Abstract
Currently, synthetic hydroxyapatite nanoparticles (HANPs) are used in nanomedicine fields. The delivery of nanomedicine to the bloodstream exposes the cardiovascular system to a potential threat. However, the possible adverse cardiovascular effects of HANPs remain unclear. Current observations using coculture models of endothelial cells and monocytes with HANPs to mimic the complex physiological functionality of the vascular system demonstrate that monocytes could play an important role in the mechanisms of endothelium dysfunction induced by the exposure to HANPs. Our transmission electron microscopy analysis revealed that both monocytes and endothelial cells could take up HANPs. Moreover, our findings demonstrated that at a subcytotoxic dose, HANPs alone did not cause direct endothelial cell injury, but they did induce an indirect activation of endothelial cells, resulting in increased interleukin-6 production and elevated adhesion molecule expression after coculture with monocytes. The potential proinflammatory effect of HANPs is largely mediated by the release of soluble factors from the activated monocytes, leading to an inflammatory response of the endothelium, which is possibly dependent on p38/c-Jun N-terminal kinase, and nuclear factor-kappa B signaling activation. The use of in vitro monocyte–endothelial cell coculture models for the biocompatibility assessment of HANPs could reveal their potential proinflammatory effects on endothelial cells, suggesting that exposure to HANPs possibly increases the risk of cardiovascular disease.
Introduction
Synthetic hydroxyapatite (HA) (Ca10[PO4]6[OH]2), a typical bioceramic with good osteoconductive and osteoinductive capabilities, has been used clinically for many years.Citation1 Currently, nanotechnology has entered the field of biomaterials, resulting in the rapid development of nano-based HA. Due to their better bioactivity, their excellent capacity to penetrate cell membranes, and their increased circulation time, HA nanoparticles (HANPs) have gradually garnered significant interest in various medical fields, such as bone tissue engineering, cardiovascular graft coating, contrast agent synthesis, drug delivery, and gene therapy.Citation2–Citation5 In these cases of therapeutic and diagnostic application, HANPs may become systemically available, which increases the risk of their exposure to the blood vessels. It has been proposed that atherosclerotic complications may occur with the continued use of HANPs.Citation3,Citation6 Thus, in terms of human health, the main potential adverse effect of HANPs on the cardiovascular system needs to be carefully assessed before reaching the clinical application stage.
It is well known that synthetic nanoparticles (NPs) can enter the vascular system intentionally by injection in the form of nanomedicines or nanodiagnostics.Citation7 Accordingly, endothelial cells (ECs), which form the inner cellular lining of the entire cardiovascular system, have direct contact with these NPs. In addition to ECs, NPs may simultaneously encounter circulating immune cells upon introduction into the blood circulation. Monocytes/macrophages are the body’s first line of defense and are recognized as important contributors to atherosclerosis via interactions with ECs. Thus, considering the fundamental role exerted by both ECs and monocytes in cardiovascular events, to understand the interaction of monocytes and ECs, both with each other and with NPs, it is very important to assess NP-induced cardiovascular effects. In recent years, several toxicology studies have demonstrated that diesel particulates can indirectly activate ECs via exposure to macrophages, with even more profound effects than those generated by direct exposure to ECs.Citation8–Citation10 Our current studies also found that silica NPs could significantly augment proinflammatory and procoagulant responses in ECs through monocyte–EC interactions.Citation11 However, to the best of our knowledge, most studies are still focused on the direct biological response of engineered HANPs to monocultures of cells in the endothelium or the immune system;Citation5,Citation12–Citation18 as such, the coculture of monocytes and ECs with HANPs has received little attention in this regard.
In this study, to model an in vivo vascular microenvironment when HANPs enter into systemic circulation, we established an in vitro coculture model using THP-1 cells (monocytes) and human umbilical vein ECs (HUVECs). By utilizing this in vitro system, we investigated both the direct and the integrated/indirect effects of NPs on ECs in the presence or absence of THP-1 cells to evaluate the potential cardiovascular toxicity of HANPs. First, HANPs were synthesized, and their physiochemical characteristics were studied. Subsequently, HANP biocompatibility was assessed at the level of specific features, including cell viability, cellular uptake, cytokine production, and cell adhesion molecule (CAM) expression in HUVECs and THP-1 cells in monoculture and in HUVECs/THP-1 cells cocultured with HANPs. Moreover, the proinflammatory effects of HANPs on ECs were compared following their exposure to direct contact with the coculture, and after exposure to monocyte-derived soluble factors. Finally, to investigate the signaling pathway activated by HANPs in ECs cocultured with THP-1 cells, we measured the stimulation of mitogen-activated protein kinases (MAPKs) and nuclear factor-kappa B (NF-κB) in HUVECs.
Materials and methods
Preparation and characterization of HANPs
HANPs were synthesized using a chemical precipitation method according to our previously published procedures.Citation19 Briefly, the pH of a Ca(NO3)2 solution was adjusted to 12 by adding a concentrated ammonia solution, and an (NH4)2HPO4 solution (pH =12) was added dropwise during vigorous stirring. A voluminous precipitate was formed. The reaction mixture was gently boiled for 10 minutes. The precipitate was allowed to settle, and it was subsequently separated from the supernatant by centrifugation. The HA precipitate was washed with distilled water, frozen at −20°C, and lyophilized. The size and shape of the HANPs were examined using transmission electron microscopy (TEM) (JEOL, Tokyo, Japan). The structure of HANPs was examined by X-ray diffraction (XRD) using a RINT2000 vertical goniometer (Rigaku Corporation, Tokyo, Japan). The powder was examined with Ni filtered CuKα radiation generated at 40 kV and 100 mA. The powders were scanned from 10°–80° 2θ with a scan speed of 2° per minute. The peak of the obtained XRD pattern was compared with standard HA in the Joint Committee on Power Diffraction Standards file available in software (No 09-0432). Energy-dispersive X-ray spectroscopy can be used to find the chemical composition of materials. Thus, for the elemental analysis, a Hitachi S-2600N-type scanning electron microscope (Hitachi Ltd, Tokyo, Japan) was equipped with an energy dispersive X-ray attachment (EDAX Inc., Mahwah, NJ, USA) to create element composition spectra. The hydrodynamic size and surface charge (zeta potential) of the HANP dispersions were characterized using the Zetasizer Nano ZS (Malvern Instruments, Malvern, UK) utilizing dynamic light scattering and electrophoretic light scattering, respectively. The specific surface area of the HANPs was determined by the Brunauer– Emmett–Teller method using a surface area analyzer (ASAP 2020; Micromeritics Instrument Corporation, Norcross, GA, USA) after pre-preparation of samples by heating at 200°C in a stream of N2 in excess of 24 hours.
