?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Glioblastoma multiforme (GBM) is the most common and most aggressive malignant primary brain tumor in humans. Current GBM treatment includes surgery, radiation therapy, and chemotherapy, sometimes supplemented with novel therapies. Despite recent advances, survival of GBM patients remains poor. Major challenges in GBM treatment are drug delivery across the blood–brain barrier, restriction of damage to healthy brain tissues, and limitation of resistance to therapies. This article reviews recent advances in the application of magnetic nanoparticles (MNPs), gold nanorods (GNRs), and carbon nanotubes (CNTs) for hyperthermia ablation of GBM. First, the article introduces GBM, its current treatment, and hyperthermia as a potential modality for the management of GBM. Second, it introduces MNPs, GNRs, and CNTs as inorganic agents to induce hyperthermia in GBM. Third, it discusses different methodologies for synthesis of each inorganic agent. Finally, it reviews in vitro and in vivo studies in which MNPs, GNRs, and CNTs have been applied for hyperthermia ablation and drug delivery in GBM.
Introduction
Glioblastoma multiforme
Gliomas are heterogeneous central nervous system tumors.Citation1 Glioblastoma multiforme (GBM) is the most frequent and malignant type of glioma, and despite advances in diagnosis and treatment of GBM, median survival of GBM patients remains less than 15 months.Citation2 There are at least two factors that make GBM treatment extremely difficult. First, the brain has limited capacity to repair itself. Second, GBM is highly invasive and resistant to therapies.Citation3
Conventional GBM therapies consist of surgery, radiotherapy (RT), and chemotherapy. Objectives of surgery range from merely confirmation of the diagnosis or alleviation of symptoms due to mass effects to aggressive attempts to improve quality of life and prolong survival of the patient.Citation3 RT is one of the oldest and most common treatment options for GBM patients. It is based on generation of electrons and free radicals by ionizing radiation to damage deoxyribonucleic acid (DNA). Early clinical trials revealed a modest, yet undeniable efficacy of RT in treating GBM. However, there are several limitations of RT including risk of necrosis, permanent neuronal damage, and radio resistance of certain tumor types ().Citation4
Figure 1 Current treatment options for glioblastoma multiforme patients.
Abbreviations: GBM, glioblastoma multiforme; HGG, high-grade glioma; RNA, ribonucleic acid.
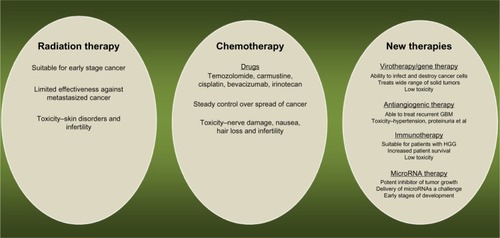
Temozolomide has been the mainline chemotherapy drug for GBM treatment for over 10 years.Citation5 Temozolomide, an oral alkylating agent, is a second-generation imidazotetrazine derivative, which exhibits cytotoxic effects by methylation of specific DNA sites.Citation5
Bevacizumab is often used as adjuvant therapy for GBM treatment. It is a humanized monoclonal antibody against vascular endothelial growth factor that act as angiogenesis inhibitor.Citation6 Side effects of chemotherapy are often severe, including nerve damage, nausea, hair loss, and infertility (). Chemotherapy in combination with other therapies, especially RT, provides the most effective treatment strategy for GBM at the moment.Citation5 For instance, a Phase III study conducted by the European Organization for Research and Treatment of Cancer and the National Cancer Institute of Canada reported that combined therapy of RT and temozolomide increased median survival time significantly when compared with RT alone.Citation7 The 5-year overall survival rate was 9.8% for the combination therapy group versus 1.9% for the RT alone group, with a median follow-up of 61 months. This and other studies are paving the way toward new treatment combinations.
Hyperthermia
Hyperthermia is a fairly new concept that finds its application in the treatment of different types of cancers and is based on generation of heat at the tumor site. This results in changes in the physiology of diseased cells, finally leading to apoptosis.Citation8
Hyperthermia treatment mechanisms involve intracellular heat stress in the temperature range of 41°C–46°C, resulting in activation and/or initiation of many intracellular and extracellular degradation mechanisms. The intracellular and extracellular effects of hyperthermia include protein misfolding and aggregation, alteration in signal transduction, induction of apoptosis, changes in potential of hydrogen (pH), and reduced perfusion and oxygenation of the tumor.Citation8
The effectiveness of hyperthermia treatment greatly depends on the temperature profile at the targeted tumor site, duration of exposure, and characteristics of cancer cells.Citation9,Citation10 Traditionally, hyperthermia treatment was performed using external devices to transfer thermal energy to cancerous tissues, either by irradiation with light or electromagnetic waves. Conventional techniques for induction of hyperthermia are ultrasound, microwaves, infrared irradiation, and tubes with hot water. However, each of these methods suffers from limitations (),Citation10 including low penetration of heat in the tumor; excessive heating of healthy tissue; thermal under-dosage in the target region; and dissipation of heat by the blood, which is especially a problem in well-vascularized tumors.Citation10,Citation11
Table 1 Types of hyperthermia therapy in cancer treatment
To overcome these limitations, magnetic materials were first proposed for hyperthermia treatment of cancer in 1957.Citation12 Many approaches have evolved since then to develop a new therapeutic system called magnetic hyperthermia therapy (MHT). MHT is based on generation of heat by magnetic nanoparticles (MNPs) when exposed to alternating magnetic fields. When MNPs are injected in the tumor and an alternating magnetic field is applied, the tumor temperature rises and results in thermal ablation of tumor cells.Citation13 MHT encompasses many salient features including externally-stimulated intracellular heating, delivery through multiple routes, potential to cross the blood–brain barrier, and antitumoral immunity.Citation13,Citation14
Nanoparticle-based hyperthermia
MNPs
In the last decade, several types of iron oxides have been explored to synthesize MNPs, including magnetite (Fe3O4), hematite (α-Fe2O3), and maghemite (γ-Fe2O3 and β-Fe2 O3).Citation15 MNPs are the most frequently investigated nanoparticles for biomedical applications because of their biocompatibility.Citation15 Among MNPs, superparamagnetic iron oxide nanoparticles (SPIONs) are the nanoparticles of choice due to their unique optical and magnetic properties, such as high paramagnetism, coercivity, magnetic susceptibility, and low Curie temperature.Citation16
Coercivity and Curie temperature are two important properties for nanoparticles. Coercivity of ferromagnetic material is a measure of intensity of the applied magnetic field that is required to reduce the magnetization of a material to zero.Citation17 Thus, coercivity measures the resistance of ferromagnetic material to demagnetize. Curie temperature is the temperature where the permanent magnetism of material changes to induced magnetism. When the Curie temperature is known, overheating of tumor tissue can be avoided, and thus side effects on healthy tissue can be limited.Citation18 Preliminary studies have suggested that MHT has potential to be applied for treatment of GBM. In fact, SPIONs have shown uniform intratumoral distribution and controlled heating of GBM without major side effects.Citation19
For the application of MNPs in MHT, their physical and chemical properties need to be optimized, which is a technological challenge. The next section discusses strategies for synthesis of different types of MNPs.
Methods for the synthesis of MNPs
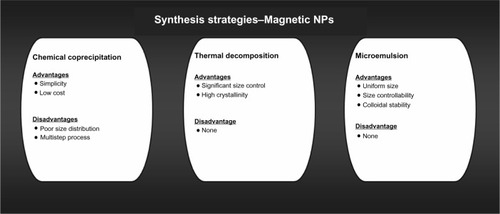
Methods that are most commonly used for MNP synthesis are chemical coprecipitation, thermal decomposition, and microemulsion (). Other less commonly used methods include hydrothermal synthesis, sonochemical synthesis, and electrochemical synthesis.Citation20 Control of size, stability, biocompatibility, and monodispersion of nanoparticles are some of the most important factors to consider before selecting a synthesis route.Citation20 The three most commonly used methods to synthesize MNPs are discussed here.
