?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The most common bone cement material used clinically today for orthopedic surgery is poly(methyl methacrylate) (PMMA). Conventional PMMA bone cement has several mechanical, thermal, and biological disadvantages. To overcome these problems, researchers have investigated combinations of PMMA bone cement and several bioactive particles (micrometers to nanometers in size), such as magnesium oxide, hydroxyapatite, chitosan, barium sulfate, and silica. A study comparing the effect of these individual additives on the mechanical, thermal, and cell functional properties of PMMA would be important to enable selection of suitable additives and design improved PMMA cement for orthopedic applications. Therefore, the goal of this study was to determine the effect of inclusion of magnesium oxide, hydroxyapatite, chitosan, barium sulfate, and silica additives in PMMA on the mechanical, thermal, and cell functional performance of PMMA. American Society for Testing and Materials standard three-point bend flexural and fracture tests were conducted to determine the flexural strength, flexural modulus, and fracture toughness of the different PMMA samples. A custom-made temperature measurement system was used to determine maximum curing temperature and the time needed for each PMMA sample to reach its maximum curing temperature. Osteoblast adhesion and proliferation experiments were performed to determine cell viability using the different PMMA cements. We found that flexural strength and fracture toughness were significantly greater for PMMA specimens that incorporated silica than for the other specimens. All additives prolonged the time taken to reach maximum curing temperature and significantly improved cell adhesion of the PMMA samples. The results of this study could be useful for improving the union of implant-PMMA or bone-PMMA interfaces by incorporating nanoparticles into PMMA cement for orthopedic and orthodontic applications.
Introduction
Joint replacement surgery is one of the greatest medical advances of modern medicine. The annual number of hip and knee replacements is now reaching 500,000 in the USA.Citation1 Two methods for implanting femoral stems in total hip arthroplasty are available.Citation2 First, the femoral stem may be press-fitted into the femoral bone. Second, it may be secured with the femoral bone using bone cement. Metals are the most common femoral stem material. Metals used in femoral stems include titanium, stainless steel, and cobalt chrome. Bone cement is used in joint replacement surgeries. The most common bone cement material used is poly(methyl methacrylate) (PMMA). PMMA cement is associated with several drawbacks that limit its efficacy, such as a strong exothermic reaction, weak radiopacity, and poor mechanical strength compared with the host bone. The most challenging issue associated with commercially available PMMA bone cements such as Cobalt® (Biomet Inc., Warsaw, IN, USA), Simplex™ (Stryker Corporation, Kalamazoo, MI, USA), and Palacos® (Heraeus Holding GmbH, Hanau, Germany) when used in total joint replacement is their poor osseointegration, ie, incorporation of the cement into the surrounding bone tissues.Citation3 Polymeric materials are known to have an exothermic reaction during polymerization. This releases heat, and can cause damage to the surrounding bone cells as well as other adjacent tissues. Other reported problems include infection and loosening of the cement at the bone-cement interface.Citation4 One way to reduce the risk of infection and loosening would be to promote growth of osteoblasts around the cemented surfaces. Such cells would eliminate contact between the bone and the environment and restrict contamination at the cemented prosthetic joint. Another way to reduce loosening would be to increase the mechanical interlocking between bone and cement.Citation5,Citation6 This can be done by enhancing the surface roughness of the PMMA cement. Several research groups have reported improvement in the biological, thermal, and mechanical properties of PMMA bone cement after incorporating various types of additive particles (AP) into the cement. Specifically, at least one biological, thermal, or mechanical benefit has been reported due to incorporation of magnesium oxide (MgO), hydroxyapatite (HAp), chitosan (CS), barium sulfate (BaSO4), or silica (SiO2) with PMMA.Citation7–Citation11 Ricker et alCitation7 investigated the influence of nanosized MgO AP on the thermal, surface, and cytocompatibility properties of PMMA cement. They reported that, compared with PMMA containing microsized MgO, PMMA with nanosized MgO reduced the harmful exothermic behavior of PMMA during solidification and also increased radiopacity and improved osteoblast function. Serbetci et alCitation8 found that addition of HAp to low-viscosity cement compositions caused an increase in compressive strength and compressive elastic modulus (addition of 7.7% [weight/weight {w/w}] and 14.3% [w/w] HAp), but caused a decrease in tensile strength. Tunney et alCitation9 found that incorporating CS into gentamicin-loaded Palacos R bone cement for use in revision surgery had no clinical antimicrobial benefit, and its detrimental effect on mechanical properties could adversely affect the longevity of the prosthetic joint.
Studies reported by Ricker et alCitation7 and Gillani et alCitation10 demonstrated that PMMA containing BaSO4 significantly altered the mechanical properties and osteoblast function associated with PMMA. Hong et alCitation11 found improved glass transition temperature, surface hardness, flexural strength, and impact strength for PMMA reinforced by SiO2 nanoparticles compared with PMMA alone.
The above studies suggested that MgO, HAp, CS, BaSO4, and SiO2 confer additional thermal, biological, and mechanical benefits to PMMA. However, we are not aware of a comparative study of the mechanical, thermal, and biological performance of PMMA with respect to that of PMMA bone cement incorporating those AP. Such research would be of interest to those working on the design of novel or improved bone cements for orthopedic applications, such as total hip arthroplasty.
