Abstract
Near-infrared fluorescence (NIRF) imaging is an attractive modality for early cancer detection with high sensitivity and multi-detection capability. Due to convenient modification by conjugating with moieties of interests, NIRF probes are ideal candidates for cancer targeted imaging. Additionally, the combinatory application of NIRF imaging and other imaging modalities that can delineate anatomical structures extends fluorometric determination of biomedical information. Moreover, nanoparticles loaded with NIRF dyes and anticancer agents contribute to the synergistic management of cancer, which integrates the advantage of imaging and therapeutic functions to achieve the ultimate goal of simultaneous diagnosis and treatment. Appropriate probe design with targeting moieties can retain the original properties of NIRF and pharmacokinetics. In recent years, great efforts have been made to develop new NIRF probes with better photostability and strong fluorescence emission, leading to the discovery of numerous novel NIRF probes with fine photophysical properties. Some of these probes exhibit tumoricidal activities upon light radiation, which holds great promise in photothermal therapy, photodynamic therapy, and photoimmunotherapy. This review aims to provide a timely and concise update on emerging NIRF dyes and multifunctional agents. Their potential uses as agents for cancer specific imaging, lymph node mapping, and therapeutics are included. Recent advances of NIRF dyes in clinical use are also summarized.
Introduction
Cancer remains a great challenge against global public health and a tremendous economic burden on society despite significant progress in comprehensive therapy. Recently, the death toll resulting from cancer in the US reached half a million in 2013. It is projected that, in 2014, the total number of newly diagnosed cancer patients will be over 1.6 million in US.Citation1 Although interventions such as radical surgical treatment, radiotherapy, and chemotherapy were applied, many patients die within a year after initial cancer diagnosis. The main inadequacy of current diagnostic imaging methods is the relatively low specificity and sensitivity in the detection of cancer at an early stage. Several imaging modalities, such as ultrasound, X-ray radiography, computed tomography (CT), magnetic resonance imaging (MRI), and positron-emission tomography scanning (PETS), are widely utilized to detect the functional and structural changes in disease areas. However, these conventional imaging modalities sometimes fail to achieve a high contrast among malignancies, benign lesions, and adjacent normal tissues.Citation2,Citation3 Thus, a novel imaging technique is imperative to enhance the theranostics and improve the surveillance of cancers at any stage.
Optical imaging holds great promise as an ideal modality for cancer imaging. Light signals emitted from biological tissues present molecular information that is related to the pathophysiological change. Near-infrared fluorescence (NIRF) imaging, with high sensitivity and multi-detection capability, is an attractive modality for early cancer detection among potential optical imaging technologies. This approach basically depends on a fluorescence probe with emissions in the NIR region. In this region (650–900 nm), lower tissue autofluorescence and less fluorescence extinction enhance deep tissue penetration with minimal background interference.Citation4
Nanotechnology significantly facilitates the development of both therapeutic and diagnostic agents. Theranostic nanomedicine, the integration between diagnosis and treatment, takes advantage of imaging and therapeutic functions to simultaneously detect and treat disease. Thus, it allows for successive determination of agent distribution, release, and efficacy, which is anticipated to achieve personalized medicine.Citation5–Citation7
Strong interest has been attracted in bioimaging and therapeutics of NIRF probes in the last two decades. Numerous novel NIRF dyes with fine photophysical properties have been developed. These dyes can easily be conjugated with various moieties such as small molecules, nucleotides, double-stranded deoxyribonucleic acid (DNA), DNA primers, amino acids, proteins, and antibodies to acquire specific targeting abilities.Citation2 Due to convenient modification by conjugating with moieties of interest, NIR dyes are ideal candidates for cancer imaging with high specificity and sensitivity. Nanoparticles (NPs) containing NIRF dyes and anticancer agents contribute to the synergistic management of cancer. Moreover, novel NIRF dyes alone can be utilized as effective agents for photothermal and photodynamic therapy.
This review aims to provide a timely and essential update on emerging NIRF dyes and multifunctional agents. Their potential uses for cancer-specific imaging, lymph node mapping, and therapeutics are included. These advances have widely extended current concepts of cancer theranostics by NIRF imaging.
Classifications of organic NIRF dyes
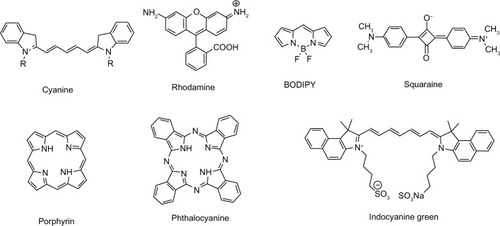
Although NIRF dyes attract intensive attention in bioimaging, only a few of them are readily available owing to poor photostability and hydrophilicity, and difficulties of signal capture in heterogeneous tissues in vivo. Extensive research has been focused on the redesign and adaptation of NIRF dyes to overcome these limitations. Great effort has been made to develop new NIRF dyes with better photostability and strong fluorescence emission. Parts of the hydrophobic dyes are improved, with stronger hydrophilicity to decrease self-aggregation. These new dyes show high potential for biomedical imaging. Herein, NIRF dyes are classified into several categories, including cyanines, rhodamine analogs, 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPYs), squaraines, phthalocyanines, and porphyrin derivatives and other related dyes in light of their NIR organic fluorophore platforms.Citation8 The basic chemical structures of these dyes are presented in .
Figure 1 Basic chemical structures of NIRF dyes.
Abbreviations: NIRF, near infrared fluorescent; BODIPY, 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene.
Cyanine dyes
Cyanine dyes were first developed by Williams in 1856. Indocyanine green (ICG), for instance, is a tricarbocyanine dye that was permitted by the US Food and Drug Administration (FDA) for medical diagnostic application over 50 years ago.Citation9 Typical cyanine dye contains two nitrogen-containing heterocycles as charged chromophores, each conjugated to the end of a polymethine that comprises an odd number of carbons. Most of these dyes display narrow absorption bands and high extinction coefficients. Some cyanines (Cy5 and Cy7) emit in the NIR region, presenting as NIRF dyes with relatively strong fluorescence intensity and extinction coefficients.Citation10 The elongation of the middle polymethine by the vinylene bond (CH=CH) results in red shift by approximately 100 nm, and the extension of the nitrogen-containing heterocycles leads to a red shift by 20 nm.Citation8 Traditional cyanine dyes display poor photostability, self-aggregation, and low quantum yields in aqueous solution, while a cyclohexenyl group replacement in the central link of polymethine significantly increases the photostability and fluorescence intensity.Citation2 Ongoing efforts in the research of cyanine dyes are focused on exploiting their value in biomedical applications.
James and coworkers constructed a series of symmetrical polymethine dyes containing different bis-N-substituted indoles, linkers, and benzindole moieties. They found that commercially available heptamethine carbocyanine IR-783 with a cyclic chloro-cyclohexene moiety showed better fluorescence-imaging ability.Citation11 IR-783 and its derivative MHI-148, have demonstrated potential as optical imaging agents both in vivo and in vitro for the rapid detection of human kidney cancer.Citation12 Fluorescent hyaluronan (HA) analogs were developed by linking different molar percentages of IR-783 derivative. Hyaluronidase-mediated HA biodegradation was not influenced by IR-783 conjugation. Moreover, this imaging probe (17% dye in HA) could detect HA fragments to study the HA uptake and degradation.Citation13 Tan et al developed two multifunctional NIRF heptamethine dyes, IR780 and IR808, with suitable optical characteristics, good biocompatibility, and with the ability to target against cancer cells.Citation14 A polyethylene glycol (PEG)ylated IR-786 derivative, synthesized by Lu et al, indicated a large stokes shift, reduced cytotoxicity, and enhanced photostability.Citation15 By functionalization with magnetic NPs and polymers, the stokes shift of IR-820 could be tuned.Citation16 New octupolar merocyanine chromophores developed by Poronik et al could also be easily tuned into the NIR region.Citation17 The colored conjugate base of 1,3-bis(dicyanomethylidene)indan also displayed the ability as an anionic NIRF dye for biomolecule imaging.Citation18
Rhodamine dyes
Rhodamine dyes belong to the class of xanthene dyes. These dyes have been extensively exploited as fluorescent probes owing to their favorable photophysical characteristics, such as great molar extinction coefficients and resistance in photobleaching. However, the fluorescence probes adapted from classic rhodamine dyes emit visible light only (500–600 nm wavelength), and are thus unavailable for in vivo bioimaging. Interestingly, their fluorescence properties can be easily modified through a ring close/open process or photo-induced electron transfer (PET).Citation19,Citation20 Thus, numerous NIR rhodamine derivatives have been developed by modifying the xanthene core, through various mechanisms to regulate fluorescence properties, such as PET, oxidation– reduction, and spiro ring opening of xanthenes.Citation8
Sun et al revealed three rhodamine derivatives that maintained their properties. They emitted fluorescence in the NIR region with great potential for biological application.Citation21 SiR680 and SiR700, synthesized via modifying the Si-rhodamine scaffold, could emit strong NIR fluorescence in aqueous media.Citation22 2-Me TeR, a NIRF dye that selectively probed reactive oxygen species (ROS) utilizing tellurium, could monitor dynamically in vivo endogenous ROS levels.Citation23 Unique NIR-absorbing xanthene chromophores could also be synthesized by modulating the HOMO-LUMO (highest occupied molecular orbital-lowest unoccupied molecular orbital) gap in xanthene dyes.Citation24
BODIPY-based NIRF probes
BODIPY, initially depicted in 1968, is advantageous for bioimaging due to high extinction coefficients and quantum yield as well as thermal and photochemical stability.Citation25 However, absorption and emission wavelengths of classical BODIPY dyes are not in the NIR region. Therefore, great efforts in seeking ideal BODIPY derivatives have been made by research groups worldwide. Currently, two main strategies are adopted to shift BODIPY into NIRF dyes, modifying the phenyl rings and merging the 3- and 5-phenyl rings with the aza-BODIPY core, which forms six-membered rings and reduces the torsion angles formed by peripheral phenyl groups and the central core.Citation26,Citation27 Enhancement of co-planarity between phenyls and the central core results in NIR bathochromic shifts, probably due to electron delocalization.Citation28
Diphenyl dithienyl aza-BODIPY was synthesized through the substitution of phenyl groups by thiophene in aza-BODIPY. Remarkable red shifts and strong NIR fluorescence could be found.Citation28 Bromo-substituted BODIPY containing thienopyrrole moieties was reported by Yang’s group. Exposure of these products in the NIR region exhibited a high singlet oxygen quantum yield that leads to photo cytotoxicity.Citation29 Sakamoto and coworkers developed a series of new alpha-bridged linear BODIPY oligomers that displayed strong absorption and high fluorescence efficiency in the NIR region.Citation30 Two novel NIRF BODIPY dyes, each containing two pyridinium groups showed DNA photocleavage ability through the production of free radicals. These implied the potential mechanism and feasibility of BODIPY-based photodynamic therapy (PDT).Citation31 DC-SPC, a BODIPY derivative, was fabricated by the classical Knoevenagel condensation method between 3, 5-dimethyl-BODIPY dyes and N-propargyl carbazole aldehyde. DC-SPC emitted in the NIR region and displayed desirable photostability. After facile functionalization into DC-SPC-PPh3, superior amphipathy, cell membrane permeability, and specific localization in mitochondria were revealed.Citation32 Stable NIR BODIPY developed via a novel pyrrole was non-cytotoxic and suitable for bio-imaging assay.Citation33
Squaraine-based NIRF probes
Squaraines (squarylium dyes), consisting of a central ring-based core and a zwitterionic structure, were first developed by Treibs and Jacob in 1965.Citation34 The oxocyclobutenolate core is linked by aromatic or heterocyclic components at both ends, which shapes a donor–acceptor–donor motif. These dyes typically emit in the red to NIR region, with distinct physical–chemical properties. However, the relatively large scale and hydrophobic features of conventional squaraines remain a grave challenge. Currently, three main strategies for the design of NIRF squaraine dyes are exploited, regulating aggregation or disaggregation of squaraine dyes, modifying squaraine dyes with specific binding moiety, and inducing squaraine formation or destruction in certain conditions. Squaraine-based probes have been developed for different targets, including ions, small molecules, and proteins through these effective strategies. For instance, bis(vinyl ruthenium)-modified squaraine dyes are synthesized through a reversible polyelectrochromic switch that regulates the NIR absorption bands. Fluorescence signal could be easily turned off or shifted deep into the NIR region.Citation35 Adding dicyanovinyls into the framework of conventional squaraines enhances NIR fluorescence properties and chemical robustness.Citation36
Phthalocyanines and porphyrin derivatives
Phthalocyanines and porphyrin derivatives are versatile functional pigments containing four isoindole or pyrrole nitrogen atoms.Citation37 There are 4n+2 π electrons in the middle of phthalocyanines. It was revealed that the π electrons are strongly delocalized around the chromophore, which renders high thermal and chemical stability that can endure intense electromagnetic radiation. Moreover, two hydrogen atoms in the central part can be readily modified by metal ions and various substituents, thus changing the physical properties. Although applications in the fields of electronics, optoelectronics, and biomedicine can be found, their emission bands are under the NIR region.Citation2 Hence, strategies such as ligating the benzene group and replacing multiple electron-donating substituents can be applied to acquire NIRF derivatives. Porphyrins and expanded porphyrins contain four or more pyrrole or heterocyclic rings. When used as new platforms for NIRF probe design, such macro-cyclic structures show the advantages of superior chemical stability and excellent photophysical properties.
