Abstract
Nanoparticles have displayed considerable promise for safely delivering therapeutic agents with miscellaneous therapeutic properties. Current progress in nanotechnology has put forward, in the last few years, several therapeutic strategies that could be integrated into clinical use by using constructs for molecular diagnosis, disease detection, cytostatic drug delivery, and nanoscale immunotherapy. In the hope of bringing the concept of nanopharmacology toward a viable and feasible clinical reality in a cancer center, the present report attempts to present the grounds for the use of cell-free nanoscale structures for molecular therapy in experimental hematology and oncology.
Introduction
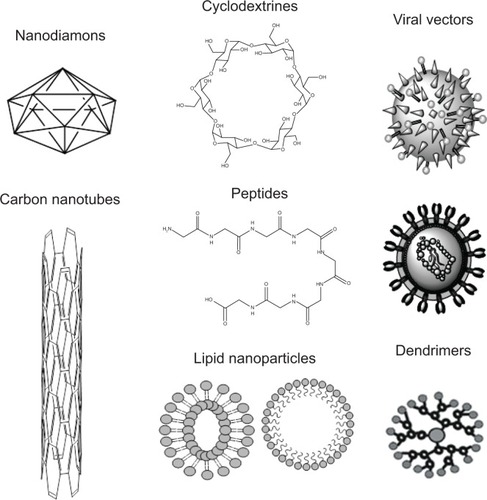
Nanopharmacology is an interdisciplinary research field, which was developed as an interaction between chemistry, engineering, biology, and medicine, and it is currently receiving growing interest in the clinic.Citation1 Progress in nanotechnology has gained attention in recent years by developing novel nanoparticle-based drugs or by discovering novel applications in early diagnostic or prognostic assays in cancer.Citation2 Multiple preclinical studies aim to improve the therapeutic index of a patient diagnosed with cancer using a wide range of nanostructures including carbon nanotubes, peptides, nanodiamonds, cyclodextrine, graphenes, liposomes, quantum dots, nanowires, and metal-based nanoparticles.Citation3,Citation4
The latest advances in nanotechnology have brought various options that could be used in the clinic by employing constructs for molecular diagnosis, disease detection, cytostatic drug delivery, and nanoscale immunotherapy.Citation5–Citation8 The United States Food and Drug Administration has approved the use of liposome-encapsulated doxorubicin (Doxil®; Janssen Products, LP, Johnson & Johnson, New Brunswick, NJ, USA) and paclitaxel attached to nanoparticles (Abraxane®; Celgene Corporation, Summit, NJ, USA)Citation9,Citation10 in cancer therapy.
In this review, we present the latest investigation on nanostructure systems with applications in hematology and oncology. The latest advancements in nanopharmacology lead to heightened expectations concerning their application in diagnostics, therapy and imaging.
Drug nanocarriers in cancer pharmacology
In the last few years, our team has shed a new light on the fieldCitation11,Citation12 by different conjugation procedures for these therapeutics. Overcoming this threshold bears major clinical significance in oncology and hematology, as developing nonviral gene delivery vehicles will bring new patient-tailored drugs within reach (). The transport of therapeutic nucleic acids through the cell membrane is inefficient mainly in experimental models and includes antisense or antigene oligonucleotides, short interference ribonucleic acid (siRNA) or micro ribonucleic acids (miRNA).Citation13,Citation14
Surgical resection in early tumor stages is the main therapeutic option for most solid malignancies, yet therapeutic benefits are frequently modest because of the high rate of tumor recurrence.Citation15 Chemotherapy and, more recently, molecular therapy, were proven to offer much more efficient therapeutic approaches for patients diagnosed with cancer.Citation2 Nevertheless, these options are most often accompanied by important systemic side effects associated with the active agent, making a direct delivery the most “elegant” and efficient therapeutic option. The direct delivery of chemotherapy drugs aims to achieve high concentrations of the cytostatic agents at the target site with minimized risk of systemic toxicity ().Citation16
In cancer chemotherapy, the clinician aims to achieve a good therapeutic index, which is the ratio of the lethal dose for 50% of the population to the minimum effective dose for 50% of the population.Citation17 However, cancer is most often characterized by multidrug resistance (MDR) and thus scientists have developed new ways to target the MDR cells.Citation18 MDR cells are known to be frequently located in hypoxic areas, distant from any blood supply, thus overcoming the natural barrier of drug efflux pump activity.Citation18 Such smart molecules may increase the drug’s bioavailability and transform an active agent from a low therapeutic candidate into a highly efficient drug. A wide variety of both organic and inorganic substances are used for engineering nanostructures, such as liposomes, micelles, nanoemulsions, polymers, quantum dots, gold, iron oxide, and even dendrimers.Citation19–Citation22 All these structures were developed in order to have a large surface area, making these particles suitable for suspension storage, as well as high drug encapsulation and extensive surface absorption capacity, which are pharmacokinetic features that are found in any current structure used in classical pharmacology.Citation23 However, the most important aspect, especially for cancer, is that nanocarriers are able to bypass the extracellular efflux activity of the adenosine triphosphate-binding cassette transporters in order to be internalized via nonspecific endocytosis,Citation24–Citation26 such as the case of immunoliposomesCitation27,Citation28 and poly (butyl)-cyanoacrylate nanoparticles.Citation29,Citation30 At the same time, the nanocarriers use surface charge-switchable polymeric magnetic nanoparticles as a safe delivery system.Citation31–Citation33 In this way, the active agent is released near the nucleus, far away from the membrane-bound P-glycoproteins, which is of paramount importance when trying to overcome the resistance to conventional chemotherapy of cancer stem-like cells.Citation34–Citation36
Ozeki et alCitation37,Citation38 have experimented with a new drug delivery model in malignant gliomas. They managed to bypass the blood–brain barrier by using a unique thermo-reversible hydrogel, composed of drug/poly(lactic-co-glycolic acid) (PLGA) microspheres. This thermo-reversible polymer is a gel at body temperature and a sol at room temperature – conditions in which the drug/PLGA microspheres dispersed in the polymer are injected into the human body. Following the procedure, a gel forms around the injection site; this keeps a high concentration of the active substance in the tumor, preventing its dispersion in adjacent healthy tissues.Citation39 Devalapally et alCitation40 used poly(epsilon caprolactone) nanostructures whose surface has been modified with poly(ethylene glycol) (PEG) before being loaded with tamoxifen and paclitaxel for the treatment of multidrug-resistant cancer cells. The results were encouraging, as this combination resulted in a lower therapeutic dose of the cytostatic agent, with important clinical applications regarding chemotherapy-related side effects.
The first groundbreaking drug was doxorubicin encapsulated in circulating liposomes (Doxil) for the treatment of Kaposi’s sarcoma in patients diagnosed with acquired immunodeficiency syndrome (AIDS).Citation41 Recently, this therapeutic option has been applied for other cancers such as breast cancer, since doxorubicin encapsulated in liposomes induces a twofold increase in intracellular drug levels when compared to standard doxorubicin treatment.Citation42 Doxil is a PEGylated liposomal drug that has a 100 nm diameter in order to prevent the interaction with plasma proteins such as opsonins and high-density lipoproteins (HDLs) and low-density lipoproteins (LDLs), or to avoid elimination by macrophages.Citation43–Citation45 After the conjugation of a liposome with PEG (a process called PEGylation), the new drug can stay in the systemic blood flow for longer periods of time due to the development of an aqueous layer on the surface of the liposome, leading to a lack of immune recognition and rejection.Citation46,Citation47 This results in the stabilization of the lipid bilayer and steric hindrance, with important consequences such as decreasing protein absorbance and recognition by the host’s macrophages.Citation48 Many reports show the ability of various gold/silver nanoparticles or carbon nanostructures to enhance the antitumor effect of certain drugs.Citation23 However, in hematology, HDL nanostructures target the scavenger receptors (B1) and promote cholesterol efflux in lymphoma cells.Citation49 Indirectly, these exogenous lipoproteins inhibit lymphoma growth and invasion by starving the malignant cell,Citation50,Citation51 thereby aiding the classic chemotherapy regimen.