Endotoxin measurement
The endotoxin content in the HANP dispersions was tested using the Limulus amebocyte lysate kinetic chromogenic assay (Sigma-Aldrich, St Louis, MO, USA). The endotoxin content in the HANP dispersions used in the study was below 1 EU/mL.
Cell preparation and culture
HUVECs were isolated from freshly obtained human umbilical cord using our previously published method.Citation20 Briefly, the umbilical vein was rinsed three times with phosphate buffered saline (PBS) containing 100 μg/mL of penicillin/streptomycin (Gibco®; Life Technologies, Carlsbad, CA, USA), filled with 0.1% collagenase I (Sigma-Aldrich) and incubated for 15 minutes at 37°C. After harvesting, the ECs were placed in 75 cm2 tissue culture flasks (Corning Incorporated, Corning, NY, USA) and grown in EC medium (ScienCell Research Labs, Carlsbad, CA, USA). HUVECs between the third and sixth passages were used in our experiments. The phenotype of the ECs was confirmed by performing immunofluorescence using monoclonal antibodies for the von Willebrand factor (vWF) (ShangHai ChangDao Biotech Co, Ltd, Shanghai, People’s Republic of China).
Human monocytes (THP-1 cells) were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, People’s Republic of China) and were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco®; Life Technologies) supplemented with 10% fetal bovine serum (Biochrom AG, Berlin, Germany) and 100 μg/mL of penicillin/streptomycin.
For the coculture of monocytes and HUVECs, THP-1 cells (1 × 106 cells/mL) were added onto confluent HUVEC layers (5 × 105 cells/mL) in six-well plates. Contact cocultures were performed in the presence or absence of HANPs for 24 hours. Meanwhile, to prevent direct cell contact between monocytes and HUVECs, at the end of the 24-hour incubation, the supernatants were collected, cleared of cells by centrifugation, and transferred to stimulate the HUVECs or monocytes for 24 hours and cultured in parallel.
Cell viability assays
Cell viability was measured by assessment of mitochondrial function using the CellTiter 96® AQueous One Solution assay (Promega Corporation, Madison, WI, USA). The solution reagent contains a tetrazolium compound (3-[4, 5-dimethylthiazol-2-yl]-5-[3-carboxymethoxyphenyl]-2-[4-sulfophenyl]-2H-tetrazolium, inner salt; MTS) and an electron coupling reagent (phenazine ethosulfate). After seeding the cells for 24 hours, differing concentrations of HANPs were added to the 96-well plates. The cultures were further incubated for 24 hours, and 20 μL of MTS agent was then directly added to each well. After a 4-hour incubation period, the absorbance of formazan was measured at 490 nm using a microplate reader (Wellscan MK3, Labsystems Dragon, Inc., Helsinki, Finland).
Particle uptake
To determine the cellular uptake and localization of the particles, HUVECs and THP-1 cells were individually exposed to HANPs (100 μg/mL) for 24 hours and analyzed by TEM. After incubation for 24 hours with HANPs, the cells were washed with a PBS solution, trypsinized, and centrifuged. Next, the cell pellets were fixed in a 0.1 M PBS solution containing 2.5% glutaraldehyde for 4 hours. The cells were dehydrated through a series of ethanol washes (70% for 15 minutes, 90% for 15 minutes, and 100% for 15 minutes, twice) and embedded in Epon–Araldite resin (polymerization at 65°C for 15 hours). Thin sections containing the cells were placed on the grids and stained for 1 minute each with 4% uranyl acetate (in acetone: water, 1:1) and 0.2% Reynolds’ lead citrate (in water), air-dried, and imaged under TEM.
Cytokine measurement
For cytokine analysis (interleukin [IL]-6, IL-8, IL-1β, and tumor necrosis factor-alpha [TNF-α]), the supernatants of HUVECs or THP-1 cells in cocultures or monocultures exposed to HANPs (100 µg/mL) were collected after 24 hours, immediately centrifuged to remove the cells, and then frozen at −80°C until the analysis was performed. The amounts of IL-6, IL-8, IL-1β, and TNF-α were quantified with an immunoassay kit (R&D Systems, Inc., Minneapolis, MN, USA) according to the manufacturer’s instructions.
Immunofluorescence flow cytometry
The levels of surface markers expressed on HUVECs were assessed using flow cytometry. After 24 hours of coculture or monoculture in the absence or presence of HANPs (100 μg/mL), HUVECs were released from the wells after washing with PBS. The following mouse antihuman monoclonal antibodies were used: intercellular adhesion molecule (ICAM-1) (cluster of differentiation [CD]54-PE; eBioscience, Inc., San Diego, CA, USA); vascular CAM-1 (VCAM-1) (CD106 fluorescein isothiocyanate isomer 1; BD Biosciences, San Jose, CA, USA); E-selectin (CD62E-APC; BD Biosciences); and vWF (BD Biosciences). Subsequently, HUVECs were collected and labeled with the abovementioned specific antibodies at room temperature for 45 minutes in the dark, washed extensively, and then subsequently fixed with 1% paraformaldehyde. All samples were analyzed using a FACScan™ flow cytometer (BD, Franklin Lakes, NJ, USA). The data were analyzed with CellQuest™ software (BD Biosciences).