Although the success rates for total hip arthroplasty are now around 97%, the problems of osteolysis and aseptic loosening have yet to be resolved.Citation12 A common cause of cemented joint failure involves loosening at the bone-cement or implant-cement interface. This begins with microfracture at the interface and progresses with cyclical physiological loading, most notably due to mixed mode stresses. Our previous studies found that addition of MgO nanoparticles to PMMA cement improved the fracture toughness of the bone/PMMA interfaces.Citation13 Additionally, our previous studies found that micron diameter polycaprolactone fiber coating on titanium surface improved the fracture toughness of the titanium/PMMA interfaces.Citation14 Increased surface roughness, local contact stress, and surface energy are some of the major mechanical factors contributing to the improvements seen with these cemented implants.Citation15–Citation18 Increased surface roughness decreases the risk of crack micromovement at the interface, thus increasing load and elongation at the fracture. Cements incorporating microsized or nanosized MgO particles have a lower elastic modulus and higher exothermic curing temperaturesCitation7 than those without MgO. Lower modulus cement can diminish local contact stresses at the bone-cement interface.Citation19 Residual stresses caused by the exothermic temperature difference can influence the fracture energies at the bone-cement interface.Citation15 The accumulation of stresses due to differences in modulus and temperature at the interface of bone-cement or implant-cement with AP can be lower than that without AP, resulting in higher interface fracture strength for interfaces with AP than those without AP. Osseointegration is another promising technique for improving cemented implants.Citation20–Citation26 Improvement of the implant interface properties to avoid loosing of the implant from the host tissue have been a focus in our research, particularly the improvement of the bone-cement and titanium-cement interfaces for the cemented implants. The suitability of using PMMA cement with AP inclusions to solve the loosening problem requires a detailed understanding of its mechanical, thermal, and cell function properties. Therefore, the goal of this study was to identify microsized to nanosized AP that would not only increase surface roughness, attenuate the exothermic reaction, and enhance cell adhesion to PMMA cement, but would also increase the strength of the implant-cement or bone-cement interface. To achieve this goal, we compared the mechanical, thermal, and biological performance of PMMA bone cement samples incorporating AP (MgO, HAp, CS, BaSO4, or SiO2).
This study focused on three research questions as follows: whether there is a significant difference in mechanical properties (flexural strength, flexural modulus, and fracture toughness) between PMMA (control) and PMMA-AP cements; whether there is a significant difference in thermal properties (maximum curing temperature and time taken to reach maximum curing temperature) between PMMA and PMMA-AP cements; and whether there is a significant difference in cell function (adhesion and proliferation) between PMMA and PMMA-AP cements.
Materials and methods
Materials
Cobalt HV was used as the PMMA cement. MgO, HAp, CS, BaSO4, and SiO2 AP were used as the additives for the PMMA bone cements. All AP were purchased from Sigma-Aldrich (St Louis, MO, USA).
Sample preparation
Mechanical tests
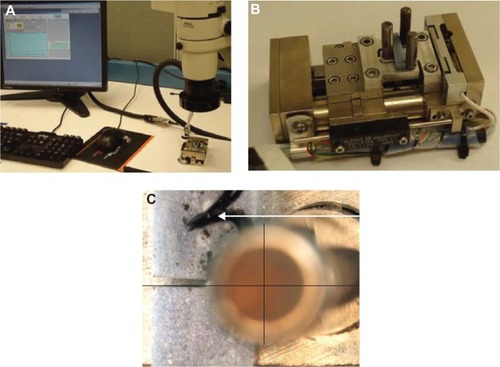
Evex mechanical test system (Evex Analytical Instruments Inc., Princeton, NJ, USA) were used for the three-point flexural and fracture tests on the different PMMA samples (). Following the manufacturer’s recommendations, PMMA specimens were prepared by hand mixing 2.2 g of PMMA powder with 1.1 mL of methyl methacrylate monomer using a powder to monomer ratio of 2:1. Ten wt% (0.22 g) of the selected AP were mixed with 1.98 g of PMMA beads to prepare PMMA with AP-added PMMA cements. The mixers were added with 1.1 mL of methyl methacrylate monomers maintaining the same powder to monomer ratio of 2:1 for preparation of the respective PMMA-AP samples (PMMA-MgO, PMMA-HAp, PMMA-CS, PMMA-BaSO4, and PMMA-SiO2). All samples were cured in a custom-made moldCitation13 at 60 kPa (the pressure used during orthopedic surgical procedures)Citation27 to a block of size 20×8×10 mm. The pressure was applied at exactly 3 minutes after the start of mixing and was sustained throughout the curing period (approximately 15 minutes).Citation18 To prepare American Society for Testing and Materials F417-78 standard flexural test samples (),Citation28 20×4×2 mm blocks were cut from the 20×8×10 mm block using an IsoMet® low-speed cutter (Buehler, Lake Bluff, IL, USA). A 4×0.012×0.5 inch wafering blade was used to cut the samples. To prepare the American Society for Testing and Materials E399-83 single-edge notch bend test samples (),Citation29 20×4×2 mm blocks were cut from a 20×8×10 mm block using the low-speed cutter. To prepare the single-edge notch bend test samples, a center notch was cut at the middle of the 20×4×2 mm cement samples using the low-speed cutter. A 4×0.012×0.5 inch wafering blade was used to cut the samples. The samples were stored in an antistatic ziplock bag at room temperature.
Figure 1 (A) Evex mechanical test system (Evex Analytical Instruments Inc., Princeton, NJ, USA) used for the three-point flexural and fracture tests in the different PMMA samples. (B) Fabricated indenter and support fixtures for flexural tests. (C) Alignment of a notched specimen during fracture tests. During the tests, two lines perpendicular to each other were drawn to align the center of the notch with the center of the roller on the indenter using a Nikon stereomicroscope and NIS BR software (Nikon, Tokyo, Japan).
Abbreviation: PMMA, poly(methyl methacrylate).