PEGylated liposomes loaded with docetaxel, and prepared using the thin film hydration method, showed enhanced in vitro cytotoxicity against A549 and B16F10 cells when compared to Taxotere® (Sanofi-Aventis, Bridgewater, NJ, USA).Citation52 The capacity of a self-nanoemulsifying drug delivery system was assessed in order to increase the bioavailability of docetaxel and, consequently, its therapeutic activity.Citation53 This study showed that a self-nanoemulsifying drug delivery system exhibited superior efficacy with low associated toxicity when compared to the commercialized formulation of this bioactive agent (Taxotere).Citation53
In recent years, gold nanoparticles have also emerged as therapeutic options for the targeted delivery of antineoplastic active substances, due to their special chemical and physical properties such as functional versatility, biocompatibility, and low toxicity.Citation54–Citation56 Apart from being of small size (30–50 nm in diameter),Citation57 naked gold nanostructures have a plasmon absorption in the near-infrared region and display strong photothermal ability. These structures lack a silica core, have a spherical shape, and have a strong and tunable absorption band between 550 nm and ~820 nm.Citation58–Citation61 These properties make them highly efficient carriers of various drugs already used in the clinic. Their effect has already been shown by our team in malignant gliomas and hepatocellular carcinoma for temozolomide, cisplatin, doxorubicin, and capecitabine.Citation62
Diamonds can provide a very efficient delivery system for some chemotherapy agents. In the last few months, nanodiamonds have emerged as potential carriers in neuro-oncology or hemato-oncology. Xi et alCitation63 have conjugated nanodiamonds with doxorubicin and used convection-enhanced delivery for supratentorial tumors in a murine model. Man et alCitation64 have also used nanodiamonds to deliver another type of anthracycline to multidrug-resistant malignant cells. They showed that acute myelogenous leukemia, often leading to patient death in the clinic because of resistance to chemotherapy, might be managed by a nanotechnology-based targeted delivery of daunorubicin to the hematological malignancies. Camptothecin is a natural hydrophobic anticancer drug that could be potentially used for breast adenocarcinoma management if delivered correctly at the tumor site.Citation65 Delivery can be achieved using nanotechnology, as is the case of the self-assembling peptide amphiphile nanofibers. Soukasene et alCitation66 proved this concept in a mouse orthotopic model of breast carcinoma. Camptothecin was also confirmed by Min et alCitation67 to be efficient in breast chemotherapy when delivered to malignant cells, by encapsulating it in modified glycol chitosan nanoparticles, thereby achieving a high concentration with minimal side effects in healthy tissues, after having used subcutaneously implanted xenografts in immunocompromised mice. Since monoclonal antibodies are increasingly used in clinical oncology, some investigators have tried to add the targeted effect of antibody-based drugs to a nanocarrier in order to obtain maximum anticancer effects with minimal side effects.Citation2,Citation23 Thus, trastuzumab was conjugated with various nanostructures, including carbon nanotubes or nanospheres.Citation68 The desired effect was achieved and, in the near future, we may expect newly described cytostatic agents in Phase I or Phase II clinical trials.
Immunotherapy is a very important part of the multimodal approach in cancer management. The immune system may also influence the outcome of a certain regimen. Ni et alCitation69 have applied this concept by developing a local vaccine after conjugating graphene oxide targeting interleukine-10 receptor. Thus, the anti-inflammatory action of interleukin-10 is blocked and the suppressive tumor microenvironment becomes a target for the immune system.
Nanovectors can be used as carriers for drugs, but also for contrast substances, with a high applicability in diagnostic medicine.Citation70 Iron oxide, gold, gadolinium, or even quantum dots represent good alternatives for radiation oncology, photodynamic therapy, or hyperthermia.Citation71,Citation72 Iron oxide has important superparamagnetic characteristics and is one of the most investigated nanostructures in diagnostics, including in lymph node imaging, the inhibition of cancer cell dissemination and stem cell trafficking, visualization of ribonucleic acid (RNA), interference and T-cell-specific labeling.Citation73–Citation77 As a contrast agent, iron oxide is especially useful for magnetic resonance imaging (MRI), and it is very sensitive in detecting solid tumors, but it has little or no applicability in lymph node micrometastasis or hematological malignancies.Citation78,Citation79 While still in the early stages, the research in this field will more than likely improve in the near future. In prostate cancer, Harisinghani et alCitation78 have already proven that the use of iron nanostructures as a contrast agent in MRIs detects over 90% of all lymph node disseminations, which is in comparison with the detection rate of 35% in classic MRIs.
Even though chemotherapy remains the most widely used and effective treatment option for disseminated malignancies, acquired or intrinsic drug resistance accounts for almost 90% of treatment failure. MDR represents the simultaneous resistance to various medications that are different both structurally and functionally, most often as a result of the drug efflux pumps that reduce the intracellular levels and thus reduce the cytotoxic effect on the cancer cell.Citation6 New nanotechnology-based theranostics are evolving and are expected to confer new strategies in overcoming the drug efflux transporters, which are findings that are presented further in the next section. The multifunctional characteristics of the nanocarriers make them very suitable for treating a heterogeneous tumor mass in comparison to classic approaches.Citation15 Nanocarriers have a preferential accumulation within the malignant cell due to the enhanced permeability and retention effect.Citation23,Citation80 Thus, the drug concentration is increased in the malignancy and reduced in the surrounding, healthy tissue. This will result in an increased efficacy of systemic therapy, with decreased side effects.Citation23,Citation70
Nanotechnology can be applied not only in chemotherapy, but also in radiation oncology, by combining radiobiology with experimental pharmacology. Malignant cells are sensitive to ionizing radiation emitted by various radioactive metals.Citation81 By delivering such substances to the primary tumor site, we may improve current radiotherapy or brachytherapy protocols. Chanda et alCitation82 have conjugated gum arabic glycoproteins to gold nanoparticles and tested this new assay on a murine model of prostate cancer. The administration of a single dose of beta-emitting irradiation increased the local administered dose up to 70 Gy and induced the regression of prostate adenocarcinoma in nude mice. Garrison et alCitation83 also used an in vivo murine model of prostate cancer and demonstrated that beta radiation emitting bombesin could be used to specifically target cancer cells.
Nanocarriers conjugated with miRNAs or anti-miRNA oligonucleotides
The human body has natural barriers for preventing a wide range of diseases, whether considering the organism/body level, the tissue–organ level, the cellular level, or even the molecular level. Thus, the simple aim of achieving highly localized drug delivery with maximal anticancer effects and minimal side effects is very troublesome, as it can be expensive, time consuming, and it does not offer any guaranteed success.Citation2,Citation23 This emphasizes the need to design highly specific carriers that can deliver highly specific active agents in order to achieve maximum efficacy with minimal toxicity. In vivo, various miRs can be delivered either by viral or nonviral carriers, depending on transfection efficiency, the safety of the receiving host, immunogenicity, or side effects.Citation84,Citation85 Nonviral carriers are nonetheless considered to be more suitable in the clinic, especially cationic transporters such as PEG. This is because it has a strong buffering ability and it can release functional genetic material into the cytosol after having induced osmotic endosome breakage.Citation86–Citation88 The main disadvantage is that PEG is not cell-specific, and one would need very high doses in order to achieve the desired concentration, leading to potentially serious side effects.Citation89,Citation90 Thus, the need to improve current knowledge in the field and to produce other ligand molecules for aptamers functionalization. Aptamers are short, single-stranded oligonucleotides formed by 30–50 bases and they express minimal or even no antigenicity and immunogenicity, making them more suitable for in vivo use in clinical hematology and oncology.Citation91–Citation93
Other nonviral vector systems may also include carbon nanomaterials, such as nanotubes or fullerenes. Our studies used nanotubes because of their unique intrinsic physical and chemical properties in an attempt to deliver siRNA in hepatocellular carcinoma cells.Citation12 The molecular analysis of the experiments has proven that p53, TNF-α, and VEGF levels were altered after siRNA transfection. This proves that carboxylated carbon nanotubes may provide an alternative to the lipid transfection system-based therapy for liver malignancies. The successful functioning of the endosomal siRNA system and followed by the release of the RNA molecules into the cytoplasm are very important for the efficient use of oncogene silencing.Citation1,Citation2,Citation13 In order for this process to be carried out with minimal side effects, tertiary complexes were developed out of nucleic acids, polycations, and a charge-reversal polymer that can pH-dependently alter its electric charge either into the positive or negative state.Citation94–Citation96 When the vector arrives into an organelle such as an endosome or a lysosome, both are known to have a pH of 5–6, the charge conversion facilitates endosomal escape through a membrane disruption process after having enhanced the so-called “proton sponge”.Citation97–Citation99 Apoptosomes represent other models of molecular self-assembly structures. In such wheel-like structures, an individual Apaf-1 protein will form a complex with cytochrome-cCitation100 before recruiting and activating procaspase-9.Citation101 This will trigger a cascade of other events, which may lead to apoptosis. Polymeric micelles are artificial structures that resemble apoptosomes and act as either drug solubilizers or carriers of antisense oligonucleotides and drug molecules.Citation102 A single-stranded oligonucleotide can recognize a target molecule on a cancer cell both through Watson–Crick base pairing with folic acid, and also through hydrophobic interactions and hydrogen bonding.Citation103 Such an oligonucleotide ligand is also known as an aptamer, which has very important properties that include its small size, a lack of immunogenicity, and ease of synthesis.Citation104,Citation105
Exosomes are vesicles ranging from 30–90 nm in diameter, and they are known to play a key role in intercellular communication.Citation106,Citation107 This communication is accomplished using various cytokines, interleukins, and a substantial amount of RNA.Citation108 RNA carried by the exosomes is mostly a RNA and miRNA messenger, with very little 18S and 28S ribosomal RNA.Citation109,Citation110 Since exosomes are used in normal cell physiology in RNA transport, researchers have attempted to use these nanostructures in gene therapy as a vector to deliver therapeutic nucleic acids to target cancer cells.Citation108,Citation111 Gene therapy aims to provide a therapeutic solution for the cause of the disease, rather than for its symptoms. Two types of vectors (either viral or nonviral) are currently available in the US, according to an online search of the National Institutes of Health database (http://clinicaltrials.gov/ct2/home). Most of the 262 ongoing trials use viral vectors, yet this approach is associated with a high toxicity and an important immunological response from the host organ. Exosomes are far more efficient because they can target cancer cells and trigger little or no immune response since they are isolated from the patient’s bodily fluids and are subsequently transferred back to the same patient after an insertion or deletion of the genetic material in vitro.Citation112–Citation114 Wahlgren et alCitation115 used exosome-delivered siRNA in order to achieve posttranscriptional gene silencing. They showed that the MAPK-1 protein was downregulated in both monocytes and lymphocytes that were cocultured with particles, which were genetically modified to carry an anti-MAPK-1 transcript.
