?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study aimed to optimize and evaluate a thermoresponsive ophthalmic in situ gel containing curcumin-loaded albumin nanoparticles (Cur-BSA-NPs-Gel). Albumin nanoparticles were prepared via a desolvation method, and the gels were prepared via a cold method. The central composite design and response surface method was used to evaluate the effects of varying Pluronic® F127 and Pluronic® F68 concentrations on the sol–gel transition temperature, which is an indicator of optimum formulations. The optimized formulation was a free-flowing liquid below 30.9°C that transformed into a semi-solid gel above 34.2°C after dilution with simulated tear fluid. Results of the in vitro release and erosion behavior study indicated that Cur-BSA-NPs-Gel achieved superior sustained-release effects and that incorporation of albumin nanoparticles exerted minimal effects on the gel structure. In addition, in vivo ophthalmic experiments employing Cur-BSA-NPs-Gel were subsequently performed in rabbits. In vivo eye irritation results showed that Cur-BSA-NPs-Gel might be considered safe for ophthalmic drug delivery. The in vivo study also revealed that the formulation could significantly increase curcumin bioavailability in the aqueous humor. In conclusion, the optimized in situ gel formulation developed in this work has significant potential for ocular application.
Introduction
Diabetes mellitus is a widely distributed chronic metabolic disorder characterized by impaired glucose metabolism caused by insulin deficiency or insulin resistance; the disease often leads to hyperglycemia and late development of vascular and neurological complications.Citation1 One of the primary complications experienced by diabetic patients is diabetic retinopathy (DR), a severe complication of diabetes mellitus and the leading cause of blindness among middle-aged adults.Citation2 DR is a complex progressive disease of the retina with multifactorial etiology. The pathogenesis of DR is fairly complex and involves different cells, molecules, and factors.Citation3 All of the major retinal cells, including endothelial, ganglion, Müller, and pigment epithelial cells, may be damaged by diabetes.Citation4 Diabetes induces maladjusted levels of metabolites, such as glucose, amino acids, and lipids, which activate retinal cells prior to damage.Citation5,Citation6 Laser photocoagulation remains the cornerstone of treatment for DR patients; however, a specific subgroup of DR patients do not respond effectively to this treatment.Citation7 This therapeutic restriction has promoted interest in developing alternative treatments to address DR. Several therapeutic modalities, including intravitreal injection, ocular local administration, and surgical ablation, are currently under investigation.Citation8,Citation9 Our study focuses on DR management with local ocular administration of drugs.
Curcumin, a traditional medicinal ingredient with poor solubility and stability, is used to provide frontline pharmacotherapy for a number of important diseases.Citation10,Citation11 Modern studies show that curcumin possesses a broad range of medicative properties, including anti-inflammatory, anticancer, antioxidant, and chemopreventive activities.Citation12 Elaborate studies of different molecular pathways modulated by curcumin have proven that the drug is a potential therapeutic and nutraceutical constituent that could be used for the prevention and treatment of a great number of diseases.Citation12,Citation13 Curcumin is proved to be effective for ophthalmic use in various ocular fundus pathologies. It inhibits proliferation of human lens epithelial cells and protects retinal cells, retinal ganglion cells, and corneal epithelial cells.Citation14–Citation16 As diabetes damages mainly the major retinal cells, including endothelial, Müller, ganglion, and pigment epithelial cells, curcumin is used as the model medicine in this study for DR treatment.
Albumin, an omnipotent protein carrier for drug delivery, has been proven to be non-immunogenic, nontoxic, biodegradable, and biocompatible.Citation17 Amino acids in the albumin molecule are linked to each other by peptide bonds, and the molecules are twisted into a reticular structure. This structure is highly conducive for carrying and encapsulating drug molecules and enables delayed drug release. This specific structure has remarkable advantages in increasing the water solubility and stability of the loaded drug.Citation18 As such, albumin is a suitable matrix material for fabricating nanoparticles (NPs) for drug delivery, particularly for drugs with poor water solubility, low bioavailability, and poor stability.Citation19 Albumin NPs (BSA-NPs) have gained considerable research attention because of their excellent capacity for combining multifarious drugs and their property of being well tolerated without serious side-effects.Citation20 Thus, we selected BSA-NPs as curcumin carriers for drug delivery to the eyes.
