Abstract
The purpose of this review is to present the most recent findings in bone tissue engineering. Special attention is given to multifunctional materials based on collagen and collagen–hydroxyapatite composites used for skin and bone cancer treatments. The multi-functionality of these materials was obtained by adding to the base regenerative grafts proper components, such as ferrites (magnetite being the most important representative), cytostatics (cisplatin, carboplatin, vincristine, methotrexate, paclitaxel, doxorubicin), silver nanoparticles, antibiotics (anthracyclines, geldanamycin), and/or analgesics (ibuprofen, fentanyl). The suitability of complex systems for the intended applications was systematically analyzed. The developmental possibilities of multifunctional materials with regenerative and curative roles (antitumoral as well as pain management) in the field of skin and bone cancer treatment are discussed. It is worth mentioning that better materials are likely to be developed by combining conventional and unconventional experimental strategies.
Introduction
Bone is one of the naturally occurring composite materials that still does not have an artificial correspondent.Citation1 The interdependence between its morphology and properties is well understood, and two types of bone structures – cortical (compact) and trabecular (spongy) – can be easily identified. These different morphologies seem to be induced by piezoelectricity, with cortical bone being a result of a mechanically assisted biomineralization process.Citation2 The arrangement of osteons along with the loading direction can be explained by piezoelectricity. Recently, Noris-Suárez et al reproduced natural biomineralization conditions in vitro. They proved that the mechanical loading of the collagenous material induces important modifications upon the mineral-deposition process. They demonstrated that once mechanical loading takes place, the collagen fibers became arched and the negative charges appear especially distributed on the compressed zones. This is why mineralization occurs predominantly on the compressed areas, even if no osteoblasts are present.Citation3
Tissue engineering is of interest for researchers especially because of the increasing need for grafting materials.Citation4 The starting monolithic materials have been continuously improved by adding different components aimed at inducing new properties or to improve existing ones.Citation5–Citation9 The most common improvements have been related to the increase of healing rate, biocompatibility, or mechanical properties, or with the inducing of new properties, such as antimicrobial, anti-inflammatory, or analgesic activity. These new properties are sought after to avoid certain undesirable side effects or infections.Citation7,Citation9–Citation11 The complex composition and morphology of bone tissues confers them remarkable properties and functionalities.Citation1,Citation12–Citation14 In the last few decades, many researchers have invested their efforts in developing new materials for bone grafting inspired in bone composition and structure.Citation15 The compositions of bone and some of the most studied bone grafts are presented in . The systematic study of bone grafts can be considered to have started in the early twentieth century, when different transplants were done (allografts and xenografts).Citation16 Nowadays, special attention is paid to the synthesis of new bone grafts based on composite (nano)materials. Also, many papers deal with the important issue of how to design these materials in order to obtain improved biological properties. Biocompatibility and biointegration are usually realized by using engineered composite (nano)materials starting from natural polymers, calcium phosphates, and bone cells.Citation5,Citation6
Table 1 Composition of bone and its substitutes
Based on the classification made by Ashby et al,Citation17 nowadays there is a gradual transition occurring from the “nano- and bio-” age to a material-design age. During the nano- and bio-age, scientists focused their attention on improving material properties by decreasing materials’ size to the nanometric scale, but also paid attention to improving biological assessment in order to be better accepted by the human and animal body.Citation18 The material-design age maintains this principal concern, but improvements are achieved by optimizing such material characteristics as porosity, hydrophilicity, pore size, distribution, and shape, etc.Citation19,Citation20 This is why there are a lot of papers dealing with material design or tissue-engineered nanobiomaterials, or even with both concepts.Citation5,Citation12,Citation13,Citation21–Citation27 The use of bone cells for obtaining bone grafts could bring some major advantages: 1) the cells could be gathered from the patient and cultured in vitro; 2) the opportunity for using available stem cells that can be differentiated under proper conditions into bone cells; and 3) bone graft seeded with bone cells has the ability of being easily invaded by new bone ingrowth, thus promoting a much faster integration and the achievement of natural bone properties in a shorter time, in safe conditions, and with less donor tissue compared with classical auto- and allografting procedures.Citation36,Citation37 Moreover, the bone graft can act as a drug-delivery system for antibiotics and consequently enhance bone ingrowth in conjunction with wound healing.Citation37
Worldwide, cancer remains the second-most common cause of death, despite the advances in prevention, early detection, and protocols of treatment. It is well known that pain continues to be the most feared complication during treatment.Citation38–Citation40 In 2008, the total number of new cancer cases, based on the International Agency for Research on Cancer, was 12,662,554 (52.26% men), while for 2030 ~21 million new cases of cancer are expected.Citation41 Mortality is strongly influenced by cancer type. The overall or mortality numbers worldwide in 2008 were 7,564,802 (~59.75% of total incidence). Among the cancer types, the best survival rates (mortality/incidence × 100) are for thyroid and testis cancer (16% and 18%, respectively), while the worst are for liver and pancreas cancer (93% and 96%, respectively).Citation42 The very low survival rate is probably strongly influenced by the high mortality induced by lung cancer (which accounts for ~18.2% of total cancer mortalities).Citation43
Cancer usually occurs in mature/old people, except osteosarcoma, which is typically diagnosed in young people (10–20 years old) and rarely in old people,Citation44,Citation45 in the extremity of the long bones, especially in the femur.Citation46 There are 45 main types of primary bone tumor, the most important being osteosarcoma (35.1% of the primary bone tumors), followed by chondrosarcoma, Ewing’s sarcoma, and chondroma. By sex, males are more exposed to bone cancer (4% incidence in males compared to 3% in females),Citation47 even though osteosarcoma develops earlier in females compared with males (by about 2 years).Citation46
Cancer treatment is mainly based on surgery and radio- and chemotherapy, but also other unconventional therapies are available: hyperthermia, targeted therapy, immuno- or phototherapy, the use of nanoparticles or stem cell transplants, or many other less used therapies.Citation48 Hyperthermia is being used more and more as complementary therapy. The main result of the application of this therapy is decreasing chemotherapeutic doses or levels of radiation needed to maintain or even improve the efficiency of the treatment.Citation49–Citation51 Also, the use of nanoparticles showed a significant antitumoral effect, alone or in association with other therapies.Citation52–Citation55 These alternative therapies are mostly in the experimental phase of research, present an exciting challenge for the present, and will probably offer solutions for cancer treatment in the future, but there are also some alternative therapies currently available for cancer treatment, such as Doxil® (Janssen, Beerse, Belgium) and Abraxane® (Celgene, Summit, NJ, USA).Citation55