Exosomes represent an important delivery system,Citation116,Citation117 which proved its efficacy in vitro for RNA and protein transport.Citation118 A good therapeutic effect with low immunogenicity was observed for siRNA.Citation1,Citation2,Citation13 In a study conducted by Alvarez-Erviti et alCitation119 the capacity to downregulate the BACE1 protein and messenger RNA levels was demonstrated using exosome-mediated siRNA delivery produced by dendritic cells. The same groupCitation119 has also engineered dendritic cells to express the exosome-specific protein, Lamp2b, fused with the peptide, rabies virus glycoprotein, which is specific for neuronal lineage cells. Thus, dendritic cells synthesized exosomes, which were loaded to exogenous siRNA. This resulted in the knockdown of BACE2. The clinical implications are of great potential in the management of Alzheimer’s disease.Citation120 Gold is a noble metal used throughout the ages of human history in all aspects of civilization, including in medical science,Citation121,Citation122 and nanotechnology-based new approaches make no exception. Polyvalent oligonucleotide-functionalized gold nanostructures have been designed to enter cancer cells without the use of a cationic cocarrier after having been functionalized with a synthetic miR sequence. The prototype of the miR mimic-gold nanoparticle construct consists of a 1–15 nm gold nanoparticle, which was functionalized with a monolayer of a double-stranded alkylthiol-modified RNA molecule of around 30 duplexes.Citation123 Hao et al further proved that a gold nanostructure could carry the mimics of miR-205, which are known to have a tumor suppressive effect, thus inhibiting cancer cell proliferation and migration.Citation123
miRNA-based therapy
miRNAs are able to modulate different pathways,Citation124,Citation125 taking into account that a single miRNA is able to target multiple genes. Various approaches were applied to assess the significance of a particular miRNA or distinct representatives from a miRNA family, while noting that miRNAs from the same family could have antagonistic biological effects.Citation126,Citation127 There is increasing evidence that attempts to explain the miRNA’s observed correlation with drug sensitivity.Citation128–Citation130
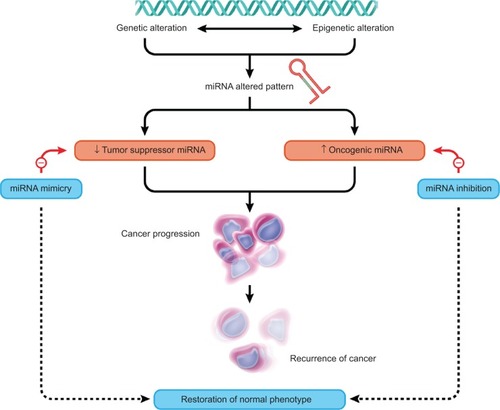
The practical implication of miRNAs in the initiation, development, and progression of cancer has led to the buildup of novel therapeutic schemes. Approaches include, among others, the inhibition of upregulated miRNAs (oncogenic role), as well as using miRNA replacement therapy by restoring the normal levels of tumor supressors miRNA. Oncogenic miRNAs are inhibited based on antisense oligonucleotides, antagomirs, sponges, or locked nucleic acid structures.Citation118 Additional approaches involve the restoration of tumor suppressor miRNA expression using miRNA mimics, based on viral or nonviral delivery systems. Both approaches have showcased favorable outcomes in preclinical and clinical studies.Citation118
Considering the significance of miRNAs in cancer and its capacity to modulate various biological pathways, miRNA mimics/inhibition asserted a new and effective therapeutic strategy in cancer.Citation1,Citation2,Citation118 Specifically, miRNAs or anti-miRNA may be used individually or in combination with chemotherapy, leading to an enhanced therapeutic response and to an improved survival rate.Citation131 In order to apply the vast potential of miRNAs for therapy, the main obstacle for the successful translation of therapeutic strategies into the clinic remains the pathway of delivery.Citation132
miRNA expression patterns can be altered by various mechanisms, including genetic and epigenetic alterations.Citation133 Correlations between miRNA expression and chromosomal abnormalities were shown to be involved in the pathogenesis of chronic lymphocytic leukemia, since miRNAs are involved in the initiation, prognosis, and chemoresistance of chronic lymphocytic leukemia.Citation134–Citation136 Concomitantly, the inhibition of the oncogenic miR-21 with antisense oligonucleotides generates a proapoptotic and antiproliferative response in vitro in different cell models, reducing tumor development and metastatic potential in vivo.Citation118 Other examples are presented in .
Table 1 Examples of miRNA therapeutic implications in hematological malignancies
Clinical implications in hematology and oncology
Nanotechnology is of major interest in clinical hematology and oncology for both therapy and diagnosis because of their unique features. These include self-assembly or the ability to make use of the enhanced permeability and retention capacity that most malignancies have as a consequence of leaky neoangiogenesis and the absence of a functional lymphatic system.Citation149 Nanostructures () can also be designed to carry useful payloads that include low molecular weight chemotherapy agents or contrast agents.Citation150,Citation151 Moreover, the newly formed structures are able to rapidly detect cancer cells, load multiple anticancer agents on their surface, and deliver the drugs rapidly at the target cell,Citation152–Citation154 while preventing their bioactive cargo degradation when the investigator chooses to use an RNA-based approach.
Table 2 Various nanostructures used in translational cancer research
Non-Hodgkin’s lymphomas are the most common lymphohematopoietic malignancies both in the US and in Europe.Citation166 One particular type is anaplastic lymphoma kinase (ALK)-positive anaplastic large cell lymphoma, which is a very aggressive T-cell lymphoma with an abnormal expression of both the ALK oncogene, as well as the surface protein, CD30.Citation167–Citation169 A nucleic acid-based knockdown of ALK gene expression has been proven to promote cell death of the malignant T-cell.Citation170,Citation171 Mori et alCitation172 have developed an RNA aptamer that specifically binds to the CD30 epitope. Zhao et alCitation173 have subsequently hypothesized that a lymphoma cell-selective delivery of a tumor gene-specific siRNA could be achieved by assembling a functional RNA nanocomplex comprising the CD30-specific aptamer and the ALK-targeted siRNA, all within a nanosized PEG-based polymer carrier. PEG-based structures are considered to be rather safe, as toxicity assays done using BALB/c mice showed little or no side effects, except for 40% accumulation in the liver.Citation174 This new approach proved that the nanocomplex could be cancer cell-selective and cancer gene-specific, with great potential in the clinic if hepatic damage can be avoided.
Another non-Hodgkin’s lymphoma with a very aggressive behavior and short-term survival is mantle cell lymphoma. This particular type of malignancy is resistant to most therapeutic approaches, including immunochemotherapy and stem cell transplantation, leading investigators to look for different salvage treatment options.Citation175–Citation178 SYK is a new target for the management of B-lineage leukemias and lymphomas,Citation179 as it regulates apoptosis by controlling activation of the phosphoinositide 3-kinase/AKT, nuclear factor-kappa B, and signal transducer and activator of transcription 3 pathways, which are all very important in the signaling of the stem cell lineage.Citation180,Citation181 Cely et alCitation182 reported a different approach by developing a nanotechnology-based platform that can be used to target a very selective SYK inhibitor for the lymphoma cell. The designed liposomal nanoparticle was the pentapeptide mimic, 1,4-bis(9-O-dihydroquinidinyl)phthalazine/hydroquinidine 1,4-phathalazinediyl diether (C16). The liposomal nanoparticle of C16 was shown to induce apoptosis of the lymphoma cell after 24 hours, providing the scientific background for an alternative treatment for refractory mantle cell lymphoma.Citation182 However, previous experience using liposomes shows that this treatment strategy is accompanied by several side effects. For patients with AIDS-related Kaposi’s sarcoma, 30% of those treated with Doxil presented with low blood counts and palmar–plantar erythrodysethesia,Citation183,Citation184 yet the clinicians easily managed these symptoms.