Western blot analysis
Protein expression was determined using Western blot. Briefly, following the incubation periods, HUVECs were washed once with ice-cold PBS and lysed in ice-cold radioimmunoprecipitation assay buffer (Applygen Technologies Inc., Beijing, People’s Republic of China) containing 1 mM of phenylmethylsulfonyl fluoride (Sigma-Aldrich) and a phosphatase inhibitor cocktail (Sigma-Aldrich) for 30 minutes. After centrifuging the lysates at 12,000 rpm at 4°C for 10 minutes, the supernatants were collected and stored at −80°C for future use. The protein concentrations of these extracts were determined by performing a bicinchoninic acid protein assay (Thermo Fisher Scientific, Waltham, MA, USA). Equal amounts of protein samples (40 μg) were separated using 10% sodium dodecyl sulfate– polyacrylamide gels (SDS-PAGE) and subsequently transferred onto nitrocellulose membranes (Amersham plc, Amersham, UK). Membranes were blocked with 5% nonfat milk buffer and incubated with anti-p-p38, anti-p-p-c-Jun N-terminal kinase (JNK), anti-JNK (1:1,000, rabbit polyclonal antibodies; Bioworld Technology, St Louis Park, MN, USA), anti-p-38 (1:1,000, rabbit polyclonal antibodies; Cell Signaling Technology, Inc., Danvers, MA, USA), and anti-β-actin (1:1,000, a mouse polyclonal antibody), (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight, washed with Tris-buffered saline and incubated with a horseradish peroxidase-conjugated antirabbit immunoglobulin (Ig)G/antimouse IgG secondary antibody at 37°C for 1 hour. The antibody-bound proteins were detected using an enhanced chemiluminescence reagent (EMD Millipore, Billerica, MA, USA). Quantification of the Western blot and electrophoretic mobility shift assay (EMSA) data were performed by measuring the intensity of the band using the ImageJ analysis program (Image J, NIH).
Electrophoretic mobility shift assay (EMSA)
EMSA was used to assess the NF-κB activation of HUVECs. Briefly, following the incubation periods, HUVEC nuclear extracts were prepared using Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific). The protein concentration of each nuclear extract was quantified using the bicinchoninic acid protein assay. Ten micrograms of nuclear protein was incubated in binding buffer containing 50 ng/μL of poly (dI · dC), 2.5% glycerol, 0.05% NP-40, 5 mM of MgCl2, and 20 fmol of Biotin end-labeled oligonucleotides at room temperature for 20 minutes. The labeled oligonucleotides had the following sequences: 5′-AGTTGA GGG GAC TTT CCC AGG C-3′ and 5′-GCC TGG GAA AGT CCC CTC AAC T-3′. Protein– deoxyribonucleic acid (DNA) complexes were separated from the free DNA probe via electrophoresis through 4% native polyacrylamide gels. Gels were dried, and protein–DNA complexes were then visualized using enhanced chemiluminescence.
Statistical analysis
The data are expressed as the means ± standard deviation or means ± standard error of the mean (SEM). Statistical comparisons of the means were performed using one-way analysis of variance with SAS 6.12 software (SAS Institute Inc., Cary, NC, USA). The differences were considered to be significant when the P-value was <0.05.
Results
Characterization and dispersion of HANPs
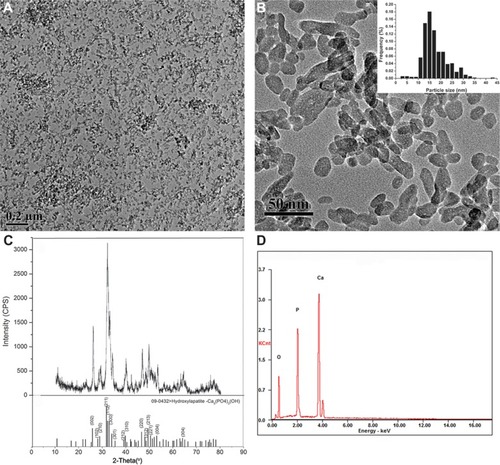
In this study, we investigated the main physicochemical properties of our synthetic HANPs. TEM analyses revealed that the HANPs were near spherical, or they exhibited slightly elongated shapes (an average of 15 nm in diameter). The size and morphology of HANPs are illustrated in . The XRD pattern of the HANPs showed that the structure of the prepared sample was similar to that of the HA standard, as shown in . Our data of the HANPs are highly consistent with the standard HA database (powder diffraction file no 09-0432), indicating that the chief inorganic phase of our synthetic NPs is an HA crystal. Furthermore, our energy-dispersive X-ray spectroscopy data show that the Ca/P ratio in our samples is close to 1.67 which is similar to the theoretical value in HA, suggesting our synthetic HAP is of high purity (). Surface charge and hydrodynamic diameter are two important properties of NP dispersions in aqueous systems. For the remainder of the study, NPs were characterized in complete cell culture medium (1:1 mixture of EC medium and RPMI 1640 medium). The hydrodynamic size of the NPs, as measured by dynamic light scattering, was approximately 248 nm, revealing that the NPs were well dispersed in the cell culture medium (). The zeta-potential of the NPs was determined and displayed a positive potential (−8.89 mV) in cell culture medium, indicating that the NPs presented a negative surface charge. Additionally, to examine the surface property of the HANPs, we estimated the Brunauer–Emmett–Teller-specific surface area. As shown in , the specific surface area of our prepared HANPs was 109 m2/g, which was significantly larger than the commercial HANPs measured by other researchers (7.4 m2/g),Citation15 indicating that the HANPs have a higher biological reactivity.Citation21–Citation23
Table 1 Characterization of the particle parameters of HANPs
Figure 1 Characterization of hydroxyapatite nanoparticles.
Notes: (A and B) Transmission electron microscopy image; inset: histogram showing size distribution; about 500 NPs were considered for the sample to obtain the size distribution histogram. (C) X-ray diffraction patterns. (D) Energy-dispersive X-ray spectroscopy spectra.
Abbreviations: CPS, counts per second; KCnt, 1,000 counts; NPs, nanoparticles.
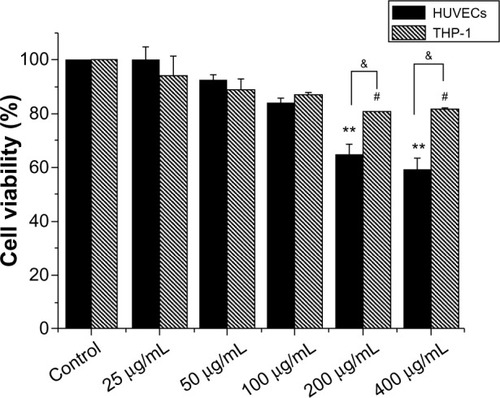
Effects of direct HANP exposure on HUVECs and THP-1 cell viability
To determine the effects of HANPs on HUVEC and THP-1 cell viability in vitro, mitochondrial function was measured using the MTS assay after culturing with serial dilutions (25 μg/mL, 50 μg/mL, 100 μg/mL, 200 μg/mL, and 400 μg/mL) of HANPs for 24 hours. As depicted in , both HUVEC and THP-1 cell viability decreased in relation to increasing concentrations of HANPs. At concentrations of 100 μg/mL or below, no significant differences were observed in the cell viability between HANP-stimulated cells and untreated cells (P>0.05). After treatment with HANPs at 200 μg/mL and 400 μg/mL, HUVEC viability dramatically decreased to 65% and 59%, respectively. Similarly, at 200 μg/mL and 400 μg/mL, HANPs also caused a significant inhibition of mitochondrial function in THP-1 cells (P<0.05).