Chemical coprecipitation
In this method, hydrolysis of a mixture of Fe2+ and Fe3+ ions in 2:1 molar ratio is carried out under inert atmosphere. Ferrous and ferric ions are mixed in a 2:1 molar ratio at pH 8–14. Magnetite precipitates after being formed according to the following reaction:
Structural characteristics, such as size, dispersion, and morphology, of MNPs mainly depend on the type of salts used, the ratio of ferric and ferrous ions, and the reaction temperature.Citation21 Coprecipitation synthesis involves two major steps: first, formation of nuclei and second, growth of nuclei. The advantage of coprecipitation synthesis is the possibility to synthesize industrial quantities of nanoparticles. Large size distribution is the most common problem associated with coprecipitation. To overcome this problem reaction conditions are varied, such as pH, temperature, nature and origin of salts, and the Fe3+/Fe2+ ratio.Citation21,Citation22
Thermal decomposition
Thermal decomposition is carried out in an organic solution and results in formation of monodispersed iron oxide nanoparticles, with significant size control and crystallinity. Thermal decomposition involves decomposition of iron complexes in the presence of surfactants and organic solvents at high temperatures.Citation23
Sun and ZhangCitation24 prepared SPIONs using thermal decomposition. Reactions of iron (III) acetylacetonate were performed at a high temperature (265°C) in the presence of phenyl ether, alcohol, oleic acid, and oleylamine to form SPIONS with a diameter of 4 nm. Hyeon et alCitation25 synthesized SPIONs with a diameter of 7–25 nm by thermal decomposition. In their study, nanoparticles were synthesized as iron oleate complexes from iron pentacarbonyl by decomposition in the presence of octyl ether and oleic acid at 100°C. Park et alCitation26 used iron salt (iron chloride [FeCl3 · 6H2O]) instead of toxic ferrous metallic compounds as precursor to form an iron-oleic complex. Iron-oleic complexes yielded monodispersions of iron oxide crystals when mixed with sodium oleate in the presence of 1-octadecene and aged for 30 minutes.
Microemulsion
Iron oxide nanoparticles generated in the presence of microemulsions yield nanoparticles of uniform size and colloidal stability. Inouye et al were the first to synthesize MNPs in microemulsion by oxidation of Fe2+ salts, and the size of MNPs was controlled by varying temperature and surfactant concentration.Citation27 Micelles have been immensely useful in synthesizing monodispersed, size-controlled nanoparticles. Lee et al used reverse micelles (micelles with head groups in the center and tails at the periphery) for the synthesis of SPIONs.Citation28 This method to generate monodispersed SPIONs is efficient, inexpensive, and large-scale, and it involves synthesis of MNPs at high temperatures using iron salts, surfactant, and solvents in varying concentrations or proportions.Citation28
Methods for the surface modification of MNPs
MNPs are susceptible to corrosion. The most common form of corrosion that occurs in MNPs is oxidation, which leads to loss of magnetism and dispersibility of nanoparticles, thus affecting their ability to induce hyperthermia. Therefore it is necessary to protect MNPs from oxidation. Methods that have been explored to protect MNPs against oxidation include surface modification by surface passivation, surfactant and polymer coating, and metal, silica, and carbon coating. A detailed review on this topic has already been published. A brief overview of methods for surface modification of MNPs is presented below.
Surface passivation by mild oxidation
Surface passivation is the protection of any material from external environmental factors, and surface passivation by mild oxidation has been used for protecting MNPs.Citation29,Citation30 Peng et al used plasma gas condensation to oxidize cobalt nanoparticles in gas phase.Citation29 Bönnemann et al used controlled air atmosphere to oxidize cobalt nanoparticles, and this resulted in the formation of an outer layer of cobalt oxide on the nanoparticle and protected it from further oxidation.Citation30
Surfactant and polymer coating
MNPs synthesized through coprecipitation carry negatively charged surfaces, causing them to agglomerate. Surfactants and polymers have been peptized, chemically anchored, or physically adsorbed on MNPs to form stable colloids.Citation31–Citation33 These stable colloids create repulsive forces to balance the magnetic and the van der Waals attractive forces acting on the nanoparticles, thus preventing nanoparticles from agglomeration. Polymers that have been used for the formation of stable colloids are poly(pyrrole), poly(aniline), poly(alkyl cyanoacrylates), poly(methylidene malonate), poly(lactic acid), poly (glycolic acid), and poly(e-caprolactone).Citation34–Citation37
Metal, silica, and carbon coating
Precious metals deposited onto MNPs protect the magnetic nanoparticle core from oxidation. Of all precious metals, gold is the metal of choice for coating MNPs due to its low reactivity. However, direct coating of gold onto MNPs is difficult because of the dissimilar nature of the two surfaces.Citation38,Citation39 Despite this complication, many researchers have been able to coat MNPs with gold by using new techniques. Ban et al synthesized gold-coated iron nanoparticles using a partial replacement reaction in a polar aprotic solvent,Citation40 Liu et al synthesized gold-coated iron nanoparticles using a reverse microemulsion method,Citation41 and Zhang et al synthesized iron (core) gold (shell) nanoparticles using a combination of wet chemistry and laser irradiation.Citation42
Gold nanorods
Gold nanorods (GNRs) exhibit optical properties that depend on size and aspect ratio. Surface plasmon resonance is the most important property of GNRs. It is a result of interaction between electrons in a conduction band of gold atoms and electric field components of incident electromagnetic radiation.Citation43 As a result of surface plasmon resonance, GNRs emit heat upon irradiation with an infrared laser. This property was used in numerous studies to develop photodynamic therapy for GBM treatment.Citation44 The most efficient strategies for the synthesis of GNRs of different aspect ratios and sizes are discussed here.
Methods for the synthesis of GNRs
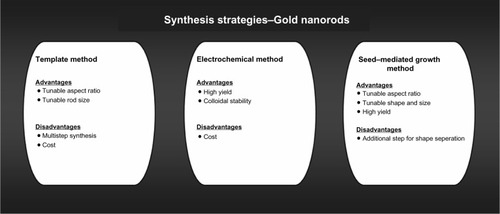
GNRs can be synthesized by either wet or dry chemistry. Wet chemistry is the preferred mode of synthesis because of its simplicity and low costs.Citation45 In the following subsections, three major synthesis methods are reviewed: the template method, the electrochemical method, and the seed-mediated growth method ().
Template method
The template method for synthesis of GNRs involves electrochemical deposition of gold onto the pores of an alumina template membrane or nanoporous polycarbonate. The method was originally used to prepare microscopic electrodes by depositing gold onto a polycarbonate membrane using electroplating. Genzel et al were the first to successfully employ the template method for the synthesis of GNRs.Citation46
The method involves four steps. In the first step, copper is sputtered onto an alumina template. In the second step, gold is electrodeposited onto the nanosized pores of alumina. In the third step, the alumina membrane and copper film are dissolved in the presence of an appropriate stabilizer. In the final step, GNRs are dispersed in water or an organic solvent using ultrasonic waves or agitation. GNRs of various diameters and lengths have been successfully generated using the template method. The diameter of nanorods is controlled by the pore diameter of the template, and the length is controlled by the quantity of gold deposited within the membrane nanopores.Citation45
Electrochemical method
The electrochemical method is the most commonly used method for the synthesis of transition metal clusters in an organic solvent. Nikoobakht et al developed the application of an electrochemical method for the synthesis of GNRs and synthesized GNRs in high yields.Citation47 The actual synthesis was conducted in an electrochemical cell containing two electrodes. In the cell, a gold metal plate was used as anode, and a platinum plate was used as cathode. The final electrolysis was carried out in the presence of a hydrophilic cationic surfactant and a controlled current mode for 30–60 minutes to successfully synthesize GNRs.Citation47
A recent study used a silver plate to control the aspect ratio of GNRs.Citation48 Silver metal reacted with gold ions to generate silver ions at the anode. The amount of silver ions and their release rates were found to affect the length of GNRs.Citation48
Seed-mediated growth method
The seed-mediated growth method has been used for decades to synthesize monodispersed metal nanoparticles. Brown et al were the first to use the seed-mediated growth method for the synthesis of GNRs by reducing auric gold (Au3+) with hydroxylamine.Citation49 One of the major biocompatibility issues of the seed-mediated growth method is the formation of nanoparticles of other shapes along with nanorods.
