Abstract
Oral cancer (oral cavity and oropharynx) is a common and aggressive cancer that invades local tissue, can cause metastasis, and has a high mortality rate. Conventional treatment strategies, such as surgery and chemoradiotherapy, have improved over the past few decades; however, they remain far from optimal. Currently, cancer research is focused on improving cancer diagnosis and treatment methods (oral cavity and oropharynx) nanotechnology, which involves the design, characterization, production, and application of nanoscale drug delivery systems. In medicine, nanotechnologies, such as polymeric nanoparticles, solid lipid nanoparticles, nanostructured lipid carriers, gold nanoparticles, hydrogels, cyclodextrin complexes, and liquid crystals, are promising tools for diagnostic probes and therapeutic devices. The objective of this study is to present a systematic review of nanotechnology-based drug delivery systems for oral cancers.
Introduction
Oral squamous cell carcinoma (OSCC) is the sixth most common cancer for both sexes worldwide, and the 5-year survival rate for this disease is approximately 50%.Citation1 In 2011, OSCC accounted for nearly 3% of all cancer cases worldwide; its estimated incidence is approximately 275,000 cases per year, with two-thirds of these cases occurring in developing countries.Citation1 In 2013, there were 41,380 estimated new cases of oral cavity and pharyngeal cancer in the US for both sexes, with 32.8% associated with the tongue, 27.5% with the mouth, 33.7% with the pharynx, and 5.9% in other parts of the oral cavity. There were also 7,890 estimated deaths in the US, of which an estimated 19.1% were new cases, with 26.2% associated with the tongue, 22.8% with the mouth, 30.4% with the pharynx, and 20.8% in other parts of the oral cavity.Citation2 OSCC is a malignant tumor of the squamous epithelium lining the oral mucosa. These tumors are malignant and tend to spread rapidly. The main causes of oral cancer include excessive alcohol intake and tobacco use.Citation3–Citation5 Exposure to sunlight is a causative factor for cancer of the lips, which is similar to that for skin cancer.Citation6–Citation9 Human papilloma virus is also a risk factor for causing oral cancer.Citation10–Citation13 Immunosuppressed patients (eg, human immunodeficiency virus [HIV] and renal transplant patients) have the highest risk factor for developing oral cancer.Citation14,Citation15
The prevalence has shown a 5.3-fold increase for men and a twofold increase for women over the past 2 decades.Citation16 In addition, the annual death toll for oral cancer in males has been rapidly increasing.Citation17 The 5-year mortality rate for oral cancer is approximately 50% worldwide,Citation18–Citation20 which signifies a poor prognosis for developing countries.Citation1 The rates for OSCC recurrence vary from 18% to 76% for patients who undergo standard treatment, and a delay in starting treatment is considered the major cause for no relevant improvement in the survival rate.Citation21
Diagnostic confirmation is only possible by biopsy and histopathological analysis prior to treatmentCitation22–Citation28 with possible prior cytological evidence,Citation29–Citation31 and lengthy and expensive diagnostic investigations that only delay the initiation of treatment should be avoided. Nevertheless, the delay in the diagnosis of oral cancer has resulted in increasing the time to treatment initiation and a consequent decrease in the survival rate of patients.Citation1
To increase the effectiveness of treatment and reduce side effects, the incorporation of nanotechnology-based drug delivery systems, such as polymeric nanoparticles (PNPs), solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs), gold nanoparticles, hydrogels, cyclodextrin complexes, and liquid crystals (LCs), represents an interesting option for drug delivery, as demonstrated in .
Table 1 Examples of drug delivery systems, compositions, and aims for cancer treatments
Pathophysiology of oral cancer
The genomic pathway plays a role in OSCC, whereby alterations in the genome result in the altered expression of proteins, chemical mediators, and enzymes.Citation32 Carcinogenesis is a process with multiple steps, each characterized by the sequential stimulation of additional genetic defects followed by clonal expansion. Due to oncogene activation and tumor suppressor gene inactivation, OSCC leads to the deregulation of cell proliferation and death. The genetic alterations include gene amplification, oncogene overexpression, mutations, deletions, and hypermethylation, leading to the inactivation of particular genes such as the p53 tumor suppressor gene.Citation33
Oncogenes do not play an important role in the cancer process, although they do play a role in initiation. Initiation transforms a normal cell into a premalignant cell, and it requires the inactivation of negative regulators in the cell (eg, tumor suppressor genes), which is considered an important event that leads to the development of malignancy. Tumor suppressor genes are most often inactivated by point mutations, deletions, and rearrangements in both gene copies.Citation34,Citation35
Mutations in p53 and p16 are involved in the carcinogenesis process. The p53 gene plays a role in maintaining genomic stability, cell cycle progression, cellular differentiation, DNA repair, and apoptosis, and p16 is involved in cell cycle regulation, including cell cycle arrest and apoptosis.Citation36
The tumor suppressor gene p53 is known to be mutated in approximately 70% of all adult solid tumors.Citation37 These p53 gene mutations have been associated with smoking and the use of tobacco in squamous cell carcinomas of the head and neck.Citation38 An in vivo study of functionally inactivated p53 in oral tumors and the restoration of p53 in oral cancer lines and tumors induced in animal models demonstrated reversal of the malignant phenotype.Citation39
Another OSCC characteristic is telomerase activity. Several oral tumors have been confirmed to have the expression of telomerase, which is strongly associated with malignancy in oral tissues. Telomerase activity has been identified in OSCC, with 80% of patients with head and neck squamous cell carcinomaCitation40 having telomerase activity, and it has been reported that most immortal OSCC cell lines have high levels of telomerase and have tumor radioresistance.Citation41,Citation42
Other chemical mediators are involved in oral cancer pain, such as endothelin-1 (ET-1), proteases, and nerve growth factor.Citation43 ET-1 is a potent vasoactive peptide that produces nociception. In oral cancer, ET-1 binds to the endothelin-B receptor and is expressed on nonmyelinating Schwann and dorsal root ganglion satellite cells.Citation44 In patients with OSCC, the ET-1 levels are higher in the tumor microenvironment, and nociception was reported with mechanical stimuli parallel to the mechanical allodynia.Citation45,Citation46 The role of ET-1 in oral cancer pain was confirmed and characterized in a mouse model by Pickering et alCitation47 and the ET-1 concentration was a more important factor than tumor volume in establishing cancer pain.
Protease-activated receptor type 2 (PAR2) is involved in oral cancer.Citation48,Citation49 This receptor is activated by serine proteases, trypsin, and tryptase.Citation50 PAR2 activates dual messenger pathways in a second step that sensitizes transient receptor potential vanilloid type-1 (TRPV1) and transient receptor potential vanilloid type-4 (TRPV4) receptors on nociceptive afferents where there is resulting TRPV1-dependent thermal hyperalgesia and TRPV4-dependent mechanical allodynia.Citation51 In OSCC, the fibroblasts in the stroma produce trypsin, and this serine protease is capable of activating PAR2 on sensory neurons. This continual release of serine proteases in the microenvironment could produce an ongoing excitation of primary nociceptive afferents, leading to mechanical allodynia in oral cancer patients.Citation49
In the microenvironment of many cancers, sensory neurons are chronically exposed to nerve growth factor (NGF).Citation1,Citation52 The acute peripheral administration of this chemical mediator leads to thermal hyperalgesia, whereas chronic administration produces mechanical allodynia.Citation53 The activity of NGF is mediated via a receptor tyrosine kinase;Citation54 thus, NGF can also facilitate the proliferation and invasion of multiple cancers,Citation55,Citation56 including oral cancer;Citation56 a process related to pain. The pain mechanism in oral cancer can be established by association with perineural involvement, with invasion and proliferation of a cancer occurring within a nerve associated with pain.Citation56,Citation57 Higher NGF levels were found in cancer tissues from oral cancer patients.Citation57
Angiogenesis is a crucial step in the processes of uncontrolled tumor proliferation and metastasis, and inhibiting angiogenesis is considered to be effective for treating oral cancer. Vascular endothelial growth factor (VEGF) is thought to be an important angiogenic factor,Citation58 and studies have shown that OSCC is associated with an elevated VEGF concentration in the serum. These higher levels of VEGF are correlated with lymph node metastasis, clinical stage, and the prognosis and treatment of OSCC.Citation59–Citation62
Cancer cells induce the development of an exaggerated inflammatory state in the stroma, which in turn promotes cancer growth, invasion, and metastasis. Inflammatory cells in the microenvironment, such as myeloid dendritic cells, macrophage subtypes (M1 and M2), mast cells, neutrophils, and T and B lymphocytes, secrete chemokines, prostaglandins, proteinases, and complement components that collectively adopt an exaggerated inflammatory state that promotes cancer growth, tissue invasion, and metastasis.Citation63–Citation65
The chemical mediators produced by an upregulation in inflammation include transforming growth factor beta (TGF-β), tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, cyclooxygenase 2 (COX-2), and matrix metalloproteinase-7 (MMP-7).Citation66,Citation67 TNF-α and IL-6 are produced by malignant keratinocytes, stromal fibroblasts, and macrophages, and these cytokines promote tumor growth by modifying the expression of cell-adhesion molecules and extracellular matrix proteins and stimulate angiogenesis.Citation68
A high level of COX-2 expression exists in stromal cells and cancerous cells at the invasive front in OSCC.Citation69,Citation70 Thus, COX-2 plays a role in the process of local invasion and metastasis.Citation62 Increased COX-2 expression in OSCC is associated with a high rate of recurrence after treatment with a poor response to radiotherapy and poor prognosis.Citation71 Matrix metalloproteinases are involved in the cell migration, angiogenesis, and proteolytic activation of growth factors, events necessary for invasion into surrounding connective tissue for neoplastic cells.Citation72,Citation73 MMP-7 plays a pivotal role in inflammatory diseases and malignant invasion by tissue remodelingCitation72,Citation74 and destroying the extracellular matrix, including the basement membrane, and this process is necessary for invasion and metastasis.Citation75 Increased MMP-7 expression has been found to be related to oral cancer.Citation72,Citation76
Nuclear factor-kappa B (NF-κB) participates in the expression of genes involved in inflammatory and immune responses, cell proliferation, and survival.Citation77 NF-κB protein levels gradually increase from the premalignant lesion stage to invasive cancer, indicating an important role for NF-κB in the early stages of carcinogenesis.Citation77,Citation78 In OSCC, reduction in NF-κB activity results in low IL levels, including those for IL-2, IL-6, and IL-8. In addition, IL-8 plays a role in the induction of the angiogenesis process.Citation79
A number of complex mechanisms are involved in the genesis and progression of oral cancer. OSSC is a multistep process in which multiple genetic events occur that alter the normal function of oncogenes and tumor suppressor genes. These events can result in the increased production of growth factors. Recent advances in the understanding of the molecular control of these various pathways will allow for more accurate diagnosis and assessment of prognosis and might lead the way for more novel approaches for treatment and prevention.