Drug-delivery systems are also used for different kinds of cancer. The most popular drug-delivery systems are based on polymers and ceramics and their composites. Polymers are by far the most used drug-delivery systems, the most used being polyethylene glycol (PEG), polyethylene oxide, poly-ε-caprolactone, chitosan, alginate, polyvinyl alcohol (PVA), polymethyl methacrylate, cellulose, etc.Citation56–Citation67 Also, proteins (collagen being the most abundant) are known as support for drug-delivery systems, but usually their high chemical and physical instability present technical problems related to synthesis and storage.Citation68–Citation71 PVA is extremely useful for chemoembolization, and in certain conditions can be loaded with various antitumoral drugs, such as cisplatin, doxorubicin, mitomycin C, and ethiodol.Citation67,Citation72–Citation74 Ceramic drug-delivery systems are also used for the treatment of bone cancer.Citation75
Collagen–hydroxyapatite composite materials
Collagen is a special class of proteins present in many tissues and organs. The history of collagen starts in 1960 with the discovery of the first representative of this class. Currently, 29 types of collagenCitation76 are known. From the point of view of distribution and biomedical applications, type I collagen is by far the most abundant and used variety. The intensive use of type I collagen can be easily explained based on the following: 1) there are a large number of type I collagen precursors (especially bovine calf); 2) the extraction technology is convenient (even native, fibrillar collagen is obtained under controlled conditions, collagen being susceptible to denaturation), because of the short extraction time with cheap reactants, especially if compared with the technology of extraction of type V collagen from bone.Citation77–Citation79 In the case of bone, a supplementary step is required, which consists of bone decalcification with hydrochloric acid and/or ethyl-enediaminetetraacetic acid.Citation80–Citation82 Once extracted, the native or denatured collagen can be stored as gel or transformed in fibers or matrices.Citation77
It is worth mentioning that type I collagen is also commercially available and used as wound dressing, especially in the case of burns,Citation83,Citation84 as a main component of many creams designed for care or treatment of skin laxity, rhytides, or photoaging,Citation85 or as a component of many engineered materials used for bone regeneration and cancer treatment.Citation70,Citation78,Citation86,Citation87 Collagen has also been used since 1980 as a drug-delivery system for ophthalmic agents (especially the antibiotics gentamicin and vancomycin),Citation88 the trend being to extend the use of this material in obtaining many other drug-delivery systems.Citation71,Citation78,Citation89
Despite intensive research efforts in the field of bone and bone grafts,Citation29,Citation90–Citation94 the properties of the materials obtained are still far from those of healthy bone.Citation95 Many types of materials have been separately attempted as bone grafts, such as ceramicsCitation32,Citation96,Citation97 and polymers,Citation98,Citation99 or combined in different manners to obtain composite materials.Citation12,Citation22,Citation23,Citation27,Citation29,Citation93,Citation100–Citation108 Collagen–hydroxyapatite (COLL/HA) composites are desired materials for bone grafting, especially due to their very good compositional similarity with bone,Citation1,Citation28 but also as drug-delivery systems.Citation109–Citation113 COLL/HA composite materials are currently extensively used as bone grafts.Citation12,Citation21,Citation33,Citation34,Citation93,Citation100–Citation108,Citation114–Citation117 Obviously, the biological properties as well as the mechanical properties are influenced by the manufacturing process. The size and crystallinity of hydroxyapatite crystals are essential parameters that influence the biological properties,Citation95,Citation118 mate rials based on smaller crystals inducing less inflammatory response.Citation95
Most studied are the porous COLL/HA composite materials, which could be considered especially for trabecular bone grafting and reconstruction, but can also be used for compact bone reconstruction. The biointegration of COLL/HA scaffolds is strongly influenced by porosity and pore size. Generally, it can be assumed that 150–200 μm pores are optimal for rapid osteointegration.Citation119 Larger pores are unwanted, because the mechanical properties of the graft drastically decrease, while narrower pores limit cell penetration inside the graft.Citation120,Citation121 Porosity and pore size can be controlled by different parameters, such as precursor concentration, drying conditions, presence of different components, etc.Citation13,Citation22 Usually, COLL/HA composite materials with high porosity are obtained from diluted, mineralized collagen gel followed by freeze-drying. Control of porosity can easily be achieved by controlling the drying process, (eg, air-drying followed by freeze-drying).Citation13 It has been proved that porosity decreases upon increase in air-drying time/extent.Citation12,Citation13 The explanation is very simple: air-drying is driven by capillary action that makes the material shrink and become denser during the evaporation of liquid water.Citation12 Conversely, capillary forces are absent in freeze-drying, which involves sublimation of frozen water, therefore maintaining the initial morphology of the porous structure. Based on literature data published by us,Citation13 the evolution of the porosity of samples obtained by combined drying is presented in , and ranges between 95% and 38%.
Table 2 Influence of preparation route and composition on the porosity of different samples
More compact composite materials are usually obtained from collagen gel by mineralization under such conditions that allow continuous material restructuring (, sample SA, COLL-PVA 1:2 A, or COLL-PVA/HA 1:2:3 A). Porosity can fall below even 5% if centrifugal sedimentation is used and only then dried in air.
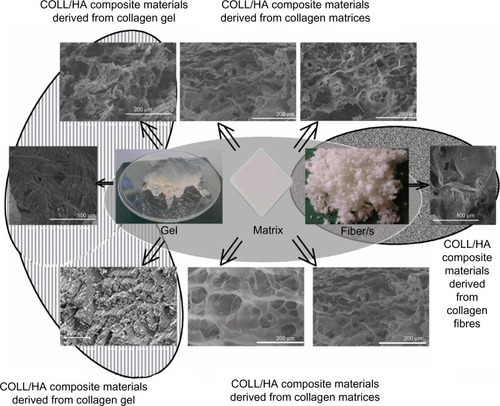
presents the morphology of some COLL/HA composite materials obtained by mineralization of collagen in different forms (gel, matrix, or fiber).Citation12,Citation21,Citation115 From collagen gel, both porous and compact materials as well as materials with intermediate porosity can be obtained. The mineralization of collagen matrix usually leads to porous composite materials. Probably, under certain conditions, collagen matrices and fibers can be processed to more compact materials. Porous composite materials have been tested as drug-delivery systems because, similarly with natural bone, the exchange rate (here the release rate) of the porous materials is higher than the release rate from compact materials.
Figure 1 Collagen (COLL) forms and their COLL/hydroxyapatite (HA) composite (nano)materials.
Notes: Reprinted from Chem Eng J.160. Ficai A, Andronescu E, Voicu G, et al. Self assembled collagen/hydroxyapatite composite materials. 794–800. Copyright (2010), with permission from Elsevier.Citation12 Reprinted from Mater Lett. 64. Ficai A, Andronescu E, Trandafir V, Ghitulica C, Voicu G. Collagen/hydroxyapatite composite obtained by electric field orientation. 541–544. Copyright (2010), with permission from Elsevier.Citation21 Adapted from Golub LM. Special Issue: Clinical Applications of Non-Antbacterial Tetracyclines Introduction. Pharmacol Res. 2011;63:99–101.Citation114
Multifunctional materials
A lot of materials have been tested as delivery support for bone-related diseases. A short review on this specific topic was recently published by Soundrapandian et al.Citation122 Most of these drug-delivery systems are based on the combination of different polymers with bioglass or calcium phosphates. Even if natural polymers are more suitable, a lot of composite materials based on synthetic polymers, such as polycaprolactone, poly(D,L-lactide), polylactide-co-glycolide (PLGA), or polymethyl methacrylate, have been also regarded with increasing interest.Citation122–Citation124 The enhanced stability of synthetic polymers in comparison with natural ones explains the higher number of composite materials based on synthetic polymer matrices. Further, the possibility of tailoring the composition of synthetic polymers enables a broader range of properties to be obtained for the final composites, including mechanical properties, drug-release rate, etc.