Their new derivatives are also promising in clinical application for cancer theranostics. Karunakaran et al developed a hydrophilic porphyrin (THPP) and its derivative (Zn-THPP). They displayed superior quantum yield and excellent free radical generation rates. In comparison with the clinical drug Photofrin, THPP exhibited higher photodynamic activity. Moreover, THPP rapidly permeated into cells and localized in the nucleus, demonstrating its potential application as a NIR probe for PDT as well as nucleus imaging.Citation38
NIRF probes based on other types of dyes
Besides cyanine dyes, rhodamine derivatives, BODIPYs, porphyrins and squaraines, some other types of NIR fluorophores have also been recently investigated. Ashitate et al demonstrated that zwitterionic NIR fluorophore ZW800-1 could provide real-time intraoperative imaging of hepatic vessels precisely during biliary tract surgery, outperforming ICG in dual-channel NIR fluorescence imaging.Citation39 When conjugated with cancer-targeting moieties, ZW800-1 provided a much improved signal-to-noise ratio (SNR) over IRDye800-CW and Cy5.5.Citation40 Hyaluronated fullerene was found to exhibit strong NIRF intensity without complex labelling with other fluorophores or isotopes. This fullerene could be applied for high-resolution NIRF imaging of tumor sites in vivo. Moreover, a remarkable regression of HCT-116 tumors but not normal tissues was found, demonstrating its potential anti-tumor activity.Citation41 Xiong et al constructed a NIRF analog of 2′,7′-dichlorofluorescein with high photostability and permeability. This dye was specifically located in the mitochondria and could be readily employed for thiol sensing via a strong turn-on NIRF signal.Citation42 Sun et al revealed that two-photon carbazole derivatives could be used as NIRF dyes for nucleic acid imaging applications.Citation43 2′-hydroxychacone derivatives of boron difluoride were synthesized with intense NIR emission in the solid state. They possessed many interesting properties that were suitable for applications in materials science as unique solid-state NIR fluorophores.Citation44 Shao et al reported a new class of NIRF dendrimeric quaterrylenedi-imide dyes exhibiting high photostability and hydrophilicity and low toxicity to normal cells.Citation45 A self-quenching composite was constructed by conjugating galactosyl human serum albumin (hGSA) and NMP1, a bacteriochlorin-based NIRF dye. The complex selectively linked to the D-galactose receptor of ovarian cancer cells and 75% of 555 peritoneal ovarian cancer metastases and was detected by hGSA-NMP1.Citation46 Although significant progress in NIRF probe design and their biological applications has been made, they are still fledgling compared to visible light optical imaging and many challenges remain in the development of clinically available NIRF dyes.
NP-based NIRF probes
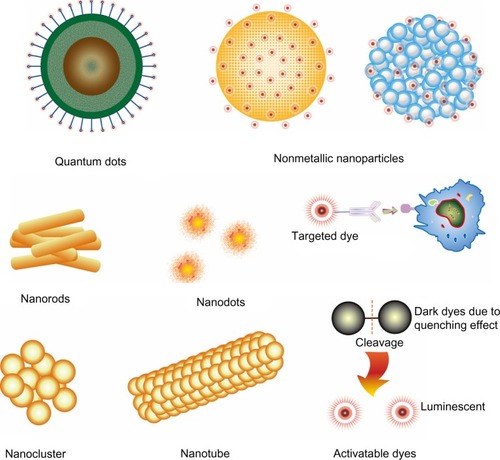
Advances in nanotechnology facilitate the development of complex multicomponent nanomaterials, significantly contributing to the fast growing biomedicine branch. Great progress has been made in the field of NP-based probes for cancer theranostics. Newly developed probes selectively accumulate in malignant lesions via the enhanced permeability and retention (EPR) effect of tumor micro-vasculatures,Citation47,Citation48 and bind to specific receptors on cancer cells through corresponding ligands in NPs. Thus, NP-based NIRF probes, such as NIRF dye-containing nonmetallic NPs, NIRF gold nanostructures, and quantum dots (QDs), attract strong interest as platforms for early-stage cancer detection (). Three main types of these fascinating probes developed in recent years are described below.
Figure 2 Structural characteristics of nanoparticle-based NIRF probes.
Notes: QDs are comprised of a core/shell structure and a coating that minimizes their potential toxicity. Furthermore, specific moieties with targeting ability can be linked to the surface. Nonmetallic nanoparticles include dye-loaded micelles and polymer-based structures that retain NIRF dyes inside or on the surface. Nanorods, nanodots, nanoclusters, and nanotubes are mainly developed using gold nanomaterials. Most of the targeted dyes are linked with moieties that have selective binding activity. Activatable NIRF dyes originally have little fluorescence emission; when disintegrated, these dyes emit strong fluorescence.
Abbreviations: NIRF, near infrared fluorescent; QDs, quantum dots.
NIRF dye-containing nonmetallic NPs
NIRF dye-containing nonmetallic NPs are based on nanomaterials of polymer and inorganic matrices that confine various NIRF dyes. Organic NIRF dyes are typically embedded through non-covalent or covalent combination to shape functional NPs with a core–shell architecture. Owing to the protective architecture, these probes show enhanced photostability and biocompatibility, low self-aggregation, bright fluorescence signal, with bioconjugation that is easily tunable.Citation49
Different routes to obtain functional NIRF nonmetallic NPs have been employed in recent years. ICG molecules with a polyethylenimine moiety were incorporated into a silica matrix, which could avoid aggregation and reduced self-quenching of ICG molecules.Citation50 Zheng et al developed a self-assembled ICG-containing nanostructure using ICG and phospholipid-PEG (PL-PEG). ICG-PL-PEG increased the NIR-dependent temperature efficiently and could target tumor cells when further linked with an antibody.Citation51 In another study, Cohen et al developed NIRF albumin NPs using human serum albumin (HSA) and a NIR dye derivative in aqueous solution.Citation52 The HSA-CANIR NPs were covalently conjugated with tumor-targeting ligands at the carboxylate site through the carbodiimide activation method. NIRF dye encapsulation within the HSA NPs significantly reduced photobleaching. Moreover, the prepared NIRF probes could specifically target colon cancer, even undistinguishable tumors that could previously only be confirmed by histopathological analysis. 1,1′-dioctadecyltetramethyl indotricarbocyanine iodide was incorporated into micelles to acquire dye-loaded NPs. The prepared NPs demarcated the tumor with high resolution, demonstrating the capability of both optical imaging and photothermal treatment.Citation53
Despite recent progress in NIRF dye-containing NPs, the characteristic discrepancies such as absorption, biodistribution, metabolism, and excretion remain high owing to large variations in the physicochemical properties. With the ongoing efforts to adapt their functions and endow multiplex imaging capability, NIRF nonmetallic nanoprobes are expected to play important roles for in vivo optical imaging.
Gold nanostructures
Numerous nanostructures with different sizes and shapes, such as nanorods, nanodots, nanoclusters, nanoshells, nanotubes, and nanocages, have been extensively investigated as candidates for Raman imaging and photoacoustic imaging (PAI) mainly due to their excellent optical properties, high stability, lower toxicity, and bioconjugation simplicity.Citation54–Citation57 Fluorescent gold nanoprobes (GNP), characterized by ultra-small size, tunable optical properties, and considerable biocompatibility, are available as targeted probes for in vivo optical imaging.Citation58
Through the modification on poly-(allylamine hydrochloride) followed by Rose Bengal (RB) molecule conjugation, a gold nanorod (GNR) platform was constructed. The RB-GNR platform exhibited significant NIR absorption and emission, excellent photostability, good biocompatibility, and specific identification of oral cancer cells.Citation59 Au-MPA was constructed by the conjugation of bluish-green fluorescence-containing gold nanoclusters (Au NCs) and a NIR organic dye, MPA, and displayed low toxicity and high affinity to tumor.Citation60 In another study, folic acid (FA) was linked to Au NCs followed by MPA conjugation (Au-FA-MPA). Doxorubicin (DOX), a clinically used anticancer drug, was then conjugated to the folate-conjugated Au NCs. Au-FA-MPA selectively targeted folate receptor (FR) positive tumors and Au-FA-DOX displayed high anti-tumor activity due to FR-mediated uptake, supporting the potential use of ligand-modified Au NCs for cancer imaging and targeted therapy.Citation61 Au-Met-MPA, which was constructed using Au NCs, methionine (Met), and MPA, also exhibited NIR tumor-targeting capability with low cytotoxicity.Citation62
Despite advances in nanotechnology, the strategy of incorporating multiple materials and/or agents into one GNP with optimal properties remains challenging. Intractable problems concerning purity, solubility, stability in physiological environments, and the extreme synthetic process of these probes limit their imaging applications in a larger scope.