Thermal test
The PMMA samples were prepared as described in the previous section. The mold used for preparing the samples for mechanical testing was also used for the thermal experiments (). The only difference was one of the side blocks in the mold used in the thermal experiment had a hole through which a thermocouple was accessed at the center of the curing PMMA cement.
Cell function tests
Two groups of samples were prepared for cell adhesion and proliferation tests. An acrylic sheet (half an inch thick) was used to create a well for the cell culture (). Holes (height 11 mm and diameter 9.525 mm) were milled using a computer numeric control machine. PMMA specimens were prepared by mixing 0.5 g of PMMA beads with 0.25 mL of methyl methacrylate. The PMMA-AP specimens were prepared by mixing 0.05 g of the selected additives with the PMMA beads and dissolving the mixture produced with 0.25 mL of methyl methacrylate. All PMMA samples, while still pliable, were divided into four parts using a knife and poured on the well. Each part of the samples was hand-pressed during curing by a flat-ended ⅜ inch highly polished round bar. All the wells were wrapped in plastic. Cell adhesion and proliferation test sample wells were kept in a bio-hood under ultraviolet light until cell culture.
Scanning electron and atomic force microscopy
Scanning electron microscopy (SEM) and atomic force microscopy (AFM) samples of the different PMMA formulations were prepared in the same acrylic wells as discussed in the previous section. A 5×5 mm coupon was cut out around the center of the well using a low-speed saw cutter to prepare the SEM and AFM samples. All wells were wrapped in plastic and stored at room temperature until analysis.
Experiments
Particle size analysis
Particle sizes were measured by the laser diffraction method using a Mastersizer MS3000 (serial number MAL1057538; Malvern Instruments Inc., Westborough, MA, USA). The MS3000 can measure the particle size distribution across a range of 0.01–3,500 μm. The samples were analyzed dry using Aero S accessories dry powder disperser (Malvern Instruments Inc., Westborough, MA, USA). Each sample was mixed well by gently turning the container upside down several times. The material was scoop-sampled and placed into the vibratory hopper of the Aero S. Consecutive repeat measurements (each 1 second in duration) were undertaken to assess the reproducibility of measurement, which is a function of the homogeneity of the material. The mass flow was adjusted until a stable and correct particle concentration was achieved at 4 bar and then kept constant for the remainder of the experiments. The maximum diameter of the particle size for 10, 50 and 90 percentage volume for each of the AP, represented by D(10), D(50), and D(90), were extracted from the MS3000 report.
Mechanical tests
The three-point bend tests on the rectangular and notched samples were conducted at room temperature and at a loading rate of 0.01 mm/sec using a tensile stage (Evex Analytical Instruments Inc., Princeton, NJ, USA) as shown in . The stage was assembled with a 300 N load cell. The specimens were mounted on the custom-made three-point bend indenter and support in the test stage during the flexural tests (). During the single-edge notch bend test, an SMZ1000 stereomicroscope with a DS-Fi-1 camera and U2 controller (Nikon, Tokyo, Japan) was used to align the notch center of the specimen and the center of the indenter round edge. shows the alignment of the single-edge notch bend specimen center with the center of the three-point bend indenter on the Evex test stage. Nikon NIS BR visualization software was used for the alignment. Load and displacement were continuously recorded using Evex nanoanalysis software until failure of the specimens. Several mechanical performance parameters were derived from the three-point bend flexural test for the various PMMA specimens. Stress and strain were calculated from load and displacement data using:
and
respectively, where P is the incremental load, d is the incremental defection, S is the standard loading span for the three-point bend specimen, B is the thickness, and W is the width of the specimen. Flexural strength, σf, was calculated using:Citation30
where Pmax is the ultimate load (force at failure). The value of the bending modulus, E, for a three-point bend specimen was measured from the slope of the stress-strain curve at the elastic region. From the single-edge notch bend test, the maximum load, Pmax, at the onset of crack extension from the notch tip was recorded from load displacement data. Pmax was used to calculate the mode I fracture toughness, KIC using the relationship:Citation31
where S is the span length (ie, the length between two support rollers), α is the normalized initial crack length (α=a/W), and f(α) is a dimensionless geometric function. The following equation was used to calculate f(α):Citation31
Thermal tests
A custom-made temperature measurement system () was used to determine the temperature changes for the different PMMA cements in an insulated acrylic mold. A four-channel DI-1000-TC thermocouple (DATAQ Instruments, Akron, OH, USA) was used to measure the temperature changes in the bone cements. The thermocouple was connected to a data acquisition device that was in turn connected to a computer utilizing InstruNet software for data collection. The PMMA sample was placed on the acrylic mold. The thermocouple needle was inserted up to the center of the sample through the side block hole. The sample was pressed from above by a set of weights equivalent to 60 kPa pressure. The temperature change was recorded by the thermocouple needle every 25 seconds for 30 minutes until the PMMA sample was completely solidified.
Cell adhesion tests
Osteoblast adhesion to the PMMA and PMMA-AP samples was investigated using mouse osteoblasts (MT3T3E1; American Type Culture Collection, Manassas, VA, USA). The cells were cultured according to American Type Culture Collection instructions using a cell viability test protocol as depicted in . Cells were seeded at a density of 3,500 cells/mL in each well and incubated under standard conditions (a humidified, 5% CO2, and 95% air environment at 37°C) for 4 hours. Cells were fixed in 10% neutral buffered formalin and stained using 4′,6-diamidino-2-phenylindole, a DNA-binding fluorescent probe. Cells were counted in five fields per substrate and images were captured with a BX41 fluorescent microscope (Olympus, Tokyo, Japan) at 100× total magnification. Cell numbers were counted using Nikon BR image processing software.