A liquid formulation based on in situ gels consisting of polymer solutions that may be administered as a liquid was recently developed. The formulation undergoes sol–gel phase transition into a semisolid gel upon exposure to certain physiological conditions, such as a change in temperature or pH or contact with a specific ion.Citation21,Citation22 Gels that undergo sol–gel phase transition caused by a temperature change are called thermoresponsive in situ gels.Citation23 Such gels have been extensively investigated as temperature-responsive materials for invertible thermoresponsive gelation under certain temperatures and concentrations.Citation24 Two of the most frequently used gel matrices are Pluronic® F127 and Pluronic® F68 (BASF SE, Ludwigshafen, Germany); these gels differ only in their ratio of polyethylene oxide (PEO) and polypropylene oxide (PPO).Citation25,Citation26 To obtain a thermoresponsive gel with an appropriate sol–gel transition temperature for an ophthalmic drug delivery system, Ma et alCitation27 incorporated Pluronic F68 into Pluronic F127 solutions to modulate the phase transition temperature. An optimum ophthalmic thermoresponsive in situ gel system must have a sol–gel transition temperature >25.0°C and convert to a semisolid gel form at precorneal temperature (34.5°C) after dilution with tear fluid. Such a property ensures that the ophthalmic thermoresponsive in situ gel can be delivered easily to the eye in drop form to prevent rapid precorneal drug loss, which is a major problem in conventional ophthalmic therapeutics. However, while hydrogels are generally suitable for water-soluble drugs, they are unsuitable for insoluble drugs, because insoluble drugs disperse in aqueous solution as suspensions, which results in poor bioavail-ability. Hence, in this work, BSA-NPs and in situ gels were combined to prolong the drug residence time and improve the ocular bioavailability of the drug.
The present study aims to develop a thermoresponsive in situ gel system containing curcumin-loaded BSA-NPs (Cur-BSA-NPs) with the desired sol–gel phase transition temperature for local ocular administration to treat DR. The central composite design and response surface methods were used to evaluate the effects of varying Pluronic F127 and Pluronic F68 concentrations on the sol–gel transition temperature and select the optimum formulation. The formulation was then evaluated in terms of sol–gel transition temperature, viscosity, in vitro release, and erosion. In vivo eye irritation and ocular availability were evaluated in rabbits. In this study, BSA-NPs and in situ gels were initially combined and applied for ophthalmic use. This combination is an innovation in ocular drug delivery systems and shows significant potential in the clinical treatment of DR.
Materials and methods
Materials
Curcumin was obtained from Shanghai Aladdin Industrial Corporation (Shanghai, People’s Republic of China). Pluronic F127 and Pluronic F68 were generously provided by BASF SE. Bovine serum albumin was purchased from Beijing Solarbio Science & Technology Co., Ltd (Beijing, People’s Republic of China).
Male and female New Zealand White rabbits weighing 2.0–2.5 kg were provided by the Laboratory Animal Center, Chongqing Medical University (People’s Republic of China).
Preparation of Cur-BSA-NPs
BSA-NPs were prepared using a desolvation method, followed by crosslinking with glutaraldehyde.Citation19 Briefly, 10 mg of albumin was dissolved in 1.0 mL of water. This aqueous phase was then desolvated with ethanol (6.0 mL). The coacervates formed were hardened for 4 hours using glutaraldehyde (0.50%, 50 μL). Organic solvents were removed using a rotary evaporator at 35.0°C. The resulting NPs were resuspended in distilled water and diluted to a metered volume of 5.0 mL.
Cur-BSA-NPs were obtained as described above, except that 4.0 mg of curcumin was dissolved in 6.0 mL of ethanol in advance. Ethanol was added dropwise to the aqueous phase at a steady rate of 1 mL per minute. The drug-loading efficiency and encapsulation efficiency of the formulation were evaluated by ultraviolet (UV) spectrophotometry (UV-2000; Unico, Shanghai, People’s Republic of China).
The particle size of each preparation was determined thrice using a Zetasizer (Malvern Instruments, Malvern, UK). The morphology of the Cur-BSA-NPs was observed by transmission electron microscopy (H-7500; Hitachi Ltd, Tokyo, Japan). A drop of the resultant Cur-BSA-NPs suspension was placed onto a carbon-coated 200 mesh copper grid, and the excess staining solution was removed using filter paper. The grid was then thoroughly air-dried for 2 hours prior to examination under the electron microscope.