Quantum dots
Semiconductor QDs are fluorescent inorganic core-shell nanocrystals with easily modified emission wavelengths and excellent extinction coefficients. Generally, the fluorescence of QDs depends on the size or shape of the intrinsic nanocrystal. QDs with a relatively large core emit longer wavelengths in the NIR region. Therefore, QDs can be finely adapted via their size and composition to obtain suitable excitation profiles and high absorption coefficients. Furthermore, QDs exhibit large Stokes shifts and can endure photobleaching.Citation63 Owing to these features, QDs have been extensively investigated for biomedical applications.Citation64 However, in vivo imaging with QDs is challenged by their potential toxicity, relatively large size, and short circulation time. To solve the potential acute and chronic toxicity of cadmium-based NIR QDs, a layer of less toxic coating is often considered in the design of QDs.Citation65,Citation66 In addition, the water solubility of QDs is crucial to their biological applications.
Xue et al systematically evaluated four different methods to enhance the water solubility of QDs (via micelles, amphiphilic polymers, nanohydrogels, and water-soluble thiols). It was demonstrated that micelles and thiols best retained the photophysical property of QDs.Citation67,Citation68 Oil-soluble NIR PbS QDs entrapped in micelles were also investigated for in vivo tumor imaging. Low cytotoxicity PbS QDs-loaded SOC (biodegradable micelles) selectively accumulated in cancer tissues through the EPR effect of SOC micelles, making them suitable for the evaluation of nanocarrier biodistribution and tumor-targeting behavior.Citation69 After modification of the QDs with a coating of silica and PEG, the ultra-stable PbS QDs were available for sentinel lymph node (SLN) imaging.Citation70 Ag2S QDs displayed strong optical signals in deep tissue with high resolution. When functionalized with PEG, the composites were found in the reticuloendothelial system and could be removed through feces without appreciable toxicity.Citation71,Citation72 Ag2S QDs terminated with a carboxylic acid group showed strong fluorescence and good photostability that is suitable for future biomedical applications.Citation73
Biocompatible core/shell QDs were constructed with Zn2+ ions to address the unexpected blue-shift of CuInS2-based nanocrystals. The prepared QDs coupled with cyclic Arg-Gly-Asp (cRGD) peptides emitted stronger signals than ZCIS/ZnS QDs in cancer detection.Citation74 CdTeS QDs coated with N-acetyl-l-cysteine (NAC) were synthesized as both bioactive ligands and a sulfur source with excellent optical properties and low toxicity. After folate-PEG decoration of the CdTeS alloyed QDs, the conjugated NIR QDs displayed good biocompatibility, excellent sensitivity, and specificity for optical imaging of tumors overexpressing the folate receptor.Citation75 Singh et al reported the functionalization of magnetic NIR QDs. When folic acid was covalently linked to these QDs, they exhibited improved specificity and unique magnetic properties.Citation76 In Gao’s study, dendron-coated InP/ZnS core/shell NIR QDs featured high stability, low cytotoxicity, the ability of extravasation, and were a suitable size for kidney excretion.Citation77 Encapsulation of phosphorothiolated oligonucleotides (5–10 nucleotides) into the QD shell resulted in excellent chemical, photonic, and colloidal stability, high quantum yields, and tunable spectral emission.Citation78 Loading QDs into deoxycholic acid-conjugated low-molecular-weight heparin micelles rendered excellent stability. These NPs were absorbed via bile acid transportation and cleared via the urinary system.Citation79
It is worth noting that NP-based NIRF probes are not limited to the three types aforementioned. Lanthanide compounds,Citation80 for example, can be synthesized by Yb(3+) chelation,Citation81 displaying NIR luminescence. Lanthanum hexaboride NPs are suitable for NIRF application since they are easy to prepare, cheaper than gold nanostructure, and have a distinct property of high photothermal conversion rate.Citation82
Multimodal NIRF probes
Although fluorescence imaging is highly sensitive to tagging a corresponding target and is reliable for molecular imaging, few anatomical details can be acquired under microscopic observation. The combinatory application of NIRF imaging and other imaging modalities that can delineate anatomical structures extends fluorometric determination of biomedical information. Various multimodal imaging schemes are designed for facile combination between NIRF probes and traditional imaging approaches that offer in vivo tumor images with excellent spatial resolution, such as MRI,Citation16,Citation83–Citation86 CT, PETS,Citation87 single photon emission computed tomography (SPECT),Citation88 PAI,Citation89 Raman imaging,Citation90,Citation91 etc. The main multimodal strategies are superparamagnetic iron oxide and gadolinium MR contrast conjugated with NIRF dyes or NPs.Citation16,Citation92–Citation94 Covalent binding of Fe3O4 and different NIR dyes, such as Cy5 and QDs, has been extensively reported.Citation95–Citation97 Xi et al combined NIR dye-labeled magnetic iron oxide NPs with a targeting moiety for urokinase plasminogen activator receptor (uPAR). The NIR830-ATF-IONP combination dramatically enhanced photoacoustic signals in tumors.Citation98 A rhodamine complex was yielded after Gd3+ chelation. The dual-modality paramagnetic/NIRF probe was feasible for both MRI and NIRF imaging to trace cell migration, homing, and differentiation in vivo.Citation99 Magnetic NPs with NIRF dyes embedded in the silica could be applied as a PET/MRI/NIRF imaging agent for biomedical imaging as well as cell tracking after being labeled with a radioisotope on NPs.Citation87 PAI, a non-invasive imaging modality that allows for combined optical signals and ultrasonography, can provide more details of biological tissues.Citation100 Both endogenous molecules and exogenous agents render this optical absorption ability. Jeon et al demonstrated the feasibility of photoacoustic cystography by NIR dyes with low toxicity.Citation101 Thus, appropriate multimodal molecular probes can exert synergistic advantages over any single modality alone.
NIRF probes with targeting abilities
Probes with targeting abilities are critical for specific and sensitive bioimaging. Although several native organic dyes display strong and intrinsic capabilities of tumor targeting, most of the NIRF dyes lack specific targeting properties, limiting further bioimaging application. Therefore, attaching targeting moieties to these dyes or NIRF dye-containing NPs is a crucial element in probe design. At present, frequently used targeting moieties are antibodies, peptides, proteins, aptamers, and small receptor ligands.Citation102 Excitingly, appropriate probe design with targeting moieties almost retains the original NIRF optical properties as well as pharmacokinetics and in vivo biodistribution.
Three main strategies are involved in designing targeted NIRF probes: single coupling between signaling and targeting moieties, target-activatable probes with a turn-on option, and molecular probes with two different targeting sites.Citation103 The interaction between activatable probes and their targets triggers a turn-on effect through oxidation or enzymatic cleavage, which disintegrates the probes into fluorescent dyes. In that circumstance, initial dark probes due to quenching effects are activated and restore the ability of fluorescence emission. Besides, NPs can also function as carriers by specific receptor identification and/or EPR effect to avoid the direct conjugation of fluorophores and target sites. Therefore, the development of probes with targeting ability holds great promise in targeted cancer imaging and related therapeutic applications.
Native NIRF dyes with tumor-targeting ability
Several native NIRF dyes have been demonstrated with unique tumor-targeting capability without conjugation to guiding moieties or incorporation into nanostructures. Currently, heptamethine indocyanine dyes such as IR-780 iodide, IR-783 (), MHI-148, and porphyrin derivatives Pz 247, are identified with preferential accumulation in cancer tissues, displaying great advantages over the common fluorescent dyes, like ICG.Citation104 Although the mechanism accounting for the specific accumulation and retention of these dyes remains unclear, the implications of mitochondrial membrane potential, organic-anion transporting polypeptides (OATPs), and EPR effect in this process have been illustrated. These natively multifunctional NIRF dyes extend our understanding in tumor-targeted imaging.
NIRF NPs with specific targeting ability
Most of the NIRF dyes require conjugation with targeting moieties to obtain specific binding abilities. These probes are first applied as fluorescent biosensors for real-time tracing of glucose,Citation105 metal ions, and anions.Citation106,Citation107 With the rapid development in bioimaging, targeted NIRF probes are introduced for cell labeling and tracking in the physiological conditionsCitation108 as well as morphological and functional evaluations in cancer cells and interstitial tissues, such as the surveillance of pH change,Citation109 hypoxia state,Citation110,Citation111 enzyme activity,Citation112,Citation113 apoptosis, and necrosis.Citation114 The pH determination relies on the unique chromophores that are susceptible to protonation and deprotonation. Syntheses of receptor-targeted NIRF probes are based on the different receptor-expressing patterns on the surface of cancer cells. A host of receptors have been exploited as ideal targets for NIRF imaging and cancer therapy, such as integrin receptor,Citation112 human epidermal growth factor receptor 2 (HER2) receptor,Citation115 folate receptor,Citation116,Citation117 transferrin receptor,Citation118 translocator protein receptor,Citation119 endothelin receptor,Citation120 somatostatin receptor,Citation121 and gastrin-releasing peptide receptor.Citation122 The targeting moieties, targets, and NP platforms that have been reported in recent years are summarized in .
Table 1 Targeted NIRF probes
In fact, receptor-targeted NIRF imaging is definitely not limited to the aforementioned receptors. Other targets, such as CXC chemokine receptor type 4 (CXCR4) and CXCR7, prostate specific membrane antigen, uPA, low-density lipoprotein (LDL) receptors, vasoactive intestinal peptide (VIP) receptors, type I insulin-like growth factor receptor (IGF1R), and estrogen receptors have also been extensively investigated for selective NIRF imaging. Meanwhile, various molecules and proteins that are involved in carcinogenesis and tumor micro-environments are included in the design of NIRF probes with specific targeting abilities.Citation123,Citation124 Upconversion materials have also been exploited for targeted cancer cell ablation.Citation125 Targeted NIRF technology provides invaluable inspiration for future biomedical application.
Target-activatable NIRF NPs
Compared with other in vivo bioimaging techniques, NIRF imaging benefits greatly from activatable probes. Specifically, these NPs initially emit little fluorescence owing to self-quenching effects. However, when the probes arrive at the target sites, enzyme cleavage occurs and the fluorophores are released, emitting strong fluorescence. Meanwhile, the non-targeting dark probes cannot be selectively activated to generate fluorescence and are quickly cleared out. Therefore, activatable probes significantly improve the signal-to-noise ratio and specificity in NIRF bioimaging applications. Activatable NIRF signals can be detected even at sub-nanomolar levels, displaying excellent sensitivity and deep tissue penetration in vivo. Great attention has been paid to the activatable strategy for highly specific targeted imaging. The discrepancies between malignancies and normal tissues, such as the concentrations of specific enzymes, pH levels, and cellular energy metabolism, were fully exploited to prepare target-activatable NIRF probes.Citation126–Citation128 Most probes are special NPs with enzyme cleavable sequences and quenching abilities through two main strategies to control probe fluorescence, PET and Förster resonance energy transfer (FRET), which result in over 99% quenching in contrast with the free fluorophores.Citation129,Citation130 These probes largely depend on the specificity of cleavable sequences used for the coupling of fluorophores and NPs. Numerous enzymes such as matriptase,Citation131 protein kinase C alpha,Citation132 uPA,Citation133 and matrix metalloproteinase (MMP),Citation134,Citation135 have been successfully used to construct activatable NPs, which might also expand to novel probe design utilizing various enzymes and energy donors, as well as energy acceptors.Citation136
Other than enzyme activation, selective and sensitive turn-on NIRF probes can be obtained by novel strategies.Citation137 Splitting the thiol group of 2,4-dinitrobenzenesulfonyl turns the aza-BODIPY with weak fluorescence to a strong NIR dye that is suitable for the selective detection of cysteine.Citation138 Cy-NiSe and Cy-TfSe were constructed as NIRF probes for thiol detection based on the cleavage of the Se-N bond. They exhibited little fluorescence due to the PET process, while glutathione addition gradually increased their fluorescence intensity.Citation139 Novel sensors could respond to endogenous thiols or H2O2 via a turn-on effect, showing the capability of selective NIRF imaging.Citation140 Huang et al reported a new strategy to detect hydrogen peroxide, glucose, and uric acid using FRET gold NPs, which was in accord with the commercially available method.Citation141 NIRF probes could also detect hydrogen sulfide through the cleavage of dinitrophenyl ether,Citation142 and carboxylic acid via a switch for fluorescence control by spirocyclization.Citation143 These probes appeared to be safe and widely applicable for the noninvasive detection of target biomolecules, making them promising candidates for in vivo imaging applications.Citation144–Citation146 These unique strategies for fluorogenic dye design also open new doors for NIRF probe discovery. Activatable NIRF imaging will become a powerful tool for the diagnosis of cancer and other diseases, and a navigation aid during surgery.