Cell proliferation tests
For the proliferation tests, the cells were cultured for an hour according to the same method described in the previous section. Proliferation was analyzed using a Click-iT kit (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA). Briefly, samples were incubated for 1 hour in the presence of ethynyl-deoxyuridine, a modified nucleotide that is incorporated into cells, replicating their DNA during the proliferation process. Samples were fixed for 5 minutes in ice-cold methanol and incubated for 30 minutes with an Click-iT® EdU Alexa-488 (Molecular Probes, Eugene, OR, USA). fluorescent tag to label the ethynyl-deoxyuridine nucleotides. After washing in phosphate-buffered saline, the samples were mounted with 80% glycerol/phosphate-buffered saline containing 4′,6-diamidino-2-phenylindole to counterstain all nuclei blue.
Scanning electron microscopy
Using a Quanta 600 field-emission gun environmental SEM with an Evex X-ray energy dispersive spectroscopy microanalysis system (FEI; Thermo Fisher Scientific), the PMMA samples prepared to characterize AP dispersion and surface topography. The prepared SEM samples were sputter-coated with gold and palladium for 1 minute.
Atomic force microscopy
Using a multimode scanning probe microscope with an AFM module (Veeco, Santa Clara, CA, USA), the PMMA samples prepared to provide quantitative evidence of the difference in surface roughness for different PMMA samples. The prepared SEM samples were sputter-coated with gold and palladium for 1 minute. All roughness values (Rq, Rm, Rmax) were measured in three areas on each substrate using installed software (Nanoscope 4.42, Veeco).
Statistical analysis
Data collated for all experimental tests were evaluated for statistical significance using one-way analysis of variance with subsampling. To determine specifically which means were significantly different, multiple comparisons were performed using Fisher’s protected least significant difference. All the tests were conducted using SAS version 9.1 (SAS Institute, Cary, NC, USA). For all analyses, statistical significance was considered as P<0.05.
Results
Particle size
shows the particle size distribution of the MgO, HAp, CS, BaSO4, and SiO2 powders obtained by laser diffraction. The average particle sizes of the AP were mainly micrometer in scale. The average particle size of MgO was lowest and CS was highest when compared with the other AP particles.
Table 1 Particle size parameters of the additive powders used in the study
Mechanical tests
The stress-strain behavior of PMMA was influenced by incorporation of AP (). The flexural strength (σf) and flexural modulus (E) under bending loading conditions was calculated from the stress-strain data for all the bone cements (). These data, along with the bar diagram (), were used to identify significant differences in flexural strength when comparing the control cement (PMMA) with the cements containing AP. shows that: the mean σf of the SiO2 specimens was significantly greater than the mean σf of all other specimens; the mean σf of the PMMA-BaSO4 specimens was significantly greater than the mean σf of the PMMA-MgO specimens; and no significant difference existed between the mean σf of the PMMA control, PMMA-CS, and PMMA-HAp. shows the differences of the values of E of additives included in the PMMA samples with respect to the values of E of PMMA. The results show that: the mean E of the SiO2 specimens was significantly greater than the mean E of all other specimens; the mean E of the PMMA and PMMA-BaSO4 specimens was significantly greater than the mean modulus of the PMMA-MgO specimens; and no significant difference existed between the mean E of PMMA-CS and PMMA-HAp. compares the fracture toughness of various composite cements with respect to the fracture toughness of PMMA. Results show that incorporation of HAp, CS, and SiO2 AP with PMMA significantly influences the fracture toughness of the PMMA. Furthermore, shows that the mean values of the fracture toughness of all AP incorporated PMMA samples were higher than the mean values of the fracture toughness of PMMA samples.
Table 2 Mechanical properties of the different cements
Figure 4 (A) Stress versus strain plots of PMMA samples tested during this study. (B) Bar diagram of the variation in flexural strength of PMMA samples due to variation of additives to PMMA.
Notes: Data are presented as the mean ± standard error of mean; n=3 for PMMA-CS; n=4 for the rest of the samples. *P<0.05 (compared with PMMA).
Abbreviations: CS, chitosan; HAp, hydroxyapatite; MgO, magnesium oxide; PMMA, poly(methyl methacrylate); BaSO4, barium sulfate; SiO2, silica.
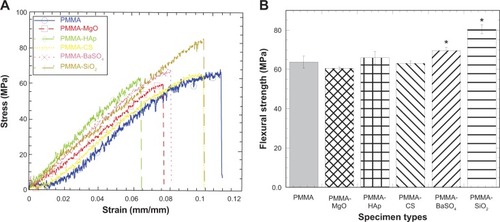
Figure 5 Bar diagram of the variation in fracture toughness of PMMA samples due to variation in additives to PMMA.
Notes: Data are presented as the mean ± standard error of the mean; n=4 for PMMA-HAp and PMMA-SiO2; n=3 for the rest of the samples. *P,0.05 (compared with PMMA).
Abbreviations: CS, chitosan; HAp, hydroxyapatite; MgO, magnesium oxide; PMMA, poly(methyl methacrylate); BaSO4, barium sulfate; SiO2, silica.