Oral cancer treatment
Treatment protocols for oral cavity cancers are generalized therapies based on stage, chemoradiation therapy, and induction chemotherapy for locally advanced disease.Citation28,Citation80 In current therapies, some anticancer drugs have been used alone or in combination for the treatment of oral cancer, such as cisplatin, cetuximab, fluorouracil, paclitaxel, docetaxel (DTX), and methotrexate.Citation81–Citation90
The oral administration of anticancer agents is preferred by patients for its convenience and potential for outpatient treatment. In addition, oral administration facilitates prolonged exposure to a cytotoxic agent.Citation91 However, low solubility in aqueous fluids, low apparent permeability, and poor bioavailability are noted as limitations for oral chemotherapy.Citation92,Citation93 Intravenous administration is the most direct, and it overcomes the variable absorption patterns of the gastrointestinal tract. Intravenous administration leads to immediate and complete bioavailability; thus, this route has the potential to be hazardous because high concentrations of drugs are delivered to normal tissues, causing greater damage to healthy tissues and increased adverse reactions.Citation94
To overcome the disadvantages of current cancer treatment techniques, the scientific community has turned toward nanotechnology to develop new and more effective nanotechnology-based drug carrier systems to optimize oral, buccal, and intravenous treatment routes.
Nanotechnology-based drug delivery systems
Nanoparticles
Nanoparticles can be defined as ultradispersed solid supramolecular structures with a submicrometer size ranging from 10 to 1,000 μm.Citation95–Citation97 The drugs can be dissolved, entrapped, encapsulated, or attached to a nanoparticle matrix, which acts as a reservoir for particulate systems and therefore plays an important role as a drug delivery system for clinical applications, particularly in oncology.Citation98,Citation99
Nanoparticles fabricated from polysaccharides, proteins, and biocompatible/biodegradable polymers, such as polyethylene glycol (PEG), poly(γ-benzyl l-glutamate) (PBLG), poly(D,L-lactide), poly(lactic acid) (PLA), poly(D,L-glycolide), poly(lactide-co-glycolide), polycyanoacrylate, chitosan, gelatin, and sodium alginate are called PNPs.Citation96,Citation100–Citation107
The nanoparticles (NPs) are mainly prepared via the dispersion of preformed polymers, the polymerization of monomers, ionic gelation, or the coacervation of hydrophilic polymers, but other methods for their generation have also been reported, such as supercritical fluid technology and particle replication in non-wetting templates (PRINT®; DeSimone Lab, Chapel Hill, NC, USA).Citation108–Citation114
NPs can improve the stability of drugs and control their targeted delivery, allowing for a constant and uniform concentration at the site of a lesion and facilitating drug extravasation into the tumor system, thus reducing side effects.Citation115–Citation117
Damascelli et al evaluated the effectiveness of the intra-arterial infusion of paclitaxel incorporated in NPs based on human albumin (albumin NPs) for use as induction chemotherapy before definitive advanced tongue cancer treatment.Citation118 Paclitaxel is a lipophilic drug; therefore, surface-active agents must be added for dissolution in organic fluids. In addition, paclitaxel causes severe allergic reactions with intravenous use. Albumin NPs are attractive formulations because they can incorporate a significant amount of drugs into a particle matrix due to the different drug-binding sites present in albumin molecules. Damascelli et al reported that the intra-arterial infusion of paclitaxel in albumin nanoparticles is reproducible and effective.Citation118
Sulfikkarali et al investigated the anti-buccal tumor effects of naringenin (NAR)-loaded nanoparticles (NARNPs) prepared in a NAR:aminoalkyl methacrylate copolymers Eudragit® (Röhm GmbH & Co. KG, Darmstadt, Germany) E:poly vinyl alcohol (1:10:10; weight (w)/w/w) ratio by a nanoprecipitation method.Citation119 NAR has promising pharmacological activity; however, it has low oral bioavailability, which is a crucial obstacle. The results of the study revealed that NARNPs have more potent antitumor effects than free NAR, preventing the formation of OSCC. In addition, NARNPs improved the biochemical status to a normal range in 7,12-dimethylbenz(a)anthracene-induced oral carcinogenesis. This result may be attributed to the fact that NAR nanoparticulates can arrive at tumor sites via a process called “enhanced permeation and retention” due to the fact that the tumor tissue vasculature is porous with leaky endothelium, which increases and sustains the drug concentration inside tumor cells over time, leading to higher antitumor efficacy compared with free NAR.Citation119
Yu et al also investigated the action of NPs against oral cancer. These authors assessed the anticancer effects of herpes simplex virus thymidine kinase (HSV-TK)-loaded PEG–PBLG nanoparticles and PEG–PBLG nanoparticle-mediated HSV-TK/ganciclovir nanoparticles toward OSCC.Citation120 HSV-TK is a good apoptosis-inducing gene; however, its transference into the tumor is critical. However, the results demonstrated that HSV-TK-loaded PEG–PBLG nanoparticles had a core-shell structure, DNA protection, and higher gene-transfer efficiency and released DNA gradually; thus, they can be used as gene carriers in future clinical applications. Furthermore, PEG–PBLG nanoparticle-mediated HSV-TK/ganciclovir had a strong anticancer effect on buccal carcinoma induced in golden hamsters.Citation120
In another study,Citation121 the potential antitumor activity of cisplatin-loaded nanoparticles based on PEG-poly(glutamic acid) block copolymers was assayed in four OSCCs. The results showed that the growth inhibitory effects of cisplatin-loaded nanoparticles were significantly less than that for free cisplatin. However, the caspase-3 and -7 cascades, which are activated by a cisplatin stimulus, induced the release of cytochrome c from mitochondria and led to an irreversible commitment to apoptotic cell death in both cisplatin- and NC-6004-treated OSC-19 cells. Other interesting data obtained from this study revealed that nephrotoxicity, a crucial side effect of cisplatin-loaded nanoparticles, is much lower than that for free cisplatin. Therefore, it can be interpreted that these NPs are as efficient against OSCC as free cisplatin but with much less renal toxicity.Citation121
Li et al prepared NPs based on biocompatible and biodegradable hyperbranched poly(ether ester) polymers that possess many hydroxyl and carboxyl functional groups available for functionalization, including the covalent attachment of drug molecules.Citation122 These hyperbranched poly(ether ester) NPs were attached to the photosensitizer chlorin(e6) (ce6), and they demonstrated an improvement in the in vitro photodynamic therapy activity over free ce6 in CAL 27 human oral cancer cells, which may be due to factors including increased cellular uptake of the photosensitizer and the disaggregating effect of covalently binding ce6 to a hydrophilic polymer that improve the quantum yield of the reactive oxygen species produced during photodynamic therapy. In addition, photosensitizer-loaded nanoparticles can reach the most sensitive subcellular sites, demonstrating a capability for treating superficial oral cancer or precancerous lesions.Citation122
Nevertheless, some studies revealed that some of the aforementioned polymers may lead to cytotoxicity after internalization into cells, restricting the use of NPs as a drug delivery system. In addition, the large-scale production of PNPs is also problematic and is not relevant for the pharmaceutical market.Citation123–Citation125
Therefore, SLNs were developed to overcome the disadvantages of PNPs because they demonstrate physical stability, protection of incorporated labile drugs from degradation, controlled release, and excellent tolerability; thus, they can be used for different routes of administration, such as parenteral, oral, dermal, ocular, pulmonary, and rectal.Citation126–Citation129
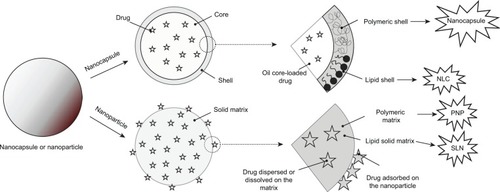
SLNs are made from solid lipids at room temperature and are stabilized by surfactant. SLNs can be obtained by a high-pressure homogenization (HPH) process that forms an average particle size of <500 nm and a low microparticle content, other production procedures that use organic solvents (HPH/solvent evaporation), or the dilution of microemulsions.Citation130–Citation132 The schematic structure of SLNs is shown in .
Figure 1 Schematic differences between nanocapsule, nanostructured lipid carrier (NLC), polymeric nanoparticle (PNP), and solid lipid nanoparticle (SLN) drug delivery systems.
Holpuch et al tested a SLN formulation as a local oral cancer chemoprevention strategy.Citation133 These authors demonstrated that SLNs composed of idarubicin hydrochloride (0.2 mg idarubicin/mL), sodium tetradecyl sulfate (0.159 mg/mL), emulsifying wax (2 mg/mL), polyoxyl 20 stearyl ether ([Brij® 78; Uniqema, Wilmington, DE, USA] 2.3 mg/mL), and D-alpha-tocopheryl PEG 1,000 succinate ([vitamin-E TPGS] 3 mg/mL) and SLNs composed of BODIPY® FL C12 (Molecular Probes, Inc., Eugene, OR, USA) (50 μg/mL), emulsifying wax (2 mg/mL), and polyoxyl 20 stearyl ether ([Brij® 78] 4.0 mg/mL) underwent internalization by OSCC cells and could provide higher final intracellular levels relative to bolus administration. Furthermore, the penetration and subsequent internalization of nanoparticles within proliferating basal layer cells demonstrates the feasibility of nanoparticle formulations for local delivery and the stabilization of oral chemopreventive compounds.Citation133
However, SLNs have some limitations because the HPH process leads to drug degradation, the coexistence of different lipid modifications and colloidal species, and a low drug-loading capacity, and because of the kinetics of the distribution processes.Citation134
To overcome these difficulties, a new generation of SLNs has emerged, ie, NLCs, which consist of solid matrix entrapping variable liquid lipid nanocompartments, as shown in . The presence of liquid lipid nanocompartments avoids solid lipid crystallization and improves the drug payload and release because these are still controlled by a surrounding solid lipid barrier.Citation135–Citation137
Aditya et al made curcumin and genistein co-loaded NLCs based on oleic acid, lecithin, glycerol monostearate, and Tween® 80 (Meryer (Shanghai) Chemical Technology Co., Ltd, Shanghai, People’s Republic of China).Citation138 These NLCs were found to be promising vehicles for the oral delivery of poorly bioaccessible molecules such as curcumin and genistein. In addition, NLCs had great effects against prostate cancer due to the enhanced intracellular uptake of NLCs by cells.Citation138 Curcumin has also shown encouraging results in in vitro and in vivo models of OSCC.Citation139 Therefore, future extensive research can determine the beneficial effects of curcumin-loaded NLCs for oral cancer treatment.