NIRF probes in cancer imaging
Tissues are optically heterogeneous due to different sizes, functional components, and microstructures. This complicates fluorescence imaging due to problems such as autofluorescence, great light absorption, and light scattering. Since the absorption and emission bands of autofluorescence in tissues peak at the near ultraviolet and yellow light regions, NIRF dyes generate less background fluorescence. Moreover, NIRF probes increase the depth of detection in mammalian tissues by several orders of magnitude.
Many NIRF probes have been designed for in vivo cancer imaging applications. Recent development of multifunctional nanomaterials has further improved the availability of NIRF imaging. Nanotechnology-based NIRF probes can efficiently target cancer sites through the EPR effect and selective linking between targeting moieties and tumor cell receptors.
Additionally, NIRF imaging is a potential strategy for intraoperative identification of malignancies. For instance, ICG can clearly identify hepatocellular carcinoma lesions and colorectal cancer liver metastases during surgery.Citation147,Citation148 Presently, intraoperative evaluation of tumor margins depends on palpation and visual inspection. NIRF probes with cancer-targeting ability can provide instrumental information in demarcating tumors. Thus, complete removal of tumors with adequate tumor-free margins maximizes the benefit of surgery and possibly decreases the risk of recurrence. Sherwinter reported his experience of transanal NIR imaging to assess mucosal perfusion at the level of the anastomoses in patients undergoing colectomy. Intraoperative high-resolution NIR mucosal angiography could be obtained easily, demonstrating the feasibility of transanal NIR angiography in surgery.Citation149
Fibroblast activation protein-alpha (FAP-α), a cell surface glycoprotein that promotes tumor growth and invasion, is ubiquitously expressed in malignancies but not in normal tissues. An activatable NIRF probe that contains a NIR dye and a quencher dye was synthesized for real-time imaging of FAP-α. This probe selectively accumulated in FAP-α expressing tumors, demonstrating its targeting ability for early cancer detection.Citation150
NIRF probes in lymph node imaging
The peripheral lymphatic system originates from lymphocapillary vessels, where foreign materials, macromolecules, and excess fluid infiltrate out of the blood. The colorless lymph fluid is then transported through collecting vessels, regional lymph nodes, and lymphatic ducts, finally passing back to the blood circulation to maintain tissue fluid homeostasis. Abnormal lymphatic function may be closely related to malignancies and venous disease.Citation151 SLNs are the initial draining lymph nodes of tumors. The presence of cancer-positive SLNs usually indicates regional metastases. Therefore, lymph node mapping is a requisite for disease staging, prognosis prediction, and decision making in cancer treatment. The identification and dissection of SLNs using methylene blue dye and radioactive colloid have become the current standard for staging cancers such as breast cancer and melanoma. However, radioactive issues, low spatial resolution, and allergic reactions to the blue dye hamper large-scale application of SLNs mapping. Real-time intraoperative imaging with a high resolution via a flexible, portable non-nuclear modality may facilitate the assessment and resection of the SLNs, and minimize surgical complications.
NIRF dyes have been investigated as a surrogate for radioactive colloid and blue dye imaging. Clear visualization of mammary lymph nodes and lymph vessels by ICG demonstrated the applicability of fluorescence-guided SLN resection. It was further confirmed that ICG and other NIR dyes had little impact on lymphatic function such as propulsive velocity and frequency.Citation152 Premixing 150 μg/mL ICG with 60 g/L albumin induced greater fluorescence intensity.Citation153 In Li’s study, QDs were employed in real-time imaging of the rat stomach lymph node basin and functioned well.Citation154
Rapid clearance of ICG in the blood limits its use for long-term tracing applications. Therefore, ICG-loaded NPs are widely considered to prolong ICG retention in vivo. Small NPs (<50 nm in diameter) are mainly retained in the lymph nodes, the selective accumulation of small NPs makes it more suitable for lymph node mapping.Citation155 ICG and LI-COR IRDye 800CW PEG were utilized for lymphatic function imaging. The LI-COR tracer remained visible for 2 weeks. Moreover, this dye produced significantly lower effects on lymphatic function than ICG, and lymph nodes were not enlarged at any time point, indicating the LI-COR tracer as a more appropriate contrast agent for longitudinal lymphatic imaging.Citation156 Jeong et al designed mannosylated liposome-encapsulated ICG (M-LP-ICG) as an optical contrast agent for SLNs mapping. M-LP-ICG had a higher ultraviolet absorbance spectrum, stronger fluorescence intensity, and better stability than LP-ICG, which made it a good contrast agent candidate for the optical imaging of the lymphatic system.Citation157 NIR nanogels prepared by the conjugation of IRDye800 and pullulan-cholesterol polymer nanogels could provide satisfactory imaging of the lymphatic system.Citation158
SLNs mapping by NIRF dye have also been implemented in numerous clinical trials. Maus et al investigated NIRF imaging in a lymphedema patient following surgery and radiotherapy to evaluate the lymphatic structure and function.Citation159 Ten cancer patients participated in NIRF-imaging-guided dissection of head and neck tumors. Real-time surveillance enabled visualization of SLNs in all ten patients, demonstrating the feasibility of NIRF imaging to detect lymph nodes in patients with head and neck cancer.Citation160 Intraoperative SLNs imaging was performed in 25 consecutive patients with biopsy-proven melanoma via NIRF probe (ICG:HSA) and technetium-99m nanocolloid. NIR fluorescent imaging demonstrated an accuracy of 98% when compared with radioactive colloid. Therefore, SLNs mapping with ICG:HSA is feasible and accurate in melanoma.Citation161 Moreover, ICG has the advantage of safety, low cost, and is an intraoperative technique that does not alter the surgical field, thus allowing for easy identification of SLNs.Citation162 Stoffels et al investigated intraoperative fluorescence imaging of lymphatic systems by ICG, SPECT/CT, and preoperative lymphoscintigraphy in 22 patients with melanoma. It was found that 11 additional SLNs were only recognized using fluorescence labeling.Citation163 Twenty-two colon cancer patients were included in another study to assess the additional value of NIRF imaging. Ninety-five percent of patients and 77 SLNs were discovered by NIRF dye, compared to 70 SLNs by blue dye.Citation164 A hybrid NIRF and radioactive tracer was evaluated for SLN imaging in 32 breast cancer patients. All 48 axillary SLNs were found by radioactive tracer and NIRF dye.Citation165 In another study including 49 consecutive breast cancer patients, the use of ICG:HSA also provided additional benefits compared to RC alone.Citation166 In 32 women with vulvar cancer, 35 SLNs in 24 patients were intraoperatively detected by NIRF imaging using ICG:HSA and ICG alone, though no significant difference was found between them.Citation167 In Rossi et al’s study, 17 out of 20 patients with cervical or endometrial carcinoma were observed with SLNs by ICG injection in the cervix, demonstrating that it is a reliable method for intraoperative SLNs mapping.Citation168 Eighteen patients with confirmed neoplasia underwent NIR laparoscopy that could transmit both white light and NIR image. Quick switch of NIRF imaging facilitated the confirmation of mesocolic lymphatic drainage patterns and the identification of SLNs.Citation169
The aforementioned studies ascertain the effectiveness of NIRF imaging in clinical settings. NIRF imaging has been experiencing explosive development and will be employed in more operating rooms and other biomedical applications. With further progress on multifunctional technologies, there will be more NIRF probes for early cancer imaging, treatment evaluation, and intraoperative tumor delineation, as well as cancer therapy.
NIRF probes in cancer therapy
In addition to targeted detection of early cancer, NIRF probes exhibit tumoricidal activities at high concentrations. Moreover, cyanine dyes and porphyrin derivatives are potential photosensitizers for photodynamic therapy in cancer treatment. These multifunctional NIRF dyes can be explored for cancer-targeted therapy after further conjugation with specific moieties. For instance, NP–drug formulations can solubilize or shield hydrophobicity or high toxicity to static agents and overcome bioavailability challenges. Further, NP systems can be used to alter pharmacokinetics and increase the percentage of drug accumulation in cancerous lesions. Their favorable size range and the ease of surface functionalization enable targeting of NPs to specific cell types at diseased sites.Citation170
Photothermal therapy
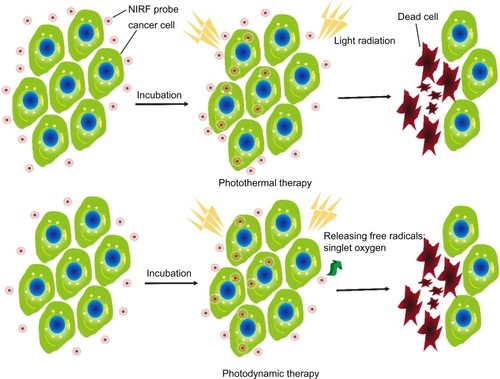
Photothermal therapy (PTT), a noninvasive treatment effective for the treatment of many diseases, has been extensively investigated in cancer treatment.Citation171,Citation172 Minimally invasive laser thermal therapy engendered by NPs or drugs, is among the most promising technologies to arrest expansion of cancerous growths with minimal morbidity and reduced toxicity (). Laser-absorbing agents or dyes are used to increase laser-induced thermal damage in the tumor.
Figure 4 Schematic illustration of PTT and PDT.
Notes: NIRF probes were incubated with cancer cells. Upon light radiation, the accumulation of NIRF probes drastically increased the efficiency of PTT through effective conversion of light energy into heat, resulting in laser-induced thermal damage to cancer cells. In PDT settings, NIRF probes facilitate the generation of cytotoxicity-free radicals as singlet oxygens, and initiate an inflammatory microenvironment that leads to cancer cell death after light radiation.
Abbreviations: NIRF, near infrared fluorescent; PDT, photodynamic therapy; PTT, photothermal therapy.