Thermal tests
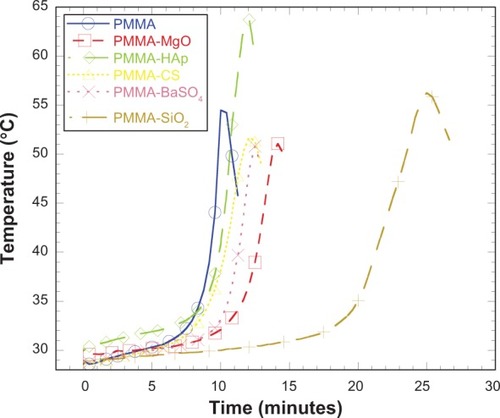
shows the variation in curing temperature with respect to time for the different PMMA samples. All samples showed the similar characteristic of temperature increase to a peak temperature (Tc) and a temperature decrease after Tc. It is also evident from the graph that the AP influenced the time taken to reach Tc, which was lowest for the PMMA samples without additives when compared with PMMA samples including additives. The PMMA cement incorporating SiO2 took the longest to reach maximum temperature. Given that thermal stress is proportional to rise in temperature, the thermal stress created in the PMMA samples must be higher than in the PMMA-AP samples. shows the curing temperature of the different samples at intervals of 0, 2.5, 5, 7.5, 10, 12.5, and 15 minutes. also shows the maximum Tc and time taken to reach Tc. MgO-containing, CS-containing, and BaSO4-containing PMMA cements showed a lower maximum temperature than PMMA samples, whereas the HAp-containing and SiO2-containing PMMA cements showed higher maximum temperatures than the PMMA samples.
Table 3 Descriptive statistics of the curing experiment data for PMMA samples
Cell adhesion tests
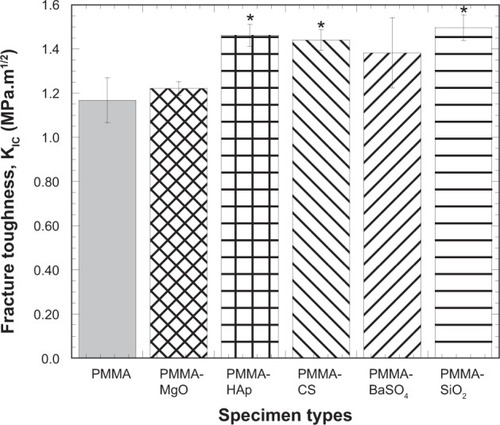
shows the mean osteoblast density for the different PMMA sample types. The bar diagram in demonstrates how the AP affected cell viability in the PMMA samples. The cell density of the PMMA specimens when additives were included was significantly greater than the cell density of PMMA alone, suggesting that MgO, HAp, CS, BaSO4, and SiO2 have a positive influence on cell activity in PMMA.
Figure 7 Bar diagram of the variation in cell density with PMMA samples due to variation in additives to PMMA.
Notes: Data are presented as the mean ± standard error of the mean; n=8. *P<0.05 versus PMMA.
Abbreviations: CS, chitosan; HAp, hydroxyapatite; MgO, magnesium oxide; PMMA, poly(methyl methacrylate); BaSO4, barium sulfate; SiO2, silica.
Cell proliferation tests
No proliferation in the cement samples was found, except for a few cells (). However, there were many instances of paired cells being found close together, suggesting cell division had occurred. It would have been better to run at least a 24-hour proliferation assay to confirm that some of the cells were dividing. Proliferation tests were conducted for an hour since the osteoblasts on a coverslip showed a good degree of proliferation (about 50%) after an hour. These results suggest that it may take longer for cells to become accustomed to the bone matrix material than for cells to acclimate to coverslips.
Figure 8 Fluorescent microscope images of different kinds of bone cements used during cell proliferation tests. (A) PMMA, (B) PMMA with MgO, (C) PMMA with HAp, (D) PMMA with CS, (E) PMMA with BaSO4, and (F) PMMA with SiO2.
Abbreviations: CS, chitosan; HAp, hydroxyapatite; MgO, magnesium oxide; PMMA, poly(methyl methacrylate); BaSO4, barium sulfate; SiO2, silica.
SEM and AFM analysis
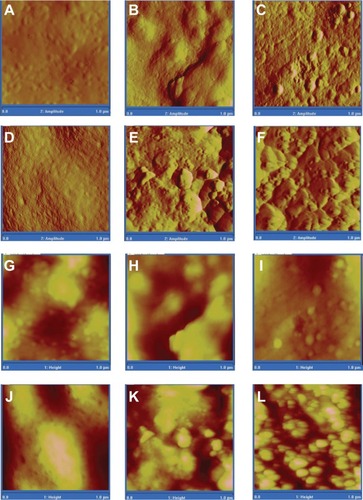
Qualitative and quantitative examination of SEM and AFM images and data for the different PMMA cements show that the pure PMMA and PMMA-CS samples were less rough than the PMMA samples incorporating other AP ( and , ).
Table 4 Atomic force microscopy RMS data for different PMMA samples
Figure 9 Scanning electron micrographs of the different types of bone cement used in cell function tests (30,000×, scale bar 3 μm). (A) PMMA, (B) PMMA with MgO, (C) PMMA with HAp, (D) PMMA with CS, (E) PMMA with BaSO4, and (F) PMMA with SiO2.
Abbreviations: CS, chitosan; HAp, hydroxyapatite; MgO, magnesium oxide; PMMA, poly(methyl methacrylate); BaSO4, barium sulfate; SiO2, silica.
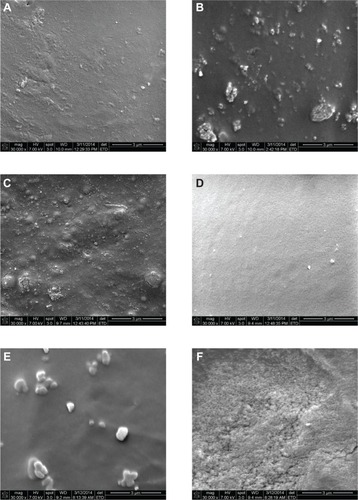
Figure 10 Atomic force micrographs of amplitude (A–F) and height (G–L) of PMMA, PMMA with MgO, PMMA with HAp, PMMA with CS, PMMA with BaSO4, and PMMA with SiO2.
