Abstract
The use of nanoparticles holds promise for medical applications, such as X-ray imaging, photothermal therapy and radiotherapy. However, the in vivo toxicity of inorganic nanoparticles raises some concern regarding undesirable side effects which prevent their further medical application. Ultrasmall sub-5.5 nm particles can pass through the barrier for renal clearance, minimizing their toxicity. In this letter we address some recent interesting work regarding in vivo toxicity and renal clearance, and discuss the possible strategy of utilizing ultrasmall nanomaterials. We propose that small hydrodynamic sized nanoclusters can achieve both nontoxic and therapeutic clinical features.
Dear editor
Biomedical applications of inorganic nanoparticles have been investigated for several years.Citation1–Citation3 It was conceived that nanoparticles hold potential for use as X-ray contrast agents, fluorescence imaging agents, photothermal therapy agents, and radiosensitizers.Citation4–Citation12 However, their toxicity, especially in vivo toxicity, is still a huge challenge for further applications in medicine.Citation13–Citation17 In earlier years, several groups have demonstrated that citric acid-coated nanoparticles are not toxic in vitro, and they demonstrated that human cells were viable and highly active even at millimolar levels.Citation18,Citation19 However, the in vitro toxicity cannot necessarily reflect the in vivo toxicity. The in vivo toxicity of nanoparticles mainly arises from accumulation of nanoparticles in the liver and spleen. Specifically, nanoparticles with sizes ranging from 10 to 250 nm show very high distributions in the liver and spleen due to reticuloendothelial system (RES) absorption.Citation20 Several groups have reported that large nanoparticles accumulate in the liver and spleen, and they are not cleared easily.Citation21–Citation23 Large nanoparticles can be absorbed rapidly by macrophages, because the small naked gold nanoparticles will first react with blood proteins and then form larger nanoparticle–protein complexes, namely protein corona.Citation24–Citation28 The high distribution in the liver and spleen can induce potential liver toxicities.Citation7,Citation27,Citation29
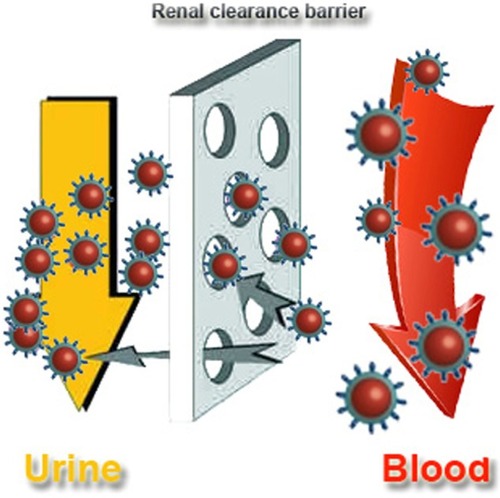
In order to decrease toxicity, one of the most effective options is to decrease the size of the nanoparticles, making them behave as supermolecules which can induce “leakage” of nanoparticles in vivo mediated by the kidney.Citation30,Citation31 Choi et al proposed that nanoparticles can be cleared when the hydrodynamic size of nanoparticles is decreased to 5.5 nm.Citation32 We can define this hydrodynamic size as the size of the renal clearance barrier (). Indeed, it has been shown that small quantum dots can present with highly efficient renal clearance.Citation33 However, only being small in size is not enough, because naked nanoparticles can still react with blood proteins and form larger complexes. For example, Hainfeld et al used X-ray imaging of 1.9 nm gold nanoparticles,Citation6 and when ultrahigh doses (2.7 g Au/kg) of gold nanoparticles are used in mice, many were found in the blood vessels. Indeed, these nanoparticles are difficult to clear. Furthermore, toxic effects were not demonstrated, but the high dose was considered to be very dangerous.
Polyethylene glycol (PEG) is widely used in coating of nanoparticles for nanomedical applications.Citation34,Citation35 However, PEG-coated sub-5 nm nanoparticles cannot be cleared by the kidney,Citation27,Citation36,Citation37 even when the hydrodynamic size of PEG-coated gold nanoparticles decreased to 3 nm.Citation36 Ultrasmall BaGdF5-based upconversion nanoparticles also cannot be cleared by the kidney.Citation38 Thus, it is clear that only having a small size is not sufficient for the clearance of nanoparticles.