Carbon nanotubes are tubes made out of graphic carbon that have very good mechanical strength, good flexibility, and excellent thermal and electrical conductivity,Citation185–Citation187 qualities that initially made them suitable candidates for novel drug design. These tubes have been conjugated with monoclonal antibodies and plasmid deoxyribonucleic acid (DNA) in order to achieve cancer cell inhibition,Citation188–Citation191 and conjugates have also been made with paclitaxel and other cytostatics.Citation192 Liu et al inhibited the growth of breast cancer by conjugating carbon nanotubes with paclitaxel, and they showed that the intravenous administration of 10 mg/kg of the new com pound enhanced the therapeutic efficacy when compared with doxorubicin-free treated mice.Citation104 Still, because of their fiber shape and size, carbon nanotubes cause cytotoxicity, inflammation, and DNA damage in vitro.Citation193–Citation196 The animal models used to study the toxic effects of carbon nanotubes demonstrated that the high concentrations needed to induce the regression of the tumor may cause chronic lung inflammation, foreign-body granulomas, or interstitial fibrosis,Citation197–Citation200 limiting their potential clinical use.
Other types of nanoparticles with biomedical application are metallic colloidal gold and silver.Citation201–Citation206 These structures are used for photothermal ablation therapy, as well as for contrast enhancers in computed tomography or X-ray diagnostics.Citation207,Citation208 Niidome et alCitation202 have reported no toxicity in their studies in a mouse model of colon adenocarcinoma after having used intravenous PEG-coated nanorods, in spite of the fact that gold may interact with intracellular proteins and modify their structure, causing autoimmune-related toxicity. Silver nanostructures are commercially available for antimicrobial use,Citation209,Citation210 yet recent data show that silver oxide may also be used in cancer research, as the nanostructure cargo can induce the regression of cancer neoangiogenesis.Citation211–Citation214 Still, toxicity limits their use because silver nanoparticles can cause destruction of the blood–brain barrier, brain degeneration, and edema,Citation215–Citation217 as well as liver failure.Citation218
Diagnostics can also be aided based on diffident nanostructures types including quantum dots or metallic core-shell nanoparticles that usually contain cadmium telluride, cadmium selenide, and either indium arsenide or indium phosphide.Citation1,Citation2 This structure is then covered by a shell of zinc sulfide and is subsequently coated with PEG in order to facilitate the attachment of various drugs, nucleic acids, or antibodies.Citation219–Citation221 These structures are very good fluorophores because of their broad-spectrum fluorescence,Citation222 and they can be used to properly identify cancer cells, as well as signal events such as peroxisome activity or the presence of certain membrane receptors.Citation223–Citation226 Toxicity in clinical use is not known in great detail, but it seems that following the removal of the coating after their exposure to oxidative environments such as the endosome,Citation227–Citation229 quantum dots may be very toxic, which may limit their clinical use ().
Conclusion
In recent years, important progress has been made in nanotechnology, with its ever-increasing applicability in basic and translational medicine, leading to the appearance of a new field known as nanomedicine. This new science deals with the engineering of various structures of nanoscale dimensions that can be properly conjugated with various highly specific targeting agents in order to be used in the clinic, for either early diagnostic purposes or for disease treatment.Citation1,Citation2
These endeavors are possible because nanoparticles have unique properties, such as a preferential accumulation in the neoplastic tissue in comparison with healthy cells.Citation1,Citation2 These particles hold great potential for possibly replacing current active agents, which have been shown to be highly inefficient, based on epigenetics and molecular pharmacology principles. This step in clinical oncology and hematology is, however, still far from being implemented in clinical practice. Nevertheless, with each experimental report, we come closer to a patient-tailored approach in order to achieve maximum anticancer effects with minimal side effects.
Acknowledgments
The study was partially financed by a POSCCE grant (709/2010) titled, “Clinical and Economical Impact of Proteome and Transcriptome Molecular Profiling in Neoadjuvant Therapy of Triple Negative Breast Cancer (BREASTIMPACT)”, and by an international grant, Romania–European Economical Space (Norway), which was awarded to Ciprian Tomuleasa (contract 1/16.01.2014). Ciprian Tomuleasa’s work was financed by an internal grant of the Iuliu Hatieganu University of Medicine and Pharmacy awarded to contract 1492/1/28.01.2014, and Cornelia Braicu’s work was supported by a National Fellowship Program, UNESCO L’Oréal “For women in science”.
Disclosure
The authors report no conflicts of interest in this work.
References
- de MoraisMGMartinsVGSteffensDPrankePda CostaJABiological applications of nanobiotechnologyJ Nanosci Nanotechnol20141411007101724730317
- LiangXJNanotechnology and cancer nanomedicineBiotechnol Adv201432466524913056
- SelvamuthukumarSVelmuruganRNanostructured lipid carriers: a potential drug carrier for cancer chemotherapyLipids Health Dis20121115923167765
- SinghSSharmaARobertsonGPRealizing the clinical potential of cancer nanotechnology by minimizing toxicologic and targeted delivery concernsCancer Res201272225663566823139207
- OrzaASoriţăuOTomuleasaCReversing chemoresistance of malignant glioma stem cells using gold nanoparticlesInt J Nanomedicine2013868970223467447
- TomuleasaCSoritauOOrzaAGold nanoparticles conjugated with cisplatin/doxorubicin/capecitabine lower the chemoresistance of hepatocellular carcinoma-derived cancer cellsJ Gastrointestin Liver Dis201221218719622720309
- RotelloVSniffing out cancer using “chemical nose” sensorsCell Cycle20098223615361619884794
- YouCCMirandaORGiderBDetection and identification of proteins using nanoparticle-fluorescent polymer ‘chemical nose’ sensorsNat Nanotechnol20072531832318654291
- LeonardRCWilliamsSTulpuleALevineAMOliverosSImproving the therapeutic index of anthracycline chemotherapy: focus on liposomal doxorubicin (Myocet)Breast200918421822419656681
- PetrelliFBorgonovoKBarniSTargeted delivery for breast cancer therapy: the history of nanoparticle-albumin-bound paclitaxelExpert Opin Pharmacother20101181413143220446855
- BraicuCGhermanCDIrimieABerindan-NeagoeIEpigallocatechin-3-Gallate (EGCG) inhibits cell proliferation and migratory behaviour of triple negative breast cancer cellsJ Nanosci Nanotechnol201313163263723646788
- NeagoeIBBraicuCMateaCEfficient siRNA delivery system using carboxilated single-wall carbon nanotubes in cancer treatmentJ Biomed Nanotechnol20128456757422852466
- ShangLNienhausKNienhausGUEngineered nanoparticles interacting with cells: size mattersJ Nanobiotechnology201412524491160
- JulianoRLMingXNakagawaOCellular uptake and intracellular trafficking of antisense and siRNA oligonucleotidesBioconjug Chem201223214715721992697
- Del BurgoLSPedrazJLOriveAdvanced nanovehicles for cancer managementDrug Discov Today2014
- BashaRSabnisNHeymKBowmanWPLackoAGTargeted nanoparticles for pediatric leukemia therapyFront Oncol2014410124860784
- SinghRMukhopadhyayKSurvival analysis in clinical trials: Basics and must know areasPerspect Clin Res20112414514822145125
- MinkoTRodriguez-RodriguezLPozharovVNanotechnology approaches for personalized treatment of multidrug resistant cancersAdv Drug Deliv Rev20136513–141880189524120655
- HedeSHuilgolN“Nano”: the new nemesis of cancerJ Cancer Res Ther20062418619517998702
- PanchapakesanBWickstromENanotechnology for sensing, imaging, and treating cancerSurg Oncol Clin N Am200716229330517560513