Chinsriwongkul et al researched NLCs based on a blend of cetyl palmitate and different liquid lipids, including soybean oil, medium-chain triglyceride, soybean oil/oleic acid (3:1) and medium-chain triglyceride/oleic acid (3:1), at a 1:1 weight ratio for the parenteral delivery of the anticancer drug all-trans retinoic acid (ATRA).Citation140 NLCs based on oleic acid enhanced the ATRA loading capacity in the NLCs; however, all ATRA-loaded NLCs had prolonged release of ATRA in addition to being more cytotoxic than the free drug in an in vitro model of leukemia and hepatic cancer cells.Citation140 ATRA-loaded NLCs could also be assessed in OSCC because retinoic acid is also effective at preventing the development of oral cancers.Citation141,Citation142
Liu et al designed DTX-loaded NLCs (DTX–NLCs) based on stearic acid, glyceryl monostearate, soya lecithin, and oleic acid prepared by the modified film ultrasonication–dispersion method.Citation143 DTX was held in the lipid core of NLCs, which results in a prolonged release that could reduce the frequency of administration. Furthermore, DTX–NLCs had more cytotoxicity than free DTX, which is likely because DTX–NLCs carry drugs into cancer cells by endocytosis and enhance intracellular drug accumulation by nanoparticle uptake.Citation143 These results are promising for cancer therapy, including that for oral cancer, because DTX provides an alternative for the management of OSCC.Citation144
Zhang et al aimed to develop three NLC formulations (NLC, PEG–NLC, distearoylphosphatidylethanolamine (DSPE)–PEG–NLC) for etoposide (VP16) and evaluate potential NLCs as an oral delivery system.Citation145 The NLCs were based on VP16 (15 mg), monostearin (100 mg), soybean oil (30 mg), and soya lecithin (70 mg); PEG–NLCs were based on VP16 (15 mg), monostearin (100 mg), soybean oil (30 mg), soya lecithin (70 mg), and PEG-40 (140 mg); and DSPE–PEG–NLCs were based on VP16 (15 mg), monostearin (100 mg), soybean oil (30 mg), soya lecithin (70 mg), PEG-40 (140 mg), and DSPE–PEG (12 mg). All NLCs were prepared by an emulsification and low-temperature solidification method. A pharmacokinetic study conducted in rats revealed that the relative bioavailability of VP16–NLCs, VP16–PEG-40–NLCs, and VP16–DSPE–NLCs was enhanced approximately 1.8-, 3.0-, and 3.5-fold, respectively, compared with a VP16 suspension.Citation145 Moreover, VP16–DSPE–NLCs showed the highest cytotoxicity against human epithelial-like lung carcinoma cells, which is likely due to NLC absorption at the cell surface and the release of VP16 close to the membrane, or NLC was internalized in cells and then released from the nanoparticles.Citation145 These formulations may also provide an alternative for the treatment of oral cancer because VP16 also appears to have action in OSCC.Citation146
Liu et al designed quercetin (QR)-loaded cationic NLCs that were based on a glycerol monostearate:medium-chain triglycerides ratio of 4:1, lecithin concentration of 3%, didodecyldimethylammonium bromide concentration of 1%, and QR concentration of 5%.Citation147 Liu et al reported that the QR-loaded cationic NLCs released QR slower than QR in solution released QR in vitro, mainly due to the slow erosion or degradation of the lipid matrix, which could prolong the residence time of the drug at the tumor site, eg, an oral cancer tumor site.Citation147
Nanoparticles based on noble metals, particularly gold, have an immense potential for cancer diagnosis and therapy based on their surface-plasmon resonance-enhanced light scattering and absorption.Citation148,Citation149
El-Sayed et al prepared gold nanoparticles (AuNPs) by the citrate reduction of chloroauric acid.Citation150 This group used a simple and inexpensive conventional microscope with proper rearrangement of the illumination system and a light collection system to image cells incubated with AuNPs or anti-epidermal growth factor receptor (EGFR) antibody-loaded AuNPs. Both types of AuNPs were then incubated with a single nonmalignant epithelial cell line, HaCaT (human keratinocytes), and two malignant epithelial cell lines, HOC 313 clone 8 and HSC 3 (human OSCC cell lines). The results showed that the scattering images and absorption spectra recorded from anti-EGFR antibody-conjugated AuNPs incubated with cancerous and noncancerous cells were different and provided a potential technique for oral cancer diagnostics.Citation150
Kah et al also investigated AuNPs for the early diagnosis of oral cancer based on surface plasmon resonance.Citation151 These authors prepared AuNPs via the reduction of 0.259 mM hydrogen tetrachloroaurate by 34 mM trisodium citrate (Sigma-Aldrich Co., St Louis, MO, USA) at a temperature of 90°C, and the AuNPs were conjugated to a monoclonal anti-EGFR antibody as a cancer biomarker for imaging via established protocols for the passive absorption of anti-EGFR on the surface of AuNPs. It was demonstrated that the use of EGFR-loaded AuNPs improved optical contrast under reflectance-mode imaging in vitro. Furthermore, the use of gold nanoparticles in surface-enhanced Raman scattering enhanced Raman spectroscopy signals for the analysis of cancer-related chemical changes in saliva.Citation151
Afifi et al used hamster buccal pouch carcinoma as a model for OSCC to study the effects of plasmonic photothermal therapy using AuNPs combined with visible laser irradiation.Citation152 AuNPs were synthesized using the citrate reduction method. The results demonstrated an amplified decrease in proliferation rates for cancer cells upon plasmonic photothermal therapy using AuNPs in addition to maintaining no adverse effects on normal cells, which can be explained by the enhanced permeability and retention effect. These findings indicate that AuNPs directly injected into hamster buccal pouch carcinomas can be used as a treatment for human OSCC in the future.Citation152
Liposomes
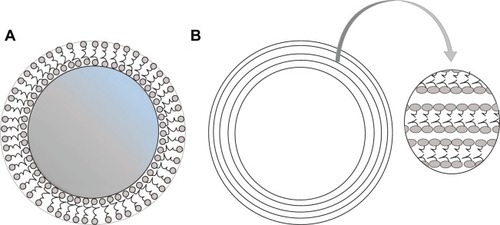
Liposomes are unilamellar or multilamellar microscopic particles composed of membrane-like lipid layers, often phospholipids and cholesterol, surrounding aqueous compartments,Citation124,Citation153 as depicted in .
Figure 2 Schematic representation of unilamellar (A) and multilamellar (B) liposomes.
Note: The arrow indicates an enlarged view of the outer layers of multilamellar liposomes.
Liposomes are the most widely used drug delivery systems for the systemic administration of many drugs for decreasing drug toxicity and increasing their accumulation at target sites.Citation154 Therefore, liposomes have been intensively studied for the delivery of chemotherapeutic drugs to improve therapeutic efficacy and decrease the toxicity to normal cells.Citation155
Furthermore, liposome-based formulations for gene therapy, such as synthetic cationic liposomal-DNA called lipoplexes, have clear potential, particularly for oral cancer treatment.Citation156
In this context, Konopka et al investigated the effects of high concentrations of fetal bovine serum on the transfection efficiency of a polycationic liposome (Metafectene™; Biontex Laboratories GmbH, München, Germany) and a polyamine reagent (GeneJammer; Agilent Technologies, Santa Clara, CA, USA) in HSC-3 and H357 human OSCC cells. The results showed that both polycationic liposomes could mediate gene delivery, which is not excessively inhibited even in the presence of 60% fetal bovine serum; therefore, they can be used in the delivery of genes in biological environments.Citation157
Figueiró Longo et al studied the effects of photodynamic therapy mediated by a liposomal formulation prepared by dimyristoyl phosphatidylcholine in the presence and absence of additives such as cholesterol or cardiolipin to release aluminum phthalocyanine chloride, a photosensitizer, in tongue tumors induced in Swiss mice.Citation158 This treatment produced intense necrosis in the tumor tissue accompanied by the infiltration of polymorphonuclear cells and thrombi formation on tumor-associated blood vessels. Thus, these results showed that photodynamic therapy mediated by a liposomal formulation of aluminum phthalocyanine chloride can be effective against chemically induced oral cancer.Citation158
Velloso et al showed that liposomal aluminum phthalocyanine chloride-based photodynamic therapy inhibits the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) signaling pathway in cultured human OSCC cells.Citation159 mTOR, a 289 kDa serine/threonine kinase located downstream of the PI3K/Akt pathway, has been shown to be a major regulator of cell growth, proliferation, migration, differentiation, and survival. In OSCC, activation of PI3K is a frequent event, and mTOR can be involved in the pathophysiology of oral cancer. Thus, these results are promising for oral cancer treatment.Citation159
Hydrogels
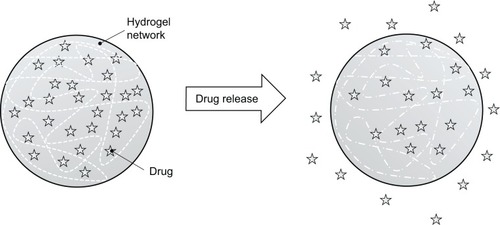
A hydrogel is a mesh of hydrophilic polymeric chains dispersed in waterCitation160 that is swellable and can release drugs for dissolution and disintegration through the spaces in their mesh, as shown in . In addition to swelling, physical properties include permeability, mechanical resistance, and surface aspects that can be modulated through structural modification.Citation161
Figure 3 Hydrophilic polymeric chains network and release the drug for dissolution through the spaces of their mesh.