Abbreviations: CS, chitosan; HAp, hydroxyapatite; MgO, magnesium oxide; PMMA, poly(methyl methacrylate); BaSO4, barium sulfate; SiO2, silica.
Discussion
MgO is an inorganic white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium. This study found that MgO nanoparticles significantly reduced the flexural strength of PMMA (). PMMA-MgO samples had the lowest flexural strength when compared with the other PMMA-AP cements. No significant change in fracture toughness values was found for PMMA-MgO samples when compared with PMMA and PMMA-BaSO4. PMMA-MgO samples had lower KIC values than the other PMMA-AP cements. MgO nanoparticles improved the thermal performance of the PMMA samples by reducing the maximum curing temperature (Tmax) of PMMA, although the Tmax for the PMMA-MgO samples was lower than for the other PMMA-AP cements ( and ). The time taken for the PMMA-MgO samples to reach Tmax was longer than for the other PMMA-AP cements, except PMMA-SiO2. No negative cell viability effect on PMMA due to addition of MgO was observed (). These results suggest that the detrimental effect of addition of MgO nanoparticle additives on the mechanical properties of PMMA bone cement could offset the thermal and biological benefits achieved by inclusion of MgO. The results of our thermal and cell viability studies are in agreement with those of Ricker et alCitation7 who also found less harmful exothermic reactions of PMMA during solidification, and increased osteoblast adhesion on PMMA incorporating MgO nanoparticles, compared with PMMA alone. To our knowledge, no studies have attempted to determine the flexural and fracture properties of MgO-containing PMMA cements.
HAp is a predominantly crystalline inorganic compound, although it may exist in amorphous forms.Citation32 In our study, HAp particles did not affect the flexural strength of PMMA (). However, the PMMA-HAp samples had a higher σf compared with the PMMA-MgO cement and a lower σf compared with PMMA-SiO2 cement, but showed no difference in σf when compared with the other PMMA-AP samples. A significantly higher KIC value was found for PMMA-HAp samples than for control PMMA and PMMA-MgO samples, but no difference in KIC was found between PMMA-HAp and other PMMA-AP samples. HAp had a negative effect on the thermal performance of PMMA samples by increasing the maximum curing temperature of PMMA. Additionally, PMMA-HAp samples showed the highest Tmax when compared with other PMMA-AP cements ( and ). The time taken for PMMA-HAp to reach Tmax was found to be higher than for PMMA cements lacking additives, lower than for PMMA-MgO and PMMA-SiO2 cements, and not different to that for PMMA-CS and PMMA-BaSO4 cements. We found no negative cell viability effect on PMMA due to addition of HAp ().
The above results suggest that a detrimental curing temperature effect could override the mechanical and biological benefits achieved by inclusion of HAp in PMMA. The results of our mechanical and thermal studies are in agreement with those of Chu et alCitation33 who found inclusion of 10%–20% HAp had significant beneficial effects on the mechanical properties of PMMA and that more than 40% HAp should be mixed into PMMA to reduce the peak curing temperature. However, the results of our thermal studies are not in agreement with those of Serbetci et alCitation8 who reported a decrease in peak curing temperature when HAp was added to PMMA. This difference is likely due to the different experimental conditions affecting heat dissipation. Our study modified the experimental conditions used by Serbetci et al to include the use of an insulating mold to minimize heat dissipation during the curing process.
CS is a natural polymer with coagulation and flocculation properties that can be used to treat organic or inorganic particulate suspensions and also to treat dissolved organic materials (including dyes and humic acid).Citation34 Like HAp particles, CS particles did not affect the flexural strength of PMMA in our study (). PMMA-CS samples also had a higher σf than the PMMA-MgO cement and a lower σf than the PMMA-SiO2 cement, with no difference in σf when compared with the other PMMA-AP samples. A significantly higher KIC value was found for PMMA-CS samples than for PMMA and PMMA-MgO, with no difference in KIC for PMMA-CS when compared with the rest of the PMMA-AP samples. In contrast with HAp, CS had a positive effect on the thermal performance of the PMMA samples by decreasing the maximum curing temperature of PMMA. Tmax for the PMMA-CS samples was lower than for the PMMA only, PMMA-HAp, and PMMA-SiO2 samples and similar to the Tmax of the remaining PMMA-AP samples ( and ). Further, PMMA-CS reached Tmax at a lower temperature than the other PMMA-AP samples. Our study found no negative cell viability effects on PMMA when CS was added (). These results suggest that CS is one of the most suitable additives for PMMA cement based on the mechanical, thermal, and biological benefits achieved. The results of our flexural tests are in agreement with those of Tunney et alCitation9 who also reported finding no significant difference in flexural strength between PMMA and PMMA containing CS. To our knowledge, no studies have attempted to determine the fracture and curing properties of PMMA cements that include CS.