Preparation of Cur-BSA-NPs-encapsulated in situ gels (Cur-BSA-NPs-Gel)
Thermoresponsive in situ gels were prepared on a weight basis using the cold method.Citation28 Briefly, proportional amounts of Pluronic F127 and Pluronic F68 were added separately into the BSA-NP suspensions, stirred periodically, and refrigerated at 4°C until homogeneous solutions were obtained.
Determination of gelation temperature
A modified test tube inversion method was employed to roughly determine the sol–gel transition temperature of the in situ gels.Citation23 A water bath was used to control the temperature, and the samples were heated at a rate of 0.5°C/min from room temperature. The test tube was inverted every minute, and the flow behavior of the gel was observed. The temperature at which the liquid in the tube remained steady for at least 30 seconds was recorded as the sol–gel transition temperature. Averages and standard deviations of each sample were determined in triplicate.
Optimization of the formulation
A central composite design was used to elucidate the main effects and interactions of the Pluronic F127 (X1) and Pluronic F68 (X2) concentrations. The sol–gel transition temperature was used as the indicator to determine the optimum formulation.Citation29
A two-factor, three-level factorial design was employed for the optimization procedure, using different Pluronic F127 and Pluronic F68 concentrations as the two independent variables. The values of three coded levels of two factors were assumed after preliminary trials (). The sol–gel transition temperatures before (R1) and after dilution (R2) of each formulation were measured as response values. Design-Expert® (Stat-Ease, Inc., Minneapolis, MN, USA) software was used to generate and evaluate the statistical experimental design.Citation30
Table 1 Experimental design of independent parameters in the central composite design
Viscosity measurement of the gels
The viscosity of the prepared formulations (10 mL of the sample) was determined using an NDJ-1 viscometer (Shanghai Tianping Instrument Technology Co., Ltd, Shanghai, People’s Republic of China). A water bath was employed to heat the samples. The viscosity of each formulation was measured at room temperature and after warming up. In situ gel containing curcumin (Cur-Gel) was prepared as a comparison to investigate the effect of BSA-NPs on the gelation temperature. All measurements were performed in triplicate.
In vitro release and erosion behavior study
In vitro release studies of Cur-BSA-NPs-Gel were performed using a dialysis tube with a molecular weight cutoff of 8,000–14,000. Two milliliters of the formulation was placed into a dialysis tube and introduced into 50 mL of simulated tear fluid (STF) maintained at 34.5°C in a shaking water bath (n=3). Sink conditions were maintained in this study.Citation31 The STF (100 mL) was composed of 0.67 g of NaCl, 0.20 g of NaHCO3, and 0.008 g of CaCl2·2H2O and diluted with deionized water to 100 g. The release medium (4 mL) was withdrawn at appropriate intervals (0.17, 0.5, 1, 2, 4, 6, 8, 12, 24, 48, 60, 72, 96, 108, 120, 132, and 144 hours) and replaced by 4 mL of fresh medium. All samples were subjected to UV analysis after proper dilution.Citation32
The erosion behavior study was conducted in a shaking water bath. Briefly, 2.0 g of each gel formulation was added into a pre-weighed vial. The samples were equilibrated at 34.5°C for 10 minutes to convert into semisolid gels prior to each measurement. STF (2 mL) preheated to 34.5°C was added into each vial as the release medium. The vials were weighed each time after STF removal. The disparities in weight of the vials between any two contiguous time points yielded the amount of dissolved gel formulation. The erosion profile was then obtained by plotting the cumulative weight of each formulation dissolved (ordinate) versus time (abscissa).Citation21,Citation27 The experiments were performed in triplicate to obtain the averages and standard deviations of each sample.