Hydrogels are attractive for oral administration because their polymeric chains can closely interact with saliva glycoproteins, causing a mucoadhesion phenomenon. There has been a great deal of interest in the use of hydrogels as chemotherapeutic drug delivery systems for drugs including paclitaxel, doxorubicin, DTX, tamoxifen, and cisplatin.Citation162–Citation171 Furthermore, studies have revealed that strategies are required to overcome the disadvantages of chemotherapeutic drugs such as cisplatin, which is usually intravenously administered, whereby 90% becomes linked to hemoproteins and 10% is free to enter into the cells.
In this context, a research group investigated the incorporation of cisplatin loaded-hydrogels called P9, CP2, MH2, and CMH2.Citation172 A stock solution of cisplatin was added stepwise to each polymer solution. The acrylic hydrogels P9 and CP2, which contain a carboxyl group, were obtained by free radical polymerization of the monomers N-acryloyl-l-phenylalanine and N-isopropylacrylamide (NIPAAm), and they were cross-linked with N,N’-ethylene-bis-acrylamide. MH2 and CMH2 hydrogels were obtained by free radical polymerization of the methacrylate monomer N-methacryloyl-l-histidine and NIPAAm, and the authors assessed the in vitro cytotoxicity of cisplatin-loaded hydrogels. They reported that P9 hydrogels could modulate the rate of cisplatin release.Citation172 P9 hydrogels have also been described as a promising platform for chemotherapeutic treatment, including that for oral cancers.Citation173
Moura et al investigated in vitro cisplatin release from chitosan hydrogels cross-linked with glycerol phosphate disodium salt and chitosan hydrogels that were ionic/covalently co-cross-linked.Citation174 Their results demonstrated that the rate of release of cisplatin from ionic cross-linked chitosan hydrogels was significantly lower than that for chitosan hydrogels ionic/covalently co-cross-linked, and the amount of drug released was also quite different (60%–70% for hydrogels containing genipin against 20% for ionic hydrogels). Despite these differences, the release profiles were similar for both types of hydrogels, with an initial burst reaching a maximum concentration at approximately 2 to 3 hours. The researchers concluded that hydrogels containing both cross-linking agents can improve the chemical and mechanical properties presented when compared with hydrogels obtained with only one of the reticulating agents,Citation174 making it attractive for the treatment of oral cancers because the release profile of the system occurs quickly, thus releasing the drug formulation before it is removed from the oral cavity by the salivary flow.Citation175
Emoto et al studied hydrogels obtained with cross-linkable hyaluronic acid for the intraperitoneal administration of cisplatin for extended retention and consequent action against peritoneal carcinomatosis.Citation176 Hyaluronic acid was dissolved in water and sodium periodate was added and stirred for 2 hours. Afterward, ethylene glycol was added to stop the reaction, and the mixture was immediately dialyzed against water. The formation and swelling kinetics of hydrogels and the in vitro release kinetics of cisplatin from hydrogels were studied. The tests showed that there was sustained cisplatin release within 4 days. The researchers also evaluated the antitumor effects of the intraperitoneal administration of cisplatin-loaded acid hyaluronic hydrogels using a mouse model of gastric cancer. They observed a significant reduction in the weight of the peritoneal nodules in the gel-cisplatin group, whereas no significant reduction was detected in a phosphate-buffered saline-cisplatin group. It was concluded that this hydrogel is desirable for retention and modulates the release of cisplatin, thus increasing its antitumor effects.Citation176
Researchers have tested a system composed of a heat-sensitive copolymer formed by PEG-poly(ε-caprolactone)-PEG (PECE) for the incorporation of suberoylanilide hydroxamic acid (SAHA) with cisplatin and subsequently evaluated the in vitro release profile of these drugs against oral carcinoma.Citation152 For the formation of hydrogels, the PECE copolymer was first completely dissolved in water and cooled to 4°C to form a colloidal solution. SAHA and cisplatin solutions were then mixed into the PECE colloidal solution to form a homogeneous solution, and the PECE concentration was maintained at 30% (w/w). The authors concluded that the SAHA-cisplatin/PECE hydrogel system with direct intratumoral injections may be a useful method for the treatment of oral cancer and other solid tumors.Citation177
Liquid crystals
LCs are materials in a differential state, demonstrating a property between a solid and a liquid. This state is called mesophase: the prefix “meso-” means “intermediate”.Citation178
LCs are divided into two categories: thermotropics, which are structured by means of temperature, and lyotropics, which occur by association with amphipathic compounds and solvents. The mesophase lyotropics are mostly lamellar, hexagonal, or cubic,Citation179 as shown in .
Figure 4 Schematic representation of lamellar (A), hexagonal (B), and cubic (C) liquid crystal mesophases.

LCs are usually based on water as a solvent, surfactant (may contain cosurfactants), and an oily phase. One of the advantages of LCs is that they can be stored for long periods because they are thermodynamically stable.Citation180
Polarized light microscopy is one of the characterization techniques that is used for the preliminary identification of mesophase LCs.Citation181 In this analysis, a sample undergoes the incidence of polarized light, which is enough to deflect light and is called anisotropic (it can be mesophase lamellar or hexagonal). However, if the latter does not bend light, it is isotropic (cubic arrangements); therefore, other techniques are needed for confirming this structure,Citation179 including small-angle X-ray scattering, small-angle neutron scattering, neutron diffraction, nuclear magnetic resonance, and cryofracture electron microscopy.Citation179,Citation182,Citation183
The LC systems significantly change the drug release profile and reduce the toxicity of drugs, improving clinical efficiency.Citation178 Hosmer et al in 2012, studied mesophase lamellar LCs formed with glycerides for the incorporation of the anticancer drug paclitaxel.Citation184 Paclitaxel is highly effective against various types of cancer, including oral cancer;Citation185–Citation187 however, it has severe adverse effects associated with systemic drug administration, including hypersensitivity reactions, thrombocytopenia, and neutropenia.Citation188,Citation189 Hosmer et alCitation184 found that, among the formulations studied, the Brij-based lamellar phase containing 20% medium-chain mono-/diglycerides maximized the delivery of paclitaxel and showed good efficacy against paclitaxel-sensitive fibroblasts; therefore, LCs may be a promising strategy for the treatment of cancers, including oral cancer.
Zeng et al developed liquid crystalline nanoparticles consisting of soy phosphatidyl choline and glycerol dioleate for the incorporation of paclitaxel using a solvent precursor method described by Rizwan et al in which a 50:50 (w/w) mixture of soy phosphatidyl choline and glycerol dioleate was agitated for 3 hours to form a uniform oily phase.Citation190,Citation191 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) was then carefully added followed by stirring at room temperature for 24 hours. The precursor of LC was added under magnetic stirring at 60°C, forming a coarse dispersion. This dispersion was subsequently homogenized using a Microfluidizer® (Microfluidics, Newton, MA, USA) at a pressure of 10,000 psi for three cycles and 30,000 psi for two cycles.Citation191 The systems were characterized by polarized light microscopy, indicating the coexistence of reversed cubic and hexagonal phases in the optimized LC matrix. Transmission electron microscopy and cryo-field emission scanning electron microscopy revealed the internal water channel and “twig-like” surface morphology of the LC matrix. Tests were performed, including pharmacokinetics in vivo, and particle size distribution, phase behavior characterization, transmission electron microscopy, and cryo-field emission scanning electron microscopy in vitro. Zeng et al concluded that these systems demonstrated potential as nanocarriers for water-insoluble drugs such as paclitaxel, improving intravenous bioavailability.Citation191
Complexes of cyclodextrins
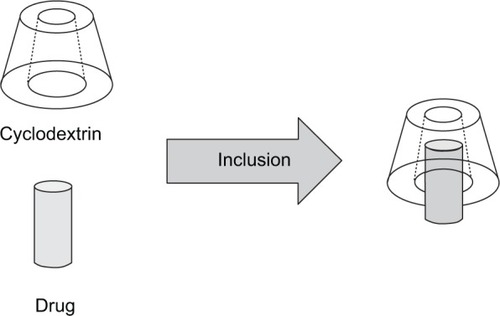
Cyclodextrins are cyclical and composed of at least six units of glucose,Citation192 resulting in a truncated cone form with a hollow cavity,Citation193 as shown in .
Although natural cyclodextrins (the best known cyclodextrins include α-, β-, and γ-cyclodextrin) are of interest for the development of pharmaceutical formulations by presenting excellent biocompatibility,Citation194 the ability to mask undesirable organoleptic properties of drugs, and the ability to increase solubility and permeability,Citation195 these compounds demonstrate limitations for the transport of drugs, enabling the loading of only lipophilic drugs by virtue of the cyclodextrin hydrophilic exterior and interior hydrophobic cavity.Citation196 Soon, natural cyclodextrins may be produced with chemical modifications in accordance with the interest in this field,Citation197 enabling the attainment of cyclodextrins with both lipophilic and conjugated polar groups, making them amphiphilic.Citation198
Cyclodextrins represent a group of excipients with great potential for use in pharmaceutical formulations. Once the bioavailability and multifunctional features of cyclodextrins are able to reduce the undesirable properties of drugs that are included in complexes, application via several routes of administration will be enabled. It is also important to highlight the ability to include drugs with solid or liquid characteristics.Citation199
Ramineni et al studied cyclodextrins for the inclusion of imiquimod to treat precancerous dysplastic lesions of the oral cavity.Citation200 This group developed a mucoadhesive film for the conveyance of a highly hydrophobic drug. To prepare these films, a polymer aqueous solution of polyvinylpyrrolidone mixed with ethanol following the addition of propylene glycol as a plasticizer was used. At the same time, a solution of carboxymethylcellulose was prepared and added to polymer aqueous solution at high speed. The polymer mixture was dried, forming films, and the antineoplastic agent was incorporated by four different methods: sonication, solubilization in linoleic acid, complex formation by co-evaporation, and solubilization in methanol and acetate buffer. The films were translucent and flexible, with the exception of those prepared with linoleic acid; therefore, the films are a promising platform for the delivery of drugs with mucoadhesion properties, which are able to be administered at the desired location in addition to sustaining the delivery of imiquimod.Citation200
Researchers have also developed a new type of hollow complex based on the combination of cyclodextrin with hyaluronic acid, which can be added to paclitaxel.Citation201 The system was innovatively performed and was associated with the pursuit of controlled drug release by complexation with cyclodextrins that are recognized by cancer cells and sensitized to enzymatic hydrolysis caused by the natural biological properties of hyaluronic acid. Under physiological conditions, paclitaxel was released slowly, demonstrating that the cyclodextrins were stable.Citation201
A group of researchersCitation202 developed α-cyclodextrins with pH-sensitive nanoparticles. To reach the specific location of a tumor that is around pH 5.7–7.8, this group prepared a system for drug targeting. These authors incorporated paclitaxel and analyzed the activity of various tumor cells in addition to conducting tests in vivo in mice with melanoma. These mice were given a single intravenous dose of paclitaxel (10 mg/kg) and were compared with a negative control that was administered saline solution, and a reduction in tumor cells was observed. Thus, it is noted that formulated nanomedicines can effectively reverse the multidrug resistance of cancer cells resistant to paclitaxel. In summary, pH-sensitive α-cyclodextrin materials can be conveniently produced by a facile acetonation process, which may be further processed into NPs with controllable size and size distribution. The results demonstrated that the systems have biocompatibility and lead to a reduction in adverse effects and improved antitumor activity.Citation202
New approaches and challenges
The ultimate goal of cancer treatment is to kill as many cancer cells as possible without affecting healthy cells. However, the ability of a drug to target specific sites in the body to achieve defined therapeutic effects needs improvement. In this context, nanodelivery systems emerge as a potential alternative for overcoming some previously encountered obstacles to efficiently target several cancer cell types because they have shown several promising characteristics, including optimal anti-oral tumor effects, which are not available with traditional chemotherapy.