Jana et al synthesized GNRs using 3.5 nm seed nanoparticles.Citation50 The yield of this method was 4%, but it was later improved by changing the pH of the growth solution from 2.8 to 5.6. The change in pH resulted in formation of GNRs with an aspect ratio between 18 and 20 and a yield of 90%.Citation51 Hu et al used various temperatures and capping agents for GNR synthesis.Citation52 It appeared that a reduced temperature and the use of hexadecyltrimethylammonium bromide as capping agent led to nanorods with an aspect ratio between 1 and 6 and a yield in the order of 50%. The study also suggested that stability of seeds, growth temperature, and concentration of surfactant influence monodispersity, yield, and aspect ratio.
The seed-mediated growth method does not only generate nanorods but also nanoparticles of various shapes. For this reason, separation of nanorods from other nanoparticles is critical. Methods such as size-selective precipitation, nanoporous filtration, and methods for extraction have successfully separated nanospheres of different sizes but are not very selective for nanorods due to their relatively large size.Citation53,Citation56 Wei et al demonstrated that size-exclusion chromatography can separate nanorods from spheres, but this method is only partly successful.Citation54 Jana used phase-separation involving surfactant-assisted ordering.Citation55 The method successfully separated single-sized nanorods from a mixture of differently-sized rods, spheres, and plates. More recently, Sharma et al demonstrated centrifugation as an efficient technique to separate nanorods from a mixture of nanorods and nanospheres.Citation56 The technique is based on the fact that shape-dependent drag causes particles to have shape-dependent sedimentation behavior.
Methods for the surface modification of GNRs
GNRs synthesized using the above mentioned methods have dispersion and biocompatible flaws and therefore need further surface modification before use as hyperthermia agents.Citation57 There are two main strategies for surface modification of GNRs: matrix entrapment and ligand exchange.
Matrix entrapment
Entrapment of GNRs in a matrix improves their dispersion and optical and chemical characteristics. Mitamura et al entrapped and dispersed GNRs in an alginate matrix using energy dispersive X-ray spectroscopy. Alginate entrapped GNRs were highly dispersed and maintained their optical character.Citation58 Apart from alginate, poly(N-isopropylacrylamide) gel has also been used to disperse GNRs in a gel matrix. Gorelikov et alCitation59 and Kumar et alCitation60 dispersed GNRs in a poly(N-isopropylacrylamide) microgel using hybridization. The resulting hybrid gel was unique in that it was able to reversibly shrink with or without infrared radiation.
Ligand exchange
Surfactants used in the seed-mediated growth method for synthesis of GNRs are toxic. Ligand exchange has been the method of choice for removal of residual surfactants from GNRs. Gentili et al used a double phase transfer process for surface functionalization of GNRs.Citation61 The process consisted of a simple one-step ligand exchange in a hydroalcoholic mixture with thiols and a phase transfer to entrap gold nanoparticles into polyethylene glycol-based polymeric nanoparticles. Thus, the obtained GNRs were lipophilic-free with a robust coating. Khanal et al synthesized GNRs stabilized by cetyltrimethylammonium bromide and subsequently exchanged the cetyltrimethylammonium bromide for a functional thiol using 4-mercaptophenol.Citation62 The method resulted in formation of anisometric GNRs that self-assembled into ring-like superstructures.
Carbon nanotubes
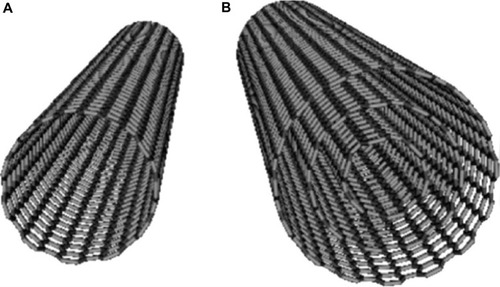
Carbon nanotubes (CNTs) were first observed in 1952 by Radushkevich and Lukyanovich, but only in 1991 was a methodology for the synthesis of CNTs described by Iijima using C60 carbon molecules.Citation63,Citation64 CNTs are most commonly synthesized from allotropes of carbon in two different forms, such as single-walled carbon nanotubes (SWNTs) and multi-walled nanotubes (MWNTs). SWNTs consist of a single tube of graphene, and MWNTs consist of several concentric tubes of graphene (). Both forms have unique physical and chemical properties that enable their application in anticancer hyperthermia therapy. CNTs generate heat upon infrared irradiation. Another salient feature of CNTs is the possibility to engineer their surface for conjugation with a wide variety of molecules.Citation64
Figure 4 Structural arrangement of carbon in (A) single-walled nanotube and (B) multiwalled nanotube.
Methods for synthesis of CNTs
There are three major techniques for the synthesis of CNTs: arc discharge, laser ablation, and chemical vapor deposition (CVD). Arc discharge and laser ablation were the initial techniques used to synthesize CNTs. Both techniques require high temperatures (generally >1,700°C) and are now more or less obsolete, replaced by CVD which is carried out at comparatively low temperatures (<800°C) and enables the synthesis of CNTs with controlled orientation, alignment, length, diameter, and density.Citation65
Arc discharge
Arch discharge is a gas phase reaction process requiring high temperatures for synthesis of CNTs. The advantage of arc discharge is that CNTs with minimal imperfections and structural defects are generated. The process conditions are different for the synthesis of MWNTs and SWNTs.
Synthesis of MWNTs
Graphite electrodes are used for synthesis of MWNTs. The electrodes have a diameter of 8–12 nm and are water cooled. The reaction atmosphere consists of helium, hydrogen, or methane. The purity and yield of CNTs are found to vary with the gas atmosphere and pressure inside the reaction vessel. Wang et al used evaporation in methane under high pressure and high arc current to obtain thick nanotubes.Citation66 Lowering the methane pressure and the current at the anode resulted in formation of thin and long MWNTs. Zhao et al used hydrogen gas instead of methane as reaction atmosphere and also obtained thin and long MWNTs.Citation67
Another technique for arc discharge deposition is the use of pulsed current rather than direct current. Parkansky et al used a single-pulse arc to produce MWNTs with a diameter of 10 nm and a length of 3 μm.Citation68 In addition to standard arc deposition, alternative methods have been used for synthesis of CNTs. Sornsuwit et alCitation69 and Montoro et alCitation70 used arc discharge in an aqueous vanadic acid solution to grow high quality SWNTs and MWNTs. Jung et al used arc discharge in the presence of liquid nitrogen to successfully grow MWNTs.Citation71
Synthesis of SWNTs
Unlike MWNTs, SWNTs cannot be synthesized in the absence of a transition metal catalyst. The most commonly used method for the synthesis of SWNTs is based on an arc discharge system fitted with a composite anode. The composite anode consists of graphite and a combination of transition metals as catalyst. The catalyst plays an important role in increasing the yield of the process while high efficiency is ensured by a constant gap between electrodes.