Starting from the well-established materials for bone grafting, different kinds of natural or synthetic components () have been added in order to induce some new functionalities. Multifunctional materials are being regarded with increasing interest for both industrial and biomedical applications.Citation125,Citation126 The multifunctional features of collagen and COLL/HA composite materials can be induced by the incorporation of various components, such as bone morphogenic protein,Citation127–Citation131 vitamins,Citation110,Citation132 bisphosphonates,Citation111,Citation133 antibiotics,Citation69,Citation112,Citation113 magnetite,Citation116 cytostatics,Citation70 or even more complex systems.Citation134 A main functionality of many of these systems is related to their ability to deliver the active component. Perhaps the most studied drug-delivery systems are those loaded with antibiotics or analgesics.Citation134 For the treatment of severe bone defects, surgical intervention might be required, because otherwise self-healing would be very slow, or even abnormal repair could happen.Citation29 The current protocols in the case of surgical intervention include the administration of antibiotics. A better alternate is to use bone grafts with antibacterial activity (for instance, an antibiotic-loaded bone graft), because the local delivery of the antibiotic reduces the systemic toxicity of these drugs.Citation135,Citation136 The use of analgesic-loaded materials is a real need in the treatment of many diseases. In some cases, drug-loaded systems are easy to apply clinically. For instance, in the case of bone cancer, resection of the tumoral tissue is often required, leaving a bone defect that needs to be filled with bone-regenerative material. Bone fillers can in fact be more complex systems incorporating pharmaceutically active substances (analgesic and/or antitumoral drugs), allowing them to be released in situ.Citation70,Citation134 Generally, the presented multifunctional systems were developed in order to assist natural repair mechanisms (bone morphogenic protein presence improves the rate of bone regeneration, bisphosphonate indirectly favors bone formation by suppressing bone resorption) or even to act as drugs (for avoiding infections [antibiotics] or even to fight against cancer [cytostatics] or other bone-related diseases). All these systems can be assimilated with drug-delivery systems and could be used to treat diseases from simple bone defects/fractures up to bone cancer. It is expected that clinical trials will be positive, because local administration will improve drug efficiency and limit side effects.Citation70,Citation137
Table 3 Common components used for inducing bone graft multifunctionality
Drug-delivery systems for bone cancer treatment
Research on cancer treatment has focused on two main areas: 1) developing new drugs, and 2) improving the activity of existing drugs by reducing their side effects. The main strategy for improving the activity of antitumoral agents is local delivery. A lot of drug-delivery systems were proposed and tested between 1991 and 2013,Citation138 such as PLGA/doxorubicin,Citation139 chitosan/paclitaxel,Citation140 polyurethane/curcumin,Citation141 chitosan/ellagic acid,Citation142 alginate/cisplatin,Citation62 poly-L-lactic acid/paclitaxel,Citation143 PLGA/isopropyl myristate/paclitaxel,Citation143 PEG–poly(aspartic acid)/adriamycin,Citation144 gelatin/doxorubicin,Citation145 hydroxyapatite/platinum complexes,Citation146,Citation147 or COLL/HA/cisplatin.Citation70
Apatite-based materials are extensively used as bone filler/grafts.Citation148–Citation150 This is why many drug-delivery systems designed for bone-disease treatment are based on hydroxyapatite. For instance, hydroxyapatite/cisplatin drug-delivery systems were obtained and tested as delivery systems of different platinum complexes.Citation147,Citation151–Citation154 Many trials were taken into account, focusing on the synthesis route, drug content, porosity, pore size, etc. Hydroxyapatite samples with different porosity fractions (58%, 76%, and 82%) and average pore sizes (15 μm, 21 μm, and 35 μm) were obtained by the gel-casting method followed by cisplatin loading.Citation147 Percentage cisplatin recovery after 168 hours increased from 21% to 28% and 42% as porosity fractions increased within the aforementioned range (58%–82%). Control of the release rate is of paramount importance, because long-term delivery could decrease cancer recurrence by reducing remnant cancerous cells.Citation70
Recently, Abe et al developed new paclitaxel-loaded hydroxyapatite/alginate composite material for the treatment of metastatic spine cancer,Citation153 which develops frequently in patients with breast cancer. Based on animal experiments, the use of paclitaxel-loaded hydroxyapatite/alginate composite beads led to 140%–150% increases in disease-free time as well as survival time compared with control animals.
Itokazu et al developed some drug-delivery systems based on hydroxyapatite and cytostatics for bone cancer treatment.Citation177–Citation179 They proved that porosity and pore size influenced the release rate of both doxorubicin and methotrexate. The implantation of these ceramic blocks at the tumor site led to a reduction in dose of the antitumor agent, and consequently the risk of systemic toxicity decreased drastically compared with conventional systemic administration. The improved contact of antitumor agents with tumoral cells is expected to reduce the recurrence and metastasis of cancer.
A gelatin/doxorubicin drug-delivery systemCitation145 was obtained and tested for the treatment of bone cancer, because doxorubicin is one of the most potent antitumor agents in use for bone cancer treatment, while the gelatin could act, after doxorubicin release, as a scaffold for bone regeneration. The classical administration route of doxorubicin is undesirable because of severe side effects. A general way to reduce side effects is to avoid intravenous administration of antitumor agents by using drug-delivery systems. In the case of bone cancer, the use of implantable gelatin/doxorubicin could be a promising way of targeted delivery of doxorubicin to tumoral tissue. The rate of delivery could be easily controlled by the degree of cross-linking and porosity.
COLL/HA–cisplatin is a remarkable material for the treatment of bone cancer because it assures two functions: targeted delivery of cisplatin and acting as a regenerative scaffold.Citation70 For this reason, samples were obtained and tested from the point of view of cisplatin-induced cytotoxicity. The delivery curve of cisplatin has two independent regions: a fast delivery up to 2 hours, followed by a sustained delivery of cisplatin up to 26 hours.Citation70 The short release time can be exploited by choosing a proper polychemotherapeutic method that includes the cisplatin release and further traditional administration of complementary cytostatics.Citation181
Bone cancer is usually associated with terrible pain.Citation182–Citation184 Up to 30% of patients with recently diagnosed cancers report pain. With the evolution of the cancer, the pain becomes more intense, and about 80% of patients with primary bone cancer and over 90% of patients with metastases to osseous structures need ever-stronger drugs for pain management.Citation39,Citation185–Citation187 Based on the World Health Organization analysis, pain intensity as well as pain management is classified at three levels. The lowest level of pain is usually treated with nonopioid and/or adjuvant drugs (aspirin and acetaminophen being extensively used), the middle and worst levels of pain need increasing doses of opioids (and also with increasing efficiency from weak [codeine, for instance] to strong opioids [morphine, for instance]) combined or not with nonopioid and/or adjuvant drugs.Citation186 In the case of severe pain, systems with immediate or sustained release are used.Citation187
Magnetite and magnetite-based materials for bone cancer treatment
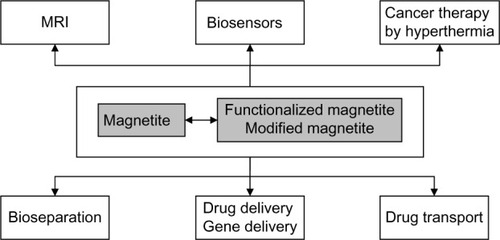
An overview of the most important applications of magnetite and magnetite-based materials is presented in . Pure magnetite is rarely used for cancer treatment, in particular because of its high tendency of agglomeration and high reactivity. This is why many researchers have attempted to functionalize its surface from simple fatty acids,Citation188 up to complex agents, such as aminophosphonic acid, diols and polyols, polyhydroxy acids, siloxanes, thioacids, etc.Citation189 As presented in , magnetite and magnetite-based materials are efficient in cancer diagnosis as well as in cancer treatment, including hyperthermia as well as drug transport and targeted delivery. The hyperthermia is produced by magnetite when a proper alternating electromagnetic field is applied. The output power and the applied frequency are essential for producing medical hyperthermia, especially in the case of deep organs/tissues.Citation190 Usually, these radiations are of low power and should induce low toxicity.Citation191
Figure 2 Applications of magnetite and magnetite-based materials.
Abbreviation: MRI, magnetic resonance imaging.
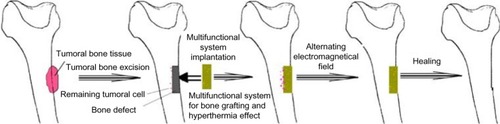
Magnetic materials proved its effectiveness in the treatment of different diseases, including cancer treatment, by combining surgery – as a conventional treatment and hyper-thermia – as an alternate route of treatment. A methodology of treating bone cancer was presented by Andronescu et al,Citation116 () and consists of two main parts. The first step is assimilated with the surgical intervention of resection of the tumoral tissue, while the second step consists of filling the resulting bone defect with multifunctional materials. Once implanted, bone healing starts due to the presence of COLL/HA composite material. The magnetic nanoparticles can be activated, externally and at any time, by applying an electromagnetic field that induces hyperthermia.
Figure 3 Schematic representation of bone cancer treatment by combined therapy (surgery and hyperthermia).