The accumulation of NIRF probes in tumor sites drastically increases the efficiency of PTT through effective conversion of light energy into heat.Citation82 It has been demonstrated that ICG promotes the absorption of NIR laser light delivered by a diode laser, inducing more thermal damage to solid tumors after laser irradiation compared to laser alone.Citation173,Citation174 In addition, local hyperthermia greatly enhances the delivery of ICG to the tumor site and interstice, thereby allowing a greater thermal ablation effect of laser therapy on the tumor cells, vasculature, and surrounding tumor matrix to induce tumor regression. Hyperthermia by ICG (37°C to 43°C within 1 minute) is also a safe approach to help doxorubicin, a chemotherapy drug, overcome multidrug resistance.Citation175 Due to limitations such as poor photostability, self-aggregation, rapid elimination from the body, and lack of target specificity, ICG is usually encapsulated into the core of a polymeric micelle. Interestingly, ICG-encapsulated micelles are still available for potential application in tumor photothermal therapy.Citation51,Citation176 Cyanine dye IR820 has optical and thermal generation properties similar to those of ICG but with improved in vitro and in vivo stability. It may be an alternative to ICG with greater stability, longer image collection times, and more predictable peak locations.Citation177
Other types of NIRF dyes, such as phthalocyanine-aggregated pluronic NPsCitation178 and IR780-loaded NPsCitation179,Citation180 are also constructed as novel agents for photothermal therapy and/or fractionated photothermal therapy for clinical use. Some of these probes have obtained promising results in preliminary experiments. Researchers will continuously focus on developing suitable NIRF platforms for photothermal applications.
Photodynamic therapy
PDT utilizes light irradiation that is enhanced by photosensitizers to exert therapeutic effects in cancer tissues. When excited by light at a certain wavelength, photosensitizers facilitate the generation of cytotoxic free radicals ().Citation3 These products affect tumor growth by destroying the abnormal neovasculature directly. They also initiate an inflammatory microenvironment that leads to cancer cell death.Citation181 The first approved photosensitizer, Photofrin, is a composite of oligomeric porphyrins that has been applied for the treatment of lung cancer, esophagus cancer, and other types of cancer. In 1993, Photofrin was applied for the first time in PDT to treat bladder tumors. It is noteworthy that current PDT using Photofrin exhibits many drawbacks that limit wide clinical application, such as low deep-tissue penetration, limited tumor specificity, and unwanted localization, especially in the skin, which leads to skin photosensitivity after sunlight exposure. Therefore, developing new photosensitizers without these limitations is imperative for PDT application.
Various NIRF sensitizers have been tested for their use in PDT, such as classical cyanines, squaraines, porphyrins, and phthalocyanines and their derivatives.Citation85 Precise and real-time demarcation of tumors provided by NIRF probes significantly increases the therapeutic efficiency of PDT. Gupta et al reported a new method to construct a multifunctional nanoplatform that could incorporate both the NR fluorophore and photosensitizer for NIRF imaging and photodynamic cancer therapy, making an ideal platform for simultaneous cancer imaging and therapy.Citation182 Karunakaran et al developed two hydrophilic porphyrins, THPP and Zn-THPP. In comparison with Photofrin, these probes have stronger intense fluorescence emission and singlet oxygen generation efficiency, showing the potential for PDT.Citation38
Photoimmunotherapy
Photoimmunotherapy (PIT) is based on cancer-targeted therapy that can selectively monitor and destroy cancer tissues. Nakajima et al developed a NIRF probe for PIT by linking a phthalocyanine dye IR700 and a monoclonal antibody. When exposed to NIR light, the conjugates that had been accumulated in the target sites induced highly specific tumoricidal activities. Selective binding avoided unnecessary injury to normal tissues. It was revealed that IR700 eventually accumulated in lysosomes. After exposure to a threshold intensity of NIR light, the conjugates immediately disrupted the outer cell membrane and lysosomes.Citation183,Citation184 Furthermore, repeated application of NIRF dyes was an effective strategy for cancer therapy without severe side effects; complete pathological remission might even be achieved.Citation185
Drug delivery
Chemotherapy has been widely applied to maximize the therapeutic outcome in cancer treatment. However, most cytotoxic drugs lack the ability of specific accumulation in tumors. In addition, various side effects may occur during the course of chemotherapy. These remain major impediments to the treatment of malignancies.Citation186 Thus, novel platforms for targeted drug delivery that are safe and effective in vivo are highly desirable. Effective delivery of chemical drugs to tumor sites is particularly appealing for the enhancement of the tumor-killing effect and the reduction of systemic toxicities.Citation187,Citation188 It was revealed that drug-loaded NP systems accumulate in tumors through the EPR effect, which increases drug bioavailability and prolongs the exposure to therapeutic agents.Citation189 Rapid uptake and retention of polymer conjugates in the lymphatic system have also been observed with low toxicity.Citation190
Mieszawska et al presented a highly complex and multifunctional hybrid polymer-lipid NP platform that incorporated diagnostic nanocrystals and two therapeutic drugs, the antiangiogenic drug sorafenib and the cytotoxic drug doxorubicin for combined cancer therapy.Citation170 The prepared NPs accumulated at the tumor sites and prevented angiogenesis, leading to cancer cell death. NIR irradiation of a light- sensitive amphiphilic copolymer cleaved the cypate-containing micelles and released cytotoxic o-nitrosobenzaldehyde that could damage the surrounding tissues.Citation191 Turner et al successfully constructed various temperature-sensitive NIRF mixtures to realize efficient drug delivery. The thermosensitive liposome composites were stable at 37°C while burst releases of encapsulated drugs at 40°C and 42°C were observed.Citation192
Currently, antibodies against biomarkers and therapeutic targets of cancer have already been developed. The crosslinking of monoclonal antibodies and NIRF dyes has also been applied for selective cancer theranostics,Citation193 such as cutaneous tumor,Citation194,Citation195 breast cancer,Citation196 ovarian cancer,Citation193 gastric cancer,Citation130 and prostate cancer.Citation197 This controlled drug delivery may potentially address the described limitations of the aforementioned chemotherapy.
Conclusion and perspectives
Over the last decade, NIRF dyes have been studied extensively for biomedical application since the NIR region is a suitable optical window for deep tissue imaging. When appropriately adapted, these dyes show the abilities of fast screening and early detection of cancer and provide invaluable guidance in cancer therapy, reducing cancer morbidity and mortality. However, conventional dyes experience photobleaching easily and barely achieve sufficient target-to-background ratio for clinical use. Recent developments in nanotechnology and imaging technologies have dramatically facilitated innovations in probe design and multichannel techniques. Many types of NPs have recently been designed and evaluated as potential contrast agents or delivery vehicles for molecular imaging. Successes in the development of receptor-specific NIR nanoprobes and their applications for cancer diagnosis and treatment monitoring have greatly inspired research interest in individualized medical interventions.
NPs provide direct protection of the NIRF dyes against degradation, improve their fluorescence characteristics, and render specific targeting abilities with enhanced signal-to-noise ratio. Although striking results in experimental studies have been obtained, NP-based NIRF probes are still far away from clinical application.Citation198 There are still many obstacles before final clinical trials, especially the pharmacokinetics and potential toxicity related to chemical or metallic materials, dosage, and surface coating.
Therefore, non-toxic, biodegradable, and biocompatible materials are highly recommended for constructing the NIR-related probes. Further development of targeted NIRF dyes and multimodal probes is expected to broaden their roles in basic cancer research and advance into clinical applications. For rapid progress in this field, interdisciplinary collaboration is still needed.
Taken together, NIRF imaging is promising for early stage cancer detection and cancer therapy, but the development of satisfactory NIRF probes remains challenging for investigators worldwide. Although there are still many hurdles before NIRF imaging can advance to clinical applications, huge opportunities and value exist in this fascinating field.
Acknowledgments
Funding was provided by the Scientific Innovative Project of Shaanxi Province, No 2012KTCL03-03.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRMaJZouZJemalACancer statistics, 2014CA Cancer J Clin201464192924399786
- LuoSZhangESuYChengTShiCA review of NIR dyes in cancer targeting and imagingBiomaterials201132297127713821724249
- YuanAWuJTangXZhaoLXuFHuYApplication of near-infrared dyes for tumor imaging, photothermal, and photodynamic therapiesJ Pharm Sci2013102162823132644
- WurthCHoffmannKBehnkeTOhnesorgeMResch-GengerUPolymer-and glass-based fluorescence standards for the near infrared (NIR) spectral regionJ Fluoresc201121395396120393786
- McCarthyJRMultifunctional agents for concurrent imaging and therapy in cardiovascular diseaseAdv Drug Deliv Rev201062111023103020654664
- JanibSMMosesASMacKayJAImaging and drug delivery using theranostic nanoparticlesAdv Drug Deliv Rev201062111052106320709124
- XieJLeeSChenXNanoparticle-based theranostic agentsAdv Drug Deliv Rev201062111064107920691229
- YuanLLinWZhengKHeLHuangWFar-red to near infrared analyte-responsive fluorescent probes based on organic fluorophore platforms for fluorescence imagingChem Soc Rev201342262266123093107
- BarnesKDShafirsteinGWebberJSKoonceNAHarrisZGriffinRJHyperthermia-enhanced indocyanine green delivery for laser-induced thermal ablation of carcinomasInt J Hyperthermia201329547447923902340
- LavisLDRainesRTBright ideas for chemical biologyACS CHem Biol20083314215518355003
- JamesNSChenYJoshiPEvaluation of polymethine dyes as potential probes for near infrared fluorescence imaging of tumors: part – 1Theranostics20133969270224019854
- YangXShaoCWangROptical imaging of kidney cancer with novel near infrared heptamethine carbocyanine fluorescent dyesJ Urol2013189270271023000848