Iijima et al were the first to synthesize SWNTs in 1993.Citation72 Later that year, Bethune et al utilized coevaporation of carbon and cobalt to generate SWNTs with a single atomic layer.Citation73 Chen et al modified the electric arc technique for the synthesis of SWNTs by using a ferrium-hydrogen arc discharge.Citation74 This new technique used hydrogen arc discharge with a carbon anode containing a 1% iron (Fe) catalyst in a mixed hydrogen-argon gas environment to produce highly crystalline SWNTs. The technique successfully generated SWNTs with a purity >90%. Fan et al further modified this technique to develop an economic process for the synthesis of SWNTs.Citation75 The technique used argon as reaction atmosphere and direct current arc discharge with charcoal as carbon source and iron sulfide and cobalt oxide as catalyst. SWNTs thus formed were highly pure with a diameter of 1.2 nm.
CVD
Arc discharge enables the generation of large quantities of CNTs with minimal imperfections but with low purity. CVD on the other hand produces CNTs with predefined properties and high purity.
CVD involves catalytic decomposition of hydrocarbon or carbon monoxide feedstock, with the aid of supported transition metal catalysts to produce CNTs. The synthesis is achieved by combining a carbon source with an energy source. Most commonly used energy and carbon sources are a plasma- and resistively-heated coil, methane, carbon monoxide, and acetylene. There are three main steps in the synthesis process.Citation76 The first step is the cracking step, where the energy source is used to crack the gas phase molecule into atomic carbon. The second step is the diffusion step, during which cracked atomic carbon diffuses toward the substrate. The third step is the heating and coating step. During this step, diffused carbon is heated and coated with the metal catalyst while it binds to the catalyst. Excellent diameter control and growth rate can be achieved with CVD. The actual process for synthesis of CNTs consists of a catalyst preparation step that is followed by the actual synthesis of the nanotube. The catalyst preparation is performed by using either sputtering or chemical etching. The temperatures for the synthesis of nanotubes by CVD are usually within the 650°C–900°C range. Typical yields for CVD are approximately 30%.Citation77
CVD has been used to create several structural forms of carbon, including SWNTs and MWNTs from well-crystallized graphite layers. CVD allows selective CNT growth in a variety of forms, such as powder and an aligned forest of CNTs.Citation78,Citation79
In the last decade, different CVD-based techniques have been developed for CNT synthesis, including plasma-enhanced CVD, thermal-chemical CVD, alcohol-catalytic CVD, vapor phase growth, aero gel-supported CVD, and laser-assisted CVD.Citation80
Hyperthermia with MNPs
Hyperthermic therapy
Hyperthermia using MNPs is often referred to as MHT. MHT involves injection of iron oxide nanoparticles inside the tumor and subsequent placement of the patient in an alternating magnetic field, which results in an increased intratumoral temperature, thermal ablation of tumor cells, and subsequent tumor shrinkage.
MHT is advantageous in two ways. First, MNPs accumulate in the tumor. Therefore, healthy tissue damage is limited. Second, MHT is noninvasive and capable of inducing hyperthermia in tumors at any location in the body. However, the efficiency of therapy is highly variable and often found to be dependent on particle properties, including size, magnetization, and Curie temperature. Curie temperature is an important parameter, indicating the maximum temperature of a magnetic particle. A good estimate of the Curie temperature allows an efficient temperature control and is thus critical to avoid overheating of tissue by MNPs. Besides particle properties, other parameters that play an important role in determining efficiency of MHT are intensity and frequency of the alternating magnetic field and the dissipation of heat from the tumor.
The effectiveness of MHT in glioma was first reported by Shinkai et al.Citation81 In their first study, the group used an ex vivo rat model implanted with glioma cell pallets and magnetic cationic liposomes. Three doses of alternating magnetic field for 60 minutes each, at intervals of 12 hours were applied to the rats. The treatment was reported to completely inhibit tumor development for up to 90 days.Citation81 In the second study, an in vivo rat model was used to examine the effect of hyperthermia in glioma tumors.Citation82 Magnetite cationic liposomes were administered to rats, and histological analysis revealed that the rats that were exposed to the magnetic field showed a homogeneous distribution of magnetite cationic liposomes, which also coincided with the necrotic regions. In the third study, an in vivo model of rat with glioma tumors was used to study antitumor immunity of rats by hyperthermia therapy.Citation83 During the primary treatment, three doses of 30 minutes each were applied at 24-hour intervals. Tumor tissue was completely eliminated in two-thirds of the rats. These rats were rechallenged with glioma cells 3 months later. Transient growth was observed in the initial 2 weeks, but the tumors disappeared in 4 weeks. The explanation for this phenomenon was promotion of anti-tumor response by activation of heat shock proteins (see below).
In the last two decades, other studies have also demonstrated the effectiveness of MHT, on its own as well as in combination with other therapies. Ito et al observed that hyperthermia promotes an anti-tumor immune response by activation of heat shock proteins.Citation84 In a later study, it was suggested that hyperthermia in combination with gene therapy is an effective strategy for the treatment of GBM.Citation85 Le et al demonstrated increased effectiveness of magnetite cationic liposomes in the destruction of GBM tissue by conjugating magnetic cationic liposomes to a specific antibody against GBM.Citation86 shows a comprehensive list of in vivo studies that evaluated SPIONs for GBM treatment.
Table 2 In vivo animal model studies to evaluate MHT using SPION for thermal ablation of GBM
Latest clinical trials in Europe suggest that the application of magnetic hyperthermia may become clinically effective soon. MagForce Nanotechnologies (Berlin, Germany) has developed a MHT system called “NanoTherm®.” The system is based on aminosilane-coated SPIONs and the application of an alternating magnetic field. This MHT system has received approval in Europe to use iron oxide MNPs for GBM treatment.Citation87
Hyperthermia as enhancer of drug delivery
The term “hyperthermia” has thus far been confined to the use of heat for therapy. However, hyperthermia has also been utilized as a novel mechanism to improve drug delivery to tumors.Citation88 The use of magnetic field to control drug release from polymeric matrices containing iron oxide was first reported nearly 30 years ago.Citation88,Citation89 The MNP design for drug delivery is unique and generally consists of an iron oxide core and a polymer shell. The polymer shell is heat sensitive, and upon heat transferred from the iron oxide core, it contracts to release the encapsulated drug. MNP systems based on this concept are able to deliver drugs in a controlled manner. Control is possible because of the sensitivity of the MNP shell to a magnetic field, which is a function of frequency and strength of alternating magnetic field.Citation90
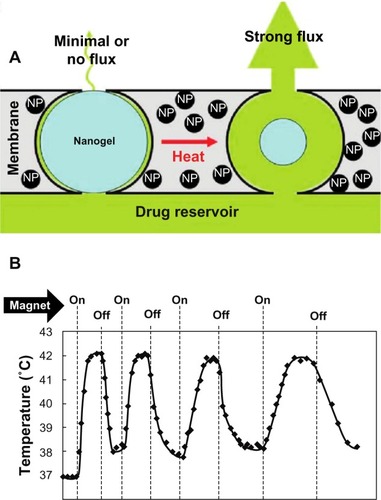
Hyperthermia-mediated release of various drugs was first demonstrated using temperature-sensitive poly(N-isopropylacrylamide) hydrogels incorporating SPIONs.Citation91 Hoare et al demonstrated on-demand release of sodium fluorescein using nanocomposite membranes of thermosensitive poly(N-isopropylacrylamide)-based nanogels and magnetite nanoparticlesCitation92 (). This study opened avenues for the development of therapeutic systems such as magnetic micro- and nanopumps, magnetic field-controlled drug delivery devices, and magnetic switches.
Figure 5 Stimulus-responsive membrane triggered in vitro (A) and magnetic triggering and differential flux of sodium fluorescein out of membrane-capped devices as a function of timeover successive on/off cycles of external magnetic field (B). Reprinted with permission from Hoare T, Santamaria J, Goya GF, et al. A magnetically triggered composite membrane for on-demand drug delivery. Nano Lett. 2009;9(10): 3651–3657. Copyright © 2009. American Chemical Society.Citation92
Abbreviation: NP, nanoparticle.