- ThorntonGMaterials Science. Watching nanoparticles growScience200330056241378137912775827
- MoghimiSMHunterACMurrayJCNanomedicine: current status and future prospectsFASEB J200519331133015746175
- UpretiMJyotiASethiPTumor microenvironment and nanotherapeuticsTransl Cancer Res20132430931924634853
- DavisMEChenZGShinDMNanoparticle therapeutics: an emerging treatment modality for cancerNat Rev Drug Discov20087977178218758474
- KopecekJKopeckováPMinkoTLuZRPetersonCMWater soluble polymers in tumor targeted deliveryJ Control Release2001741–314715811489491
- ShenFChuSBenceAKQuantitation of doxorubicin uptake, efflux, and modulation of multidrug resistance (MDR) in MDR human cancer cellsJ Pharmacol Exp Ther200832419510217947497
- ParkJWHongKKirpotinDBAnti-HER2 immunoliposomes: enhanced efficacy attributable to targeted deliveryClin Cancer Res2002841172118111948130
- YuBMaoYYuanYTargeted drug delivery and cross-linking induced apoptosis with anti-CD37 based dual-ligand immunoliposomes in B chronic lymphocytic leukemia cellsBiomaterials201334266185619323726226
- LinYPanYShiYHuangXJiaNJiangJYDelivery of large molecules via poly(butyl cyanoacrylate) nanoparticles into the injured rat brainNanotechnology2012231616510122460562
- DuanJMansourHMZhangYReversion of multidrug resistance by co-encapsulation of doxorubicin and curcumin in chitosan/poly(butyl cyanoacrylate) nanoparticlesInt J Pharm20124261–219320122274587
- ShenJMGaoFYYinTcRGD-functionalized polymeric magnetic nanoparticles as a dual-drug delivery system for safe targeted cancer therapyPharmacol Res201370110211523376353
- ShenJMGuanXMLiuXYLanJFChengTZhangHXLuminescent/magnetic hybrid nanoparticles with folate-conjugated peptide composites for tumor-targeted drug deliveryBioconjug Chem20122351010102122486419
- ShenJMYinTTianXZGaoFYXuSSurface charge-switchable polymeric magnetic nanoparticles for the controlled release of anticancer drugACS Appl Mater Interfaces20135157014702423815399
- MiklášováNFischer-FodorELönneckePAntiproliferative effect of novel platinum (II) and palladium (II) complexes on hepatic tumor stem cells in vitroEur J Med Chem201249414722305340
- FlorianISTomuleasaCSoritauOCancer stem cells and malignant gliomas. From pathophysiology to targeted molecular therapyJ BUON2011161162321674845
- TomuleasaCSoritauORus-CiucaDFunctional and molecular characterization of glioblastoma multiforme-derived cancer stem cellsJ BUON201015358359120941832
- OzekiTKanekoDHashizawaKImaiYTagamiTOkadaHCombination therapy of surgical tumor resection with implantation of a hydrogel containing camptothecin-loaded poly(lactic-co-glycolic acid) microspheres in a C6 rat glioma modelBiol Pharm Bull201235454555022466559
- OzekiTHashizawaKKanekoDImaiYOkadaHTreatment of rat brain tumors using sustained-release of camptothecin from poly(lactic-co-glycolic acid) microspheres in a thermoreversible hydrogelChem Pharm Bull (Tokyo)20105891142114720823591
- AraiTJokiTAkiyamaMNovel drug delivery system using thermoreversible gelation polymer for malignant gliomaJ Neurooncol200677191516292493
- DevalapallyHDuanZSeidenMVAmijiMMModulation of drug resistance in ovarian adenocarcinoma by enhancing intracellular ceramide using tamoxifen-loaded biodegradable polymeric nanoparticlesClin Cancer Res200814103193320318483388
- RaimundoKBiskupiakJGoodmanMSilversteinSAscheCCost effectiveness of liposomal doxorubicin vs. paclitaxel for the treatment of advanced AIDS-Kaposi’s sarcomaJ Med Econ201316560661323425295
- ThierryARVigéDCoughlinSSBelliJADritschiloARahmanAModulation of doxorubicin resistance in multidrug-resistant cells by liposomesFASEB J1993765725798097173
- BitounisDFanciullinoRIliadisACiccoliniJOptimizing druggability through liposomal formulations: new approaches to an old conceptISRN Pharm2012201273843222474607
- KlibanovALMaruyamaKTorchilinVPHuangLAmphipathic polyethyleneglycols effectively prolong the circulation time of liposomesFEBS Lett199026812352372384160
- MaruyamaKYudaTOkamotoAKojimaSSuginakaAIwatsuruMProlonged circulation time in vivo of large unilamellar liposomes composed of distearoyl phosphatidylcholine and cholesterol containing amphipathic poly(ethylene glycol)Biochim Biophys Acta19921128144491390877
- PapahadjopoulosDAllenTMGabizonASterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacyProc Natl Acad Sci U S A1991882411460114641763060
- UsterPSAllenTMDanielBEMendezCJNewmanMSZhuGZInsertion of poly(ethylene glycol) derivatized phospholipid into pre-formed liposomes results in prolonged in vivo circulation timeFEBS Lett19963862–32432468647291
- HatakeyamaHAkitaHHarashimaHThe polyethyleneglycol dilemma: advantage and disadvantage of PEGylation of liposomes for systemic genes and nucleic acids delivery to tumorsBiol Pharm Bull201336689289923727912
- MooberryLKNairMParanjapeSMcConathyWJLackoAGReceptor mediated uptake of paclitaxel from a synthetic high density lipoprotein nanocarrierJ Drug Target2010181535819637935
- BhattacharyaRMukherjeePBiological properties of “naked” metal nanoparticlesAdv Drug Deliv Rev200860111289130618501989
- Oraki KohshourMMirzaieSZeinaliMAblation of breast cancer cells using trastuzumab-functionalized multi-walled carbon nanotubes and trastuzumab-diphtheria toxin conjugateChem Biol Drug Des201483325926524118702
- ManjappaASGoelPNVekatarajuMPIs an alternative drug delivery system needed for docetaxel? The role of controlling epimerization in formulations and beyondPharm Res201330102675269323756759
- SeoYGKimDHRamasamyTDevelopment of docetaxel-loaded solid self-nanoemulsifying drug delivery system (SNEDDS) for enhanced chemotherapeutic effectInt J Pharm20134521–241242023707964
- TempletonACWuelfingWPMurrayRWMonolayer-protected cluster moleculesAcc Chem Res2000331273610639073
- ConnorEEMwamukaJGoleAMurphyCJWyattMDGold nanoparticles are taken up by human cells but do not cause acute cytotoxicitySmall20051332532717193451
- BocaSCPotaraMGabudeanAMJuhemABaldeckPLAstileanSChitosan-coated triangular silver nanoparticles as a novel class of biocompatible, highly effective photothermal transducers for in vitro cancer cell therapyCancer Lett2011311213114021840122
- SuarasanSFocsanMManiuDAstileanSGelatin-nanogold bioconjugates as effective plasmonic platforms for SERS detection and taggingColloids Surf B Biointerfaces201310347548123261569
- GabudeanAMBiroDAstileanSHybrid plasmonic platforms based on silica-encapsulated gold nanorods as effective spectroscopic enhancers for Raman and fluorescence spectroscopyNanotechnology2012234848570623138835
- FocsanMArdeleanIICraciunCAstileanSInterplay between gold nanoparticle biosynthesis and metabolic activity of cyanobacterium Synechocystis sp. PCC 6803Nanotechnology2011224848510122072064
- PotaraMManiuDAstileanSThe synthesis of biocompatible and SERS-active gold nanoparticles using chitosanNanotechnology2009203131560219597258
- BocaSCAstileanSDetoxification of gold nanorods by conjugation with thiolated poly(ethylene glycol) and their assessment as SERS-active carriers of Raman tagsNanotechnology2010212323560120463383
- AldeaMDPetrushevBSoritauOMetformin plus sorafenib highly impacts temozolomideresistant glioblastoma stem-like cellsJ BUON20141925021124965413
- XiGRobinsonEMania-FarnellBConvection-enhanced delivery of nanodiamond drug delivery platforms for intracranial tumor treatmentNanomedicine201410238139123916888
- ManHBKimHKimHJSynthesis of nanodiamond-daunorubicin conjugates to overcome multidrug chemoresistance in leukemiaNanomedicine201410235936923916889
- CirpanliYBilensoyELale DoğanACalişSComparative evaluation of polymeric and amphiphilic cyclodextrin nanoparticles for effective camptothecin deliveryEur J Pharm Biopharm2009731828919442723
- SoukaseneSToftDJMoyerTJAntitumor activity of peptide amphiphile nanofiber-encapsulated camptothecinACS Nano20115119113912122044255
- MinKHParkKKimYSHydrophobically modified glycol chitosan nanoparticles-encapsulated camptothecin enhance the drug stability and tumor targeting in cancer therapyJ Control Release2008127320821818336946