Figure 2 Cytotoxicity of hydroxyapatite nanoparticles to HUVEC and THP-1 cells.
Notes: Cells were exposed to increasing doses of hydroxyapatite nanoparticles for 24 hours, and cytotoxicity was determined by the MTS assay. Normal HUVECs or THP-1 cells without nanoparticle treatment served as control. Results are presented in mean ± standard error of the mean of the three independent experiments; each was carried out in triplicate. #P<0.05; **P<0.01 versus control; &P<0.05 significant difference between the compared groups.
Abbreviations: HUVECs, human umbilical vein endothelial cells; MTS, 3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium.
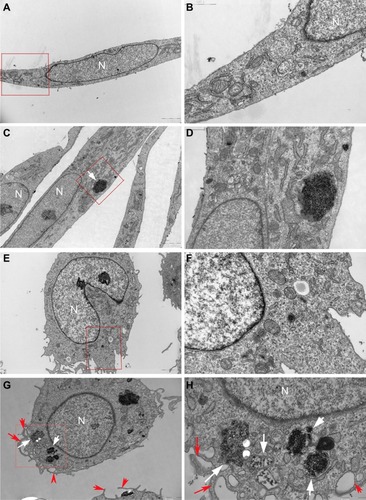
Cellular uptake and localization of HANPs
It is known that cellular uptake is not only a critical step in gene carrier or intracellular drug delivery, but it is also directly related to the biological impact of NPs. To follow the internalization and intracellular localization of HANPs, HUVECs and THP-1 cells were exposed to the HANPs at a low toxic dose (100 μg/mL) and were then examined using TEM. As shown in , most of the HANPs in HUVECs and THP-1 cells were confined to vesicles and lysosomes within the cytoplasm and did not enter the nucleus. Moreover, based on the imaging, HUVECs retained their cellular morphology without damage following the uptake of HANPs. In contrast, in HANP-stimulated THP-1 cells, several pseudopodia and large vacuoles appeared, indicating the activated state of the cell.
Figure 3 Uptake of HANPs by HUVEC and THP-1 cells.
Notes: TEM micrographs of cells exposed for 24 hours to HANPs. (A and B) HUVECs without any treatment; (C and D) HUVECs treated with HANPs; (E and F) THP-1 cells without any treatment; (G and H) THP-1 cells treated with HANPs. (A, C, E and G) Overall cell morphology (scale bar: 2 μm). (B, D, F and H) Higher magnification of cells in red boxed areas (scale bar: 1 μm). White arrows denote nanoparticles. Red arrows indicate the protrusion of the plasma membrane for phagocytosis.
Abbreviations: N, nucleus; HANPs, hydroxyapatite nanoparticles; HUVECs, human umbilical vein endothelial cells; TEM, transmission electron microscopy.
Cytokine production by HANPs in monoculture and cocultures
To obtain a more realistic assessment of the effects of HANPs on the human vascular system, this study simulated the human vasculature using a coculture model composed of ECs and monocytes. A proinflammatory response was first investigated after the cocultures were exposed to HANPs for 24 hours. Responses were compared with those of HUVECs or monocyte monocultures at the same condition. Four patterns of cytokine release (TNF-α, IL-1β, IL-6, and IL-8) were observed. As shown in , THP-1 cells exposed to HANPs exhibited significant increases in TNF-α (up to 15-fold) and IL-1β (up to twofold) in both monoculture and cocultures; however, cocultures with HUVECs had no appreciable effects on the levels of TNF-α and IL-1β compared with monocultures stimulated with HANPs (P>0.05). Moreover, in HUVECs exposed to HANPs, no relevant increases were observed for any cytokine, whereas in the cocultures of HUVECs/THP-1 cells with HANPs, a strong increase of IL-6 production was induced ().
Figure 4 Proinflammatory cytokine production induced by HANPs in mono- and cocultures.
Notes: (A) TNF-α release; (B) IL-1β release; (C) IL-6 release; (D) IL-8 release. Normal HUVECs or THP-1 cells or cocultures without NPs treatment served as control. Results are presented as the mean ± standard error of the mean of three independent experiments, each of which was carried out in triplicate. *P<0.05; **P<0.01 versus control; &P<0.05 significant difference between compared groups.
Abbreviations: TNF-α, tumor necrosis factor-alpha; EC, endothelial cells; HANPs, hydroxyapatite nanoparticles; (HANPs+THP-1)s+EC, human umbilical vein endothelial cells treated with supernatants of hydroxyapatite nanoparticle-stimulated THP-1 cells; (HANPs+ECs)+THP-1, THP-1 cells treated with the supernatants of hydroxyapatite nanoparticle-stimulated human umbilical vein endothelial cells; IL, interleukin; NPs, nanoparticles.
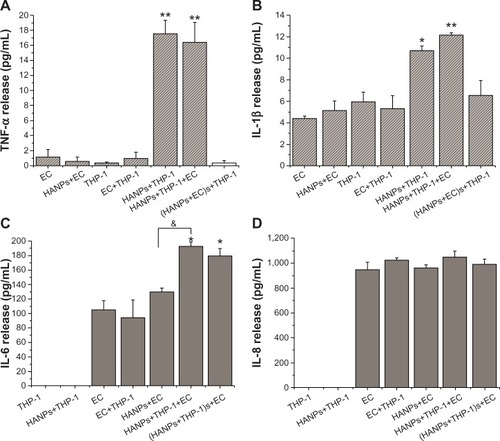
To clarify whether cell-derived soluble factors induced by HANPs are responsible for secondary proinflammatory cytokine release in cocultures, HUVECs and THP-1 cells were pretreated with HANPs for 24 hours, and the cell-free supernatant was collected to stimulate another cell type for 24 hours. As shown in , the supernatants of HANP-stimulated THP-1 cells exhibited a significant increase in IL-6 release from HUVECs, whereas the supernatants of HANP-stimulated HUVECs revealed no effects on TNF-α and IL-1β release from the THP-1 cells, suggesting that HANPs might cause an indirect activated effect on HUVECs by monocyte-derived soluble factors.