Thus, the US Food and Drug Administration (FDA) recently approved a clinical trial of a nanoparticle-based system to use in humans for treatment of solid tumors.Citation203 Furthermore, Yang et al in 2003, evaluated targeted delivery to cervical lymph nodes by perioral cancer submucosal injection of cucurbitacin BE polylactic acid nanoparticles (CuBE-PLA-NPs) and their clinical therapy efficacy. The results showed that the drug concentrations in cervical lymph nodes after CuBE-PLA-NP injection were far higher than those in the control group. Furthermore, the drug concentrations in the blood in the CuBE-PLA-NP group were far lower than those in the control group.Citation204
Hence, in the near future, oncologists and patients will benefit from suitable nanotechnology-based drug delivery systems that could lead to improved therapeutic outcomes with reduced costs. There are few clinical studies on oral cancer in the field of nanotechnology, but nanotechnology is also predicted to alter health care in dentistry, with novel methods of identifying the cancer as well as customization of a patient’s therapeutic profile.Citation205
Further studies are needed to turn concepts of nanotechnology into practical applications and to elucidate correct drug doses and ideal release from these systems for the treatment of several cancers with different molecular and cellular mechanisms.
Acknowledgments
The authors are grateful to FAPESP (São Paulo, Brazil), CNPq (Brasília, Brazil), and PADC/FCF-UNESP (Araraquara, Brazil) for research fellowships.
Disclosure
The authors report no conflicts of interest in this work.
References
- JemalABrayFCenterMMFerlayJWardEFormanDGlobal cancer statisticsCA Cancer J Clin2011612699021296855
- SiegelRNaishadhamDJemalACancer statistics, 2013CA Cancer J Clin2013631113023335087
- EpsteinJBZhangLRosinMAdvances in the diagnosis of oral premalignant and malignant lesionsJ Can Dent Assoc2002681061762112410942
- LlewellynCDJohnsonNWWarnakulasuriyaKARisk factors for oral cancer in newly diagnosed patients aged 45 years and younger: a case-control study in Southern EnglandJ Oral Pathol Med200433952553215357672
- ChitapanaruxILorvidhayaVSittitraiPOral cavity cancers at a young age: analysis of patient, tumor and treatment characteristics in Chiang Mai University HospitalOral Oncol2006421838816249113
- BinnieWHRankinKVMackenzieICEtiology of oral squamous cell carcinomaJ Oral Pathol198312111296403682
- DouglassCWGammonMDReassessing the epidemiology of lip cancerOral Surg Oral Med Oral Pathol19845766316426377170
- de RosaIStaibanoSLo MuzioLPotentially malignant and malignant lesions of the lip. Role of silver staining nucleolar organizer regions, proliferating cell nuclear antigen, p53, and c-myc in differentiation and prognosisJ Oral Pathol Med199928625225810426197
- FriersonHFJrCooperPHPrognostic factors in squamous cell carcinoma of the lower lipHum Pathol19861743463543957335
- NäsmanAAttnerPHammarstedtLIncidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma?Int J Cancer2009125236236619330833
- HafkampHCSpeelEJHaesevoetsAA subset of head and neck squamous cell carcinomas exhibits integration of HPV 16/18 DNA and overexpression of p16INK4A and p53 in the absence of mutations in p53 exons 5–8Int J Cancer2003107339440014506739
- RaginCCTaioliESurvival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysisInt J Cancer200712181813182017546592
- HuangBChenHFanMA postulated role for human papillomavirus (HPV) in the transformation and proliferation of oral squamous cell carcinoma (OSCC)Med Hypotheses20087051041104317910995
- PetersenPEOral cancer prevention and control – the approach of the World Health OrganizationOral Oncol2009454–545446018804412
- JonesDLRankinKVOral cancer and associated risk factorsCappelliDPMobleyCCPrevention in Clinical Oral Health CareSt Louis, MOMosby Elsevier20086877
- SuCCYangHFHuangSJLianIeBDistinctive features of oral cancer in Changhua County: high incidence, buccal mucosa preponderance, and a close relation to betel quid chewing habitJ Formos Med Assoc2007106322523317389167
- ChenCJYouSLLinLHHsuWLYangYWCancer epidemiology and control in Taiwan: a brief reviewJpn J Cancer Res200232SupplS66S81
- KantolaSParikkaMJokinenKPrognostic factors in tongue cancer – relative importance of demographic, clinical and histopathological factorsBr J Cancer200083561461910944601
- SankaranarayananRMasuyerESwaminathanRFerlayJWhelanSHead and neck cancer: a global perspective on epidemiology and prognosisAnticancer Res1998186B477947869891557
- CarvalhoALMagrinJKowalskiLPSites of recurrence in oral and oropharyngeal cancers according to the treatment approachOral Dis20039311211812945592
- ShahJPLydiattWTreatment of cancer of the head and neckCA Cancer J Clin19954563523687583907
- OnofreMASpostoMRNavarroCMMottaMETurattiEAlmeidaRTPotentially malignant epithelial oral lesions: discrepancies between clinical and histological diagnosisOral Dis1997331481529467356
- BhalangKSuesuwanADhanuthaiKSannikornPLuangjarmekornLSwasdisonSThe application of acetic acid in the detection of oral squamous cell carcinomaOral Surg Oral Med Oral Pathol Oral Radiol Endod2008106337137618547833
- EpsteinJBSilvermanSJrEpsteinJDLonkySABrideMAAnalysis of oral lesion biopsies identified and evaluated by visual examination, chemiluminescence and toluidine blueOral Oncol200844653854417996486
- Wilder-SmithPLeeKGuoSIn vivo diagnosis of oral dysplasia and malignancy using optical coherence tomography: preliminary studies in 50 patientsLasers Surg Med200941535335719533765
- KochFPKaemmererPWBiesterfeldSKunkelMWagnerWEffectiveness of autofluorescence to identify suspicious oral lesions – a prospective, blinded clinical trialClin Oral Investig2010156975982
- KochFPKunkelMBiesterfeldSWagnerWDiagnostic efficiency of differentiating small cancerous and precancerous lesions using mucosal brush smears of the oral cavity – a prospective and blinded studyClin Oral Investig2010155763769
- ScullyCCancerOral and Maxillofacial Medicine: The Basis of Diagnosis and Treatment3rd edNew York, NYChurchill Livingstone Elsevier2013204217
- CsikarJAravaniAGodsonJDayMWilkinsonJIncidence of oral cancer among South Asians and those of other ethnic groups by sex in West Yorkshire and England, 2001–2006Br J Oral Maxillofac Surg2013511252922495403
- WeinbergMAEstefanDJAssessing oral malignanciesAm Fam Physician20026571379138411996421
- HomerJJGreenmanJStaffordNDAngiogenic cytokines in serum and plasma of patients with head and neck squamous cell carcimonaClin Otolaryngol Allied Sci200025657057611123175
- WeinbergRATumor suppressor genesScience19912545035113811461659741
- MehrotraRVasstrandENIbrahimSORecent advances in understanding carcinogenicity of oral squamous cell carcinoma: from basic molecular biology to latest genomic and proteomic findingsCancer Genomics Proteomics200414283294
- KhanZBisenPSOncoapoptotic signaling and deregulated target genes in cancers: special reference to oral cancerBiochim Biophys Acta20131836112314523602834
- VogelsteinBKinzlerKWThe multistep nature of cancerTrends Genet1993941381418516849
- MehrotraRYadavSOral squamous cell carcinoma: etiology, pathogenesis and prognostic value of genomic alterationsIndian J Cancer2006432606616790942
- HollsteinMSidranskyDVogelsteinBHarrisCCp53 mutations in human cancersScience1991253501549531905840
- BrennanJABoyleJOKochWMAssociation between cigarette smoking and mutation of the p53 gene in squamous-cell carcinoma of the head and neckN Engl J Med1995332117127177854378
- SchantzSPBasic science advances in head and neck oncology: the past decadeSemin Surg Oncol19951132722797638515
- CalifanoJAhrendtSAMeiningerGWestraWHKochWMSidranskyDDetection of telomerase activity in oral rinses from head and neck squamous cell carcinoma patientsCancer Res19965624572057228971181
- MiyoshiYTsukinokiKImaizumiTTelomerase activity in oral cancerOral Oncol199935328328910621849
- BrachmanDGBeckettMGravesDHarafDVokesEWeichselbaumRRp53 mutation does not correlate with radiosensitivity in 24 head and neck cancer cell linesCancer Res19935316366736698339273
- HaranoNOnoKHidakaKKaiANakanishiOInenagaKDifferences between orofacial inflammation and cancer painJ Dent Res201089661562020332329
- PetersCMLindsayTHPomonisJDEndothelin and the tumorigenic component of bone cancer painNeuroscience200412641043105215207337
- PickeringVJordanRCSchmidtBLElevated salivary endothelin levels in oral cancer patients – a pilot studyOral Oncol2007431374116757207
- SchmidtBLPickeringVLiuSPeripheral endothelin A receptor antagonism attenuates carcinoma-induced painEur J Pain200711440641416807013
- PickeringVJay GuptaRQuangPJordanRCSchmidtBLEffect of peripheral endothelin-1 concentration on carcinoma-induced pain in miceEur J Pain200812329330017664075
- NybergPYlipalosaariMSorsaTSaloTTrypsins and their role in carcinoma growthExp Cel Res2006312812191228