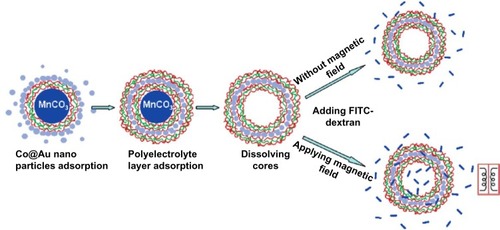
A more recent design of a magnetic drug delivery platform is magnetic composite nanoparticles consisting of multiple metals. Behrens et al demonstrated the potential of ferromagnetic cobalt nanoparticles in modulating the permeability of polyelectrolyte microcapsules.Citation93 In this study, ferromagnetic gold-coated cobalt nanoparticles were embedded inside polymeric walls using the layer-by-layer self-assembly technique. The polymer capsules consisted of eleven layers, with one layer of ferromagnetic gold-coated cobalt nanoparticles and ten layers of polyelectrolyte bilayers. The polymer capsule design was found to be ideal for the magnetic control of the permeability of the composite nanoparticle.
Another interesting type of MNPs for drug delivery is iron-core and silica-shell nanoparticles. This nanoparticle design is unique because it protects biomolecules that are encapsulated in the shell from the environment. The core-shell nanoparticles resemble extracellular vesicles. Extracellular vesicles are small granules that are produced by cells in our body to deliver biological materials to recipient cells.Citation94
More recently, implantable microchips have been developed on the basis of MNPs, such as a novel drug-delivery chip for magnetically-controlled release of anti-epileptic drugsCitation95,Citation96 (). The chip was made of an electrically-conductive flexible polyethylene terephthalate substrate that contained drug-loaded magnetic SPION-core-silica-shell nanoparticles.
Figure 6 Layer-by-layer self-assembly and permeability test for microcapsules embedded with ferromagnetic gold-coated cobalt nanoparticles under an oscillating magnetic field. Reprinted with permission from Zonghuan L, Malcolm PD, Zhanhu G, et al. Magnestic switch of permeability for polyelectrolyte microcapsules embedded with nanoparticles. Langmuir. 2005;21:2042–2050. Copyright © 2005. American Chemical Society.Citation109
Abbreviations: Co@Au, ferromagnetic gold-coated cobalt; FITC, fluorescein isothiocyanate; MnCO3, manganese carbonate.
Hyperthermia with GNRs
Hyperthermic therapy
Gold nanoparticles possess unique optical properties of which surface plasmon resonance is the most important. Surface plasmon resonance is the collective oscillation of electrons in a solid or liquid state stimulated by incident light. In the case of gold nanoparticles, absorption of infrared light induces surface plasmon resonance that is converted into heat. GNRs have the greatest potential for biomedical applications because of their high light absorption efficiency per unit volume. GNRs exhibit two distinct surface plasmon oscillations: a strong band in the near infrared region, corresponding to electron oscillation along the long axis (longitudinal band) and a weak band in the visible region (transverse band). Only the longitudinal band is sensitive to size changes and can be shifted from the visible to the near infrared region by adjusting the aspect ratio (length/width) during synthesis.Citation97
GNRs have attracted great interest for hyperthermic therapy due to superior biocompatibility. The effectiveness of GNRs in hyperthermic anti-tumor therapy has been demonstrated.Citation97 GNRs have been conjugated with molecules that carry sequences that can bind to cancer biomarkers. These GNRs specifically target and destroy tumors. Oral cancers are known to overexpress folate receptors, and therefore many studies utilized GNR conjugated with folic acid to target oral cancer cells. The study successfully ablated oral cancer cells in a targeted manner.Citation98 Choi et al used PEGylated GNRs conjugated with an Arg-Gly-Asp sequence to specifi-cally bind to αvβ3 integrins expressed on GBM cells.Citation99 The study demonstrated that (Arg-Gly-Asp)-GNRs are able to circulate for prolonged periods of time and bind to GBM cell surface, thus targeting GNRs to the GBM tumor. Fernandez et al developed an optical hyperthermia method for treatment of GBM by irradiation of GNRs by laser light and was successful in ablating GBM cells in vitro.Citation100 Oli et al used folate-conjugated GNRs to specifically target GBM cells.Citation101 The study showed that folate-conjugated GNRs can successfully target and kill GBM cells upon excitation with a near infrared laser.
Hyperthermia as enhancer of drug delivery
GNRs have also been studied as enhancers of drug delivery. GNRs offer tunable and localized surface plasmon resonance, which makes them highly localized heat sources when irradiated with a laser. The heat generated by GNRs is used for hyperthermic cancer therapy and/or to trigger the release of a drug.
Although drug delivery using GNRs is a new field, there are two major studies that investigated the use of GNRs to deliver doxorubicin to GBM. Agarwal et al were the first to study remotely triggered release of doxorubicin in GBM tumors using thermosensitive liposomes and GNRs.Citation102 PEGylated GNRs triggered release of doxorubicin from thermosensitive liposomes in a mouse tumor model of human GBM when irradiated with near infrared light. The stimulation resulted in significantly increased efficacy when compared to nontriggered or nonthermosensitive PEGylated liposomes. More recently, Xiao et al studied GNRs conjugated with doxorubicin and cyclo(Arg-Gly-Asp-D-Phe-Cys) peptide for drug delivery in GBM.Citation103 Doxorubicin was covalently conjugated to PEGylated GNRs via a hydrazone bond. Flow cytometric analysis revealed that GNRs conjugated with doxorubicin and cyclo(Arg-Gly-Asp-D-Phe-Cys) peptide exhibited a higher cellular uptake and cytotoxicity than nontargeted GNRs conjugated with doxorubicin in human GBM cells.
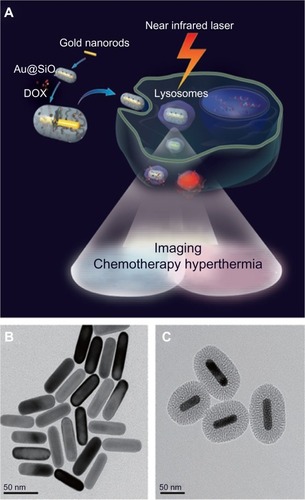
Zhang et al evaluated the potential of mesoporous silica-coated GNRs for cancer theranostics in a proof of concept study in human alveolar adenocarcinoma cells.Citation104 The core of GNRs functioned both as an agent that allowed noninvasive imaging as well as a hyperthermic agent while the outer mesoporous silica shell encapsulated a high drug load, thus posing itself as an effective drug carrier ().
Figure 7 Mechanism of action of mesoporous silica-coated gold nanorods (A), gold nanorods without coating (B), and mesoporous silica-coated GNRs (C).
Notes: The core of GNRs functioned both as an agent that allowed noninvasive imaging as well as a hyperthermic agent while the outer mesoporous silica shell encapsulated a high drug load, thus posing itself as an effective drug carrier. Reproduced from Zhang Z, Wang L, Wang J, et al. Mesoporous silica-coated gold nanorods as a light-mediated multifunctional theranostic platform for cancer treatment. Adv Mater. 2012;24(11):1418–1423.Citation104 Copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Abbreviations: Au@SiO2, silica-coated gold nanorods; DOX, doxorubicin; GNRs, gold nanorods.