In vivo eye irritation test
The in vivo eye irritation test of the Cur-BSA-NPs-Gel was conducted in five New Zealand White rabbits. All tests were carried out in the identical laboratory with constant artificial lighting. After 60 minutes of acclimatization in restrainer boxes, 50 μL of the Cur-BSA-NPs-Gel formulation was instilled into the conjunctival sac of the rabbit’s right eye; the left eye was kept without manipulation (control). The test eye was observed at 0, 0.08, 0.17, 0.5, 1, 6, 12, 24, 48, and 72 hours to compare changes in the cornea, iris, conjunctiva, secretion, and chemosis with the control. Eye irritation levels were scored using the modified Draize test.Citation33
In vivo study
In vivo experiments were performed on New Zealand White rabbits of either sex weighing 2.0–2.5 kg, free from any signs of inflammation or gross abnormality. Ten albino rabbits were divided equally into two groups. Each group was treated in one eye with either Cur-BSA- NPs-Gel as the experimental group or the curcumin suspension (Cur-sus) as the control group. The test solution (50 μL) was instilled into the conjunctival sac of the albino rabbits. The rabbits were then maintained in an upright position using restraining boxes. Aqueous humor (200 μL) was withdrawn at predetermined time points after anesthetization of the rabbits.Citation23
Curcumin content in the aqueous humor was assayed by high-performance liquid chromatography (HPLC) (Agilent 1100; Agilent Technologies, Santa Clara, CA, USA). The instrument had a 20 μL injection volume and was coupled to a UV detector for detection at 422 nm. A Hypersil™ BDS C18 column (15 cm × 4.5 mm; Elite Analytical Instrument Co., Ltd, Dalian, People’s Republic of China) with a particle size of 5 μm was used. The mobile phase consisted of 730 mL of methanol and 270 mL of ultra-pure water containing 1.0% citric acid and was pumped at a flow rate of 1.0 mL/min. The samples were prepared by mixing 20 μL of HCl (0.1 mmol/L) and 2 mL of ethyl acetate into the aqueous humor for 4 minutes. This mixture was centrifuged for 10 minutes at 12,000 rpm. The supernatant was transferred into a fresh tube and then dried under reduced pressure. The residue was dissolved in 50 μL of the mobile phase by swirl mixing for 1 minute, and then centrifuged once more. The supernatant was then analyzed for curcumin content using HPLC.Citation34 Different concentrations of curcumin in the aqueous humor (7.8125, 250, and 4,000 ng/mL) were determined for bioanalytical method validation. DAS2.0, issued by the China Food and Drug Administration for pharmacokinetic study, was applied to calculate relevant pharmacokinetic parameters.
Results and discussion
Preparation of Cur-BSA-NPs-Gel
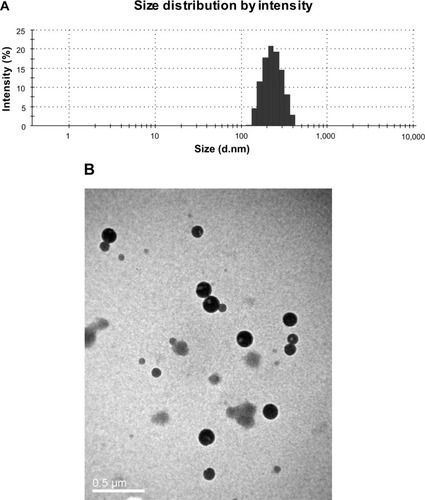
A light yellow, translucent suspension of Cur-BSA-NPs was obtained. The size distribution of the Cur-BSA-NPs () showed a mean size of 221.2 nm. The morphology of the Cur-BSA-NPs () exhibited a normal spherical shape with no aggregation or degradation. The drug-loading efficiency and encapsulation efficiency of the formulation were 4.07%±0.08% and 85.48%±1.03% respectively, indicating excellent drug-loading efficiency. All of the in situ gels prepared by the cold method were transparent and showed good fluidity at room temperature. Thus, the developed formulation may conveniently be stored without a refrigerator, which could promote patient compliance.
Optimization of the formulation
The sol–gel transition temperatures obtained prior to dilution, which were determined from 13 experimental runs generated by the central composite design, ranged from 30.23°C to 35.07°C. By contrast, the sol–gel transition temperatures obtained after dilution ranged from 34.06°C to 38.20°C. The effects of the independent variables (X1, X2) on the sol–gel transition temperatures were evaluated using the gelation temperatures before and after dilution (R1, R2), and the following models were obtained:
clearly shows the linear effects of Pluronic F127 concentration (X1) and Pluronic F68 concentration (X2) on the sol–gel transition temperatures before (R1) and after (R2) dilution. All of the results indicate that the sol–gel transition temperature decreased with increasing X1 and decreasing X2. We selected the optimal formulation at an average ocular surface temperature of about 34.5°C; this formulation consisted of 26% (w/w) Pluronic F127 and 4% (w/w) Pluronic F68 as the gel matrix. The optimized formulation was a free-flowing liquid at <30.9°C and converted to a semisolid gel at >34.2°C after dilution with STF.