Endothelial adhesion molecule expression induced by HANPs in monoculture and cocultures
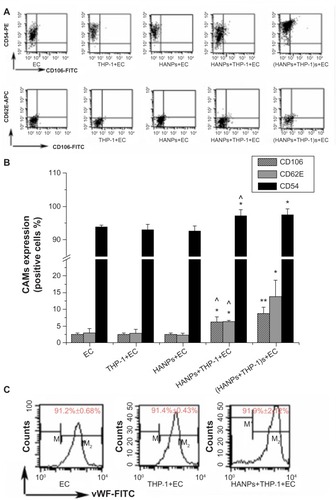
To further evaluate EC activation in HANP-stimulated cocultures, endothelial adhesion molecule expression (CD106, CD54, and CD62E) was measured using flow cytometry. As shown in , HANPs alone did not induce CD54, CD106, or CD62E expression; however, when cocultured with THP-1 cells, HANPs significantly increased the percentage of HUVECs that expressed CD54, CD106, and CDD62E. In addition, the supernatants of HANP-stimulated monocytes also increased the expression of CD54, CD106, and CD62E. To investigate whether HUVEC monolayers in HANP-stimulated coculture would become more adherent for leukocytes, flow cytometry was performed to examine the vWF expression of HUVECs. As shown in , the vWF expression of separated HUVECs from the HANP-treated coculture was decreased to 91.9%±2.12%, slightly lower than the untreated HUVECs (99.2%±0.69%) (P<0.05), indicating that there might be some monocyte adhesion to HUVECs.
Figure 5 CAMs expression induced by HANPs in HUVECs in mono- and cocultures.
Notes: (A) Dot-plot of flow cytometry analysis showing the expressions of CD54, CD106, and CD62E on HUVECs in mono-and cocultures exposed to HANPs for 24 hours. (B) Bar graph shows the percentage of CD54-, CD106-, and CD62E-positive cells in NP-stimulated HUVECs in monoculture or in coculture. (C) The vWF expression on HUVECs in HANP-stimulated cocultures. Normal HUVECs served as the negative control. Data represent the means ± standard error of the means; n=3. *P<0.05; **P<0.01 versus control; ^P<0.05 versus HUVECs with HANPs.
Abbreviations: CD, cluster of differentiation; EC, endothelial cells; FITC, fluorescein isothiocyanate isomer 1; HANPs, hydroxyapatite nanoparticles; vWF, von Willebrand factor; CAMs, cell adhesion molecules; HUVECs, human umbilical vein endothelial cells; NP, nanoparticle; n, number.
Involvement of p38, JNK, and NF-κB in the activation of HUVECs in HANP-stimulated cocultures
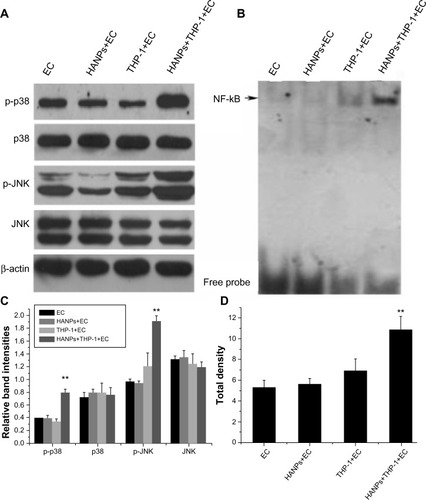
To clarify the possible signaling pathway of the proinflammatory effects of HANPs on HUVECs in coculture, the activation levels of JNK, p38, and NF-κB were detected in HANP-stimulated cocultures. As depicted in , the cocultures had significantly elevated JNK and p38 MAPK phosphorylation levels after treatment with HANPs when compared with ECs cultured alone. Similarly, NF-κB was significantly activated in HANP-stimulated HUVEC cocultures compared with HUVECs in monoculture ().
Figure 6 The activation of JNK, p38, and NF-κB by HANPs in HUVECs in mono- and cocultures.
Notes: (A) The activation of JNK and p38 by HANPs in HUVECs in mono- and cocultures. Aliquots of cell lysates were separated by SDS-PAGE and analyzed for protein expression by Western blotting, as described in section 2. β-actin was used as an internal control to monitor for equal loading. (B) The activation of NF-κB by HANPs in HUVECs in mono- and cocultures. (C) The relative density of the bands normalized to beta-actin by gray value analysis (β-actin as control); (D) The total density of the NF-κB band by gray value analysis. NF-κB DNA-binding activity was assayed by electrophoretic mobility shift assay as described in the Materials and methods section. **Represents a statistically significant difference from EC (P<0.01).
Abbreviations: JNK, c-Jun N-terminal kinase; EC, endothelial cells; HANPs, hydroxyapatite nanoparticles; NF-κB, nuclear factor-kappa B; HUVECs, human umbilical vein endothelial cells; SDS-Page, sodium dodecyl sulfate polyacrylamide gel electrophoresis.
Discussion
Recently, more efforts have been focused on the possibility of utilizing synthetic HANPs for multiple applications, such as magnetic resonance imaging, antimicrobial treatments, drug delivery, and gene transfection; however, their biocompatibility and toxicity remain unclear.Citation3,Citation6 A major limitation of in vitro cell models in investigations of NP-induced biological effects is that common monoculture systems do not represent a realistic model of how NPs will interact with a specific organ of the body due to the lack of cellular interactions and extracellular signal communications that are important in an in vivo situation.Citation24 The present study using coculture models of ECs and monocytes with HANPs to mimic the complex physiological functionality of the vascular system demonstrate that monocytes could play an important role in the mechanisms of endothelium dysfunction induced by HANP exposure. According to our findings, at a subcytotoxic dose, direct HANP exposure did not cause EC injury, but when cocultured with THP-1 cells, we observed a significant inflammatory activation of HUVECs. This process is possibly dependent on the action of monocyte-derived soluble factors via p38/JNK MAPK and NF-κB activation. Thus, in the present study, we observed a series of cellular and molecular interactions initiated by HANPs between monocytes and ECs that resulted in an inflammatory cascade through a complex network of pathways.