Hyperthermia with CNTs
Hyperthermia using CNTs is a new field compared to MNP-and GNR-mediated hyperthermia. CNTs exhibit unique physical and chemical properties that hold promise for drug delivery and cancer therapy.Citation105 The mechanisms of selective tumor targeting with CNTs are currently being explored due to their impressive ability to convert near infrared light into heat. This intrinsic property of CNTs opens new avenues for the development of novel nanostructures for cancer phototherapy.Citation105
Markovic et al used CNTs and graphene nanoparticles for hyperthermic ablation of human glioma cells in vitro.Citation106 The study showed that graphene nanoparticles triggered cell death by inducing oxidative stress, and CNTs triggered cell death via mitochondrial membrane depolarization. Wang et al incubated GBM cells with anti-CD133 monoclonal antibody-conjugated SWNTs,Citation107 and irradiation with near infrared laser light resulted in targeted ablation of GBM cells while the viability of control cells remained unaffected. In a second study, Wang et al generated anti-GD2-conjugated CNTs to target GD2 receptors present on the surface of neuroblastoma cells.Citation108 Neuroblastoma cells were pretreated with anti-GD2-conjugated CNTs and were then irradiated with a near infrared laser. Postexposure analysis revealed necrosis among GD2-positive cells, whereas GD2-negative (control) cells remained unaffected. These studies suggest that SWNTs and MWNTs possess unique optical properties that have potential to develop novel therapies for the treatment of GBM.
Conclusion
GBM is an aggressive tumor type with very low survival rates. Many factors attribute to the low survival rates; some are inherent while others are treatment dependent. Conventional therapies lack specificity for cancer cells and therefore lead to side effects. Conventional therapies in combination with alternative or adjuvant therapies provide a better treatment strategy but still lack the efficacy and potential that is needed for effective treatment of GBM.
Conventional hyperthermia with external devices has been used for a long time but lacks accurate temperature control, leading to overheating and damage of healthy tissue or dissipation of heat from the tissue. Hyperthermia using nanoparticles is a novel concept that enables controlled heating of tumor tissue. Nanoparticle-based hyperthermia can have direct therapeutic effects and enhance drug delivery in a single therapy, making it a therapy with two advantages for cancer treatment.
MNPs, GNRs, and CNTs are the most promising nanoparticles for hyperthermic therapy in GBM. Among MNPs, SPIONs are the nanoparticles of choice for treatment of GBM. The salient features of SPIONs are:
ease of synthesis;
superior biocompatibility; and
relatively low cost.
Cationic liposomes and aminosilane-coated SPIONs have been found to be effective in ablating GBM in the presence of an alternating magnetic field. MagForce Nanotechnolgies has commercialized MHT for the treatment of GBM after successful Phase I, II, and III trials.
In addition to high treatment efficacy, many studiesCitation81,Citation82 have also confirmed an antitumor immune response after hyperthermia, based on elevated expression of heat shock proteins, which is an added advantage.
Core-shell SPIONs, when incorporated with drugs and stimulated with alternating magnetic field, are capable of drug delivery in a controlled manner. Layer-by-layer self-assembly is the preferred method for synthesis of core-shell SPIONs. Drug delivery and hyperthermia using SPIONs is promising yet challenging. Advantages of SPION-based hyperthermia are:
ability to cross the blood–brain barrier;
delivery through multiple routes;
minimal or no side effects;
noninvasive procedure; and
antitumoral immunity.
Some of the challenges on the other hand are moderate to low absorption rates, monitoring temperature distribution, self-regulation of heating, and precise control of intratumoral temperature.
Gold nanoparticles exhibit exceptional physical and optical properties including surface plasmon resonance. These properties enabled the development of novel photothermal therapies for the treatment of GBM. GNRs encompass better properties than gold nanospheres including superior biocompatibility and higher light absorption per unit volume.
GNRs are prepared by either wet or dry chemistry. Wet chemistry is the preferred mode due to the simple and inexpensive nature of synthesis. Most investigated wet chemistry-based methods for GNR synthesis are:
template;
electrochemical; and
seed-mediated growth.
When GNRs are intravenously injected in in vivo models of GBM and irradiated with near infrared laser light, they cause significant thermal ablation of tumor cells via necrosis and/or apoptosis. This procedure is successful in reducing tumor volume but often leads to nonspecific heating of surrounding healthy tissue. Conjugation of GNRs with cell surface targeting antibodies or aptamers can overcome these nonspecific heating effects. Studies have successfully used αvβ3 integrins and folate receptors to target GNRs to GBM cells.
Core-shell GNRs are versatile nanocarriers for drug delivery. Studies have successfully delivered doxorubicin as model drug to GBM cells using targeted as well as nontargeted approaches.
In summary, core-shell versions of GNR are capable of:
controlled drug delivery;
hyperthermia; and
combinational therapy.
Development of multifunctional systems based on core-shell MNPs and GNRs with individual functions acting in a coordinated way is critical to optimize therapeutic efficacy and safety of therapeutic regimes, and could provide more opportunities for on-demand therapy and pave the road toward personalized medicine.
CNTs and graphene nanoparticles exhibit an impressive ability to convert near infrared light into heat. This intrinsic property of CNTs is utilized to develop hyperthermic therapy for GBM treatment. The studies suggest that CNTs and graphene nanoparticles can effectively ablate glioma cells in a targeted as well as a nontargeted manner. Although CNTs are successful in cancer phototherapy, further studies are needed to elucidate all mechanisms involved in CNT-mediated anticancer therapy.
Among MNPs, GNRs, and CNTs, SPIONs are promising in the development of MHT, GNRs are promising in the development of photodynamic therapy, and CNTs have potential in the development of photothermal therapy for the treatment of GBM.
Disclosure
The authors report no conflicts of interest in this work.
References
- BrandesAATosoniAFranceschiEReniMGattaGVechtCGlioblastoma in adultsCrit Rev Oncol Hematol200867213915218394916
- WenPYKesariSMalignant gliomas in adultsN Engl J Med2008359549250718669428
- IacobGDincaEBCurrent data and strategy in glioblastoma multiformeJ Med Life20092438639320108752
- PirothMDGagelBPinkawaMStanzelSAsadpourBEbleMJPostoperative radiotherapy of glioblastoma multiforme: analysis and critical assessment of different treatment strategies and predictive factorsStrahlenther Onkol20071831269570218040615
- StuppRMasonWPvan den BentMJEuropean Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy GroupsNational Cancer Institute of Canada Clinical Trials GroupRadiotherapy plus concomitant and adjuvant temozolomide for glioblastomaNew Eng J Med20053521098799615758009
- MoustakasAKreislTNNew treatment options in the management of glioblastoma multiforme: a focus on bevacizumabOnco Targets Ther20103273820616955