Figure 2 Response surface plot showing concentration effect of Pluronic® F127 (X1) and Pluronic® F68 (X2) on sol–gel transition temperature. Sol–gel transition temperatures of in situ gels before (A) and after (B) dilution both increased with the increased concentration of Pluronic F68 and decreased concentration of Pluronic F127.
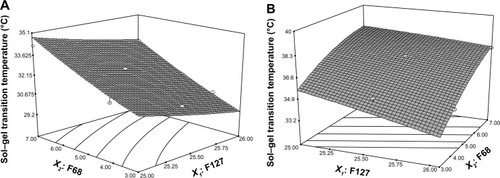
As shown in , the sol–gel transition temperatures tended to decrease with increasing Pluronic F127 concentration. This phenomenon was attributed to changes in the amphiphilic properties of PEO and PPO in the Pluronic F127 molecules brought about by variations in either temperature or Pluronic concentration. The polymers possess amphiphilic structures attributable to the hydrophobic propylene oxide and the hydrophilic ethylene oxide. In aqueous solutions, amphiphilic block copolymer molecules are able to assemble into micelles. Above a certain concentration defined as the critical micelle concentration, the polymer molecules assemble and form micelles. Micelle formation is strongly temperature dependent. At temperatures below the critical micelle temperature, both ethylene and propylene oxide blocks are hydrated; PPO is relatively soluble in aqueous solutions. As the temperature increases, the PPO chains become less soluble than the PEO chains, which results in hydrophobic interactions among the PPOs and formation of spherical micelles composed of a dehydrated PPO core with an outer tumid hydrous PEO shell. Such micelles are difficult to distribute separately in the solution and instead come into contact and entangle with other micelles, this entanglement results in a three-dimensional network structure.Citation35
Viscosity measurement of the gels
The variation in viscosity under different conditions is an important rheological parameter to consider in the utilization and in vivo performance of thermoresponsive in situ gels. Formulations with excessively high viscosity are difficult to administer.Citation30 By contrast, drainage increases if the formulation viscosity is too low. As such, the optimum preparation should possess lower viscosities under storage conditions and higher viscosities in physiological environments.
The viscosity values obtained by the viscometer are given in . At temperatures <31.0°C, both Cur-BSA-NPs-Gel and Cur-Gel showed low viscosities, which indicates that the formulations could be instilled into the eye easily without need for storage in a refrigerator. The viscosities of Cur-BSA-NPs-Gel and Cur-Gel slightly decreased after dilution with STF, but their viscosities at 34.5°C were apparently higher than those at 25°C. This phenomenon indicates that the formulations may potentially prolong the contact time of curcumin in the eye.
Table 2 Viscosity of Cur-BSA-NPs-Gel and Cur-Gel (n=3)
In vitro release and erosion behavior study
A dialysis tube method and a membraneless approach were employed to study the in vitro release and erosion behavior, respectively, of Cur-BSA-NPs-Gel. Given the unique anatomical configuration and physiological characteristics of the eyes, ophthalmic gels are continuously diluted by tear fluid, and the drug must permeate through the cornea to produce an effect. The dialysis tube method allows interpretation of the release behavior of a drug for ophthalmic drug delivery but it cannot adequately study gel erosion behaviors. The membraneless approach is more appropriate for gel erosion behavior studies. Previous investigations have suggested that NPs exhibit burst release. After combination with in situ gels, the NP-loaded thermosensitive gels exhibited reduced burst release, which results from a change in diffusion mechanism. The NPs are initially released from the gel matrix followed by curcumin, resulting in postponed release behavior.
shows a typical plot of the cumulative drug release of the in situ gel formulations versus time. The release study indicated that incorporation of BSA-NPs significantly enhanced the dissolution rate and cumulative release percentage of curcumin compared with the gel alone. Compared with the Cur-BSA-NPs, the combination of BSA-NPs and gel prolonged the release time, which would result in prolonged effects after application in the eyes.
Figure 3 Release curve (A) and erosion curve (B) of different formulations.
Note: Most error bars are smaller than the symbols.
Abbreviations: Cur-BSA-NPs, curcumin-loaded albumin nanoparticles; Cur-BSA-NPs-Gel, gel containing curcumin-loaded albumin nanoparticles; Cur-Gel, gel containing curcumin.