In this study, HUVECs and THP-1 cells were used to evaluate the cytotoxic effect of HANPs by the MTS assay. ECs and monocytes were selected because NPs intravenously administered into the systemic circulation are the most common route of exposure to nanomedicine. At concentrations of 200 μg/mL and greater, HANPs became cytotoxic in both cell types (). Exposure to a subcytotoxic dose can help identify potential health effects of NPs other than those due to cytotoxicity;Citation25 thus, we chose to work with a lower particle concentration in the following studies (100 μg/mL). Under these conditions, neither cell type exhibited dramatic cell death after a 24-hour treatment with NPs. Given that it has been demonstrated that the cellular uptake of NPs plays a pivotal role in the alteration of various cellular functions, such as cell viability and the inflammatory response,Citation26 the internalization and intracellular localization of HANPs were subsequently examined by TEM. In this assay, different cellular uptake behaviors were observed in HUVECs and THP-1 cells after treatment with HANPs for 24 hours (). In both cell lines, the HANPs were tightly packed and largely confined to spherical vesicles resembling lysosomes within the cytoplasm. Importantly, after HANP uptake, HUVECs did not display a change of morphology (–). In contrast, a prominent change in THP-1 cells was observed, which included increased phagocytic activity, as demonstrated by large vacuoles and multiple pseudopodia of the plasma membrane, suggesting the potential immune activation of these particular cells (–).
Cytokines are important regulators of immune, inflammatory, and vascular reactions, and they play critical roles in cardiovascular disease.Citation27 Among these cytokines, TNF-α and IL-1β are generally markers of monocyte activation. Moreover, IL-6 is clinically considered a bio-marker of endothelial dysfunction and an independent risk factor for atherosclerosis.Citation28,Citation29 As a next step, to further investigate the impact of NP uptake on the cell activation status, we measured the concentrations of TNF-α, IL-1β, IL-6, and IL-8 present in HUVECs and THP-1 cell supernatants after direct exposure to HANPs. These experiments demonstrated that increased levels of TNF-α and IL-1β were released in THP-1 cells after treatment with HANPs (), whereas no proinflammatory cytokines were released in HUVECs alone ().
Since the interaction of the monocytes and ECs that line the blood vessels is a critical process in vascular homeostasis and in pathological conditions of inflammation or thrombosis, the monocyte–EC coculture model is frequently used to assess the biocompatibility of synthetic materials, such as vascular grafts, artificial hearts, and coronary-artery stents for vascular application.Citation30–Citation34 Recently, some researchers have attempted to use an in vitro coculture model of immune cells and ECs to evaluate the inflammatory potential of nanomaterials, and they found that exposure to these NPs (for example, titanium dioxide [TiO2] or silicone dioxide [SiO2]) in direct-contact cocultures led to dramatically elevated levels of proinflammatory cytokines.Citation10,Citation35 Here, we found that the coculture of HUVEC/THP-1 cells resulted in an approximately twofold increase in IL-6 concentrations compared with the individual cell types alone (). Separating the two cell types and treating HUVECs with conditioned media from HANP-exposed THP-1 cells also significantly increased the release of IL-6, indicating that the induction of IL-6 in HUVECs may be largely dependent on the HANP-activated monocytes (). Previous studies have reported that noncontact cocultures of ECs and macrophages/monocytes strongly induced more potent inflammatory cytokine release in ECs upon silica particle exposure compared with monocultures alone.Citation36,Citation37 However, coculture or treatment with the supernatants from HANP-stimulated HUVECs did not increase the levels of TNF-α and IL-1β in THP-1 cells when compared with the THP-1 cells directly exposed to HANPs, indicating that the HANP-stimulated ECs were relatively ineffective on monocyte activation ().
When undergoing activation, apart from IL-6, ECs also express various CAMs, such as ICAM-1 (CD54), VCAM-1 (CD106), and E-selectins (CD62E). Generally, ICAM-1 is constitutively present in ECs, and its expression is increased by proinflammatory stimuli.Citation38 VCAM-1 and E-selectin are not routinely expressed under physiologic conditions, but they can be induced in activated endothelium. The expression of VCAM-1 and E-selectin is important in the initial steps of monocyte recruitment to atherosclerotic lesions and in the rolling of monocytes on the endothelial surface, whereas ICAM-1 is involved in the firm adhesion step in leukocyte infiltration.Citation39,Citation40 CAM expression and established markers of endothelial dysfunction have been clinically considered to be associated with cardiovascular risk factors, and they may predict the development of cardiovascular disease.Citation41 Thus, to further verify the contribution of monocytes in EC activation induced by HANP exposure, CAM expression in HUVECs was measured using flow cytometry. Consistent with proinflammatory cytokine results after exposure to HANPs, cocultures of ECs and monocytes had significantly greater increased levels of VCAM-1, E-selectin and ICAM-1 expression compared with HUVECs cultured alone. vWF is considered to be a classic marker of ECs, because it has been found only in ECs, megakaryocytes, and platelets.Citation42 Thus, we assessed the THP-1 cells’ adhesion to HUVECs by cell- specific surface markers (vWF). Our data showed that the vWF expression of separated HUVECs from the HANP-treated coculture was slightly lower than that of the HUVECs from the untreated coculture (P<0.05), indicating that HANPs may elicit an attachment of monocytes to HUVECs. Additionally, HUVECs incubated with supernatants of HANP-stimulated monocytes expressed levels of VCAM-1, E-selectin, and ICAM-1 that were similar to those generated by the direct-contact coculture system, suggesting that monocyte-derived soluble factors were responsible for the activation of ECs (). Among the soluble mediators possibly involved, TNF-α and IL-1β, which are primarily produced by monocytes and are well-known inducers of EC activation, were good candidates.Citation43 Taken together, these results suggest that direct exposure to HANPs could result in TNF-α and IL-1β production in THP-1 cells and that both cytokines, once released, might stimulate IL-6 generation and CAM expression by HUVECs. These results were consistent with those from previous studies that demonstrated that the release of TNF-α and IL-1β from particle-activated monocytes/macrophages might be the critical determinants for increased synthesis of IL-6 or CAM expression in ECs.Citation7,Citation9,Citation36