- BaruaSYooJWKolharPWakankarAGokarnYRMitragotriSParticle shape enhances specificity of antibody-displaying nanoparticlesProc Natl Acad Sci U S A201311093270327523401509
- NiGWangYWuXWangXChenSLiuXGraphene oxide absorbed anti-IL10R antibodies enhance LPS induced immune responses in vitro and in vivoImmunol Lett2012148212613223064239
- JaganathanHMitraSSrinivasanSDaveBGodinBDesign and in vitro evaluation of layer by layer siRNA nanovectors targeting breast tumor initiating cellsPLoS One2014 Apr 294e9198624694753
- DavisMEThe first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinicMol Pharm20096365966819267452
- van VlerkenLEAmijiMMMulti-functional polymeric nanoparticles for tumour-targeted drug deliveryExpert Opin Drug Deliv20063220521616506948
- SunCSzeRZhangMFolic acid-PEG conjugated superparamagnetic nanoparticles for targeted cellular uptake and detection by MRIJ Biomed Mater Res A200678355055716736484
- MedarovaZPhamWFarrarCPetkovaVMooreAIn vivo imaging of siRNA delivery and silencing in tumorsNat Med200713337237717322898
- GunnJWallenHVeisehOA multimodal targeting nanoparticle for selectively labeling T cellsSmall20084671271518528851
- VeisehOKievitFMGunnJWRatnerBDZhangMA ligand-mediated nanovector for targeted gene delivery and transfection in cancer cellsBiomaterials200930464965718990439
- LewinMCarlessoNTungCHTat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cellsNat Biotechnol200018441041410748521
- HarisinghaniMGBarentszJHahnPFNoninvasive detection of clinically occult lymph-node metastases in prostate cancerN Engl J Med2003348252491249912815134
- WadajkarASMenonJUKadapureTTranRTYangJNguyenKTDesign and application of magnetic-based theranostic nanoparticle systemsRecent Pat Biomed Eng201361475723795343
- VlasovaMARytkönenJRiikonenJNanocarriers and the delivered drug: Effect interference due to intravenous administrationEur J Pharm Sci2014
- BabincováMKontrisovaKDurdíkSBergemannCSourivongPRadiation enhanced efficiency of combined electromagnetic hyperthermia and chemotherapy of lung carcinoma using cisplatin functionalized magnetic nanoparticlesPharmazie201469212813124640602
- ChandaNKanPWatkinsonLDRadioactive gold nanoparticles in cancer therapy: therapeutic efficacy studies of GA-198AuNP nanoconstruct in prostate tumor-bearing miceNanomedicine20106220120919914401
- GarrisonJCRoldTLSieckmanGLIn vivo evaluation and small-animal PET/CT of a prostate cancer mouse model using 64Cu bombesin analogs: side-by-side comparison of the CB-TE2A and DOTA chelation systemsJ Nucl Med20074881327133717631556
- El-AneedAAn overview of current delivery systems in cancer gene therapyJ Control Release200494111414684267
- MintzerMASimanekEENonviral vectors for gene deliveryChem Rev2009109225930219053809
- BoussifOLezoualc’hFZantaMAA versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimineProc Natl Acad Sci U S A19959216729773017638184
- SwamiAGoyalRTripathiSKEffect of homobifunctional crosslinkers on nucleic acids delivery ability of PEI nanoparticlesInt J Pharm20093741–212513819446769
- OhYKSuhDKimJMChoiHGShinKKoJJPolyethylenimine-mediated cellular uptake, nucleus trafficking and expression of cytokine plasmid DNAGene Ther20029231627163212424615
- XuPLiSYLiQBiodegradable cationic polyester as an efficient carrier for gene delivery to neonatal cardiomyocytesBiotechnol Bioeng200695589390317001632
- FloreaBIMeaneyCJungingerHEBorchardGTransfection efficiency and toxicity of polyethylenimine in differentiated Calu-3 and nondifferentiated COS-1 cell culturesAAPS Pharm Sci200243E12
- EllingtonADSzostakJWIn vitro selection of RNA molecules that bind specific ligandsNature199034662878188221697402
- TuerkCGoldLSystematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymeraseScience199024949685055102200121
- JenisonRDGillSCPardiAPoliskyBHigh-resolution molecular discrimination by RNAScience19942635152142514297510417
- LeeYMiyataKObaMCharge-conversion ternary polyplex with endosome disruption moiety: a technique for efficient and safe gene deliveryAngew Chem Int Ed Engl200847285163516618528828
- XuPVan KirkEAZhanYMurdochWJRadoszMShenYTargeted charge-reversal nanoparticles for nuclear drug deliveryAngew Chem Int Ed Engl200746264999500217526044
- ShenYZhouZSuiMCharge-reversal polyamidoamine dendrimer for cascade nuclear drug deliveryNanomedicine (Lond)2010581205121721039198
- RozemaDBEkenaKLewisDLLoomisAGWolffJAEndosomolysis by masking of a membrane-active agent (EMMA) for cytoplasmic release of macromoleculesBioconjug Chem2003141515712526692
- MiyataKObaMNakanishiMPolyplexes from poly(aspartamide) bearing 1,2-diaminoethane side chains induce pH-selective, endosomal membrane destabilization with amplified transfection and negligible cytotoxicityJ Am Chem Soc200813048162871629419006313
- LiuXZhangJLynnDMPolyelectrolyte multilayers fabricated from ‘charge-shifting’ anionic polymers: a new approach to controlled film disruption and the release of cationic agents from surfacesSoft Matter2008481688169519122876
- BaoQShiYApoptosome: a platform for the activation of initiator caspasesCell Death Differ2007141566516977332
- MannaPGhoshMGhoshJDasJSilPCContribution of nano-copper particles to in vivo liver dysfunction and cellular damage: role of IκBα/NF-κB, MAPKs and mitochondrial signalNanotoxicology20126112121319953
- JeongJHParkTGNovel polymer-DNA hybrid polymeric micelles composed of hydrophobic poly(D,L-lactic-co-glycolic acid) and hydrophilic oligonucleotidesBioconjug Chem201112691792311716682
- KataokaKItakaKNishiyamaNYamasakiYOishiMNagasakiYSmart polymeric micelles as nanocarriers for oligonucleotides and siRNA deliveryNucleic Acids Symp Ser (Oxf)2005491718
- LiuJLuYFast colorimetric sensing of adenosine and cocaine based on a general sensor design involving aptamers and nanoparticlesAgnew Chem Int Ed Engl20054519094
- YangHLiuHKangHTanWEngineering target-responsive hydrogels based on aptamer-target interactionsJ Am Chem Soc2008130206320632118444626
- MarcusMELeonardJNFedExosomes: engineering therapeutic biological nanoparticles that truly deliverPharmaceuticals (Basel)20136565968023894228
- EL AndaloussiSMägerIBreakefieldXOWoodMJExtracellular vesicles: biology and emerging therapeutic opportunitiesNat Rev Drug Discov201312534735723584393
- KowalJTkachMThéryCBiogenesis and secretion of exosomes201429116125
- ValadiHEkströmKBossiosASjöstrandMLeeJJLötvallJOExosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cellsNat Cell Biol20079665465917486113
- NazarenkoIRuppAKAltevogtPExosomes as a potential tool for a specific delivery of functional moleculesMethods Mol Biol2013104949551123913240
- TicknerJAUrquhartAJStephensonSARichardDJO’ByrneKJFunctions and therapeutic roles of exosomes in cancerFront Oncol2014412724904836
- LehrmanSVirus treatment questioned after gene therapy deathNature1999401675351751810524611
- KogaKMatsumotoKAkiyoshiTPurification, characterization and biological significance of tumor-derived exosomesAnticancer Res2005256A3703370716302729
- CastanottoDRossiJJThe promises and pitfalls of RNA-interference-based therapeuticsNature2009457722842643319158789
- WahlgrenJDeLKarlsonTBrisslertMPlasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytesNucleic Acids Res20124017e13022618874
- Alvarez-ErvitiLSeowYYinHBettsCLakhalSWoodMJDelivery of siRNA to the mouse brain by systemic injection of targeted exosomesNat Biotechnol201129434134521423189
- El-AndaloussiSLeeYLakhal-LittletonSExosome-mediated delivery of siRNA in vitro and in vivoNat Protoc20127122112212623154783
- BraicuCCalinGABerindan-NeagoeIMicroRNAs and cancer therapy - from bystanders to major playersCurr Med Chem201320293561357323834177
- GarzonRMarcucciGCroceCMTargeting microRNAs in cancer: rationale, strategies and challengesNat Rev Drug Discov201091077578920885409
- JichaGACarrSAConceptual Evolution in Alzheimer’s Disease: Implications for Understanding the Clinical Phenotype of Progressive Neurodegenerative DiseaseJ Alzheimers Dis201019125327220061643
- FreddiGRomàn-PumarJLEvidence-based medicine: what it can and cannot doAnn Ist Super Sanita2011471222521430334
- SinghSLokeYKDrug safety assessment in clinical trials: methodological challenges and opportunitiesTrials20121313822906139