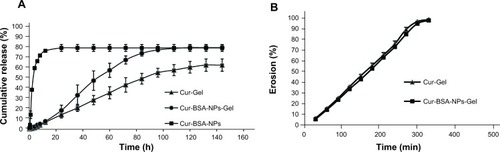
shows the gel erosion behavior obtained using the membraneless method. The behaviors of Cur-BSA-NPs-Gel and Cur-Gel were similar, which demonstrates that incorporation of BSA-NPs into the gel has no effect on the gel structure. Moreover, the gel erosion profiles of both formulations were linear until approximately 95% of the gel had dissolved.
In vivo eye irritation test
Changes in the cornea, iris, conjunctiva, secretion, and chemosis were carefully observed and recorded after the formulations were applied into the conjunctival sac of the rabbit’s eye. As shown in , no lesion formation was observed during the test. Cur-BSA-NPs-Gel did not irritate the rabbit eyes, as indicated by the total score of 0 obtained from eye irritation evaluations. Hence, Cur-BSA-NPs-Gel may be considered safe for ophthalmic drug delivery.
Table 3 Scores for the evaluation of Cur-BSA-NPs-Gel in rabbits, eyes
In vivo study
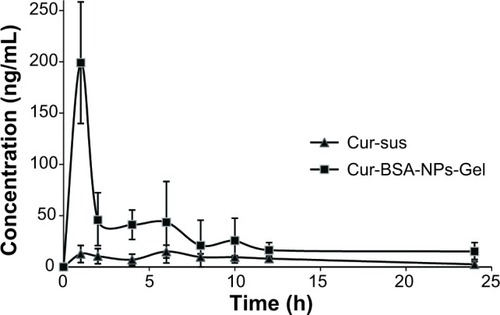
The curcumin concentrations in the aqueous humor of New Zealand White rabbits obtained at various time points after instillation of 50 μL of Cur-BSA-NPs-Gel and Cur-sus are shown in . Curcumin contents in the aqueous humor of rabbits administered Cur-BSA-NPs-Gel were significantly higher at all time points than those obtained after instillation of the Cur-sus. The pharmacokinetic parameters of curcumin in the aqueous humor after instillation of the two formulations are shown in . The maximum curcumin concentration in the aqueous humor observed after Cur-BSA-NPs-Gel administration was 5.6-fold higher than that obtained after administration of the Cur-sus. Calculation of the area under the curve of different formulations indicated that ocular bioavailability in the aqueous humor increased 4.4-fold after application of Cur-BSA-NPs-Gel compared with that observed after administration of the Cur-sus. This result indicated that Cur-BSA-NPs-Gel significantly enhanced the ocular bioavailability of curcumin (P<0.01).
Table 4 Pharmacokinetic parameters of curcumin in rabbit aqueous humor
Figure 4 Concentration of curcumin in rabbit aqueous humor at various time points after instillation of different formulations.
Note: n=5, mean ± standard deviation.
Abbreviations: Cur-BSA-NPs-Gel, gel containing curcumin-loaded albumin nano-particles; Cur-sus, curcumin suspension.
Bioanalytical method validation parameters were statistically analyzed. The mean extract coefficient of recovery was 94.74%±6.16%, the mean method coefficient of recovery was 89.22%±5.41%, and the mean relative standard deviations of within-day and day-to-day precision were 3.78% and 3.94%, respectively.
The results thus far indicate that Cur-BSA-NPs-Gel could significantly increase the curcumin content absorbed by the eyes, resulting in better ophthalmic bioavailability of curcumin compared with the use of curcumin suspension.
Rabbit eyes punctured by a syringe needle and treated with Cur-BSA-NPs-Gel showed better recovery and less swelling, corneal opacity, and redness compared with eyes treated with Cur-sus. This finding indicates that the proposed formulation may be used to treat other eye diseases.