Note: With kind permission from Springer Science+Business Media: J Mater Sci—Mater M., Synthesis and characterization of collagen/hydroxyapatite:magnetite composite material for bone cancer treatment. 21, 2010, 2237–2242, Andronescu E, Ficai M, Voicu G, Ficai D, Maganu M, Ficai A, .Citation116
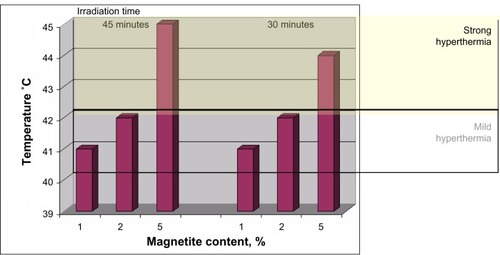
Even if only a few materials are based on COLL/HA–Fe3O4,Citation116,Citation134 perhaps, due to the high sensibility of the collagenous structure, their potential is great. The work realized by Andronescu et alCitation116 presents the preparation of different COLL/HA–Fe3O4 with a 1:4 ratio of COLL:HA and 1%, 2%, and 5% magnetite. The in vitro studies revealed that mild hyperthermia is produced even at low magnetite content. In the case of COLL/HA–Fe3O4 with 1% magnetite, the maximum temperature reached was ~41°C, which means mild hyperthermia, while at 5% magnetite the maximum temperature exceeded 45°C (). All these data were obtained using thermostated samples (37°C) at 150 kHz.
Figure 4 Hyperthermia versus content of magnetite.
Note: Data from Andronescu E et al.Citation116
The aforementioned methodology of bone cancer treatment can be easily adapted for more complex material drug-delivery systems, such as COLL/HA–Fe3O4–cyto-static, COLL/HA–Fe3O4–analgesic, COLL/HA–Fe3O4–Me (Me = Au, Ag), COLL/HA–Fe3O4–Me–cytostatic, COLL/HA–Fe3O4–Me–analgesic, or even COLL/HA–Fe3O4–Me–analgesic–cytostatic.Citation134 These multifunctional materials assure the convergence of conventional (surgery and chemotherapy) and alternative (hyperthermia, antitumoral effect of some metallic nanoparticles, phototherapy, and pain management due to the presence of analgesics) routes of bone cancer treatment. It is expected that due to the unconventional component of bone cancer treatment (as well as the targeted delivery of chemotherapeutic drugs) that the content of chemotherapeutic drugs will decrease and consequently systemic toxicity will be minimized.
For instance, Campbell et alCitation192 synthesized quasicubic magnetite/silica core–shell nanoparticles that proved to be enhanced magnetic resonance imaging (MRI) contrast agents for cancer imaging. The synthesis of Fe3O4@SiO2 was performed from prefabricated magnetite nanoparticles by controlled hydrolysis of Tetraethylorthosilicate with the formation of a silica network onto the magnetite nanoparticles. Using these quasicubic magnetite/silica nanoparticles, in vitro and in vivo experiments were carried out. Based on the in vivo experiments on mice infected with PC3 human prostate cancer cells, the change in MRI signal was up to 80% for 100 μg/mL Fe, a value that is significantly higher than reported results obtained with other materials, which reach up to only 15%–20%. The presence of silica led to a higher uptake of PC3 prostate cancer cells compared with pure magnetite. PC3 prostate cancer cell viability decreased once the content of Fe increased from 0 to 100 μg/mL.Citation191
Treating bone cancer with magnetite and/or magnetite-based materials has also been attempted with different materials, such as polymethyl methacrylate/Fe3O4, Citation193,Citation194 HA/Fe3O4, Citation195–Citation198 glass- and bioglass-based composites,Citation199,Citation200 and complex polymer/ceramic composite materials with various magnetite content.Citation116,Citation201,Citation202 Based on the literature survey, most materials designed for bone cancer treatment by hyperthermia are based on calcium phosphates or bioglass and magnetite.
Conclusion and perspectives
Cancer remains the second-most common cause of death in the world, despite advances in prevention and early detection and newer treatment protocols. The development of new antitumoral agents as well as the development of more efficient treatment strategies are current pursuits for scientists. Chitosan and PEG have been intensively studied for drug delivery in many applications, including cancer treatment. Only a few papers have dealt with collagen-based support materials, most probably because of the high chemical sensibility of this protein in comparison with chitosan, PEG, alginate, etc. It is expected that the use of collagen for the preparation of drug-delivery systems of cytostatics will be continued in the future. Expected applications are bone cancer treatment by using composite materials based on collagen and calcium phosphates, skin cancer treatment by using collagen-based polymeric materials, or even colon cancer, collagen being a good carrier through the stomach.
Acknowledgments
The authors recognize financial support from the European Social Fund through the POSDRU/89/1.5/S/54785 project “Postdoctoral Program for Advanced Research in the Field of Nanomaterials”. Catarina Marques thanks FCT for the fellowship grant (SFRH/BD/78355/2011). The support from CICECO, University of Aveiro, is also acknowledged.
Disclosure
The authors report no conflicts of interest in this work.
References
- FratzlPWeinkamerRNature’s hierarchical materialsProg Mater Sci20075212631334
- SilvaCCThomaziniDPinheiroAGCollagen-hydroxyapatite films: piezoelectric propertiesMat Sci Eng B Solid State Mater Adv Technol200186210218
- Noris-SuarezKLira-OlivaresJFerreiraAMIn Vitro Deposition of Hydroxyapatite on Cortical Bone Collagen Stimulated by Deformation-Induced PiezoelectricityBiomacromolecules2007894194817261065
- von WalterskirchenMThe U.S. Market for Medical Devices—Opportunities and Challenges for Swiss Companies2004 Available from: http://www.swissbusinesshub.com/photos/news/SwissMedtech.pdfAccessed January 7, 2014
- FicaiMAndronescuEFicaiDVoicuGFicaiASynthesis and characterization of COLL-PVA/HA hybrid materials with stratified morphologyColloids Surf B Biointerfaces20108161461920828998
- ZhangLHTangPFZhangWXuMWangYEffect of Chitosan as a Dispersant on Collagen-Hydroxyapatite Composite MatricesTissue Eng Part C Methods201016717919364274
- MelvilleAJRodriguez-LorenzoLMForsytheJSGrossKADrug delivery behaviour of hydroxyapatite and carbonated apatiteBioceramics200416529532
- KimYHSongBGKimSRKimKJDrug loaded porous hydroxyapatite and its in vitro releaseBioceramics200517423426
- LarenaACaceresDAVicarioCFuentesARelease of a chitosan-hydroxyapatite composite loaded with ibuprofen and acetyl-salicylic acid submitted to different sterilization treatmentsAppl Surf Sci2004238518522
- ParkJLakesRSBiomaterials: An introductionNew YorkSpringer2007
- DernerRAndersonACThe bone morphogenic proteinClin Pediatric Med Surg200522607618
- FicaiAAndronescuEVoicuGSelf assembled collagen/hydroxyapatite composite materialsChem Eng J2010160794800
- AndronescuEVoicuGFicaiMMohoraIATruscaRFicaiACollagen/hydroxyapatite composite materials with desired ceramic propertiesJ Electr Microscopy (Tokyo)201160253259
- IlieAAndronescuEFicaiDNew approaches in layer by layer synthesis of collagen/hydroxyapatite composite materialsCent Eur J Chem20119283289
- VranceanuMDSabanRAntoniacIAlbuMMiculescuFDevelopment and characterization of novel porous collagen based biocomposite for bone tissue regenerationUPB Sci Bull, Series B201274145156