It is a widely accepted view that intracellular MAPK signaling cascades probably play an important role in the pathogenesis of cardiovascular disease.Citation39 To clarify the possible signaling pathway of the proinflammatory effects of HANPs on HUVECs in coculture, we detected the activation levels of JNK, p38, and NF-κB. p38 and JNK belong to the MAPK superfamily and are stress-activated serine/threonine protein kinases with major functions in apoptosis, cytokine production, transcriptional regulation, and inflammation.Citation44 Various inflammatory cytokines are capable of activating p38 and JNK MAPK, which has been shown to regulate inflammation and induce endothelial dysfunction.Citation45,Citation46 In addition, NF-κB is also a critical transcription factor involved in the transcription of proinflammatory cytokines and the expression of CAMs, such as E-selectin, VCAM-1, and ICAM-1.Citation47,Citation48 Our results clearly demonstrated that HANPs alone could not induce JNK, p38, or NF-κB activation in HUVECs; however, HANP-stimulated cocultures significantly elevated the phosphorylation levels of p38/JNK MAPK and the activity of NF-κB, suggesting that the activation of JNK, p38, and NF-κB in ECs by exposure to HANPs required the involvement of monocytes. It has been demonstrated that IL-1β mainly induces proinflammatory cytokine release, such as IL-6 gene expression in HUVECs, which is a p38/JNK-dependent process.Citation49 However, TNF-α more profoundly affected the expression of CAMs via the NF-κB pathway.Citation49 TNF-α and IL-1β release by HANP-stimulated monocytes may induce p38/JNK MAPK and NF-κB activation in HUVECs, suggesting that HANPs can indirectly induce ECs’ inflammatory response by activating monocytes. However, in this study, although we have found that HANPs could directly induce TNF-α and IL-1β release from monocytes, and that these cytokines released by HANP-stimulated monocytes play a critical role in monocyte-mediated inflammatory activation in HUVECs, we cannot rule out other potential proinflammatory mechanisms of HANP-activated monocytes against HUVECs. The detailed mechanism of this phenomenon needs to be further investigated.
Conclusion
In summary, our data provide evidence that HANPs could be taken up by both monocytes and ECs, and that HANP phagocytosis caused an inflammatory response in monocytes, but not in ECs. In addition, although HANPs had no direct effect on endothelial inflammation, we determined that HANPs induced an indirect activation of ECs, resulting in increased IL-6 production and elevated adhesion molecule expression after coculture with monocytes. The potential proinflammatory effect of HANPs is primarily mediated by the release of soluble factors from activated monocytes, which leads to an inflammatory response of the endothelium that is possibly dependent on p38/JNK MAPK and NF-κB signaling activation. Thus, the use of the in vitro monocyte–EC coculture model on the biocompatibility assessment of HANPs could reveal their potential proinflammatory effects on ECs, suggesting that exposure to HANPs could increase the risk of cardiovascular disease.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (no 81271700, no 81201191, no 31070843), and the Shanghai Sci-Tech Committee Foundation (13DZ2291100).
Disclosure
The authors report no conflicts of interest in this work.
References
- AulakhTSJayasekeraNKuiperJHRichardsonJBLong-term clinical outcomes following the use of synthetic hydroxyapatite and bone graft in impaction in revision hip arthroplastyBiomaterials20093091732173819136150
- ZhouHLeeJNanoscale hydroxyapatite particles for bone tissue engineeringActa Biomater2011772769278121440094
- FoxKTranPATranNRecent advances in research applications of nanophase hydroxyapatiteChemphyschem201213102495250622467406
- KadonoHFuruzonoTMasudaMIn vivo evaluation of hydroxyapatite nanocoating on polyester artificial vascular grafts and possibility as soft-tissue compatible materialASAIO J2010561616620051831
- AshokanAChandranPSadanandanARDevelopment and haematotoxicological evaluation of doped hydroxyapatite based multimodal nanocontrast agent for near-infrared, magnetic resonance and X-ray contrast imagingNanotoxicology20126665266621780855
- UskokovićVUskokovićDPNanosized hydroxyapatite and other calcium phosphates: chemistry of formation and application as drug and gene delivery agentsJ Biomed Mater Res B Appl Biomater201196115219121061364
- DonaldsonKIDuffinRLangrishJPNanoparticles and the cardiovascular system: a critical reviewNanomedicine (Lond)20138340342323477334
- KristovichRKnightDALongJFWilliamsMVDuttaPKWaldmanWJMacrophage-mediated endothelial inflammatory responses to airborne particulates: impact of particulate physicochemical propertiesChem Res Toxicol200417101303131215487890
- WeldyCSWilkersonHWLarsonTVStewartJAKavanaghTJDIESEL particulate exposed macrophages alter endothelial cell expression of eNOS, iNOS, MCP1, and glutathione synthesis genesToxicol In Vitro20112582064207321920430
- ShawCARobertsonSMillerMRDiesel exhaust particulate – exposed macrophages cause marked endothelial cell activationAm J Respir Cell Mol Biol201144684085120693402
- LiuXXueYDingTSunJEnhancement of proinflammatory and procoagulant responses to silica particles by monocyte-endothelial cell interactionsPart Fibre Toxicol201293622985792
- NadraIBoccacciniARPhilippidisPEffect of particle size on hydroxyapatite crystal-induced tumor necrosis factor alpha secretion by macrophagesAtherosclerosis200819619810517350022