- LamDKSchmidtBLSerine proteases and protease-activated receptor 2-dependent allodynia: a novel cancer pain pathwayPain2010149226327220189717
- RussoASohUJTrejoJProteases display biased agonism at protease-activated receptors: location matters!Mol Interv200992879619401541
- AmadesiSCottrellGSDivinoLProtease-activated receptor 2 sensitizes TRPV1 by protein kinase Cepsilon- and A-dependent mechanisms in rats and miceJ Physiol2006575Pt 255557116793902
- ZhengJTianXSunYLuDYangWpH-sensitive poly (glutamic acid) grafted mesoporous silica nanoparticles for drug deliveryInt J Pharm2013450129630323598077
- DavisBMLewinGRMendellLMJonesMEAlbersKMAltered expression of nerve growth factor in the skin of transgenic mice leads to changes in response to mechanical stimuliNeuroscience19935647897927506820
- KleinRJingSQNanduriVO’RourkeEBarbacidMThe trk proto-oncogene encodes a receptor for nerve growth factorCell19916511891971849459
- GlinskyGVIvanovaABVinnitskyVBArumäeUSiigurJNeumanTNerve growth factor may be the metastasis growth factorAnn N Y Acad Sci19874966566593496831
- KolokythasACoxDPDekkerNSchmidtBLNerve growth factor and tyrosine kinase A receptor in oral squamous cell carcinoma: is there an association with perineural invasion?J Oral Maxillofac Surg20106861290129520363547
- YeYDangDZhangJNerve growth factor links oral cancer progression, pain, and cachexiaMol Cancer Ther20111091667167621750223
- OkadaYUenoHKatagiriMExperimental study of antiangiogenic gene therapy targeting VEGF in oral cancerOdontology2010981525920155508
- RiedelFGötteKSchwalbJWirtzHBerglerWHörmannKSerum levels of vascular endothelial growth factor in patients with head and neck cancerEur Arch Otorhinolaryngol2000257633233610993554
- ShangZJLiJRLiZBCirculating levels of vascular endothelial growth factor in patients with oral squamous cell carcinomaInt J Oral Maxillofac Surg200231549549812418564
- MărgăritescuCPiriciDSimionescuCVEGF and VEGFRs expression in oral squamous cell carcinomaRom J Morphol Embryol200950452754819942948
- SalimiMEsfahaniMHabibzadehNChange in nicotine-induced VEGF, PGE2 AND COX-2 expression following COX inhibition in human oral squamous cancerJ Environ Pathol Toxicol Oncol201231434935623394447
- ColottaFAllavenaPSicaAGarlandaCMantovaniACancer-related inflammation, the seventh hallmark of cancer: links to genetic instabilityCarcinogenesis20093071073108119468060
- Del PreteAAllavenaPSantoroGFumaruloRCorsiMMMantovaniAMolecular pathways in cancer-related inflammationBiochem Med (Zagreb)201121326427522420240
- KampDWShacterEWeitzmanSAChronic inflammation and cancer: the role of the mitochondriaOncology (Williston Park)201125540041041321710835
- MignognaMDFedeleSLo RussoLLo MuzioLBucciEImmune activation and chronic inflammation as the cause of malignancy in oral lichen planus: is there any evidence?Oral Oncol200440212013014693234
- LiTJCuiJCOX-2, MMP-7 expression in oral lichen planus and oral squamous cell carcinomaAsian Pac J Trop Biomed201368640643
- GeorgakopoulouEAAchtariMDAchtarisMFoukasPGKotsinasAOral lichen planus as a preneoplastic inflammatory modelJ Biomed Biotechnol2012201275962622675259
- PontesHAPontesFSFonsecaFPNuclear factor kappa B and cyclooxygenase-2 immunoexpression in oral dysplasia and oral squamous cell carcinomaAnn Diagn Pathol2013171455022818026
- MauroALipariLLeoneAExpression of cyclooxygenase-1 and cyclooxygenase-2 in normal and pathological human oral mucosaFolia Histochem Cytobiol201048455556321478098
- Goulart FilhoJANonakaCFda Costa MiguelMCde Almeida FreitasRGalvãoHCImmunoexpression of cyclooxygenase-2 and p53 in oral squamous cell carcinomaAm J Otolaryngol2009302899419239949
- ImpolaUUittoVJHietanenJDifferential expression of matrilysin-1 (MMP-7), 92 kD gelatinase (MMP-9), and metalloelastase (MMP-12) in oral verrucous and squamous cell cancerJ Pathol20042021142214694517
- VuTHWerbZMatrix metalloproteinases: effectors of development and normal physiologyGenes Dev200014172123213310970876
- WernerJARathckeIOMandicRThe role of matrix metalloproteinases in squamous cell carcinomas of the head and neckClin Exp Metastasis200219427528212090467
- EgebladMWerbZNew functions for the matrix metalloproteinases in cancer progressionCancer20022316117411990853
- VairaktarisESerefoglouZYapijakisCHigh gene expression of matrix metalloproteinase-7 is associated with early stages of oral cancerAnticancer Res2007274B2493249817695544
- KarinMNuclear factor-kappa B in cancer development and progressionNature2006441709243143616724054
- BindhuOSRamadasKSebastianPPillaiMRHigh expression levels of nuclear factor kappa B and gelatinases in the tumorigenesis of oral squamous cell carcinomaHead Neck2006281091692516823875
- RichmondANf-kappa B, chemokine gene transcription and tumour growthNat Rev Immunol20022966467412209135
- de VisscherJGBehandeling en prognose van het mondholtecarcinoom [Treatment and prognosis of oral cancer]Ned Tijdschr Tandheelkd20081154192198 Dutch18512517
- AdelsteinDJLiYAdamsGLAn intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancerJ Clin Oncol2003211929812506176
- BachaudJMCohen-JonathanEAlzieuCDavidJMSerranoEDaly-SchveitzerNCombined postoperative radiotherapy and weekly cisplatin infusion for locally advanced head and neck carcinoma: final report of a randomized trialInt J Radiat Oncol Biol Phys199636599910048985019
- BaselgaJTrigoJMBourhisJPhase II multicenter study of the antiepidermal growth factor receptor monoclonal antibody cetuximab in combination with platinum-based chemotherapy in patients with platinum-refractory metastatic and/or recurrent squamous cell carcinoma of the head and neckJ Clin Oncol200523245568557716009950
- BonnerJAHarariPMGiraltJRadiotherapy plus cetuximab for squamous-cell carcinoma of the head and neckN Engl J Med2006354656757816467544
- CatimelGVerweijJMattijssenVDocetaxel (Taxotere): an active drug for the treatment of patients with advanced squamous cell carcinoma of the head and neck. EORTC Early Clinical Trials GroupAnn Oncol1994565335377918125
- ClavelMVermorkenJBCognettiFRandomized comparison of cisplatin, methotrexate, bleomycin and vincristine (CABO) versus cisplatin and 5-fluorouracil (CF) versus cisplatin (C) in recurrent or metastatic squamous cell carcinoma of the head and neck. A phase III study of the EORTC Head and Neck Cancer Cooperative GroupAnn Oncol1994565215267522527
- HaddadRSonisSPosnerMRandomized phase 2 study of concomitant chemoradiotherapy using weekly carboplatin/paclitaxel with or without daily subcutaneous amifostine in patients with locally advanced head and neck cancerCancer2009115194514452319634161
- VermorkenJBMesiaRRiveraFPlatinum-based chemotherapy plus cetuximab in head and neck cancerN Engl J Med2008359111116112718784101
- VermorkenJBRemenarEvan HerpenCCisplatin, fluorouracil, and docetaxel in unresectable head and neck cancerN Engl J Med2007357171695170417960012
- VermorkenJBTrigoJHittROpen-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapyJ Clin Oncol200725162171217717538161
- TerwogtJMSchellensJHHuininkWWBeijnenJHClinical pharmacology of anticancer agents in relation to formulations and administration routesCancer Treat Rev19992528310110395834
- AgüerosMRuiz-GatónLVauthierCCombined hydroxypropyl-beta-cyclodextrin and poly(anhydride) nanoparticles improve the oral permeability of paclitaxelEur J Pharm Sci200938440541319765652
- DevalapallyHChakilamAAmijiMMRole of nanotechnology in pharmaceutical product developmentJ Pharm Sci200796102547256517688284
- KruijtzerCMBeijnenJHSchellensJHImprovement of oral drug treatment by temporary inhibition of drug transporters and/or cytochrome P450 in the gastrointestinal tract and liver: an overviewOncologist20027651653012490739
- KumarGPRajeshwarraoPNonionic surfactant vesicular systems for effective drug delivery – an overviewActa Pharm Sin B201114208219
- ManmodeASSakarkarDMMahajanNMNanoparticles-tremendous therapeutic potential: a reviewInternational Journal of Pharm Tech Research20091410201027
- NehaBGaneshBPreetiKDrug delivery to the brain using polymeric nanoparticles: a reviewInternational Journal of Pharmaceutical and Life Sciences201323107132
- ChoKWangXNieSChenZGShinDMTherapeutic nanoparticles for drug delivery in cancerClin Cancer Res20081451310131618316549
- CouvreurPNanoparticles in drug delivery: past, present and futureAdv Drug Deliv Rev2013651212322580334
- KamimuraMFurukawaTAkiyamaS-INagasakiYEnhanced intracellular drug delivery of pH-sensitive doxorubicin/poly(ethylene glycol)-block-poly(4-vinylbenzylphosphonate) nanoparticles in multi-drug resistant human epidermoid KB carcinoma cellsBiomater Sci201314361367
- DuFMengHXuKCPT loaded nanoparticles based on beta-cyclodextrin-grafted poly(ethylene glycol)/poly(L-glutamic acid) diblock copolymer and their inclusion complexes with CPTColloids Surf B Biointerfaces201411323023624096159
- RavikumaraNRTiyaboonchaiWMadhusudhanBFabrication and characterization of genistein encapsulated poly (D, L) lactic acid nanoparticles for pharmaceutical applicationCurr Nanosci201392293302