- GallieWETorontoMBThe histrory of a bone graftJ Bone Joint Surg Am1914s2–12201212
- AshbyMFerreiraPSchodekDNanomaterials, Nanotechnologies and Design: An Introduction for Engineers and ArchitectsAmsterdamElsevier2009
- Chris ArtsJJVerdonschotNSchreursBWBumaPThe use of a bioresorbable nano-crystalline hydroxyapatite paste in acetabular bone impaction graftingBiomaterials2006271110111816098583
- RushSMBone graft substitutes: OsteobiologicsClin Podiatr Med Surg20052261963016213384
- KarageorgiouVKaplanDPorosity of 3D biomaterial scaffolds and osteogenesisBiomaterials2005265474549115860204
- FicaiAAndronescuETrandafirVGhitulicaCVoicuGCollagen/hydroxyapatite composite obtained by electric field orientationMater Lett201064541544
- FicaiAAndronescuEVoicuGGhitulicaCFicaiDThe influence of collagen support and ionic species on the morphology of collagen/hydroxyapatite composite materialsMater Charact201061402407
- FicaiAAndronescuEVoicuGManzuDFicaiMLayer by layer deposition of hydroxyapatite onto the collagen matrixMat Sci Eng C Mater Biol Appl20092922172220
- WangHLiYZuoYLiJMaSChengLBiocompatibility and osteogenesis of biomimetic nano-hydroxyapatite/polyamide composite scaffolds for bone tissue engineeringBiomaterials2007283338334817481726
- JiangDZhangJCalcium phosphate with well controlled nanostructure for tissue engineeringCurr Appl Phys20099
- RoviraAAmedeeJBareilleRRabaudMColonization of a calcium phosphate elastin-solubilized peptide-collagen composite material by human osteoblastsBiomaterials199617153515408853125
- VenugopalJPrabhakaranMPZhangYZLowSChoonATRamakrishnaSBiomimetic hydroxyapatite-containing composite nanofibrous substrates for bone tissue engineeringPhilos Trans A Math Phys Eng Sci20103682065208120308115
- FratzlPGuptaHSPaschalisEPRoschgerPStructure and mechanical quality of the collagen–mineral nano-composite in boneJ Mater Chem20041421152123
- MuruganRRamakrishnaSDevelopment of nanocomposites for bone graftingCompos Sci Technol20056523852406
- CiobanuCSAndronescuEVasileBSTruscaRPredoiDPreliminary studies on hydroxyapatite doped with europiumUPB Sci Bull, Series B2011736370
- DorozhkinSVNanosized and nanocrystalline calcium orthophosphatesActa Biomater2010671573419861183
- DorozhkinSVBioceramics of calcium orthophosphatesBiomaterials2010311465148519969343
- MuruganRRamakrishnaSBioresorbable composite bone paste using polysaccharide based nano hydroxyapatiteBiomaterials2004253829383515020158
- FicaiMAndronescuEFicaiAVoicuGVasileBSPoly Bis-GMA/HA based hybrid composite materialsUPB Sci Bull, Series B2011737584
- GloriaADe SantisRAmbrosioLPolymer-based composite scaffolds for tissue engineeringJ Appl Biomater Biomech20108576720740467
- RoseFRAJOreffoROCBone tissue engineering: hope vs hypeBiochem Biophys Res Commun20022921711890663
- ScholzMSBlanchfieldJPBloomLDThe use of composite materials in modern orthopaedic medicine and prosthetic devices: A reviewCompos Sci Technol20117117911803
- Marec-BerardPDelafosseCFoussatCCancer-related bone pain in childrenArch Pediatrie200512191198
- MantyhPThe science behind metastatic bone painEur J Cancer: Supplements2006448
- MercadanteSMalignant bone pain: pathophysiology and treatmentPain1997691189060007
- Cancer Research UKCancer worldwide – the global picture2008 Available from: http://www.cancerresearchuk.org/cancer-info/cancerstats/world/the-global-pictureAccessed January 7, 2014
- FotuhMAMacarySFactors affecting mechanical water shutoffJ Petrol Technol2001535859
- FerlayJShinHRBrayFFormanDMathersCParkinDMGlobocan 2008Cancer Incidence and Mortality WorldwideLyonInternational Agency for Research on Cancer2010
- RiesLAGEisnerMPKosaryCLHankeyBFMillerBACleggLMariottoAFeuerEJEdwardsBKSEER Cancer Statistics Review, 1975–2001National Cancer InstituteBethesda, MD http://seer.cancer.gov/csr/1975_2001/2004
- Canadian Cancer SocietyCanadian Cancer Statistics 2004TorontoNational Cancer Institute of Canada2004
- FraumeniJFJrStature and malignant tumors of bone in childhood and adolescenceCancer1967209679735229526
- BleyerAO’LearyMBarrRRiesLCancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age, Including SEER Incidence and Survival: 1975–2000Bethesda (MD)National Cancer Institute2006
- OrentasRHodgeJWBD JohnsonBDCancer Vaccines and Tumor ImmunityHoboken (NJ)John Wiley and Sons2008
- PurushothamSRamanujanRVThermoresponsive magnetic composite nanomaterials for multimodal cancer therapyActa Biomater2010650251019596094
- LubbeASBergemannCHuhntWPreclinical experiences with magnetic drug targeting: Tolerance and efficacyCancer Res19965646947018840986
- GuptaRBajpaiAKMagnetically Guided Release of Ciprofloxacin from Superparamagnetic Polymer NanocompositesJ Biomater Sci Polym Ed20112289391820566063
- XuRMaJSunXCAg nanoparticles sensitize IR-induced killing of cancer cellsCell Res2009191031103419621033
- GallegoÓPuntesVWhat can nanotechnology do to fight cancer?Breast Cancer Res Treat20068788795
- KattiKVKannanRKattiKHybrid gold nanoparticles in molecular imaging and radiotherapyCzechoslovak J Phys200656D23D34
- TanakaTDecuzziPCristofanilliMNanotechnology for breast cancer therapyBiomed Microdevices2008
- LiechtyWBKryscioDRSlaughterBVPeppasNAPolymers for Drug Delivery SystemsAnnu Rev Chem Biomol Eng2010114917322432577
- GhoshSGhoshSRecent research and development in synthetic polymer-based drug delivery systemsJournal of Chemical Research20044241246
- ApicellaACappelloBDelnobileMAPoly(ethylene oxide)-based delivery systemsPolym Drugs Drug Adm1994545111125
- JacksonJKMinWCruzTFA polymer-based drug delivery system for the antineoplastic agent bis(maltolato)oxovanadium in miceBr J Cancer1997757101410209083337
- SuzukiYMakinoYMucosal drug delivery using cellulose derivatives as a functional polymerJ Control Release1999621–210110710518641
- AryalSHuCMJZhangLPolymer–cisplatin conjugate nanoparticles for acid-responsive drug deliveryACS Nano20104125125820039697
- CafaggiSRussoEStefaniRLeardiRCaviglioliGParodiBPreparation and evaluation of nanoparticles made of chitosan or N-trimethyl chitosan and a cisplatin-alginate complexJ Controll Release2007121110123
- HanHDSongCKParkYSA chitosan hydrogel-based cancer drug delivery system exhibits synergistic antitumor effects by combining with a vaccinia viral vaccineInt J Pharm2008350273417897800
- SooPLChoJGrantJHoEPicluette-MillerMAllenCDrug release mechanism of paclitaxel from a chitosan-lipid implant system: Effect of swelling, degradation and morphologyEur J Pharm Biopharm20086914915718164931
- TaHTDassCRDunstanDEInjectable chitosan hydrogels for localised cancer therapyJ Controll Release2008126205216
- JunpingWTakayamaKNagaiTMaitaniYPharmacokinetics and antitumor effects of vincristine carried by microemulsions composed of PEG-lipid, oleic acid, vitamin E and cholesterolInt J Pharm20032511–2132112527171
- KieferMVAlbertMMcNallyMChemoembolization of intrahepatic cholangiocarcinoma with cisplatinum, doxorubicin, mito-mycin C, ethiodol, and polyvinyl alcohol: a 2-center studyCancer201111771498150521425151
- ArmentanoIDottoriMFortunatiEMattioliSKennyJMBiodegradable polymer matrix nanocomposites for tissue engineering: A reviewPolym Degrad Stab20109521262146