Conclusion
In this study, a thermoresponsive in situ gel system based on Pluronic F127 and Pluronic F68 and containing Cur-BSA-NPs was developed for ophthalmic drug delivery. The physicochemical properties of Cur-BSA-NPs-Gel showed temperature-responsive features. Thus, this formulation, which transforms into a gel when exposed to eye temperature, may be applied as eye drops. The sol–gel transition temperature depended on the Pluronic F127 and Pluronic F68 concentrations; increases in Pluronic F127 content decreased the sol–gel transition temperature of the formulation, whereas increases in Pluronic F68 increased the sol–gel transition temperature. The optimized Cur-BSA-NPs-Gel formulation obtained from this study was composed of 26% (w/w) Pluronic F127 and 4% (w/w) Pluronic F68 as the gelling matrix. In vitro release and erosion behavior studies showed that incorporation of BSA-NPs significantly enhanced the dissolution rate of curcumin without affecting the gel properties. In vivo eye irritation tests of Cur-BSA-NPs-Gel demonstrated that the formulation may be considered safe for ophthalmic use. Cur-BSA-NPs-Gel could also enhance the ophthalmic bioavailability of curcumin in rabbit eyes and remarkably reduce the administration frequency compared with other suspension formulations. Thus, the Cur-BSA-NPs-Gel system proposed in this work shows potential for use as an ophthalmic delivery system with prolonged drug residence time and improved ocular bioavailability.
Acknowledgments
This project was supported by Natural Science Foundation Project of CQ CSTC (No. cstc2012jjA10021), the National Research Foundation for the Doctoral Program of Higher Education of China (No. 20125503120003), Scientific and Technological Research Program of Chongqing Municipal Education Commission (Grant No. KJ110323 and KJ120307), Chongqing Board of Health Project (2013-2-060), Chongqing Yuzhong District Science and Technology Project (No. 20100203), Students’ Research and Innovation Experimental Project of Chongqing Medical University (201244, 201229, 201217), and Students’ Scientific Research Fund of “star of HIFU” (XS201309).
Disclosure
The authors report no conflicts of interest in this work.
References
- AlghadyanAADiabetic retinopathy – an updateSaudi J Ophthalmol20112529911123960911
- SivaprasadSGuptaBCrosby-NwaobiREvansJPrevalence of diabetic retinopathy in various ethnic groups: a worldwide perspectiveSurv Ophthalmol201257434737022542913
- OlaMSNawazMISiddiqueiMMAl-AmroSAbu El-AsrarAMRecent advances in understanding the biochemical and molecular mechanism of diabetic retinopathyJ Diabetes Complications2012261566422226482
- AgrawalSSNaqviSGuptaSKSrivastavaSPrevention and management of diabetic retinopathy in STZ diabetic rats by Tinospora cordifolia and its molecular mechanismsFood Chem Toxicol20125093126313222687550
- BartlettHEEperjesiFNutritional supplementation for type 2 diabetes: a systematic reviewOphthalmic Physiol Opt200828650352319076553
- GrantMBDavisMICaballeroSFeoktistovIBiaggioniIBelardinelliLProliferation, migration, and ERK activation in human retinal endothelial cells through A(2B) adenosine receptor stimulationInvest Ophthalmol Vis Sci20014292068207311481274
- PaiAEl ShafeiMMMohammedOAZAl HashimiMCurrent concepts in intravitreal drug therapy for diabetic retinopathySaudi J Ophthalmol201024414314923960892
- MaiaOOJrTakahashiBSCostaRAScottIUTakahashiWYCombined laser and intravitreal triamcinolone for proliferative diabetic retinopathy and macular edema: one-year results of a randomized clinical trialAm J Ophthalmol20091472291297e218929352
- ChoiKSChungJKLimSHLaser photocoagulation combined with intravitreal triamcinolone acetonide injection in proliferative diabetic retinopathy with macular edemaKorean J Ophthalmol2007211111717460426
- MosieniakGAdamowiczMAlsterOCurcumin induces permanent growth arrest of human colon cancer cells: link between senescence and autophagyMech Ageing Dev2012133644445522613224
- OhSWChaJYJungJECurcumin attenuates allergic airway inflammation and hyper-responsiveness in mice through NF-kappaB inhibitionJ Ethnopharmacol2011136341442120643202