- PanyamJZhouWZPrabhaSSahooSKLabhasetwarVRapid endo-lysosomal escape of poly(DL-lactide-co-glycolide) nanoparticles: implications for drug and gene deliveryFASEB J200216101217122612153989
- RenFChenRWangYSunYJiangYLiGPaclitaxel-loaded poly(n-butylcyanoacrylate) nanoparticle delivery system to overcome multidrug resistance in ovarian cancerPharm Res201128489790621184150
- KooHMinKHLeeSCEnhanced drug-loading and therapeutic efficacy of hydrotropic oligomer-conjugated glycol chitosan nanoparticles for tumor-targeted paclitaxel deliveryJ Control Release2013172382383124035978
- LeeKYMooneyDJAlginate: properties and biomedical applicationsProg Polym Sci201237110612622125349
- DasRKKasojuNBoraUEncapsulation of curcumin in alginatechitosan-pluronic composite nanoparticles for delivery to cancer cellsNanomedicine20106115316019616123
- WangHZhaoPLiangXFolate-PEG coated cationic modified chitosan – cholesterol liposomes for tumor-targeted drug deliveryBiomaterials201031144129413820163853
- MohanrajVJChenYNanoparticles – a reviewTropical Journal of Pharmaceutical Research200651561573
- FanWYanWXuZNiHFormation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation techniqueColloids Surf B Biointerfaces201290212722014934
- MakadiaHKSiegelSJPoly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrierPolymers (Basel)2011331377139722577513
- DhandaDSTyagiPMirvishSSKompellaUBSupercritical fluid technology based large porous celecoxib-PLGA microparticles do not induce pulmonary fibrosis and sustain drug delivery and efficacy for several weeks following a single doseJ Control Release2013168323925023562638
- PetrosRADeSimoneJMStrategies in the design of nanoparticles for therapeutic applicationsNat Rev Drug Discov20109861562720616808
- ChuKSSchorzmanANFinnissMCNanoparticle drug loading as a design parameter to improve docetaxel pharmacokinetics and efficacyBiomaterials201334338424842923899444
- MahapatroASinghDKBiodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccinesJ Nanobiotechnology2011915522123084
- Brannon-PeppasLBlanchetteJONanoparticle and targeted systems for cancer therapyAdv Drug Deliv Rev201264206212
- BrewerEColemanJLowmanAEmerging technologies of polymeric nanoparticles in cancer drug deliveryJ Nanomater20112011408675
- DamascelliBPatelliGLLanocitaRA novel intraarterial chemotherapy using paclitaxel in albumin nanoparticles to treat advanced squamous cell carcinoma of the tongue: preliminary findingsAJR Am J Roentgenol2003181125326012818869
- SulfikkaraliNKrishnakumarNManoharanSNirmalRMChemopreventive efficacy of naringenin-loaded nanoparticles in 7,12-dimethylbenz(a)anthracene induced experimental oral carcinogenesisPathol Oncol Res201319228729623233294
- YuDWangAHuangHChenYPEG-PBLG nanoparticle-mediated HSV-TK/GCV gene therapy for oral squamous cell carcinomaNanomedicine (Lond)20083681382119025455
- EndoKUenoTKondoSTumor-targeted chemotherapy with the nanopolymer-based drug NC-6004 for oral squamous cell carcinomaCancer Sci2013104336937423216802
- LiPZhouGZhuXPhotodynamic therapy with hyperbranched poly(ether-ester) chlorin(e6) nanoparticles on human tongue carcinoma CAL-27 cellsPhotodiagnosis Photodyn Ther201291768222369732
- Dos SantosFKOyafusoMHKiillCPDaflon-GremiaoMPChorilliMNanotechnology-based drug delivery systems for treatment of hyperproliferative skin diseases – a reviewCurr Nanosci201391159167
- Ribeiro de SouzaALKiillCPdos SantosFKNanotechnology-based drug delivery systems for dermatomycosis treatmentCurr Nanosci201284512519
- ParveenSMisraRSahooSKNanoparticles: a boon to drug delivery, therapeutics, diagnostics and imagingNanomedicine20128214716621703993
- SoutoEBDoktorovováSChapter 6 – solid lipid nanoparticle formulations pharmacokinetic and biopharmaceutical aspects in drug deliveryMethods Enzymol200946410512919903552
- WissingSKayserOMüllerRSolid lipid nanoparticles for parenteral drug deliveryAdv Drug Deliv Rev20045691257127215109768
- MehnertWMäderKSolid lipid nanoparticles: production, characterization and applicationsAdv Drug Deliv Rev200147216519611311991
- MüllerRHMäderKGohlaSSolid lipid nanoparticles (SLN) for controlled drug delivery – a review of the state of the artEur J Pharm Biopharm200050116117710840199
- PatelMSoutoEBSinghKKAdvances in brain drug targeting and delivery: limitations and challenges of solid lipid nanoparticlesExpert Opin Drug Deliv201310788990523550609
- ShiSJZhongZRLiuJZhangZRSunXGongTSolid lipid nanoparticles loaded with anti-microRNA oligonucleotides (AMOs) for suppression of microRNA-21 functions in human lung cancer cellsPharm Res20122919710921732152
- KakkarVSinghSSinglaDKaurIPExploring solid lipid nanoparticles to enhance the oral bioavailability of curcuminMol Nutr Food Res201155349550320938993
- HolpuchASHummelGJTongMNanoparticles for local drug delivery to the oral mucosa: proof of principle studiesPharm Res20102771224123620354767
- BattagliaLGallarateMLipid nanoparticles: state of the art, new preparation methods and challenges in drug deliveryExpert Opin Drug Deliv20129549750822439808
- FangJYFangCLLiuCHSuYHLipid nanoparticles as vehicles for topical psoralen delivery: solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC)Eur J Pharm Biopharm200870263364018577447
- LinYKHuangZRZhuoRZFangJYCombination of calcipotriol and methotrexate in nanostructured lipid carriers for topical deliveryInt J Nanomedicine2010511712820309398
- KovacevicASavicSVuletaGMüllerRKeckCPolyhydroxy surfactants for the formulation of lipid nanoparticles (SLN and NLC): effects on size, physical stability and particle matrix structureInt J Pharm20114061–216317221219990
- AdityaNShimMLeeILeeYImMHKoSCurcumin and genistein coloaded nanostructured lipid carriers: In vitro digestion and antiprostate cancer activityJ Agric Food Chem20136181878188323362941
- ZlotogorskiADayanADayanDChaushuGSaloTVeredMNutraceuticals as new treatment approaches for oral cancer – I: curcuminOral Oncol201349318719123116961
- ChinsriwongkulAChareanputtakhunPNgawhirunpatTNanostructured lipid carriers (NLC) for parenteral delivery of an anticancer drugAAPS Pharm Sci Tech2012131150158
- WangJDaiYHuangYAll-trans retinoic acid restores gap junctional intercellular communication between oral cancer cells with upregulation of Cx32 and Cx43 expressions in vitroMed Oral Patol Oral Cir Bucal2013184e569e57723524428
- LiuSlZhongSSYeDXChenWTZhangZYDengJRepression of G protein-coupled receptor family C group 5 member A is associated with pathologic differentiation grade of oral squamous cell carcinomaJ Oral Pathol Med2013421076176823651229
- LiuDLiuZWangLZhangCZhangNNanostructured lipid carriers as novel carrier for parenteral delivery of docetaxelColloids Surf B Biointerfaces201185226226921435845
- IidaSShimadaJSakagamiHCytotoxicity induced by docetaxel in human oral squamous cell carcinoma cell linesIn Vivo201327332133223606687
- ZhangTChenJZhangYShenQPanWCharacterization and evaluation of nanostructured lipid carrier as a vehicle for oral delivery of etoposideJ Pharm Sci2011433174179
- MishimaKInoueKHayashiYOverexpression of extracellular-signal regulated kinases on oral squamous cell carcinomaOral Oncol200238546847412110341
- LiuLTangYGaoCCharacterization and biodistribution in vivo of quercetin-loaded cationic nanostructured lipid carriersColloids Surf B Biointerfaces201411512513124333909
- KangBMackeyMAEl-SayedMANuclear targeting of gold nanoparticles in cancer cells induces DNA damage, causing cytokinesis arrest and apoptosisJ Am Chem Soc201013251517151920085324
- WangFWangYCDouSXiongMHSunTMWangJDoxorubicin-tethered responsive gold nanoparticles facilitate intracellular drug delivery for overcoming multidrug resistance in cancer cellsACS Nano2011553679369221462992
- El-SayedIHHuangXEl-SayedMASurface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancerNano Lett20055582983415884879
- KahJCKhoKWLeeCGEarly diagnosis of oral cancer based on the surface plasmon resonance of gold nanoparticlesInt J Nanomedicine20072478579818203445
- AfifiMMEl SheikhSMAbdelsalamMMTherapeutic efficacy of plasmonic photothermal nanoparticles in hamster buccal pouch carcinomaOral Surg Oral Med Oral Pathol Oral Radiol2013115674375123454046
- MezeiMGulasekharamVLiposomes – a selective drug delivery system for the topical route of administration. Lotion dosage formLife Sci19802618147314776893068
- LianTHoRJTrends and developments in liposome drug delivery systemsJ Pharm Sci200190666768011357170
- AllenTMCullisPRDrug delivery systems: entering the mainstreamScience200430356651818182215031496
- Pedroso de LimaMCSimõesSPiresPFanecaHDüzgüneşNCationic lipid–DNA complexes in gene delivery: from biophysics to biological applicationsAdv Drug Deliv Rev200147227729411311996
- KonopkaKFallahBMonzon-DullerJOverlidNDüzgünesNSerum-resistant gene transfer to oral cancer cells by Metafectene and GeneJammer: application to HSV-tk/ganciclovir-mediated cytotoxicityCell Mol Biol Lett200510345547016217556