- AlbuMGGhicaMVFicaiATitorencuIPopaLInfluence of Freeze-Drying Process on Porosity and Kinetics Release of Collagen-Doxycycline MatricesProceedings of the 3rd International Conference on Advanced Materials and Systems2010181186
- AndronescuEFicaiAGeorgianaMCollagen-hydroxyapatite/Cisplatin Drug Delivery Systems for Locoregional Treatment of Bone CancerTechnol Cancer Res Treat20131227528423547973
- TitorencuIAlbuMGPopaLCollagen-Doxycycline Matrices Used in Tissue EngineeringFarmacia201159794802
- KayalSRamanujanRVDoxorubicin loaded PVA coated iron oxide nanoparticles for targeted drug deliveryMat Sci Eng C Mater Biol Appl201030484490
- JadhavKRPacharaneSSKoshyPVKadamVJSmart polymers and their role in drug delivery: A reviewCurr Drug Ther20105250261
- MedeirosSFSantosAMFessiHElaissariAStimuli-responsive magnetic particles for biomedical applicationsInt J Pharm201140313916120951779
- GeninFYLuoPDashAKinventorsBerkeley Advanced Biomaterials, Inc., assigneeHydroxyapatite based drug delivery implant for cancer treatment United States patent US 6767550 B17302004
- ScheggBHülsmeierAJRutschmannCMaagCHennetTCore glycosylation of collagen Is initiated by two β(1-O)galactosyltransferasesMol Cell Biol200929494395219075007
- AlbuMGTitorencuIGhicaMVCollagen gels and matrices for biomedical applicationsPignatelloRBiomaterials Applications for NanomedicineRijeka, CroatiaInTech2011
- AlbuMGTitorencuIGhicaMVCollagen-based Drug Delivery Systems for Tissue EngineeringPignatelloRBiomaterials Applications for NanomedicineRijeka, CroatiaInTech2011
- NagaiTSuzukiNIsolation of collagen from fish waste material—skin, bone and finsFood Chem200068277281
- MillerEJMartinGRPiezKAPowersMJCharacterization of Chick Bone Collagen and Compositional Changes Associated with MaturationJ Biol Chem19672425481548912325363
- Castro-CesenaABNovitskayaEEPhadkeAVargheseSMcKittrickJIsolation of Collagen from Cortical Bovine Bone for Preparation of Porous Collagen Sponges. Mechanics of Biological Systems and MaterialsConference Proceedings of the Society for Experimental Mechanics Series20137378
- KubokiYShimokawaHOnoTSasakiSDetection of collagen degradation products in boneCalcif Tissue Res1973123033124201031
- SinghOGuptaSSSoniMMosesSShuklaSMathurRKCollagen dressing versus conventional dressings in burn and chronic wounds: a retrospective studyJ Cutan Aesthet Surg201141121621572675
- KleinMBEngravLHHolmesJHManagement of facial burns with a collagen/glycosaminoglycan skin substitute-prospective experience with 12 consecutive patients with large, deep facial burnsBurns200531325726115774278
- AustMCFernandesDKolokythasPKaplanHMVogtPMPercutaneous collagen induction therapy: an alternative treatment for scars, wrinkles, and skin laxityPlast Reconstr Surg200812141421142918349665
- AlbuMGCollagen gels and matrices for biomedical applicationsLambert Academic PublishingSaarbrücken2011
- AlbuMGTrandafirVSufletDMChitanuGCBudrugeacPTitorencuIBiocomposites based on collagen and phosphorylated dextran for bone regenerationJ Mater Res20122710861096
- PhienneyRBSchwartzSDLeeDAMondinoBJCollagen-shields delivery of gentamicin and vancomycinArch Ophtalmol198810615991604
- RuszczakZFriessWCollagen as a carrier for on-site delivery of antibacterial drugsAdv Drug Deliv Rev2003551679169814623407
- Bandyopadhyay-GhoshSBone as a Collagen-hydroxyapatite Composite and its RepairTrends Biomater Artif Organs200822112120
- LickorishDRamshawJAMWerkmeisterJAGlattauerVHowlettCRCollagen-hydroxyapatite composite prepared by biomimetic processJ Biomed Mater Res A200468A192714661245
- KikuchiMItohSIchinoseSShinomiyaKTanakaJSelf-organization mechanism in a bone-like hydroxyapatite/collagen nanocomposite synthesized in vitro and its biological reaction in vivoBiomaterials2001221705171111396873
- CuiF-ZLiYGeJSelf-assembly of mineralized collagen compositesMater Sci Eng R Rep200757127
- FicaiAAndronescuEFicaiDSonmezMAlbuMGVoicuGMimicking the morphology of long boneCent Eur J Chem20121019491953
- WahlDACzernuszkaJTCollagen-hydroxyapatite composites for hard tissue repairEur Cell Mater200611435616568401
- HabrakenWJWolkeJGJansenJACeramic composites as matrices and scaffolds for drug delivery in tissue engineeringAdv Drug Deliv Rev2007594–523424817478007
- DorozhkinSVAmorphous calcium (ortho)phosphatesActa Biomater201064457447520609395
- Girones MoleraJMendezJASan RomanJBioresorbable and non-resorbable polymers for bone tissue engineeringCurr Pharm Des201218182536255722512444
- BeachleyVKatsanevakisEZhangNWenXJHighly aligned polymer nanofiber structures: fabrication and applications in tissue engineeringJayakumarRNairSVBiomedical Applications of Polymeric Nanofibers246HeidelbergSpringer2012171212
- TienWBChenMTYaoPCEffects of pH and temperature on micro-structure and morphology of hydroxyapatite/collagen composites synthe-sized in vitroMater Sci Eng C Mater Biol Appl20123220962102
- SangLHuangJLuoDMChenZHLiXDBone-like nanocomposites based on self-assembled protein-based matrices with Ca2+ capturing capabilityJ Mater Sci Mater Med2010212561256820582716
- SwethaMSahithiKMoorthiASrinivasanNRamasamyKSelvamuruganNBiocomposites containing natural polymers and hydroxyapatite for bone tissue engineeringInt J Biol Macromol20104711420361991
- SzpalskiCWetterauMBarrJWarrenSMBone tissue engineering: current strategies and techniques–part I: ScaffoldsTissue Eng Part B Rev201218424625722029448
- GardinCFerroniLFaveroLNanostructured biomaterials for tissue engineered bone tissue reconstructionInt J Mol Sci201213173775722312283
- KhanFDahmanYA novel approach for the utilization of biocellulose nanofibres in polyurethane nanocomposites for potential applications in bone tissue implantsDes Monomers Polym2012151129
- CanalCGinebraMPFibre-reinforced calcium phosphate cements: a reviewJ Mech Behav Biomed Mater2011481658167122098867
- Wagoner JohnsonAJHerschlerBAA review of the mechanical behavior of CaP and CaP/polymer composites for applications in bone replacement and repairActa Biomater201171163020655397
- ChiaraGLetiziaFLorenzoFNanostructured biomaterials for tissue engineered bone tissue reconstructionInt J Mol Sci20121373775722312283
- OtsukaMHiranoRBone cell activity responsive drug release from biodegradable apatite/collagen nano-composite cements-In vitro dissolution medium responsive vitamin K2 releaseColloids Surface B Biointerfaces201185338342
- WangGMostafaNZIncaniVKucharskiCUludagHBisphosphonate-decorated lipid nanoparticles designed as drug carriers for bone diseasesJ Biomed Mater Res A2012100368469322213565
- TsourvakasSLocal antibiotic therapy in the treatment of bone and soft tissue infectionsDanillaSSelected topics in plastic reconstructive surgeryRijeka, CroatiaInTech20121744
- MartinsVCGoissisGRibeiroACMarcantônioEJrBetMRThe controlled release of antibiotic by hydroxyapatite: anionic collagen compositesArtif Organs19982232152219527282
- Amaro MartinsVCGoissisGNonstoichiometric hydroxyapatite-anionic collagen composite as support for the double sustained release of gentamicin and orfloxacin/ciprofloxacinArtif Organs200024322423010759646