- ScheelJWeimansSThiemannAHeislerEHermannMExposure of the murine RAW 264.7 macrophage cell line to hydroxyapatite dispersions of various composition and morphology: assessment of cytotoxicity, activation and stress responseToxicol In Vitro200923353153819444930
- Grandjean-LaquerriereATabaryOJacquotJInvolvement of toll-like receptor 4 in the inflammatory reaction induced by hydroxyapatite particlesBiomaterials200728340040417010424
- MotskinMWrightDMMullerKHydroxyapatite nano and microparticles: correlation of particle properties with cytotoxicity and biostabilityBiomaterials200930193307331719304317
- OkadaMMasudaMTanakaRMiyatakeKKurodaDFuruzonoTPreparation of hydroxyapatite-nanocrystal-coated stainless steel, and its cell interactionJ Biomed Mater Res A200886358959617994561
- PezzatiniSMorbidelliLSolitoRNanostructured HA crystals up-regulate FGF-2 expression and activity in microvascular endothelium promoting angiogenesisBone200741452353417681892
- ZhaoXNgSHengBCCytotoxicity of hydroxyapatite nanoparticles is shape and cell dependentArch Toxicol20138761037105222415765
- XieGSunJZhongGLiuCWeiJHydroxyapatite nanoparticles as a controlled-release carrier of BMP-2: absorption and release kinetics in vitroJ Mater Sci Mater Med20102161875188020300953
- LiuXSunJEndothelial cells dysfunction induced by silica nanoparticles through oxidative stress via JNK/P53 and NF-kappaB pathwaysBiomaterials201031328198820920727582
- KangMLimCHHanJHComparison of toxicity and deposition of nano-sized carbon black aerosol prepared with or without dispersing sonicationToxicol Res201329212112724278638
- ZhaoXHengBCXiongSIn vitro assessment of cellular responses to rod-shaped hydroxyapatite nanoparticles of varying lengths and surface areasNanotoxicology20115218219421609137
- XuZLiuCWeiJSunJEffects of four types of hydroxyapatite nanoparticles with different nanocrystal morphologies and sizes on apoptosis in rat osteoblastsJ Appl Toxicol201232642943522162110
- CliftMJGehrPRothen-RutishauserBNanotoxicology: a perspective and discussion of whether or not in vitro testing is a valid alternativeArch Toxicol201185772373120499226
- WanRMoYZhangXChienSTollerudDJZhangQMatrix metalloproteinase-2 and -9 are induced differently by metal nanoparticles in human monocytes: The role of oxidative stress and protein tyrosine kinase activationToxicol Appl Pharmacol2008233227628518835569
- OhWKKimSChoiMCellular uptake, cytotoxicity, and innate immune response of silica-titania hollow nanoparticles based on size and surface functionalityACS Nano2010495301531320698555
- KoflerSNickelTWeisMRole of cytokines in cardiovascular diseases: a focus on endothelial responses to inflammationClin Sci (Lond)2005108320521315540988
- DesseinPHJoffeBISinghSBiomarkers of endothelial dysfunction, cardiovascular risk factors and atherosclerosis in rheumatoid arthritisArthritis Res Ther200573R634R64315899050
- HouTTieuBCRaySRoles of IL-6-gp130 signaling in vascular inflammationCurr Cardiol Rev20084317919219936194
- PuFRWilliamsRLMarkkulaTKHuntJAExpression of leukocyte-endothelial cell adhesion molecules on monocyte adhesion to human endothelial cells on plasma treated PET and PTFE in vitroBiomaterials200223244705471812361609
- RoseSLBabenseeJEProcoagulant phenotype of endothelial cells after coculture with biomaterial-treated blood cellsJ Biomed Mater Res A200572326927815657946
- MartinesiMBruniSStioMTrevesCBacciTBorgioliFBiocompatibility evaluation of surface-treated AISI 316L austenitic stainless steel in human cell culturesJ Biomed Mater Res A200780113114516983653
- McDonaldSMMathesonLAMcBaneJEUse of monocyte/endothelial cell co-cultures (in vitro) and a subcutaneous implant mouse model (in vivo) to evaluate a degradable polar hydrophobic ionic polyurethaneJ Cell Biochem2011112123762377221826703
- LiuXXueYSunJIndirect induction of endothelial cell injury by PU- or PTFE-mediated activation of monocytesJ Biomater Sci Polym Ed201021131783179720557688
- SchanenBCKarakotiASSealSDrakeDR3rdWarrenWLSelfWTExposure to titanium dioxide nanomaterials provokes inflammation of an in vitro human immune constructACS Nano2009392523253219769402
- HersethJRefsnesMLågMHetlandGSchwarzePIL-1beta as a determinant in silica-induced cytokine responses in monocyte-endothelial cell co-culturesHum Exp Toxicol200827538739918715885
- NapierskaDThomassenLCVanaudenaerdeBCytokine production by co-cultures exposed to monodisperse amorphous silica nanoparticles: the role of size and surface areaToxicol Lett201221129810422445670
- LawsonCWolfSICAM-1 signaling in endothelial cellsPharmacol Rep2009611223219307690
- MuslinAJMAPK signalling in cardiovascular health and disease: molecular mechanisms and therapeutic targetsClin Sci (Lond)2008115720321818752467
- MestasJLeyKMonocyte-endothelial cell interactions in the development of atherosclerosisTrends Cardiovasc Med200818622823219185814
- VermaSAndersonTJFundamentals of endothelial function for the clinical cardiologistCirculation2002105554654911827916
- SilvermanMDZamoraDOPanYCell adhesion molecule expression in cultured human iris endothelial cellsInvest Ophthalmol Vis Sci200142122861286611687530
- MakóV1CzúczJWeiszhárZProinflammatory activation pattern of human umbilical vein endothelial cells induced by IL-1β, TNF-α, and LPSCytometry A2010771096297021290470
- DongCDavisRJFlavellRAMAP kinases in the immune responseAnnu Rev Immunol200220557211861597
- ZarubinTHanJActivation and signaling of the p38 MAP kinase pathwayCell Res2005151111815686620
- MongPYPetrulioCKaufmanHLWangQActivation of Rho kinase by TNF-alpha is required for JNK activation in human pulmonary microvascular endothelial cellsJ Immunol2008180155055818097057
- RichmondANf-kappa B, chemokine gene transcription and tumour growthNat Rev Immunol20022966467412209135
- CollinsTReadMANeishASWhitleyMZThanosDManiatisTTranscriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancersFASEB J19959108999097542214
- KuldoJMWestraJAsgeirsdóttirSADifferential effects of NF-{kappa}B and p38 MAPK inhibitors and combinations thereof on TNF-{alpha}- and IL-1{beta}-induced proinflammatory status of endothelial cells in vitroAm J Physiol Cell Physiol20052895C1229C123915972838