- Molina-JijonETapiaEZazuetaCCurcumin prevents Cr(VI)-induced renal oxidant damage by a mitochondrial pathwayFree Radic Biol Med20115181543155721839166
- WahlHTanLGriffithKChoiMLiuJRCurcumin enhances Apo2L/TRAIL-induced apoptosis in chemoresistant ovarian cancer cellsGynecol Oncol2007105110411217174384
- HuYHHuangXRQiMXHouBYCurcumin inhibits proliferation of human lens epithelial cells: a proteomic analysisJ Zhejiang Univ Sci B201213540240722556179
- BurugulaBGaneshBSChintalaSKCurcumin attenuates staurosporine-mediated death of retinal ganglion cellsInvest Ophth Vis Sci201152742634273
- RitchRNatural compounds: evidence for a protective role in eye diseaseCan J Ophthalmol200742342543817508040
- ZhaoDZhaoXZuYPreparation, characterization, and in vitro targeted delivery of folate-decorated paclitaxel-loaded bovine serum albumin nanoparticlesInt J Nanomedicine2010566967720957218
- MieleESpinelliGPMieleETomaoFTomaoSAlbumin-bound formulation of paclitaxel (Abraxane ABI-007) in the treatment of breast cancerInt J Nanomedicine200949910519516888
- ZhangLHouSMaoSWeiDSongXLuYUptake of folate-conjugated albumin nanoparticles to the SKOV3 cellsInt J Pharm20042871–215516215541922
- ZuYZhangYZhaoXZhangQLiuYJiangROptimization of the preparation process of vinblastine sulfate (VBLS)-loaded folate-conjugated bovine serum albumin (BSA) nanoparticles for tumor-targeted drug delivery using response surface methodology (RSM)Int J Nanomedicine2009432133320054435
- NanjawadeBKManviFVManjappaASIn situ-forming hydrogels for sustained ophthalmic drug deliveryJ Control Release2007122211913417719120
- HsiueGHChangRWWangCHLeeSHDevelopment of in situ thermosensitive drug vehicles for glaucoma therapyBiomaterials200324132423243012699680
- AsasutjaritRThanasanchokpibullSFuongfuchatAVeeranondhaSOptimization and evaluation of thermoresponsive diclofenac sodium ophthalmic in situ gelsInt J Pharm20114111–21283521459137
- JeongBKimSWBaeYHThermosensitive sol–gel reversible hydrogelsAdv Drug Deliv Rev2002541375111755705
- NieSHsiaoWLPanWYangZThermoreversible Pluronic F127-based hydrogel containing liposomes for the controlled delivery of paclitaxel: in vitro drug release, cell cytotoxicity, and uptake studiesInt J Nanomedicine2011615116621499415
- KimEYGaoZGParkJSLiHHanKrhEGF/HP-beta-CD complex in poloxamer gel for ophthalmic deliveryInt J Pharm20022331–215916711897420
- MaWDXuHWangCNieSFPanWSPluronic F127-g-poly(acrylic acid) copolymers as in situ gelling vehicle for ophthalmic drug delivery systemInt J Pharm20083501–224725617961940
- El-KamelAHIn vitro and in vivo evaluation of Pluronic F127-based ocular delivery system for timolol maleateInt J Pharm20022411475512086720
- ChopraSMotwaniSKIqbalZTalegaonkarSAhmadFJKharRKOptimisation of polyherbal gels for vaginal drug delivery by Box-Behnken statistical designEur J Pharm Biopharm200767112013117270408
- WeiGXuHDingPTLiSMZhengJMThermosetting gels with modulated gelation temperature for ophthalmic use: the rheological and gamma scintigraphic studiesJ Control Release2002831657412220839
- AbashzadehSDinarvandRSharifzadehMHassanzadehGAminiMAtyabiFFormulation and evaluation of an in situ gel forming system for controlled delivery of triptorelin acetateEur J Pharm Sci201144451452121946260
- GiovagnoliSTsaiTDeLucaPPFormulation and release behavior of doxycycline–alginate hydrogel microparticles embedded into Pluronic F127 thermogels as a potential new vehicle for doxycycline intradermal sustained deliveryAAPS Pharm Sci Tech2010111212220
- BozdagSGumusKGumusOUnluNFormulation and in vitro evaluation of cysteamine hydrochloride viscous solutions for the treatment of corneal cystinosisEur J Pharm Biopharm200870126026918590953
- SongZFengRSunMCurcumin-loaded PLGA-PEG-PLGA triblock copolymeric micelles: preparation, pharmacokinetics and distribution in vivoJ Colloid Interface Sci2011354111612321044788
- KloudaLMikosAGThermoresponsive hydrogels in biomedical applicationsEur J Pharm Biopharm2008681344517881200