategy for diabetic patientsPloS One201382e5763523451253
- PaneeJMonocyte chemoattractant protein 1 (MCP-1) in obesity and diabetesCytokine201260111222766373
- ParkSLakattaEGRole of inflammation in the pathogenesis of arterial stiffnessYonsei Med J201253225826122318811
- SchoberAChemokines in vascular dysfunction and remodelingArterioscler Thromb Vasc Biol200828111950195918818421
- GillitzerRGoebelerMChemokines in cutaneous wound healingJ Leukoc Biol200169451352111310836
- GravesDTJiangYValenteAJThe expression of monocyte chemoattractant protein-1 and other chemokines by osteoblastsFront Biosci19994D571D58010393126
- PiemontiLLeoneBENanoRHuman pancreatic islets produce and secrete MCP-1/CCL2: relevance in human islet transplantationDiabetes2002511556511756323
- KriegelMARathinamCFlavellRAPancreatic islet expression of chemokine CCL2 suppresses autoimmune diabetes via tolerogenic CD11c+ CD11b+ dendritic cellsProc Natl Acad Sci U S A201210993457346222328150
- ParkSYWangXChenZOptical imaging of pancreatic beta cells in living mice expressing a mouse insulin I promoter-firefly luciferase transgeneGenesis2005432808616108006
- HaraMWangXKawamuraTTransgenic mice with green fluorescent protein-labeled pancreatic beta-cellsAm J Physiol Endocrinol Metab20032841E177E18312388130
- GrossmanEJLeeDDTaoJGlycemic control promotes pancreatic beta-cell regeneration in streptozotocin-induced diabetic micePloS One201051e874920090914
- MeinelAJGermershausOLuhmannTMerkleHPMeinelLElectrospun matrices for localized drug delivery: current technologies and selected biomedical applicationsEur J Pharm Biopharm201281111322342778
- JiWSunYYangFBioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applicationsPharm Res20112861259127221088985
- SillTJvon RecumHAElectrospinning: applications in drug delivery and tissue engineeringBiomaterials200829131989200618281090
- TibbittMWAnsethKSDynamic microenvironments: the fourth dimensionSci Transl Med20124160160ps24
- PrewitzMSeibFPPompeTWernerCPolymeric biomaterials for stem cell bioengineeringMacromol Rapid Commun201233171420143122887752
- BakerBMChenCSDeconstructing the third dimension: how 3D culture microenvironments alter cellular cuesJ Cell Sci2012125Pt 133015302422797912
- YannasIVEmerging rules for inducing organ regenerationBiomaterials201334232133023092865
- EdalatFSheuIManoucheriSKhademhosseiniAMaterial strategies for creating artificial cell-instructive nichesCurr Opin Biotechnol201223582082522705446
- ChungSKingMWDesign concepts and strategies for tissue engineering scaffoldsBiotechnol Appl Biochem201158642343822172105
- UrbschatAObermullerNHaferkampABiomarkers of kidney injuryBiomarkers201116Suppl 1S22S3021707441
- ElmarakbyAASullivanJCRelationship between oxidative stress and inflammatory cytokines in diabetic nephropathyCardiovasc Ther2012301495920718759
- CirilloPSautinYYKanellisJSystemic inflammation, metabolic syndrome and progressive renal diseaseNephrol Dial Transplant20092451384138719208772
- RohJDSawh-MartinezRBrennanMPTissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodelingProc Natl Acad Sci U S A2010107104669467420207947
- OhtsukiKHayaseMAkashiKKopiwodaSStraussHWDetection of monocyte chemoattractant protein-1 receptor expression in experimental atherosclerotic lesions: an autoradiographic studyCirculation2001104220320811447087
- YadavASainiVAroraSMCP-1: chemoattractant with a role beyond immunity: a reviewClin Chim Acta201041121–221570157920633546
- DeshmaneSLKremlevSAminiSSawayaBEMonocyte chemoattractant protein-1 (MCP-1): an overviewJ Interferon Cytokine Res200929631332619441883
- SchrijversBFDe VrieseASFlyvbjergAFrom hyperglycemia to diabetic kidney disease: the role of metabolic, hemodynamic, intracellular factors and growth factors/cytokinesEndocr Rev2004256971101015583025