- WangWCameronAGKeSDeveloping fluorescent hyaluronan analogs for hyaluronan studiesMolecules20121721520153422314377
- TanXLuoSWangDSuYChengTShiCA NIR heptamethine dye with intrinsic cancer targeting, imaging and photosensitizing propertiesBiomaterials20123372230223922182749
- LuCDasSMagutPKLiMEl-ZahabBWarnerIMIrradiation induced fluorescence enhancement in PEGylated cyanine-based NIR nano- and mesoscale GUMBOSLangmuir20122840144151442322957476
- YenSKJanczewskiDLakshmiJLDesign and synthesis of polymer-functionalized NIR fluorescent dyes – magnetic nanoparticles for bioimagingACS Nano2013786796680523869722
- PoronikYMHuguesVBlanchard-DesceMGrykoDTOctupolar merocyanine dyes: a new class of nonlinear optical chromophoresChemistry201218309258926622730217
- HeoJLimCKWhangDRSelf-deprotonation and colorization of 1,3-bis(dicyanomethylidene)indan in polar media: a facile route to a minimal polymethine dye for NIR fluorescence imagingChemistry201218288699870422689413
- WangTZhaoQJHuHGSpirolactonized Si-rhodamine: a novel NIR fluorophore utilized as a platform to construct Si-rhodamine-based probesChem Comm201248708781878322836301
- ChenXPradhanTWangFKimJSYoonJFluorescent chemosensors based on spiroring-opening of xanthenes and related derivativesChem Rev201211231910195622040233
- SunYQLiuJLvXLiuYZhaoYGuoWRhodamine-inspired far-red to near-infrared dyes and their application as fluorescence probesAngew Chem Int Ed Engl201251317634763622674799
- KoideYUranoYHanaokaKDevelopment of NIR fluorescent dyes based on Si-rhodamine for in vivo imagingJ Am Chem Soc2012134115029503122390359
- KoideYKawaguchiMUranoYA reversible near-infrared fluorescence probe for reactive oxygen species based on Te-rhodamineChem Comm201248253091309322344329
- Sibrian-VazquezMEscobedoJOLowryMFronczekFRStronginRMField effects induce bathochromic shifts in xanthene dyesJ Am Chem Soc201213425105021050822642754
- BoensNLeenVDehaenWFluorescent indicators based on BODIPYChem Soc Rev20124131130117221796324
- ZhangTZhuXWongWKTamHLWongWYLight-harvesting ytterbium(III)-porphyrinate-BODIPY conjugates: synthesis, excitation-energy transfer, and two-photon-induced near-infrared-emission studiesChemistry201319273974823165692
- SarmaTPandaPKSetsuneJBis-naphthobipyrrolylmethene derived BODIPY complex: an intense near-infrared fluorescent dyeChem Comm201349849806980824030220
- ZhangXYuHXiaoYReplacing phenyl ring with thiophene: an approach to longer wavelength aza-dipyrromethene boron difluoride (Aza-BODIPY) dyesJ Org Chem201277166967322111977
- YangYGuoQChenHZhouZGuoZShenZThienopyrrole-expanded BODIPY as a potential NIR photosensitizer for photodynamic therapyChem Comm201349383940394223536148
- SakamotoNIkedaCYamamuraMNabeshimaTalpha-Bridged BODIPY oligomers with switchable near-IR photoproperties by external-stimuli-induced foldamer formation and disruptionChem Comm201248404818482022302032
- WangJHouYLeiWDNA photocleavage by a cationic BODIPY dye through both singlet oxygen and hydroxyl radical: new insight into the photodynamic mechanism of BODIPYsChemphyschem201213112739274722619214
- ZhangXXiaoYQiJLong-wavelength, photostable, two-photon excitable BODIPY fluoro-phores readily modifiable for molecular probesJ Org Chem201378189153916023984818
- JiangXDGaoRYueYSunGTZhaoWA NIR BODIPY dye bearing 3,4,4a-trihydroxanthene moietiesOrg Biomol Chem201210346861686522829188
- McEwenJJWallaceKJSquaraine dyes in molecular recognition and self-assemblyChem Comm2009426339635119841773
- ChenJWinterRFStudies on a vinyl ruthenium-modified squaraine dye: multiple visible/near-infrared absorbance switching through dye- and substituent-based redox processesChemistry20121834107331074122807257
- GaoFPLinYXLiLLSupramolecular adducts of squaraine and protein for noninvasive tumor imaging and photothermal therapy in vivoBiomaterials20143531004101424169004
- JiangJNgDKA decade journey in the chemistry of sandwich-type tetrapyrrolato-rare Earth complexesAcc Chem Res2009421798818767871
- KarunakaranSCBabuPSMadhuriBIn vitro demonstration of apoptosis mediated photodynamic activity and NIR nucleus imaging through a novel porphyrinACS Chem Biol20138112713223092119
- AshitateYStockdaleAChoiHSLaurenceRGFrangioniJVReal-time simultaneous near-infrared fluorescence imaging of bile duct and arterial anatomyJ Surg Res2012176171321816414
- ChoiHSGibbsSLLeeJHTargeted zwitterionic near-infrared fluorophores for improved optical imagingNat Biotechnol201331214815323292608
- KwagDSParkKOhKTLeeESHyaluronated fullerenes with photoluminescent and antitumoral activityChem Comm201349328228423174913
- XiongXSongFChenGConstruction of long-wavelength fluorescein analogues and their application as fluorescent probesChemistry201319216538654523589345
- SunYZhaoYLiuXTRenAMFengJKYuXQTheoretical investigation of the two-photon absorption properties of 3,6-bis(4-vinylpyridinium) carbazole derivatives – new biological fluorescent probesJ Mol Model20121862357236721989961
- D’AleoAGachetDHeresanuVGiorgiMFagesFEfficient NIR-light emission from solid-state complexes of boron difluoride with 2′- hydroxychalcone derivativesChemistry20121840127641277222933268
- ShaoPBaiMPhotostable, hydrophilic and functional near infrared quaterrylenediimide-cored dendrimers for biomedical imagingChem Comm201248769498950022896838
- AlexanderVMSanoKYuZGalactosyl human serum albumin-NMP1 conjugate: a near infrared (NIR)-activatable fluorescence imaging agent to detect peritoneal ovarian cancer metastasesBioconjug Chem20122381671167922799539
- GreishKEnhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targetingMethods Mol Biol2010624253720217587
- MaedaHMacromolecular therapeutics in cancer treatment: the EPR effect and beyondJ Control Release2012164213814422595146
- PellachMGrinbergIMargelSNear IR fluorescent polystyrene/albumin core/shell nanoparticles for specific targeting of colonic neoplasmsMacromol Biosci201212111472147922976925
- QuanBChoiKKimYHKangKWChungDSNear infrared dye indocyanine green doped silica nanoparticles for biological imagingTalanta20129938739322967569
- ZhengXZhouFWuBChenWRXingDEnhanced tumor treatment using biofunctional indocyanine green-containing nanostructure by intratumoral or intravenous injectionMol Pharm20129351452222332810
- CohenSMargelSEngineering of near IR fluorescent albumin nanoparticles for in vivo detection of colon cancerJ Nanobiotechnol20121036
- ShanGWeisslederRHilderbrandSAUpconverting organic dye doped core-shell nano-composites for dual-modality NIR imaging and photo-thermal therapyTheranostics20133426727423606913
- ZhangXBlochSAkersWAchilefuSNear-infrared molecular probes for in vivo imagingCurr Protoc Cytom2012 Chapter 12:Unit12.27
- TianLGandraNSingamaneniSMonitoring controlled release of payload from gold nanocages using surface enhanced Raman scatteringACS Nano2013754252426023577650
- RautSRichRFudalaRResonance energy transfer between fluorescent BSA protected Au nanoclusters and organic fluorophoresNanoscale20136138539124201559
- AntarisALRobinsonJTYaghiOKUltra-low doses of chirality sorted (6,5) carbon nanotubes for simultaneous tumor imaging and photothermal therapyACS Nano2013743644365223521224
- WangJMooreJLaulheSNantzMAchilefuSKangKAFluorophore-gold nanoparticle complex for sensitive optical biosensing and imagingNanotechnology201223909550122327387
- WangJHWangBLiuQBimodal optical diagnostics of oral cancer based on Rose Bengal conjugated gold nanorod platformBiomaterials201334174274428323489924
- ChenHLiBWangCCharacterization of a fluorescence probe based on gold nanoclusters for cell and animal imagingNanotechnology201324505570423307109
- ChenHLiSLiBFolate-modified gold nanoclusters as near-infrared fluorescent probes for tumor imaging and therapyNanoscale20124196050606422930451
- ChenHLiBRenXMultifunctional near-infrared-emitting nano-conjugates based on gold clusters for tumor imaging and therapyBiomaterials201233338461847622951103
- LuYSuYZhouYIn vivo behavior of near infrared-emitting quantum dotsBiomaterials201334174302430823489928
- KawaboriMKurodaSSugiyamaTIntracerebral, but not intravenous, transplantation of bone marrow stromal cells enhances functional recovery in rat cerebral infarct: an optical imaging studyNeuropathology201232321722622007875
- HelleMCassetteEBezdetnayaLVisualisation of sentinel lymph node with indium-based near infrared emitting Quantum Dots in a murine metastatic breast cancer modelPloS one201278e4443322952979
- GuYPCuiRZhangZLXieZXPangDWUltrasmall near-infrared Ag2Se quantum dots with tunable fluorescence for in vivo imagingJ Am Chem Soc20121341798222148738
- XueBCaoJDengDFour strategies for water transfer of oil-soluble near-infrared-emitting PbS quantum dotsJ Mater Sci Mater Med201223372373222311073
- DengDXiaJCaoJForming highly fluorescent near-infrared emitting PbS quantum dots in water using glutathione as surface-modifying moleculeJ Colloid Interface Sci2012367123424022122944
- CaoJZhuHDengDIn vivo NIR imaging with PbS quantum dots entrapped in biodegradable micellesJ Biomed Mater Res A2012100495896822275223
- WangDQianJCaiFHeSHanSMuY‘Green’-synthesized near-infrared PbS quantum dots with silica-PEG dual-layer coating: ultrastable and biocompatible optical probes for in vivo animal imagingNanotechnology2012232424570122641266
- ZhangYHongGZhangYAg2S quantum dot: a bright and biocompatible fluorescent nanoprobe in the second near-infrared windowACS nano2012653695370222515909
- ZhangYZhangYHongGBiodistribution, pharmacokinetics and toxicology of Ag2S near-infrared quantum dots in miceBiomaterials201334143639364623415643
- JiangPZhuCNZhangZLTianZQPangDWWater-soluble Ag(2)S quantum dots for near-infrared fluorescence imaging in vivoBiomaterials201233205130513522484042
- GuoWChenNTuYSynthesis of Zn-Cu-In-S/ZnS core/shell quantum dots with inhibited blue-shift photoluminescence and applications for tumor targeted bioimagingTheranostics2013329910823422883
- XueBDengDWCaoJSynthesis of NAC capped near infrared-emitting CdTeS alloyed quantum dots and application for in vivo early tumor imagingDalton Trans201241164935494722451225
- SinghNCharanSSanjivKSynthesis of tunable and multifunctional Ni-doped near-infrared QDs for cancer cell targeting and cellular sortingBioconjug Chem201223342143022304752
- GaoJChenKLuongRA novel clinically translatable fluorescent nanoparticle for targeted molecular imaging of tumors in living subjectsNano Lett201212128128622172022
- DengZSamantaANangreaveJYanHLiuYRobust DNA-functionalized core/shell quantum dots with fluorescent emission spanning from UV-vis to near-IR and compatible with DNA-directed self-assemblyJ Am Chem Soc201213442174241742723036133
- KhatunZNurunnabiMChoKJLeeYKOral delivery of near-infrared quantum dot loaded micelles for noninvasive biomedical imagingACS Appl Mater Interfaces2012483880388722839507
- MenelaouMOuharrouFRodriguezLRoubeauOTeatSJAliaga-AlcaldeNDy(III)- and Yb(III)-curcuminoid compounds: original fluorescent single-ion magnet and magnetic near-IR luminescent speciesChemistry20121837115451154922851519