- StuppRHegiMEMasonWPEuropean Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology GroupsNational Cancer Institute of Canada Clinical Trials GroupEffects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trialLancet Oncol200910545946619269895
- HildebrandtBWustPAhlersOThe cellular and molecular basis of hyperthermiaCrit Rev Oncol Hematol2002431335612098606
- WustPHildebrandtBSreenivasaGHyperthermia in combined treatment of cancerLancet Oncol20023848749712147435
- JordanAThermotherapy and nanomedicine: between vision and realityBaronzioGFHagerEDHyperthermia in Cancer Treatment: A Primer3rd edNew York, NYSpringer20066063
- ElliottDGHaskinsGMRandRWSnowHDinventorsInduction heating method for use in causing necrosis of neoplasm United States Patent US4545368 A1081985
- GilchristRKMedalRShoreyWDHanselmanRCParrottJCTaylorCBSelective inductive heating of lymph nodesAnn Surg1957146459660613470751
- ItoAShinkaiMHondaHKobayashiTMedical application of functionalized magnetic nanoparticlesJ Biosci Bioeng2005100111116233845
- MornetSVasseurSGrassetFMagnetic nanoparticle design for medical applicationsProg Solid State Chem2006342–4237247
- FarajiMYaminiYRezaeeMMagnetic nanoparticles: synthesis, stabilization, functionalization, characterization, and applicationsJ Iran Chem Soc201071137
- Petri-FinkAHofmannHSuperparamagnetic iron oxide nanoparticles (SPIONs): from synthesis to in vivo studies – a summary of the synthesis, characterization, in vitro, and in vivo investigations of SPIONs with particular focus on surface and colloidal propertiesIEEE Trans Nanobioscience20076428929718217622
- Editors of Encyclopaedia BritannicaCurie point [webpage on the Internet]Encyclopaedia Britannica Available from: http://www.britannica.com/EBchecked/topic/146902/Curie-pointAccessed October 14, 2013
- MartirosyanKSThermosensitive magnetic nanoparticles for self-controlled hyperthermia cancer treatmentJ Nanomed Nanotechnol20123612
- JordanAScholzRMaier-HauffKThe effect of thermotherapy using magnetic nanoparticles on rat malignant gliomaJ Neurooncol200678171416314937
- LuAHSalabasELSchüthFMagnetic nanoparticles: synthesis, protection, functionalization, and applicationAngew Chem Int Ed Engl20074681222124417278160
- WuWHeQJiangCMagnetic iron oxide nanoparticles: synthesis and surface functionalization strategiesNanoscale Res Lett200831139741521749733
- SuhSKYuetKHwangDKBongKWDoylePSHattonTASynthesis of nonspherical superparamagnetic particles: in situ coprecipitation of magnetic nanoparticles in microgels prepared by stop-flow lithographyJ Am Chem Soc2012134177337734322462394
- Perez De BertiIOCagnoliMVPecchiGAlternative low-cost approach to the synthesis of magnetic iron oxide nanoparticles by thermal decomposition of organic precursorsNanotechnology2013241717560123548801
- SunSZengHSize-controlled synthesis of magnetite nanoparticlesJ Am Chem Soc2002124288204820512105897
- HyeonTLeeSSParkJChungYNaHBSynthesis of highly crystalline and monodisperse maghemite nanocrystallites without a size-selection processJ Am Chem Soc200112351127981280111749537
- ParkJAnKHwangYUltra-large-scale syntheses of monodisperse nanocrystalsNat Mater200431289189515568032
- InouyeKEndoROtsukaYMiyashiroKKanekoKIshikawaTOxygenation of ferrous ions in reversed micelle and reversed microemulsionJ Phys Chem198286814651469
- LeeYLeeJBaeCJLarge-scale synthesis of uniform and crystalline magnetite nanoparticles using reverse micelles as nanoreactors under reflux conditionsAdv Functional Mat2005153503509
- PengDLSumiyamaKHiharaTYamamuroSKonnoTJMagnetic properties of monodispersed Co/CoO clustersPhys Rev B200061431033109
- BönnemannHBrijouxWBrinkmannRA size-selective synthesis of air stable colloidal magnetic cobalt nanoparticlesInorganica Chimica Acta2003350617624
- MassartRPreparation of aqueous magnetic liquids in alkaline and acidic mediaMagn IEEE Trans198117212471248
- ShenLLaibinisPEHattonTABilayer surfactant stabilized magnetic fluids: synthesis and interactions at interfacesLangmuir1999152447453
- SousaMHTourinhoFANew electric double-layered magnetic fluids based on copper, nickel, and zinc ferrite nanostructuresJ Phys Chem B2001105611681175
- WanMLiJSynthesis and electrical–magnetic properties of polyaniline compositesJ Polym Sci A2003361527992805
- ButterworthMDBellSAArmesSPSimpsonAWSynthesis and characterization of polypyrrole–magnetite–silica particlesJ Colloid Interf Sci199618319199
- AstiGSolziMGhidiniMInfluence of domain walls on the singular point detection of energy losses in hard magnetic materialsJ Magn Magn Mater2005290–291Pt 1533535
- BarrattGColloidal drug carriers: achievements and perspectivesCell Mol Life Sci2003601213712613656
- ChoSJIdroboJCOlamitJLiuKBrowningNDKauzlarichSMGrowth mechanisms and oxidation-resistance of gold-coated iron nanoparticlesChem Mater2005171231813186
- CaruntuDCushingBLCaruntuGO’ConnorCJAttachment of gold nanograins onto colloidal magnetite nanocrystalsChem Mater2005171333983402
- BanZBarnakovYALiFGolubVOO’ConnorCJThe synthesis of core–shell iron@gold nanoparticles and their characterizationJ Mater Chem20051546604662
- LiuQXuZFinchJAEgertonRA novel two-step silica-coating process for engineering magnetic nanocompositesChem Mater1998101239363940
- ZhangJPostMVeresTLaser-assisted synthesis of superparamagnetic Fe@Au core-shell nanoparticlesJ Phys Chem B2006110147122712816599475
- ChenHShaoLLiQWangJGold nanorods and their plasmonic propertiesChem Soc Rev2013422679272423128995
- YoungJKFigueroaERDrezekRATunable nanostructures as photothermal theranostic agentsAnn Biomed Eng201240243845922134466
- VigdermanLKhanalBPZubarevRFunctional gold nanorods: synthesis, self-assembly, and sensing applicationsAdv Mater201224364811484122740090
- GenzelLMartinTPKreibigUDielectric function and plasma resonances of small metal particlesZ Physik B1975214339346
- NikoobakhtBWangZLEl-SayedMASelf-Assembly of gold nanorodsJ Phys Chem B20001043686358640
- KimJYAhCSJangDJControlled aspect ratios of gold nanorods in reduction-limited conditionsJ Nanomater201140585317
- BrownKRWalterDGNatanMJSeeding of colloidal Au nanoparticle solutions. 2. Improved control of particle size and shapeChem Mater2000122306313
- JanaNRGearheartLMurphyCJSeed mediated growth approach for shape-controlled synthesis of spheroidal and rod-like gold nanoparticles using a surfactant templateAdv Mater2001131813891393
- JanaNRGearheartLMurphyCJWet chemical synthesis of high aspect ratio gold nanorodsJ Phys Chem B20011051940654067
- HuMHillyardPHartlandGVKoselTPerez-JusteJMulvaneyPDetermination of the elastic constants of gold nanorods produced by seed-mediated growthNano Lett200441224932497
- WuWHuangJWuLTwo-step size- and shape- separation of biosynthesized gold nanoparticlesSep Purif Technol2013106117122
- WeiGTLiuFKWangCRCShape separation of nanometer gold particles by size exclusion chromatographyAnal Chem199971112085209121662743
- JanaNRNanorod shape separation using surfactant assisted self-assemblyChem Commun20031519501951
- SharmaVParkKSrinivasaraoMShape separation of gold nanorods using centrifugationProc Natl Acad Sci U S A2009106134981498519255445
- MitamuraKImaeTFunctionalization of gold nanorods toward their applicationsPlasmonics200942330
- MitamuraKImaeTSaitoNTakaiOFabrication and structure of alginate gel incorporating gold nanorodsJ Phys Chem C20081122416422
- GorelikovIFieldLMKumachevaEHybrid microgels photoresponsive in the near-infrared spectral rangeJ Am Chem Soc200412649159381593915584708