- GolubLMSpecial Issue: Clinical Applications of Non-Antbacterial Tetracyclines IntroductionPharmacol Res2011639910120937387
- FicaiAAndronescuEVoicuGAlbuMGIlieABiomimetically synthesis of collagen/hydroxyapatite composite materialsMat Plast201047205208
- AndronescuEFicaiMVoicuGFicaiDMaganuMFicaiASynthesis and characterization of collagen/hydroxyapatite:magnetite composite material for bone cancer treatmentJ Mater Sci—Mater M2010212237224220372983
- FicaiAAndronescuEGhitulicaCColagen/Hydroxyapatite Interactions in Composite BiomaterialsMat Plast2009461115
- LiuXZhaoMLuJMaJWeiJWeiSCell responses to two kinds of nanohydroxyapatite with different sizes and crystallinitiesInt J Nanomed2012712391250
- GaloisLMainardDBone ingrowth into two porous ceramics with different pore sizes: An experimental studyActa Orthop Belg20047059860315669463
- YunokiSIkomaTMonkawaAControl of pore structure and mechanical property in hydroxyapatite/collagen composite using unidirectional ice growthMater Lett2006609991002
- YunokiSIkomaTTsuchiyaAFabrication and mechanical and tissue ingrowth properties of unidirectionally porous hydroxyapatite/collagen compositeJ Biomed Mater Res B200780B166173
- SoundrapandianCSaBDattaSOrganic-inorganic composites for bone drug deliveryAaps Pharmscitech2009101158117119842042
- VenugopalJPrabhakaranMPLowSNanotechnology for nanomedicine and delivery of drugsCurr Pharm Design20081421842200
- SandersWESandersCCToxicity of Antibacterial Agents: Mechanism of Action on Mammalian CellsAnnu Rev Pharmacol1979195383
- LichterJAVan VlietKJRubnerMFDesign of antibacterial surfaces and interfaces: Polyelectrolyte multilayers as a multifunctional platformMacromolecules2009422285738586
- SchottnerGHybrid sol-gel-derived polymers: Applications of multifunctional materialsChem. Mater2001131034223435
- KaitoTMyouiASaitoNPotentiation of the activity of bone morphogenetic protein-2 in bone regeneration by a PLA–PEG/hydroxyapatite compositeBiomaterials2005261737915193882
- FengBHuDXZhangYDAccelerated Bone Regeneration by Chitosan/Nanometer Hydroxyapatite/Collagen Composite Incorporating BMP-7 Mimetic PeptideJ Hard Tissue Biol201221481487
- LiJFLinZYZhengQXRepair of rabbit radial bone defects using true bone ceramics combined with BMP-2-related pep tide and type I collagenMater Sci Eng C Mater Biol Appl201030812721279
- LiuLRZhangLHWangFJZhangQQThe study of collagen-HA composite with incorporated BMP as bone tissue engineering scaffoldZhangXDAdvanced Biomaterials VI: ASBM6: Proceedings of the 6th Asian Symposium on Biomedical Materials, Emei, Chengdu, China, July 19–22, 2004 DürntenSwitzerlandTrans Tech2005261264
- SotomeSUemuraTKikuchiMChenJItohSTanakaJSynthesis and in vivo evaluation of a novel hydroxyapatite/collagen-alginate as a bone filler and a drug delivery carrier of bone morpho-genetic proteinMaterials Science & Engineering C-Biomimetic and Supramolecular Systems2004243341347
- OtsukaMKuninagaTOtsukaKHiguchiWIEffect of nanostructure on biodegradation behaviors of self-setting apatite/collagen composite cements containing vitamin K2 in ratsJ Biomed Mater Res B Appl Biomater200679117618416680714
- WangGBabadagliMEUludagHBisphosphonate-derivatized liposomes to control drug release from collagen/hydroxyapatite scaffoldsMol Pharm2011841025103421557579
- FicaiAAndronescuEGhitulicaCDFicaiDVoicuGAlbuMGProcess for preparing multifunctional composite materials with possible applicability in the treatment of bone cancer RO 2010 0001171/20101124
- TuJYuMLuYPreparation and antibiotic drug release of mineralized collagen coatings on titaniumJ Mater Sci Mater Med201223102413242322669283
- RobinsonDHMaugerJWDrug delivery systemsAm J Hosp Pharm19914810S14231772110
- SzaszMHajduMPestiNIn Vitro Efficiency of Vancomycin Containing Experimental Drug Delivery SystemsActa Microbiol Immunol Hung201360446146824292089
- ZhangFWuJKangDZhangHBDevelopment of a complex hydrogel of hyaluronan and PVA embedded with silver nanoparticles and its facile studies on Escherichia coliJ Biomat Sci-Polym E20132414101425
- WadajkarASBhavsarZKoCYMultifunctional particles for melanoma-targeted drug deliveryActa Biomater2012882996300422561668
- Ruel-GariepyEShiveMBicharaAA thermosensitive chitosan-based hydrogel for the local delivery of paclitaxelEur J Pharm Biopharm200457536314729080
- NagarajanSReddyBSTsibouklisJIn vitro effect on cancer cells: synthesis and preparation of polyurethane membranes for controlled delivery of curcuminJ Biomed Mater Res A20119941041722021188
- KimSLiuYGaberMWBumgardnerJDHaggardWOYangYDevelopment of chitosan-ellagic acid films as a local drug delivery system to induce apoptotic death of human melanoma cellsJ Biomed Mater Res B Appl Biomater200990114515518985785
- DhanikulaABPanchagnulaRLocalized paclitaxel deliveryInt J Pharm19991838510010361159
- FukushimaSMachidaMAkutsuTRoles of adriamycin and adriamycin dimer in antitumor activity of the polymeric micelle carrier systemColloid Surfaces B199916227236
- FanHYDashAKEffect of cross-linking on the in vitro release kinetics of doxorubicin from gelatin un-plantsInt J Pharm200121310311611165098
- IafiscoMPalazzoBMarchettiMSmart delivery of antitumoral platinum complexes from biomimetic hydroxyapatite nanocrystalsJ Mater Chem20091983858392
- NetzDJASepulvedaPPandolfelliVCPotential use of gelcasting hydroxyapatite porous ceramic as an implantable drug delivery systemInt J Pharm200121311712511165099
- BainoFBiomaterials and implants for orbital floor repairActa Biomater201173248326621651997
- DorozhkinSVCalcium Orthophosphate-Based BioceramicsMaterials2013638403942
- TardeiCSpataruMAlbuFMStoleriuSIonceaAFabrication and Characterization of Porous Tri-Calcium Phosphate Ceramic Micro-spheresRev Rom Mater2013434147 Romanian
- UchidaAShintoYArakiNOnoKSlow Release of Anticancer Drugs from Porous Calcium Hydroxyapatite CeramicJ Orthop Res1992104404451314896
- PalazzoBIafiscoMLaforgiaMBiomimetic hydroxyapatite-drug nanocrystals as potential bone substitutes with antitumor drug delivery propertiesAdv Funct Mater20071721802188
- BarrougAGlimcherMJHydroxyapatite crystals as a local delivery system for cisplatin: adsorption and release of cisplatin in vitroJ Orthop Res200220227428011918306
- IafiscoMMargiottaNSilica xerogels and hydroxyapatite nanocrystals for the local delivery of platinum-bisphosphonate complexes in the treatment of bone tumors: A mini-reviewJ Inorg Biochem201211723724722824154
- NiuXFFengQLWangMBGuoXDZhengQXPorous nano-HA/collagen/PLLA scaffold containing chitosan microspheres for controlled delivery of synthetic peptide derived from BMP-2J Control Release200913411111719100794
- SotomeSUemuraTKikuchiMSynthesis and in vivo evaluation of a novel hydroxyapatite/collagen-alginate as a bone filler and a drug delivery carrier of bone morphogenetic proteinMat Sci Eng C-Bio S200424341347
- VisserRArrabalPMBecerraJRinasUCifuentesMThe effect of an rhBMP-2 absorbable collagen sponge-targeted system on bone formation in vivoBiomaterials200930112032203719155065
- ValimakiVVAroHTMolecular basis for action of bioactive glasses as bone graft substituteScand J Surg2006959510216821652