BaSO4 is an inorganic compound with a white opaque appearance and is used as a radiopacifying agent. We found that the modes of fracture for both PMMA cements and PMMA cement containing BaSO4 ceramic microparticles were brittle. The same phenomena was also observed by Gillani et al.Citation10 In contrast, the values for fracture strength and Young modulus of PMMA and PMMA containing BaSO4 reported by Gillani et alCitation10 were four to five times lower than those values found in our study. This difference is due to the difference of the mechanical tests to find those values: tension tests were conducted, whereas bending tests were conducted during our study. Young modulus under the tensile loading of PMMA and PMMA containing BaSO4 reported by Gillani et alCitation10 were 4–5 times lower than those values under the bend loading in this study. Like HAp and CS particles, BaSO4 particles did not affect the flexural strength of PMMA (). Moreover, PMMA-BaSO4 samples had a higher σf than the other PMMA-AP samples, except for PMMA-SiO2. The KIC values of the PMMA-CS samples were not significantly different from that of PMMA or the other PMMA-AP samples. Like MgO and CS, BaSO4 had a positive effect on the thermal performance of PMMA samples by decreasing the maximum curing temperature of PMMA. The Tmax for the additionally, the time taken for PMMA-BaSO4 to reach Tmax was lower than for the other PMMA-AP samples. This study found no negative cell viability effect on PMMA due to addition of BaSO4 (). These results suggest that BaSO4 is also a suitable additive for PMMA based on the mechanical, thermal, and biological benefits achieved. The results of our thermal and cell viability studies are in agreement with those of Ricker et alCitation7 who also found less harmful exothermic reactions during solidification of PMMA and increased osteoblast adhesion on PMMA-BaSO4 samples compared with PMMA alone. The results of our fracture tests are in agreement with those of Ginebra et alCitation35 who also found increased KIC values for PMMA due to addition of BaSO4.
SiO2 is an inorganic component. We found that SiO2 particles significantly increased the σf and KIC values of PMMA (). PMMA-SiO2 samples had the highest σf and KIC values when compared with other PMMA-AP cements. SiO2 had a negative effect on the thermal performance of PMMA samples by increasing the maximum curing temperature of PMMA. The Tmax for PMMA-SiO2 samples was higher than that of the other PMMA-AP cements, except for PMMA-HAp ( and ). Also, the time taken for PMMA-SiO2 to reach Tmax was higher than for the other PMMA and PMMA-AP cements. We found no negative cell viability effect on PMMA when SiO2 was included (). These results suggest that the detrimental curing temperature effect of addition of SiO2 additives into PMMA bone cement could offset any mechanical and biological benefits achieved. The results of our flexural test and fracture test results are in agreement with those of Hong et alCitation11 and Chan et alCitation36 who reported increased flexural strength and fracture toughness of PMMA-SiO2 cements when compared with PMMA alone. To our knowledge, no studies have been done to determine the curing properties of PMMA cements incorporating SiO2.
The differences in cell density between the various PMMA samples are due to differences in AP type, dispersion, and topography, and can be seen easily in the SEM images (). The differences in cell density between the different PMMA samples are also due to differences in surface roughness, which can be seen easily in the AFM images and data (, and ). This observation is in agreement with that of Webster et alCitation37 who also reported that surface roughness enhances osteoblast and osteoclast function, thereby improving orthopedic/dental implant efficacy.
Only one concentration (10% [w/w]) of the selected additives to PMMA cement was evaluated in this study. We also conducted studies using other concentrations (2%, 6%, and 10%) of PMMA additives and similarly evaluated their mechanical, thermal, and cell function activity. The result of these studies will be published separately. The authors chose to evaluate 10% (w/w) of the selected additives in this study because this concentration can produce enhanced surface roughness at the implant-cement interface and bone-cement interface. This allows the surface roughness and interface fracture toughness relationship to be evaluated, which cannot be done using a low concentration of AP (2% and 6%) with PMMA. We also used 10% (w/w) of the selected additives to develop and compare the cell viability protocols and data with the published literature for MgO and BaSO4.Citation7 This study included only one thermal test on PMMA-AP samples because an identical result was found from thermal tests on two control (PMMA) specimens. Therefore, the authors assumed the thermal test data for PMMA-AP samples would vary little. Our study was also limited to only one cell function (adhesion) test. Other cell function tests, including proliferation, alkaline phosphatase, and calcein labeling experiments using different PMMA samples are currently under way.
The novelty of this comparative study is that it attempted to determine the relative influence of microsized MgO, HAp, CS, BaSO4, and SiO2 particles on the mechanical, thermal, and biological properties of traditional bone cements used for orthopedic applications. We also developed a novel experimental protocol for curing tests on PMMA-type samples. The novelty of the setup is that the curing experiment can be conducted at constant pressure (60 kPa) and boundary temperature. Additionally, the cured sample blocks for the setup can also be used for mechanical testing. The results of this study can be used to design modified PMMA cements for orthopedic application.
Conclusion
CS and BaSO4 are more suitable additives compared with other AP based on the mechanical, thermal, and biological benefits achieved by their inclusion in PMMA. MgO had detrimental effects on the mechanical properties of PMMA cement, HAp and SiO2 had detrimental effects on the curing properties of PMMA cement, AP had no significant effect on the modulus of PMMA, and AP significantly improved osteoblast adhesion of PMMA.
Acknowledgments
This research was supported by grant 8P20GM103447 from the US National Institutes of Health and an on-campus faculty grant program from the University of Central Oklahoma Office of Research and Grants. Special thanks are due to Dr Timothy W Teske, Orthopedic Surgery, Enid, OK, USA, for demonstrating the PMMA cement-preparation protocols.
Disclosure
The authors report no conflicts of interest in this work.