- TeraiTUranoYIzumiSKojimaHNaganoTA practical strategy to create near-infrared luminescent probes: conversion from fluorescein-based sensorsChem Comm201248232840284222222313
- LaiBHChenDHLaB6 nanoparticles with carbon-doped silica coating for fluorescence imaging and near-IR photothermal therapy of cancer cellsActa Biomater2013977556756323542555
- NiDZhangJBuWDual-targeting upconversion nanoprobes across the blood-brain barrier for magnetic resonance/fluorescence imaging of intracranial glioblastomaACS Nano2014821231124224397730
- YehCSSuCHHoWYTumor targeting and MR imaging with lipophilic cyanine-mediated near-infrared responsive porous Gd silicate nanoparticlesBiomaterials201334225677568823639532
- QiaoXFZhouJCXiaoJWWangYFSunLDYanCHTriple-functional core-shell structured upconversion luminescent nanoparticles covalently grafted with photosensitizer for luminescent, magnetic resonance imaging and photodynamic therapy in vitroNanoscale20124154611462322706800
- ChenHWangZMaXZongSCuiYMagnetically controllable dual-mode nanoprobes for cell imaging with an onion-liked structureTalanta201311697898424148504
- KimJSKimYHKimJHDevelopment and in vivo imaging of a PET/MRI nanoprobe with enhanced NIR fluorescence by dye encapsulationNanomedicine20127221922922175235
- GuoYYuanHChoHHigh efficiency diffusion molecular retention tumor targetingPloS one201383e5829023505478
- PanDCaiXKimBStacyAJWangLVLanzaGMRapid synthesis of near infrared polymeric micelles for real-time sentinel lymph node imagingAdv Healthc Mater20121558258923184793
- SatpathyMWangLZielinskiRActive targeting using HER-2- affibody-conjugated nanoparticles enabled sensitive and specific imaging of orthotopic HER-2 positive ovarian tumorsSmall Epub8272013
- O’HanlonCEAmedeKGO’HearMRJanjicJMNIR-labeled perfluoropolyether nanoemulsions for drug delivery and imagingJ Fluor Chem2012137273322675234
- SharmaPBengtssonNEWalterGAGadolinium-doped silica nanoparticles encapsulating indocyanine green for near infrared and magnetic resonance imagingSmall20128182856286822744832
- WeiXWangWChenKPreparation and characterization of ZnS:Tb, Gd and ZnS:Er,Yb,Gd nanoparticles for bimodal magnetic-fluorescent imagingDalton Trans20134251752175923160019
- MaYTongSBaoGGaoCDaiZIndocyanine green loaded SPIO nanoparticles with phospholipid-PEG coating for dual-modal imaging and photothermal therapyBiomaterials201334317706771423871538
- WatePSBanerjeeSSJalota-BadhwarACellular imaging using biocompatible dendrimer-functionalized graphene oxide-based fluorescent probe anchored with magnetic nanoparticlesNanotechnology2012234141510123010805
- LiJJiangHYuZMultifunctional uniform core-shell Fe3O4@mSiO2 mesoporous nanoparticles for bimodal imaging and photothermal therapyChem Asian J20138238539123225542
- MaQNakaneYMoriYMultilayered, core/shell nanoprobes based on magnetic ferric oxide particles and quantum dots for multimodality imaging of breast cancer tumorsBiomaterials201233338486849422906608
- XiLGrobmyerSRZhouGQianWYangLJiangHMolecular photoacoustic tomography of breast cancer using receptor targeted magnetic iron oxide nanoparticles as contrast agentsJ Biophotonics Epub1122012
- WangXYJuSLiCNon-invasive imaging of endothelial progenitor cells in tumor neovascularization using a novel dual-modality paramagnetic/near-infrared fluorescence probePloS one2012711e5057523226317
- GutrathBSBeckmannMFBuchkremerASize-dependent multispectral photoacoustic response of solid and hollow gold nanoparticlesNanotechnology2012232222570722571960
- JeonMKimJKimCPhotoacoustic cystographyJ Vis Exp201376e5034023792925
- ChengKChengZNear infrared receptor-targeted nanoprobes for early diagnosis of cancersCurr Med Chem201219284767478522873665
- BaiMBornhopDJRecent advances in receptor-targeted fluorescent probes for in vivo cancer imagingCurr Med Chem201219284742475822873663
- YangXShiCTongRNear IR heptamethine cyanine dye- mediated cancer imagingClin Cancer Res201016102833284420410058
- KhanFPickupJCNear-infrared fluorescence glucose sensing based on glucose/galactose-binding protein coupled to 651-Blue OxazineBiochem Biophys Res Comm2013438348849223928160
- KarCAdhikariMDRameshADasGNIR- and FRET-based sensing of Cu2+ and S2- in physiological conditions and in live cellsInorg Chem201352274375223302031
- ZhuWHuangXGuoZWuXYuHTianHA novel NIR fluorescent turn-on sensor for the detection of pyrophosphate anion in complete water systemChem Comm201248121784178622218364
- ArmenteroMTBossolascoPCovaLLabeling and tracking of human mesenchymal stem cells using near-infrared technologyMethods Mol Biol20131052132823640251
- FanLFuYJLiuQLLuDTDongCShuangSMNovel far-visible and near-infrared pH probes based on styrylcyanine for imaging intracellular pH in live cellsChem Comm20124891112021120423047294
- OkudaKOkabeYKadonosonoT2-Nitroimidazole-tricarbocyanine conjugate as a near-infrared fluorescent probe for in vivo imaging of tumor hypoxiaBioconjug Chem201223332432922335430
- XuKWangFPanXHigh selectivity imaging of nitroreductase using a near-infrared fluorescence probe in hypoxic tumorChem Comm201349252554255623423494
- ShenDBaiMTangRDual fluorescent molecular substrates selectively report the activation, sustainability and reversibility of cellular PKB/Akt activitySci Rep20133169723603888
- ChengTCRofflerSRTzouSCAn activity-based near-infrared glucuronide trapping probe for imaging beta-glucuronidase expression in deep tissuesJ Am Chem Soc201213463103311022239495
- ThakurMLZhangKPaudyalBTargeting apoptosis for optical imaging of infectionMol Imaging Biol201214216317121538153
- BehnkeTMathejczykJEBrehmRTarget-specific nanoparticles containing a broad band emissive NIR dye for the sensitive detection and characterization of tumor developmentBiomaterials201334116017023072943
- ZhengCZhengMGongPIndocyanine green-loaded biodegradable tumor targeting nanoprobes for in vitro and in vivo imagingBiomaterials201233225603560922575835
- KelderhouseLEChelvamVWayuaCDevelopment of tumor-targeted near infrared probes for fluorescence guided surgeryBioconju Chem201324610751080
- YueJLiuSWangRTransferrin-conjugated micelles: enhanced accumulation and antitumor effect for transferrin-receptor-overexpressing cancer modelsMol Pharm Epub662012
- WyattSKManningHCBaiMPreclinical molecular imaging of the translocator protein (TSPO) in a metastases model based on breast cancer xenografts propagated in the murine brainCurr Mol Med201212445846622348613
- ChenKYapLPParkRA Cy5.5-labeled phage-displayed peptide probe for near-infrared fluorescence imaging of tumor vasculature in living miceAmino acids20124241329133721212998
- BunschotenABuckleTKuilJTargeted non-covalent self-assembled nanoparticles based on human serum albuminBiomaterials201233386787522024362
- CaiQYYuPBesch-WillifordCNear-infrared fluorescence imaging of gastrin releasing peptide receptor targeting in prostate cancer lymph node metastasesProstate201373884285423280511
- WuPGaoYZhangHCaiCAptamer-guided silver-gold bimetallic nanostructures with highly active surface-enhanced Raman scattering for specific detection and near-infrared photothermal therapy of human breast cancer cellsAnal Chem201284187692769922925013
- SuganamiAToyotaTOkazakiSPreparation and characterization of phospholipid-conjugated indocyanine green as a near-infrared probeBioorg Med Chem Lett201222247481748523122858
- XuQCZhangYTanMJAnti-cAngptl4 Ab-conjugated N-TiO(2)/NaYF(4) :Yb,Tm nanocomposite for near infrared-triggered drug release and enhanced targeted cancer cell ablationAdv Healthc Mater20121447047423184779
- GuoJDuCShanLComparison of near-infrared fluorescent deoxyglucose probes with different dyes for tumor diagnosis in vivoContrast Media Mol Imaging20127328930122539399
- WangZZhangXHuangPDual-factor triggered fluorogenic nanoprobe for ultrahigh contrast and subdiffraction fluorescence imagingBiomaterials201334266194620123721793
- SunCWangPLiLA new near-infrared neutral pH fluorescent probe for monitoring minor pH changes and its application in imaging of HepG2 cellsAppl Biochem Biotech Epub10192013
- HeXWangYWangKChenMChenSFluorescence resonance energy transfer mediated large stokes shifting near-infrared fluorescent silica nanoparticles for in vivo small-animal imagingAnal Chem201284219056906423017033
- ShimizuYTemmaTHaraIMicelle-based activatable probe for in vivo near-infrared optical imaging of cancer biomoleculesNanomedicine201410118719523811292
- DengDZhangDLiYAchilefuSGuYGold nanoparticles based molecular beacons for in vitro and in vivo detection of the matriptase expression on tumorBiosens Bioelectron20134921622123770391
- ToitaRMoriTNaritomiYFluorometric detection of protein kinase Calpha activity based on phosphorylation-induced dissociation of a polyion complexAnal Biochem2012424213013622342947
- WangJWheelerDZhangJZAchilefuSKangKANIR fluorophore-hollow gold nanosphere complex for cancer enzyme-triggered detection and hyperthermiaAdv Exp Med Biol201376532332822879051
- MyochinTHanaokaKKomatsuTTeraiTNaganoTDesign strategy for a near-infrared fluorescence probe for matrix metalloproteinase utilizing highly cell permeable boron dipyrrometheneJ Am Chem Soc201213433137301373722830429
- AkersWJXuBLeeHDetection of MMP-2 and MMP-9 activity in vivo with a triple-helical peptide optical probeBioconjug Chem201223365666322309692
- HuangXLanTZhangBRenJGold nanoparticle-enzyme conjugates based FRET for highly sensitive determination of hydrogen peroxide, glucose and uric acid using tyramide reactionAnalyst2012137163659366622745932
- MaityDGovindarajuTA turn-on NIR fluorescence and colourimetric cyanine probe for monitoring the thiol content in serum and the glutathione reductase assisted glutathione redox processOrg Biomol Chem201311132098210423306953
- JiangXDZhangJShaoXZhaoWA selective fluorescent turn-on NIR probe for cysteineOrg Biomol Chem201210101966196822302088
- WangRChenLLiuPZhangQWangYSensitive near-infrared fluorescent probes for thiols based on Se-N bond cleavage: imaging in living cells and tissuesChemistry20121836113431134922829328
- YuanLLinWZhaoSA unique approach to development of near-infrared fluorescent sensors for in vivo imagingJ Am Chem Soc201213432135101352322816866
- HuangXWangJLiuHLanTRenJQuantum dot-based FRET for sensitive determination of hydrogen peroxide and glucose using tyramide reactionTalanta2013106798423598098
- CaoXLinWZhengKHeLA near-infrared fluorescent turn-on probe for fluorescence imaging of hydrogen sulfide in living cells based on thiolysis of dinitrophenyl etherChem Comm20124885105291053122992474
- YuanLLinWYangYChenHA unique class of near-infrared functional fluorescent dyes with carboxylic-acid-modulated fluorescence ON/OFF switching: rational design, synthesis, optical properties, theoretical calculations, and applications for fluorescence imaging in living animalsJ Am Chem Soc201213421200121122176300
- Karton-LifshinNAlbertazziLBendikovMBaranPSShabatD“Donor-two-acceptor” dye design: a distinct gateway to NIR fluorescenceJ Am Chem Soc201213450204122042023194283
- Kisin-FinferEShabatDNew repertoire of ‘donor-two-acceptor’ NIR fluorogenic dyesBioorg Med Chem201321123602360823541837