- HaoLPatelPCAlhasanAHGiljohannDAMirkinCANucleic acid-gold nanoparticle conjugates as mimics of microRNASmall20117223158316221922667
- BhayaniMKCalinGALaiSYFunctional relevance of miRNA sequences in human diseaseMutat Res20127311–2141922085809
- MunkerRCalinGAMicroRNA profiling in cancerClin Sci (Lond)2011121414115821526983
- FabbriMCalinGAEpigenetics and miRNAs in human cancerAdv Genet201070879920920746
- SantarpiaLNicolosoMCalinGAMicroRNAs: a complex regulatory network drives the acquisition of malignant cell phenotypeEndocr Relat Cancer2010171F51F7519843580
- CalinGACroceCMChronic lymphocytic leukemia: interplay between noncoding RNAs and protein-coding genesBlood2009114234761477019745066
- GarzonRCalinGACroceCMMicroRNAs in CancerAnnu Rev Med20096016717919630570
- NegriniMNicolosoMSCalinGAMicroRNAs and cancer – new paradigms in molecular oncologyCurr Opin Cell Biol200921347047919411171
- VisoneRCroceCMMiRNAs and cancerAm J Pathol200917441131113819264914
- MauritzKHPeckhamHPRestoration of grasping functions in quadriplegic patients by Functional Electrical Stimulation (FES)Int J Rehabil Res1987104 Suppl 557613509753
- PalmeroEIde CamposSGCamposMMechanisms and role of microRNA deregulation in cancer onset and progressionGenet Mol Biol201134336337021931505
- ZhouXXWangXRole of microRNAs in chronic lymphocytic leukemia (Review)Mol Med Rep20138371972523900739
- CalinGACimminoAFabbriMMiR-15a and miR-16-1 cluster functions in human leukemiaProc Natl Acad Sci U S A2008105135166517118362358
- RaoEJiangCJiMThe miRNA-17~92 cluster mediates chemoresistance and enhances tumor growth in mantle cell lymphoma via PI3K/AKT pathway activationLeukemia20122651064107222116552
- LiebermanRCalifanoPRoentgenographic manifestations of gout. A case reportJ Am Podiatr Med Assoc19877795065093668829
- VenturiniLBattmerKCastoldiMExpression of the miR-17-92 polycistron in chronic myeloid leukemia (CML) CD34+ cellsBlood2007109104399440517284533
- RommerASteinleitnerKHacklHOverexpression of primary microRNA 221/222 in acute myeloid leukemiaBMC Cancer20131336423895238
- Gimenes-TeixeiraHLLucena-AraujoARDos SantosGAIncreased expression of miR-221 is associated with shorter overall survival in T-cell acute lymphoid leukemiaExp Hematol Oncol2013211023566596
- GarzonRHeaphyCEHavelangeVMicroRNA 29b functions in acute myeloid leukemiaBlood2009114265331534119850741
- MarcucciGMaharryKSMetzelerKHClinical role of microRNAs in cytogenetically normal acute myeloid leukemia: miR-155 upregulation independently identifies high-risk patientsJ Clin Oncol201331172086209323650424
- Akbari MoqadamFLange-TurenhoutEAAriësIMPietersRden BoerMLMiR-125b, miR-100 and miR-99a co-regulate vincristine resistance in childhood acute lymphoblastic leukemiaLeuk Res201337101315132123915977
- AqeilanRICalinGACroceCMmiR-15a and miR-16-1 in cancer: discovery, function and future perspectivesCell Death Differ201017221522019498445
- PekarskyYSantanamUCimminoATcl1 expression in chronic lymphocytic leukemia is regulated by miR-29 and miR-181Cancer Res20066624115901159317178851
- MosakhaniNMustjokiSKnuutilaSDown-regulation of miR-181c in imatinib-resistant chronic myeloid leukemiaMol Cytogenet2013612723866735
- LiuYSongYMaWZhengWYinHDecreased microRNA-30a levels are associated with enhanced ABL1 and BCR-ABL1 expression in chronic myeloid leukemiaLeuk Res201337334935623287430
- MatsumuraYMaedaHA new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancsCancer Res19864612 Pt 1638763922946403
- GreishKEnhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targetingMethods Mol Biol2010624253720217587
- ZyslerRDLimaEJrVasquez MansillaMA new quantitative method to determine the uptake of SPIONs in animal tissue and its application to determine the quantity of nanoparticles in the liver and lung of Balb-c mice exposed to the SPIONsJ Biomed Nanotechnol20139114214523627077
- PradosJMelguizoCPerazzoliGApplication of nanotechnology in the treatment and diagnosis of gastrointestinal cancers: review of recent patentsRecent Pat Anticancer Drug Discov201491213423676104
- De JongWHBormPJDrug delivery and nanoparticles: applications and hazardsInt J Nanomedicine20083213314918686775
- LeucutaSENanotechnology for delivery of drugs and biomedical applicationsCurr Clin Pharmacol20105425728020925643
- AlexisFPridgenEMLangerRFarokhzadOCNanoparticle technologies for cancer therapyHandb Exp Pharmacol2010197558620217526
- FarokhzadOCChengJTeplyBATargeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivoProc Natl Acad Sci U S A2006103166315632016606824
- LeePCChiouYCWongJMPengCLShiehMJTargeting colorectal cancer cells with single-walled carbon nanotubes conjugated to anticancer agent SN-38 and EGFR antibodyBiomaterials201334348756876523937913
- ShiJMaRWangLThe application of hyaluronic acid-derivatized carbon nanotubes in hematoporphyrin monomethyl ether-based photodynamic therapy for in vivo and in vitro cancer treatmentInt J Nanomedicine201382361237323843694
- LiXGaoJYangYNanomaterials in the application of tumor vaccines: advantages and disadvantagesOnco Targets Ther2013662963423776336
- LiuZFanACRakhraKSupramolecular stacking of doxorubicin on carbon nanotubes for in vivo cancer therapyAngew Chem Int Ed Engl200948417668767219760685
- CoelhoSCRochaSJuzenasPGold nanoparticle delivery-enhanced proteasome inhibitor effect in adenocarcinoma cellsExpert Opin Drug Deliv201310101345135223937147
- LiKWenSLarsonACMultifunctional dendrimer-based nanoparticles for in vivo MR/CT dual-modal molecular imaging of breast cancerInt J Nanomedicine201382589260023888113
- FarrokhTakinECiofaniGPuleoGLBarium titanate core – gold shell nanoparticles for hyperthermia treatmentsInt J Nanomedicine201382319233123847415
- WangHJYangLYangHYAntineoplastic activities of protein-conjugated silver sulfide nano-crystals with different shapesJ Inorg Biochem20101041879119906430
- PietiläMLehenkariPKuvajaPMortalin antibody-conjugated quantum dot transfer from human mesenchymal stromal cells to breast cancer cells requires cell-cell interactionExp Cell Res2013319182770278023928292
- ZhangCJiXZhangYOne-pot synthesized aptamer-functionalized CdTe:Zn2+ quantum dots for tumor-targeted fluorescence imaging in vitro and in vivoAnal Chem201385125843584923682757
- Daldrup-LinkHERummenyEJIhssenBKienastJLinkTMIron-oxide-enhanced MR imaging of bone marrow in patients with non-Hodgkin’s lymphoma: differentiation between tumor infiltration and hypercellular bone marrowEur Radiol20021261557156612042968
- DelsolGThe 2008 WHO lymphoma classificationAnn Pathol200828Spec No 1(1)S20S24 French18984289
- FaliniBPileriSZinzaniPLALK+ lymphoma: clinic-pathological findings and outcomeBlood19999382697270610194450
- SteinHFossHDDürkopHCD30(+) anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical featuresBlood200096123681369511090048
- RitterUDamm-WelkCFuchsUBohleRMBorkhardtAWoessmannWDesign and evaluation of chemically synthesized siRNA targeting the NPM-ALK fusion site in anaplastic large cell lymphoma (ALCL)Oligonucleotides200313536537315000827
- PivaRChiarleRManazzaADAblation of oncogenic ALK is a viable therapeutic approach for anaplastic large-cell lymphomasBlood2006107268969716189272
- MoriTOguroAOhtsuTNakamuraYRNA aptamers selected against the receptor activator of NF-kappaB acquire general affinity to proteins of the tumor necrosis factor receptor familyNucleic Acids Res200432206120612815562003
- ZhaoNBagariaHGWongMSZuYA nanocomplex that is both tumor cell-selective and cancer gene-specific for anaplastic large cell lymphomaJ Nanobiotechnology20119221281497
- SemeteBBooysenLLemmerYIn vivo evaluation of the biodistribution and safety of PLGA nanoparticles as drug delivery systemsNanomedicine20106566267120230912
- WitzigTECurrent treatment approaches for mantle-cell lymphomaJ Clin Oncol200523266409641416155027
- WilliamsMEConnorsJMDreylingMHMantle cell lymphoma: report of the 2010 Mantle Cell Lymphoma Consortium WorkshopLeuk Lymphoma2011521243321133727