References
- The Anderson Orthopaedic ClinicTotal hip replacement2009 Available from: http://www.andersonclinic.com/specialties/total-hip-replacement-arlington-alexandria-virginia.phpAccessed April 21, 2014
- AnYHDraughnRAMechanical Testing of Bone and the Bone-Implant InterfaceBoca Raton, FL, USACRC Press2000
- BarraletJELilleyKJGroverLMFarrarDFAnsellCGbureckUCements from nanocrystalline hydroxyapatiteJ Mater Sci Mater Med20041540741115332608
- LewisGAlternative acrylic bone cement formulations for cemented arthroplasties: present status, key issues, and future prospectsJ Biomed Mater Res B Appl Biomater20088430131917588247
- MannKAEdidinAAOrdwayNRManleyMTFracture toughness of CoCr alloy-PMMA cement interfacesJ Biomed Mater Res1997382112199283966
- OhashiKLRomeroACMcGowanPDMaloneyMTAdhesion and reliablity of interfaces in cemented total joint arthroplastiesJ Orthop Res1998167057149877395
- RickerALiu-SnyderPWebsterTJThe influence of nano MgO and BaSO4 particle size additives on properties of PMMA bone cementInt J Nanomedicine20083112513218488423
- SerbetciKKorkusuzFHasirciNThermal and mechanical properties of hydroxyapatite impregnated acrylic bone cementsPolym Test200423145155
- TunneyMMBradyAJBuchananFNeweCDunneNJIncorporation of chitosan in acrylic bone cement: effect on antibiotic release, bacterial biofilm formation and mechanical propertiesPaper presented at the 21st European Conference on BiomaterialsBrighton, UKSeptember 9–13, 2007
- GillaniRErcanBQiaoAWebsterTNanofunctionalized zirconia and barium sulfate particles as bone cement additivesInt J Nanomedicine2010511120161983
- HongRYFuHPZhangYJSurface-modified silica nanoparticles for reinforcement of PMMAJ Appl Polym Sci200710521762184
- LiuYPYuGRLiKYuanFIs there another possible approach to inhibit wear particles-induced inflammatory osteolysis?Med Hypotheses20117628028221067868
- KhandakerMLiYMorrisTMgO micro/nano particles for the improvement of cement-bone interfaceJ Biomech2013461035103923332232
- KhandakerMUtsahaKCMorrisTInterfacial fracture toughness of titanium-cement interfaces: effects of fibers and loading anglesInt J Nanomedicine201491689169724729704
- ZorMKüçükMAksoySResidual stress effects on fracture energies of cement-bone and cement-implant interfacesBiomaterials2002231595160111922465
- RamanirakaNRakotomananaLLeyvrazPThe fixation of the cemented femoral component. Effects of stem stiffness, cement thickness and roughness of the cement-bone surfaceJ Bone Joint Surg Br20008229730310755444
- ZelleJJanssenDPeetersSBrouwerCVerdonschotNMixed-mode failure strength of implant–cement interface specimens with varying surface roughnessJ Biomech20114478078321074772
- GrahamJRiesMPruittLEffect of bone porosity on the mechanical integrity of the bone-cement interfaceJ Bone Joint Surg Am200385A1901190814563796
- FunkMJLitskyASEffect of cement modulus on the shear properties of the bone-cement interfaceBiomaterials199819156115679830981
- GittensRAMcLachlanTOlivares-NavarreteRThe effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiationBiomaterials2011323395340321310480
- ImBJLeeSWOhNTexture direction of combined microgrooves and submicroscale topographies of titanium substrata influence adhesion, proliferation, and differentiation in human primary cellsArch Oral Biol20125789890522189250
- LeeHJKimDNParkSLeeYKohWGMicropatterning of a nanoporous alumina membrane with poly(ethylene glycol) hydrogel to create cellular micropatterns on nanotopographic substratesActa Biomater201171281128921056702
- MartínezEEngelEPlanellJASamitierJEffects of artificial micro-and nano-structured surfaces on cell behaviourAnn Anat200919112613518692370
- McNamaraLEBurchmoreRRiehleMOThe role of microtopography in cellular mechanotransductionBiomaterials2012332835284722248989
- ProdanovLte RietJLamersEThe interaction between nanoscale surface features and mechanical loading and its effect on osteoblast-like cells behaviorBiomaterials2010317758776520647152
- VenkatsuryaPKGiraseBMisraRDPesacretaTCSomaniMCKarjalainenLPThe interplay between osteoblast functions and the degree of nanoscale roughness induced by grain boundary grooving of nanograined materialsMater Sci Eng C Mater Biol Appl201232330340
- RiesMDRauscherLAHoskinsSLottDRichmanJALynchFIntramedullary pressure and pulmonary function during total knee arthroplastyClin Orthop Relat Res19983561541609917680
- American Society for Testing and Materials InternationalAnnual book of ASTM standards. Section 8D790-03 Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating MaterialsWest Conshohocken, PA, USAAmerican Society for Testing and Materials International2006
- American Society for Testing and Materials InternationalAnnual book of ASTM standards. Section 3E399-90 Metals Test Methods and Analytical ProceduresWest Conshohocken, PA, USAAmerican Society for Testing and Materials International2001
- CowinSCBone Mechanics Handbook2nd edBoca Raton, FL, USACRC Press2001
- LucksanasomboolPHiggsWAJHiggsRSwainMVFracture toughness of bovine bone: influence of orientation and storage mediaBiomaterials2001223127313211603584
- DumitriuSPolymeric BiomaterialsBoca Raton, FL, USACRC Press2001
- ChuKTOshidaYHancockEBKowolikMJBarcoTZuntSLHydroxyapatite/PMMA composites as bone cementsBiomed Mater Eng2004148710514757957
- GuibalEVan VoorenMDempseyBRoussyJA review of the use of chitosan for the removal of particulate and dissolved contaminantsSep Sci Technol20064124872514
- GinebraMPAlbuixechLFernández-BarragánEMechanical performance of acrylic bone cements containing different radiopacifying agentsBiomaterials2002231873188211950058
- ChanKSLeeYDNicolellaDPFurmanBRWellinghoffSRawlsRImproving fracture toughness of dental nanocomposites by interface engineering and micromechanicsEng Fract Mech2007741857187118670579
- WebsterTJSiegelRWBiziosRNanoceramic surface roughness enhances osteoblast and osteoclast functions for improved orthopaedic/dental implant efficacyScr Mater20014416391642