- MenéndezGOPichelMESpagnuoloCCJares-ErijmanEANIR fluorescent biotinylated cyanine dye: optical properties and combination with quantum dots as a potential sensing devicePhotochem Photobiol Sci201312223624022972309
- van der VorstJRHuttemanMMieogJSNear-infrared fluorescence imaging of liver metastases in rats using indocyanine greenJ Surg Res2012174226627121396660
- MoritaYSakaguchiTUnnoNDetection of hepatocellular carcinomas with near-infrared fluorescence imaging using indocyanine green: its usefulness and limitationInt J Clin Oncol201318223224122200990
- SherwinterDATransanal near-infrared imaging of colorectal anastomotic perfusionSurg Laparosc Endosc Percutan Tech201222543343623047388
- LiJChenKLiuHActivatable near-infrared fluorescent probe for in vivo imaging of fibroblast activation protein-alphaBioconjug Chem20122381704171122812530
- Sevick-MuracaEMTranslation of near-infrared fluorescence imaging technologies: emerging clinical applicationsAnn Rev Med20126321723122034868
- AldrichMBDavies-VennCAngermillerBConcentration of indocyanine green does not significantly influence lymphatic function as assessed by near-infrared imagingLymphat Res Biol2012101202422416911
- WeilerMKassisTDixonJBSensitivity analysis of near-infrared functional lymphatic imagingJ Biomed Opt201217606601922734775
- LiPSunPYangWZhangXReal-time mapping of rat stomach lymph nodes by quantum dotsScand J Gastroenterol201247445446022300390
- ProulxSTLucianiPChristiansenAUse of a PEG-conjugated bright near-infrared dye for functional imaging of rerouting of tumor lymphatic drainage after sentinel lymph node metastasisBiomaterials201334215128513723566803
- WeilerMDixonJBDifferential transport function of lymphatic vessels in the rat tail model and the long-term effects of Indocyanine Green as assessed with near-infrared imagingFront Physiol2013421523966950
- JeongHSLeeCMCheongSJThe effect of mannosylation of liposome-encapsulated indocyanine green on imaging of sentinel lymph nodeJ Liposome Res201323429129723738810
- NohYWKongSHChoiDYNear-infrared emitting polymer nanogels for efficient sentinel lymph node mappingACS Nano2012697820783122862428
- MausEATanICRasmussenJCNear-infrared fluorescence imaging of lymphatics in head and neck lymphedemaHead Neck201234344845322311465
- van der VorstJRSchaafsmaBEVerbeekFPNear-infrared fluorescence sentinel lymph node mapping of the oral cavity in head and neck cancer patientsOral Oncol2013491151922939692
- GilmoreDMKhullarOVGiouxSEffective low-dose escalation of indocyanine green for near-infrared fluorescent sentinel lymph node mapping in melanomaAnn Surg Oncol20132072357236323440551
- van der VorstJRSchaafsmaBEVerbeekFPDose optimization for near-infrared fluorescence sentinel lymph node mapping in patients with melanomaBr J Dermatol20131681939823078649
- StoffelsIvon der StuckHBoyCIndocyanine green fluorescence-guided sentinel lymph node biopsy in dermato-oncologyJ Dtsch Dermatol Ges2012101515722103392
- SchaafsmaBEVerbeekFPvan der VorstJREx vivo sentinel node mapping in colon cancer combining blue dye staining and fluorescence imagingJ Surg Res2013183125325723391167
- SchaafsmaBEVerbeekFPRietbergenDDClinical trial of combined radio- and fluorescence-guided sentinel lymph node biopsy in breast cancerBr J Surg201310081037104423696463
- PolomKMurawaDNowaczykPRhoYSMurawaPBreast cancer sentinel lymph node mapping using near infrared guided indocyanine green and indocyanine green – human serum albumin in comparison with gamma emitting radioactive colloid tracerEur J Surg Oncol201238213714222130469
- SchaafsmaBEVerbeekFPPetersAANear-infrared fluorescence sentinel lymph node biopsy in vulvar cancer: a randomised comparison of lymphatic tracersBJOG2013120675876423418877
- RossiECIvanovaABoggessJFRobotically assisted fluorescence-guided lymph node mapping with ICG for gynecologic malignancies: a feasibility studyGynecol Oncol20121241788221996262
- CahillRAAndersonMWangLMLindseyICunninghamCMortensenNJNear-infrared (NIR) laparoscopy for intraoperative lymphatic road-mapping and sentinel node identification during definitive surgical resection of early-stage colorectal neoplasiaSurg Endosc201226119720421853392
- MieszawskaAJKimYGianellaASynthesis of polymer-lipid nanoparticles for image-guided delivery of dual modality therapyBioconjug Chem20132491429143423957728
- LiMYangXRenJQuKQuXUsing graphene oxide high near-infrared absorbance for photothermal treatment of Alzheimer’s diseaseAdv Mater201224131722172822407491
- HuSHChenYWHungWTChenIWChenSYQuantum-dot-tagged reduced graphene oxide nanocomposites for bright fluorescence bioimaging and photothermal therapy monitored in situAdv Mater201224131748175422422734
- RadziROsakiTTsukaTPhotodynamic hyperthermal therapy with indocyanine green (ICG) induces apoptosis and cell cycle arrest in B16F10 murine melanoma cellsJ Vet Med Sci201274554555122146339
- ShafirsteinGBaumlerWHenningsLJIndocyanine green enhanced near-infrared laser treatment of murine mammary carcinomaInt J Cancer201213051208121521484791
- TangYMcGoronAJIncreasing the rate of heating: a potential therapeutic approach for achieving synergistic tumour killing in combined hyperthermia and chemotherapyInt J Hyperthermia201329214515523350792
- WuLFangSShiSDengJLiuBCaiLHybrid polypeptide micelles loading indocyanine green for tumor imaging and photothermal effect studyBiomacromolecules20131493027303323941524
- Fernandez-FernandezAManchandaRLeiTComparative study of the optical and heat generation properties of IR820 and indocyanine greenMol Imaging20121129911322469238
- LimCKShinJLeeYDPhthalocyanine-aggregated polymeric nanoparticles as tumor-homing near-infrared absorbers for photothermal therapy of cancerTheranostics20122987187923082099
- GutweinLGSinghAKHahnMAFractionated photothermal antitumor therapy with multidye nanoparticlesInt J Nanomed20127351357
- YueCLiuPZhengMIR-780 dye loaded tumor targeting theranostic nanoparticles for NIR imaging and photothermal therapyBiomaterials201334286853686123777910
- QianJWangDCaiFZhanQWangYHeSPhotosensitizer encapsulated organically modified silica nanoparticles for direct two-photon photodynamic therapy and in vivo functional imagingBiomaterials201233194851486022484045
- GuptaAWangSPeraPMultifunctional nanoplatforms for fluorescence imaging and photodynamic therapy developed by post-loading photosensitizer and fluorophore to polyacrylamide nanoparticlesNanomedicine20128694195022115602
- NakajimaTSanoKMitsunagaMChoykePLKobayashiHReal-time monitoring of in vivo acute necrotic cancer cell death induced by near infrared photoimmunotherapy using fluorescence lifetime imagingCancer Res201272184622462822800710
- MitsunagaMNakajimaTSanoKKramer-MarekGChoykePLKobayashiHImmediate in vivo target-specific cancer cell death after near infrared photoimmunotherapyBMC Cancer20121234522873679
- MitsunagaMNakajimaTSanoKChoykePLKobayashiHNear-infrared theranostic photoimmunotherapy (PIT): repeated exposure of light enhances the effect of immunoconjugateBioconjug Chem201223360460922369484
- ZandbergWFBakhtiariABErnoZPhotothermal release of small molecules from gold nanoparticles in live cellsNanomedicine20128690891522100758
- GaneshSIyerAKGattaccecaFMorrisseyDVAmijiMMIn vivo biodistribution of siRNA and cisplatin administered using CD44-targeted hyaluronic acid nanoparticlesJ Control Release2013172369970624161254
- ChengYYuSZhenXWangXWuWJiangXAlginic acid nanoparticles prepared through counterion complexation method as a drug delivery systemACS Appl Mater Interfaces20124105325533223020277
- KhullarOVGrisetAPGibbs-StraussSLNanoparticle migration and delivery of Paclitaxel to regional lymph nodes in a large animal modelJ Am Coll Surg2012214332833722225645
- BagbyTRDuanSCaiSLymphatic trafficking kinetics and near-infrared imaging using star polymer architectures with controlled anionic characterEur J Pharm Sci201247128729422546180
- CaoJHuangSChenYNear-infrared light-triggered micelles for fast controlled drug release in deep tissueBiomaterials201334266272628323721796
- TurnerDCMoshkelaniDShemeshCSLucDZhangHNear-infrared image-guided delivery and controlled release using optimized thermosensitive liposomesPharm Res20122982092210322451250
- XuMRettigMPSudlowGPreclinical evaluation of Mab CC188 for ovarian cancer imagingInt J Cancer201213161351135922130973
- DayKEBeckLNHeathCHHuangCCZinnKRRosenthalELIdentification of the optimal therapeutic antibody for fluorescent imaging of cutaneous squamous cell carcinomaCancer Biol Ther201314327127723298904
- DayKEBeckLNDeepNLKovarJZinnKRRosenthalELFluorescently labeled therapeutic antibodies for detection of microscopic melanomaLaryngoscope2013123112681268923616260
- GongHKovarJLBakerBNear-infrared fluorescence imaging of mammalian cells and xenograft tumors with SNAP-tagPloS one201273e3400322479502
- KimSHParkGHyunHNear-infrared lipophilic fluorophores for tracing tissue growthBiomed Mater20138101411023353894
- MerianJGravierJNavarroFTexierIFluorescent nanoprobes dedicated to in vivo imaging: from preclinical validations to clinical translationMolecules20121755564559122576228
- DuYAnSLiuLSerial non-invasive monitoring of renal disease following immune-mediated injury using near-infrared optical imagingPloS one201279e4394123049742
- CaoJWanSTianJFast clearing RGD-based near-infrared fluorescent probes for in vivo tumor diagnosisContrast Media Mol Imaging20127439040222649045
- KeereweerSMolIMKerrebijnJDTargeting integrins and enhanced permeability and retention (EPR) effect for optical imaging of oral cancerJ Surg Oncol2012105771471821952950
- IftimiaNIyerAKHammerDXFluorescence-guided optical coherence tomography imaging for colon cancer screening: a preliminary mouse studyBiomed Opt Express20123117819122254178
- ZhangLWangKZhaoFNear infrared imaging of EGFR of oral squamous cell carcinoma in mice administered arsenic trioxidePloS one201279e4625523029451
- AgnesRSBroomeAMWangJVermaALavikKBasilionJPAn optical probe for noninvasive molecular imaging of orthotopic brain tumors overexpressing epidermal growth factor receptorMol Cancer Ther201211102202221122807580
- KeereweerSMolIMVahrmeijerALDual wavelength tumor targeting for detection of hypopharyngeal cancer using near-infrared optical imaging in an animal modelInt J Cancer201213171633164022234729
- LeeCMJangDCheongSJOptical imaging of MMP expression and cancer progression in an inflammation-induced colon cancer modelInt J Cancer201213181846185322287125
- ChenYPullambhatlaMBanerjeeSRSynthesis and biological evaluation of low molecular weight fluorescent imaging agents for the prostate-specific membrane antigenBioconjug Chem201223122377238523157641
- ZhouJTsaiYTWengHReal-time detection of implant-associated neutrophil responses using a formyl peptide receptor-targeting NIR nanoprobeInt J Nanomed2012720572068
- PauliJLichaKBerkemeyerJNew fluorescent labels with tunable hydrophilicity for the rational design of bright optical probes for molecular imagingBioconjug Chem20132471174118523758616