- DelmonteAGhielminiMSessaCBeyond monoclonal antibodies: new therapeutic agents in non-Hodgkin’s lymphomasOncologist200914551152519411316
- HessGNew combinations for mantle cell lymphoma: concerted action neededLancet Oncol201112431531621440504
- UckunFMQaziSSpleen tyrosine kinase as a molecular target for treatment of leukemias and lymphomasExpert Rev Anticancer Ther20101091407141820836676
- UckunFMEkROJanSTChenCLQaziSTargeting SYK kinase-dependent anti-apoptotic resistance pathway in B-lineage acute lymphoblastic leukaemia (ALL) cells with a potent SYK inhibitory pentapeptide mimicBr J Haematol2010149450851720151979
- UckunFMGoodmanPMaHDibirdikIQaziSCD22 EXON 12 deletion as a pathogenic mechanism of human B-precursor leukemiaProc Natl Acad Sci U S A201010739168521685720841423
- CelyIYivSYinQTargeting mantle cell lymphoma with anti-SYK nanoparticlesJ Anal Oncol2012111923730399
- MuggiaFMHainsworthJDJeffersSPhase II study of liposomal doxorubicin in refractory ovarian cancer: antitumor activity and toxicity modification by liposomal encapsulationJ Clin Oncol19971539879939060537
- MuggiaFMClinical efficacy and prospects for use of pegylated liposomal doxorubicin in the treatment of ovarian and breast cancersDrugs199754Suppl 422299361958
- ShenCBrozenaAHWangYDouble-walled carbon nanotubes: challenges and opportunitiesNanoscale20113250351821042608
- HuangNWangHZhaoJLuiHKorbelikMZengHSingle-wall carbon nanotubes assisted photothermal cancer therapy: animal study with a murine model of squamous cell carcinomaLasers Surg Med201042963864820949599
- ThakareVSDasMJainAKPatilSJainSCarbon nanotubes in cancer theragnosisNanomedicine (Lond)2010581277130121039202
- JiSRLiuCZhangBCarbon nanotubes in cancer diagnosis and therapyBiochim Biophys Acta201018061293520193746
- LiuZTabakmanSWelsherKDaiHCarbon nanotubes in biology and medicine: in vitro and in vivo detection, imaging and drug deliveryNano Res2009228512020174481
- LiuZChenKDavisCDrug delivery with carbon nanotubes for in vivo cancer treatmentCancer Res200868166652666018701489
- ChaudhuriPSoniSSenguptaSSingle-walled carbon nanotube-conjugated chemotherapy exhibits increased therapeutic index in melanomaNanotechnology201021202510219955607
- SatsangiARoySSSatsangiRKVadlamudiRKOngJLDesign of a Paclitaxel prodrug conjugate for active targeting of an enzyme upregulated in breast cancer cellsMol Pharm20141161906191824847940
- SinghSNalwaHSNanotechnology and health safety – toxicity and risk assessments of nanostructured materials on human healthJ Nanosci Nanotechnol2007793048307018019130
- RadomskiAJuraszPAlonso-EscolanoDNanoparticle-induced platelet aggregation and vascular thrombosisBr J Pharmacol2005146688289316158070
- SayesCMLiangFHudsonJLFunctionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitroToxicol Lett2006161213514216229976
- ShvedovaAACastranovaVKisinERExposure to carbon nanotube material: assessment of nanotube cytotoxicity using human keratinocyte cellsJ Toxicol Environ Health A200366201909192614514433
- WarheitDBLaurenceBRReedKLRoachDHReynoldsGAWebbTRComparative pulmonary toxicity assessment of single-wall carbon nanotubes in ratsToxicol Sci200477111712514514968
- RavichandranPPeriyakaruppanASadanandanBInduction of apoptosis in rat lung epithelial cells by multiwalled carbon nanotubesJ Biochem Mol Toxicol200923533334419827037
- ReddyARReddyYNKrishnaDRHimabinduVPulmonary toxicity assessment of multiwalled carbon nanotubes in rats following intratracheal instillationEnviron Toxicol201227421121920862737
- MullerJHuauxFMoreauNRespiratory toxicity of multi-wall carbon nanotubesToxicol Appl Pharmacol2005207322123116129115
- LooCLinAHirschLNanoshell-enabled photonics-based imaging and therapy of cancerTechnol Cancer Res Treat200431334014750891
- NiidomeTYamagataMOkamotoYPEG-modified gold nanorods with a stealth character for in vivo applicationsJ Control Release2006114334334716876898
- KawanoTYamagataMTakahashiHStabilizing of plasmid DNA in vivo by PEG-modified cationic gold nanoparticles and the gene expression assisted with electrical pulsesJ Control Release2006111338238916487614
- HainfeldJFSlatkinDNSmilowitzHMThe use of gold nanoparticles to enhance radiotherapy in micePhys Med Biol20044918N309N31515509078
- SimonTBoca-FarcauSGabudeanAMBaldeckPAstileanSLED-activated methylene blue-loaded Pluronic-nanogold hybrids for in vitro photodynamic therapyJ Biophotonics2013611–1295095923893922
- BocaSRuginaDPinteaABarbu-TudoranLAstileanSFlower-shaped gold nanoparticles: synthesis, characterization and their application as SERS-active tags inside living cellsNanotechnology201122505570221178234
- O’NealDPHirschLRHalasNJPayneJDWestJLPhoto-thermal tumor ablation in mice using near infrared-absorbing nanoparticlesCancer Lett2004209217117615159019
- WaldmanSAFortinaPSurreySHyslopTKrickaLJGravesDJOpportunities for near-infrared thermal ablation of colorectal metastases by guanylyl cyclase C-targeted gold nanoshellsFuture Oncol20062670571617155897
- ShahverdiARFakhimiAShahverdiHRMinaianSSynthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coliNanomedicine20073216817117468052
- SondiISalopek-SondiBSilver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteriaJ Colloid Interface Sci2004275117718215158396
- CoulterJAHylandWBNicolJCurrellFJRadiosensitising nanoparticles as novel cancer therapeutics – pipe dream or realistic prospect?Clin Oncol (R Coll Radiol)2013251059360323876527
- SatapathySRMohapatraPPreetRSilver-based nanoparticles induce apoptosis in human colon cancer cells mediated through p53Nanomedicine (Lond)2013881307132223514434
- KempMMKumarAMousaSGold and silver nanoparticles conjugated with heparin derivative possess anti-angiogenesis propertiesNanotechnology2009204545510419822927
- SriramMIKanthSBKalishwaralalKGurunathanSAntitumor activity of silver nanoparticles in Dalton’s lymphoma ascites tumor modelInt J Nanomedicine2010575376221042421
- GrosseSEvjeLSyversenTSilver nanoparticle-induced cytotoxicity in rat brain endothelial cell cultureToxicol In Vitro201327130531322954533
- SharmaHSSharmaANeurotoxicity of engineered nanoparticles from metalsCNS Neurol Disord Drug Targets2012111658022229317
- PowersCMLevinEDSeidlerFJSlotkinTASilver exposure in developing zebrafish produces persistent synaptic and behavioral changesNeurotoxicol Teratol201133232933221035540
- HussainSMHessKLGearhartJMGeissKTSchlagerJJIn vitro toxicity of nanoparticles in BRL 3A rat liver cellsToxicol In Vitro200519797598316125895
- SmithAMDaveSNieSTrueLGaoXMulticolor quantum dots for molecular diagnostics of cancerExpert Rev Mol Diagn20066223124416512782
- TangMXingTZengJUnmodified CdSe quantum dots induce elevation of cytoplasmic calcium levels and impairment of functional properties of sodium channels in rat primary cultured hippocampal neuronsEnviron Health Perspect2008116791592218629314
- HardmanRA toxicologic review of quantum dots: toxicity depends on physiochemical and environmental factorsEnviron Health Perspect2006114216517216451849
- ChanWCMaxwellDJGaoXBaileyREHanMNieSLuminescent quantum dots for multiplexed biological detection and imagingCurr Opin Biotechnol2002131404611849956
- DubertretBSkouridesPNorrisDJNoireauxVBrivanlouAHLibchaberAIn vivo imaging of quantum dots encapsulated in phospholipid micellesScience200229855991759176212459582
- GaoXCuiYLevensonRMChungLWNieSIn vivo cancer targeting and imaging with semiconductor quantum dotsNat Biotechnol200422896997615258594
- LidkeDSNagyPHeintzmannRQuantum dot ligands provide new insights into erbB/HER receptor-mediated signal transductionNat Biotechnol200422219820314704683
- WuXLiuHLiuJImmunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dotsNat Biotechnol2003211414612459735
- HoshinoAFujiokaKOkuTQuantum dots targeted to the assigned organelle in living cellsMicrobiol Immunol2004481298599415611617
- ShioharaAHoshinoAHanakiKSuzukiKYamamotoKOn the cyto-toxicity caused by quantum dotsMicrobiol Immunol200448966967515383704
- HoshinoAHanakiKSuzukiKYamamotoKApplications of T-lymphoma labeled with fluorescent quantum dots to cell tracing markers in mouse bodyBiochem Biophys Res Commun20043141465314715244