- KumarVRRSamalAKSreeprasadTSPradeepTGold nanorods grown on microgels leading to hexagonal nanostructuresLangmuir200723178667866917637011
- GentiliDOriGFranchiniMCDouble phase transfer of gold nanorods for surface functionalization and entrapment into PEG-based nanocarriersChem Commun20093958745876
- KhanalBPZubarevERRings of nanorodsAngew Chem Int Edit2007461321952198
- RadushkevichLVLukyanovichVMAbout structure of carbon created at thermal decomposition of carbon monoxide on iron contactJ Phys Chem1952268895
- IijimaSHelical microtubules of graphitic carbonNature19913545658
- KumarMAndoYChemical vapor deposition of carbon nanotubes: a review on growth mechanism and mass productionJ Nanosci Nanotechnol20101063739375820355365
- WangMZhaoXOhkohchiMAndoYCarbon nanotubes grown on the surface of cathode deposit by arc dischargeFullerene Sci Technol19964510271039
- ZhaoXLWangMOhkohchiMAndoYMorphology of carbon nanotubes prepared by carbon arcJpn J Appl Phys199635Pt 144514456
- ParkanskyNBoxmanRLAlterkopBZontagILereahYBarkayZSingle-pulse arc production of carbon nanotubes in ambient airJ Phys D Appl Phys2004371927152719
- SornsuwitNMaaithongWStudy of multi-walled carbon nanotubes synthesis using liquid nitrogen and the purificationPoster presented at: Asian Symposium for Precision Engineering and NanotechnologyNovember 6–9, 2007Gwangju, South Korea
- MontoroLALofranoRCZRosolenJMSynthesis of single-walled and multi-walled carbon nanotubes by arc-water methodCarbon200543200203
- JungSHKimMRJeongSHHigh-yield synthesis of multi-walled carbon nanotubes by arc discharge in liquid nitrogenAppl Phys A2003762285286
- IijimaSIchihashiTSingle-shell carbon nanotubes of 1-nm diameterNature1993363603605
- BethuneDSKiangCHDe VriesMSCobalt-catalysed growth of carbon nanotubes with single-atomic-layer wallsNature1993363605607
- ChenBZhaoXInoueSAndoYFabrication and dispersion evaluation of single-wall carbon nanotubes produced by FH-arc discharge methodJ Nanosci Nanotechnol20101063973397720355400
- FanWWLuYKBaoWRZhaoJFanBYPreparation of coal-based carbon nanotube with FeS-Co2O3 as catalystJ Mat Eng200810363365
- MoshkalyovSAMoreauALDGuttiérrezHRCottaMASwartJWCarbon nanotubes growth by chemical vapor deposition using thin film nickel catalystMater Sci Eng B2004112147153
- Dikonimos MakrisTHGiorgiLGiorgiRCVD synthesis of carbon nanotubes on different substratesNato Sci Ser II Math20062225960
- RenZFHuangZWXuJWSynthesis of large arrays of well-aligned carbon nanotubes on glassScience19982825391110511079804545
- FanSChaplineMGFranklinNRTomblerTWCassellAMDaiHJSelf-oriented regular arrays of carbon nanotubes and their field emission propertiesScience199928354015125149915692
- NessimGDProperties, synthesis, and growth mechanisms of carbon nanotubes with special focus on thermal chemical vapor depositionNanoscale201021306132320820718
- ShinkaiMYanaseMHondaHWakabayashiTYoshidaJKobayashiTIntracellular hyperthermia for cancer using magnetite cationic liposomes: in vitro studyJpn J Cancer Res19968711117911839045948
- YanaseMShinkaiMHondaHWakabayashiTYoshidaJKobayashiTIntracellular hyperthermia for cancer using magnetite cationic liposomes: ex vivo studyJpn J Cancer Res19978876306329310134
- YanaseMShinkaiMHondaHWakabayashiTYoshidaJKobayashiTIntracellular hyperthermia for cancer using magnetite cationic liposomes: an in vivo studyJpn J Cancer Res19988944634699617354
- ItoAShinkaiMHondaHHeat shock protein 70 expression induces antitumor immunity during intracellular hyperthermia using magnetite nanoparticlesCancer Immunol Immunother2003522808812594571
- ItoAHondaHKobayashiTCancer immunotherapy based on intracellular hyperthermia using magnetite nanoparticles: a novel concept of “heat-controlled necrosis” with heat shock protein expressionCancer Immunol Immunother200655332032816133113
- LeBShinkaiMKitadeTPreparation of tumor-specific magnetoliposomes and their application for hyperthermiaJ Chem Eng Jpn20013416672
- http://www.nanostart.de/en/news/press-releases/portfolio/archive/2011/873-nanostart-holding-magforce-reimbursement-of-nanotherm-therapy
- KostJNoeckerRKunicaELangerRMagnetically controlled release systems: effect of polymer compositionJ Biomed Mater Res19851989359403880352
- EdelmanERKostJBobeckHLangerRRegulation of drug release from polymer matrices by oscillating magnetic fieldsJ Biomed Mater Res198519167834077873
- KumarCSMohammadFMagnetic nanomaterials for hyperthermia-based therapy and controlled drug deliveryAdv Drug Deliv Rev201163978980821447363
- BrazelCSMagnetothermally-responsive nanomaterials: combining magnetic nanostructures and thermally-sensitive polymers for triggered drug releasePharm Res200926364465619005741
- HoareTSantamariaJGoyaGFA magnetically triggered composite membrane for on-demand drug deliveryNano Lett20099103651365719736912
- BehrensSBönnemannHMatoussevitchNAir-stable Co-, Fe-, and Fe/Co-nanoparticles and ferrofluidsPhys Chem20062201340
- AtaiNABalajLVan VeenHHeparin blocks transfer of extra-cellular vesicles between donor and recipient cellsJ Neurooncol201311534335124002181
- LuZProutyMDGuoZGolubVOKumarCSLvovYMMagnetic switch of permeability for polyelectrolyte microcapsules embedded with Co@Au nanoparticlesLangmuir20052152042205015723509
- ZhangHPanDZouKHeJDuanXA novel core–shell structured magnetic organic–inorganic nanohybrid involving drug-intercalated layered double hydroxides coated on a magnesium ferrite core for magnetically controlled drug releaseJ Mater Chem20091930693077
- JainPKEl-SayedIHEl-SayedMAAu nanoparticles target cancerNano Today2007211829
- MehdizadehAPandeshSShakeri-ZadehThe effects of folate-conjugated gold nanorods in combination with plasmonic photothermal therapy on mouth epidermal carcinoma cellsLasers Med Sci972013
- ChoiJYangJParkJSpecific near-IR absorption imaging of glioblastomas using integrin-targeting gold nanorodsAdv Funct Mater201121610821088
- Fernandez CabadaTde PabloCSSerranoAMGuerrero FdelPOlmedoJJGomezMRInduction of cell death in a glioblastoma line by hyperthermic therapy based on gold nanorodsIntl J Nanomedicine2012715111523
- OliMAptamer conjugated gold nanorods for targeted nanothermal radiation of glioblastoma cancer cellsYoung Sci J2010381825
- AgarwalAMackeyMAEl-SayedMABellamkondaRVRemote triggered release of doxorubicin in tumors by synergistic application of thermosensitive liposomes and gold nanorodsACS Nano2011564919492621591812
- XiaoYHongHMatsonVZGold nanorods conjugated with doxorubicin and cRGD for combined anticancer drug delivery and PET imagingTheranostics20122875776822916075
- ZhangZWangLWangJMesoporous silica-coated gold nanorods as a light-mediated multifunctional theranostic platform for cancer treatmentAdv Mater201224111418142322318874
- FabbroCAli-BoucettaHDa RosTKostarelosKBiancoAPratoMTargeting carbon nanotubes against cancerChem Commun2012483339113926
- MarkovicZMHarhaji-TrajkovicLMTodorovic-MarkovicBMIn vitro comparison of the photothermal anticancer activity of graphene nanoparticles and carbon nanotubesBiomaterials20113241121112921071083
- WangCHChiouSHChouCPChenYCHuangYJPengCAPhotothermolysis of glioblastoma stem-like cells targeted by carbon nanotubes conjugated with CD133 monoclonal antibodyNanomedicine201171697920620237
- WangCHHuangYJChangCWHsuWMPengCAIn vitro photothermal destruction of neuroblastoma cells using carbon nanotubes conjugated with GD2 monoclonal antibodyNanotechnology2009203131510119597244
- ZonghuanLMalcolmPDZhanhuGMagnestic switch of permeability for polyelectrolyte microcapsules embedded with nanoparticlesLangmuir2005212042205015723509