- Figueiró LongoJPMuehlmannLAVellosoNVEffects of photodynamic therapy mediated by liposomal aluminum-phthalocyanine chloride on chemically induced tongue tumorsChemotherapy20121103
- VellosoNVMuehlmannLALongoJPFAluminum-phthalocyanine chloride-based photodynamic therapy inhibits PI3K/Akt/Mtor pathway in oral squamous cell carcinoma cells in vitroChemotherapy20121107
- BahramMHoseinzadehFFarhadiKSaadatMNajafi-MoghaddamPAfkhamiASynthesis of gold nanoparticles using pH-sensitive hydrogel and its application for colorimetric determination of acetaminophen, ascorbic acid and folic acidColloids Surf A Physicochem Eng Asp2014441517524
- GiriTKThakurAAlexanderAAjazuddinBadwaikHTripathiDKModified chitosan hydrogels as drug delivery and tissue engineering systems: present status and applicationsActa Pharm Sin B201225439449
- Ruel-GariépyEShiveMBicharaAA thermosensitive chitosan-based hydrogel for the local delivery of paclitaxelEur J Pharm Biopharm2004571536314729080
- ObaraKIshiharaMOzekiYControlled release of paclitaxel from photocrosslinked chitosan hydrogels and its subsequent effect on subcutaneous tumor growth in miceJ Control Release20051101798916289419
- KonishiMTabataYKariyaMIn vivo anti-tumor effect through the controlled release of cisplatin from biodegradable gelatin hydrogelJ Control Release200392330131314568411
- GurskiLAJhaAKZhangCJiaXFarach-CarsonMCHyaluronic acid-based hydrogels as 3D matrices for in vitro evaluation of chemotherapeutic drugs using poorly adherent prostate cancer cellsBiomaterials200930306076608519695694
- GaoYRenFDingBA thermo-sensitive PLGA-PEG-PLGA hydrogel for sustained release of docetaxelJ Drug Target201119751652720883085
- HaoTLiZQiaoMFanQZhangNChenDPreparation of docetaxel hydrogels for subcutaneous injection and their release profile in vitroChinese Pharmaceutical Journal20092415 Chinese
- ShakerDSGhorabMKKlingnerATeiamaMSIn-situ injectable thermosensitive gel based on poloxamer as a new carrier for tamoxifen citrateInt J Pharm Pharm Sci20135Suppl 4429437
- KonishiMTabataYKariyaMIn vivo anti-tumor effect of dual release of cisplatin and adriamycin from biodegradable gelatin hydrogelJ Control Release2005103171915710496
- DeurlooMMKopWvan TellingenOBartelinkHBeggAIntratumoural administration of cisplatin in slow-release devices: II. Pharmacokinetics and intratumoural distributionCancer Chemother Pharmacol19912753473531998994
- TauroJRGemeinhartRAExtracellular protease activation of chemotherapeutics from hydrogel matrices: a new paradigm for local chemotherapyMol Pharm20052543543816196497
- CasolaroMCiniRDel BelloBFerraliMMaellaroECisplatin/hydrogel complex in cancer therapyBiomacromolecules200910494494919254026
- FangLWangHZhouLYuDFOXO3a reactivation mediates the synergistic cytotoxic effects of rapamycin and cisplatin in oral squamous cell carcinoma cellsToxicol Appl Pharmacol2011251181521092744
- MouraMJGilMHFigueiredoMMDelivery of cisplatin from thermosensitive co-cross-linked chitosan hydrogelsEur Polym J201349925042510
- Salamat-MillerNChittchangMJohnstonTPThe use of mucoadhesive polymers in buccal drug deliveryAdv Drug Deliv Rev200557111666169116183164
- EmotoSYamaguchiHKameiTIntraperitoneal administration of cisplatin via an in situ cross-linkable hyaluronic acid-based hydrogel for peritoneal dissemination of gastric cancerSurg Today201444591992623887848
- LiJGongCFengXBiodegradable thermosensitive hydrogel for SAHA and DDP delivery: therapeutic effects on oral squamous cell carcinoma xenograftsPloS One201274e3386022529899
- FormarizTPUrbanMCCda Silva JúniorAAGremiãoMPDde OliveiraAGMicroemulsões e fases líquidas cristalinas como sistemas de liberação de fármacos [Microemulsion and liquid crystals as drug delivery systems]Revista Brasileira de Ciências Farmacêuticas200541301313 Portuguese
- ChorilliMPrestesPSRigonRBLeonardiGRChiavacciLAScarpaMVDesenvolvimento de sistemas líquido-cristalinos empregando silicone fluido de co-polímero glicol e poliéter funcional siloxano [Development of liquid-crystalline systems using silicon glycol copolymer and polyether functional siloxane]Quim Nova20093210361040 Portuguese
- PrestesPSChorilliMChiavacciLAScarpaMVLeonardiGRPhysicochemical characterization and rheological behavior evaluation of the liquid crystalline mesophases developed with different siliconesJ Dispers Sci Technol2009311117123
- ManaiaEBKaminskiRCKSoaresCPLiquid crystalline formulations containing modified surface TiO2 nanoparticles obtained by sol–gel processJ Solgel Sci Technol2012632251257
- de Castro SantanaRFasolinLHda CunhaRLEffects of a cosurfactant on the shear-dependent structures of systems composed of biocompatible ingredientsColloids Surf A Physicochem Eng Asp20123985463
- EzrahiSAserinAGartiNAggregation behavior in one-phase (Winsor IV) microemulsion systemsKumarPMittalKLHandbook of Microemulsion Science and TechnologyNew York, NYMarcel Dekker, Inc1999185246
- HosmerJMShinSHNornooAZhengHLopesLBInfluence of internal structure and composition of liquid crystalline phases on topical delivery of paclitaxelJ Pharm Sci201110041444145520957759
- MyoungHHongSDKimYYHongSPKimMJEvaluation of the anti-tumor and anti-angiogenic effect of paclitaxel and thalidomide on the xenotransplanted oral squamous cell carcinomaCancer Lett2001163219120011165754
- KioiMShimamuraTNakashimaHIL-13 cytotoxin has potent antitumor activity and synergizes with paclitaxel in a mouse model of oral squamous cell carcinomaInt J Cancer200912461440144819065664
- EckardtASinikovicBHofeleCBremerMReuterCPreoperative paclitaxel/carboplatin radiochemotherapy for stage III/IV resectable oral and oropharyngeal cancer: seven-year follow-up of a phase II trialOncology2008733–419820318424883
- CheungMCPantanowitzLDezubeBJAIDS-related malignancies: emerging challenges in the era of highly active antiretroviral therapyOncologist200510641242615967835
- FergusonTWilckenNVaggRGhersiDNowakATaxanes for adjuvant treatment of early breast cancerCochrane Database Syst Rev20074CD00442117943815
- RizwanSAssmusDBoehnkeAPreparation of phytantriol cubosomes by solvent precursor dilution for the delivery of protein vaccinesEur J Pharm Biopharm2011791152221237267
- ZengNHuQLiuZPreparation and characterization of paclitaxel-loaded DSPE-PEG-liquid crystalline nanoparticles (LCNPs) for improved bioavailabilityInt J Pharm20124241–2586622240390
- MartinsMRFMVeigaFPromotores de permeação para a liberação transdérmica de fármacos: uma nova aplicação para as ciclodextrinas [Permeation enhancers in transdermal drug delivery systems: a new application of cyclodextrins]Revista Brasileira de Ciências Farmacêuticas20023813354 Portuguese
- LiSPurdyWCCyclodextrins and their applications in analytical chemistryChem Rev199292614571470
- ZhangJMaPXCyclodextrin-based supramolecular systems for drug delivery: recent progress and future perspectiveAdv Drug Deliv Rev20136591215123323673149
- ConcheiroAAlvarez-LorenzoCChemically cross-linked and grafted cyclodextrin hydrogels: from nanostructures to drug-eluting medical devicesAdv Drug Deliv Rev20136591188120323631979
- SzejtliJOsaTComprehensive Supramolecular Chemistry. Vol 3: CyclodextrinsOxfordElsevier Science Ltd1996 SzejtliJIntroduction and general overview of cyclodextrin chemistryChem Rev19969851743175411848947
- VeigaFJBComplexos de Inclusão com Ciclodextrinas Hidrófilas: Caracterização Físico-Química e Biofarmacêutica[Inclusion complexes with hydrophilic cyclodextrins: Physico-chemical and biopharmaceutical characterization] [doctoral thesis]CoimbraUniversity of Coimbra1996 Portuguese
- SallasFDarcyRAmphiphilic cyclodextrins – advances in synthesis and supramolecular chemistryEuropean J Org Chem20086957969
- HirayamaFUekamaKCyclodextrin-based controlled drug release systemAdv Drug Deliv Rev199936112514110837712
- RamineniSKCunninghamLLDziublaTDPuleoDADevelopment of imiquimod-loaded mucoadhesive films for oral dysplasiaJ Pharm Sci2013102259360323192692
- JingJSzarpak-JankowskaAGuillotRPignot-PaintrandIPicartCAuzély-VeltyRCyclodextrin/paclitaxel complex in biodegradable capsules for breast cancer treatmentChem Mater2013251938673873
- HeHChenSZhouJCyclodextrin-derived pH-responsive nanoparticles for delivery of paclitaxelBiomaterials201334215344535823591391
- EiferACThaxtonCSNanoparticle therapeutics: FDA approval, clinical trials, regulatory pathways, and case studyHurstSJBiomedical Nanotechnology: Methods and ProtocolsNew YorkHumana Press2011235338
- YangKWenYWangCClinical application of anticancer nanoparticles targeting metastasis foci of cervical lymph nodes in patients with oral carcinomaHua Xi Kou Qiang Yi Xue Za Zhi2003216447450 Chinese14732978
- HullLCFarrellDGrodzinskiPHighlights of recent developments and trends in cancer nanotechnology research-View from NCI Alliance for Nanotechnology in CancerBiotechnol Adv Epub1282013