- PapapoulosSEThe role of bisphosphonates in the prevention and treatment of osteoporosisAm J Med1993955A48S52S8256796
- TitorencuIAlbuMGAntonFGeorgescuAJingaVVCollagen-dexamethasone and collagen-D3 scaffolds for bone tissue engineeringMol Cryst Liq Cryst20125551208217
- ZouQLiYBZhangLZuoYLiJFLiJDAntibiotic delivery system using nano-hydroxyapatite/chitosan bone cement consisting of berberineJ Biomed Mater Res A200989A1108111718767062
- ChenTWangRXuLQNeohKGKangETCarboxymethyl chitosan-functionalized magnetic nanoparticles for disruption of biofilms of Staphylococcus aureus and Escherichia coliInd Eng Chem Res20125113164172
- TanakaKItoAKobayashiTIntratumoral injection of immature dendritic cells enhances antitumor effect of hyperthermia using magnetic nanoparticlesInt J Cancer200511662463315825167
- Di MarcoMSadunCPortMGuilbertICouvreurPDubernetCPhysicochemical characterization of ultrasmall superparamagnetic iron oxide particles (USPIO) for biomedical application as MRI contrast agentsInt J Nanomedicine20072460962218203428
- MahmoudiMSimchiAImaniMMilaniASStroevePOptimal design and characterization of superparamagnetic iron oxide nanoparticles coated with polyvinyl alcohol for targeted delivery and imagingJ Phys Chem B200811246144701448118729404
- AriasJLReddyLHCouvreurPMagnetoresponsive squalenoyl gemcitabine composite nanoparticles for cancer active targetingLangmuir200824147512751918540685
- GuoSLiDZhangLLiJWangEMonodisperse mesoporous superparamagnetic single-crystal magnetite nanoparticles for drug deliveryBiomaterials200930101881188919135248
- AnJYuanXYLuoQZWangDSPreparation of chitosan-graft-(methyl methacrylate)/Ag nanocomposite with antimicrobial activityPolymer International20105916270
- SahniGGopinathPJeevanandamPA novel thermal decomposition approach to synthesize hydroxyapatite-silver nanocomposites and their antibacterial action against GFP-expressing antibiotic resistant E. coliColloids Surf B Biointerfaces201310344144723261564
- SamaniSHossainalipourSMTamizifarMRezaieHRIn vitro antibacterial evaluation of sol-gel-derived Zn-, Ag-, and (Zn + Ag)-doped hydroxyapatite coatings against methicillin-resistant Staphylococcus aureusJ Biomed Mater Res A2013101122223022847909
- TorresNOhSApplefordMDeanDDStability of antibacterial self-assembled monolayers on hydroxyapatiteActa Biomater2010683242325520188873
- NedelcuI-AFicaiASonmezMFicaiDOpreaOAndronescuESilver based materials for biomedical applicationsCurr Org Chem2014184173182
- BagiCMTargeting of therapeutic agents to bone to treat metastatic cancerAdv Drug Deliver Rev2005579951010
- PorterJRRuckhTTPopatKCBone Tissue Engineering: A Review in Bone Biomimetics and Drug Delivery StrategiesBiotechnol Progr20092515391560
- DassCREkETHChoongPFMHuman xenograft osteosarcoma models with spontaneous metastasis in mice: clinical relevance and applicability for drug testingJ Cancer Res Clin Oncol2007133319319817031670
- ChangCHKuoTFLinCCTissue engineering-based cartilage repair with allogenous chondrocytes and gelatin-chondroitin-hyaluronan tri-copolymer scaffold: A porcine model assessed at 18, 24, and 36 weeksBiomaterials2006271876188816278014
- VenkatesanJPallelaRBhatnagarIKimSKChitosan-amylopectin/hydroxyapatite and chitosan-chondroitin sulphate/hydroxyapatite composite scaffolds for bone tissue engineeringInt J Biol Macromol20125151033104222947451
- ItokazuMEsakiMYamamotoKTanemoriTKasaiTLocal drug delivery system using ceramics: vacuum method for impregnating a chemotherapeutic agent into a porous hydroxyapatite blockJ Mater Sci-Mater M199910424925215348159
- ItokazuMKumazawaSWadaEWenyiYSustained release of adriamycin from implanted hydroxyapatite blocks for the treatment of experimental osteogenic sarcoma in miceCancer Lett1996107111188913261
- ItokazuMSugiyamaTOhnoTWadaEKatagiriYDevelopment of porous apatite ceramic for local delivery of chemotherapeutic agentsJ Biomed Mater Res19983945365389492212
- CarrleDBielackSSCurrent strategies of chemotherapy in osteosarcomaInt Orthop20063044545116896870
- LugerNMachDSevcikMMantyhPBone cancer pain: from model to mechanism to therapyJ Pain Symptom Manage200529S324615907645
- FoleyKMAdvances in Cancer PainArch Neurol19995641341710199328
- The Bone and Cancer FoundationManaging Pain Related to Cancer and BoneNew YorkBone and Cancer Foundation2011
- Bone and Cancer FoundationManaging Pain Related to Cancer and BoneNew YorkBone and Cancer Foundation2011
- PharoGHZhouLPharmacologic management of cancer painJ Am Osteopath Assoc2005105S21S2816368904
- NersesyanHSlavinKVCurrent approach to cancer pain management: availability and implications of different treatment optionsTher Clin Risk Manag20073338140018488078
- GrumezescuAMAndronescuEFicaiASynthesis, characterization and biological evaluation of a Fe3O4/C12 core/shell nanosystemLett Appl Nanobioscience201213135
- JadhavSABongiovanniRSynthesis and organic functionalization approaches for magnetite (Fe3O4) nanoparticlesAdv Mater Lett201235356361
- LacroixLMCarreyJRespaudMA frequency-adjustable electromagnet for hyperthermia measurements on magnetic nanoparticlesRev Sci Instrum200879909390919044430
- KowalCDBertinoJRPossible benefits of hyperthermia to chemotherapyCancer Res197939622852289376118
- CampbellJLAroraJCowellSFQuasi-cubic magnetite/silica core-shell nanoparticles as enhanced MRI contrast agents for cancer imagingPLoS One20116e2185721747962
- KawashitaMKawamuraKLiZPMMA-based bone cements containing magnetite particles for the hyperthermia of cancerActa Biomater2010683187319220197125
- LiZKawamuraKKawashitaMKudoTAKanetakaHHiraokaMIn vitro assessment of poly(methylmethacrylate)-based bone cement containing magnetite nanoparticles for hyperthermia treatment of bone tumorJ Biomed Mater Res A2012100102537254522528664
- TampieriAD’AlessandroTSandriMIntrinsic magnetism and hyperthermia in bioactive Fe-doped hydroxyapatiteActa Biomater20128284385122005331
- SinghRKEl-FiqiAMPatelKDKimHWA novel preparation of magnetic hydroxyapatite nanotubesMater Lett201275130133
- GopiDAnsariMTShinyjoyEKavithaLSynthesis and spectroscopic characterization of magnetic hydroxyapatite nanocomposite using ultrasonic irradiationSpectrochim Acta A Mol Biomol Spectrosc20128724525022177219
- MurakamiSHosonoTJeyadevanBKamitakaharaMIokuKHydrothermal synthesis of magnetite/hydroxyapatite composite material for hyperthermia therapy for bone cancerJ Ceram Soc Jpn20081161357950954
- SinghRKSrinivasanAKothiyalGPEvaluation of CaO-SiO2-P2O5-Na2O-Fe2O3 bioglass-ceramics for hyperthermia applicationJ Mater Sci Mater Med200920Suppl 1S147S15118560766
- BretcanuOVerneEBioactive ferrimagnetic glass-ceramics for magnetic induction hyperthermiaNanoScience and Technology InstituteTechnical Proceedings of the 2005 NSTI Nanotechnology Conference and Trade ShowBoca Raton (Fl)CRC2005266269
- BehereiHHAbdel-AalMSShaltoutAAEl-MagharbyABiophysiochemical characterization of anticancer nano-ceramic polymer scaffold for bone graftingPharma Chem201241544551
- KokuboTPreparation and properties of composite ceramics for biomedical applicationsJ Jpn Soc Powder Powder Metall199037324328 Japanese