To overcomes these obstacles, ultrasmall nanoclusters or particles with stable ligands have been proposed. Specifically, nanoparticles with small endogenous ligands would be highly desirable. For example, sub-5 nm gold nanoparticles with glutathione (GSH) ligands, a kind of endogenous small molecule, are highly efficient in renal clearance.Citation36 Meanwhile, the sub-2 nm GSH-protected Au nanoclusters are also highly efficient in renal clearance and they also show low toxicity after injection.Citation39 Even after 30 days, the GSH-protected gold nanoclusters did not show any significant toxicity. Using 1.2 nm gold nanoparticles as cores, the GSH-protected gold nanoparticles can be cleared by the kidney even at the dose of 60 μM.Citation40 In contrast, bovine serum albumin (BSA)-protected Au nanoclusters in a hydrodynamic size of 6 nm cannot pass the renal clearance barrier and show minor liver toxicities. In another independent work, the renal clearance of dithiolated polyaminocarboxylate-coated gold nanoparticles was reported.Citation41 In this work, the hydrodynamic size was as large as 6.6 nm, higher than the proposed value of 5.5 nm for the renal clearance barrier, but the nanoparticles can still induce a highly efficient renal clearance.Citation41 Therefore, this illustrates that stable ligands are as important as the hydrodynamic size in renal clearance of nanoparticles.
With the exception of gold-based nanomaterials as mentioned above, other ultrasmall nanomaterials such as carbon nanomaterials and semiconducting quantum dots have also been widely investigated. For example, Huang et al have shown that amine-functionalized carbon dots in a 4.1 nm hydrodynamic size can be cleared renally.Citation42 However, carboxylated graphene quantum dots in 3–6 nm hydrodynamic sizes cannot go through the renal clearance barrier and thus cause an appreciable distribution in the liver and spleen. It is not yet clear why these graphene quantum dots cannot pass through the renal clearance barrier, but one possible reason could be their instability exogenously. As such, it is still necessary to obtain renally-clearable graphene quantum dots with stable ligands for further medical applications. A similar phenomenon was observed using ultrasmall semiconducting nanoparticles. Polyvinylpyrrolidone-protected Gd2O3 nanoparticles in a 2.9 nm ultrasmall size can achieve efficient renal clearance.Citation43 However, 1.5 nm Ag2Se nanoparticles cannot be metabolized by the kidney.Citation44
Besides this, it is interesting to control the clearance of nanoparticles mediated by macrophages. For example, Chou et al used DNA to control the biological delivery and clearance of inorganic nanoparticles by organizing them into colloidal superstructures.Citation45 The nanoparticles behave as building blocks whose size, surface chemistry and assembly architecture dictate the overall superstructure design. These superstructures are able to interact with cells and tissues as a function of their designs, but subsequently degrade into building blocks that can escape biological sequestrations. Thereby, this design realizes successful clearance of nanoparticles resulting in intact biofunctions.
Despite some significant advances with small nanoparticles, some difficulties still exist. When the core size of the nanoparticles is decreased to sub-3 nm, the physical and chemical properties of some materials might be lost or altered. For gold-based nanomaterials, the surface plasmon resonance of gold disappears when diameters are decreased to sub-5 nm.Citation46 Instead, unique electronic structures and optical properties are introduced.Citation47,Citation48 In this situation, the photothermal efficiency of nanoparticles will be considerably affected due to sharply decreased absorption. As for quantum dots, the quantum dots will be easily quenched or bleached with their sizes decreased to sub-5 nm.Citation49 Meanwhile, as the quantum size effect is induced by small sizes, the band gap of quantum dots will be widened and the wavelength for photoluminescence will shift to the region of shorter wavelengths.Citation50 Taking carbon nanomaterials into account with their size deceased to sub-5 nm, the band gap of graphene nanosheets or graphene quantum dots increases to 2–3 eV according to band gap engineering, while the fluorescence of carbon nanotubes will disappear.Citation51–Citation54 As the diameter is decreased to sub-5 nm levels, magnetic properties of some magnetic materials such as Fe3O4 will be influenced. Meanwhile, it would be more difficult to dope them with other elements. Lots of upconversion materials will lose their fluorescence characteristics with their size decreased to 5 nm. At the size of sub-5 nm, strong surface activities can jeopardize applications, with surface modifications as a typical example. Another challenge lies in how to monitor concentrations of ultrasmall organic nanoparticles, because it is still unclear whether nanoparticles will be broken down in vivo, and in what quantity. To be specific, when nanoparticles are injected into mice, lots of particles will interact with proteins and may then be broken down in vivo. In this case, it will be very difficult to determine how many nanoparticles still stay in the body, making the related clearance complicated. In summary, obtaining ultrasmall particles with good physical and chemical properties for medical applications is still an unmet need and remains a challenge for further research.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No 81000668), Natural Science Foundation of Tianjin (Grant No 13JCQNJC13500), the Subject Development Foundation of Institute of Radiation Medicine, CAMS (Grant No SF1207, SZ1336), and PUMC Youth Fund and the Fundamental Research Funds for the Central Universities (Grant No 3332013043).
Disclosure
The authors report no conflicts of interest in this work.
References
- LeutwylerWKBürgiSLBurglHSemiconductor clusters, nanocrystals, and quantum dotsScience19962715251933937
- ChanWCNieSQuantum dot bioconjugates for ultrasensitive nonisotopic detectionScience19982815385201620189748158
- BruchezMMoronneMGinPWeissSAlivisatosAPSemiconductor nanocrystals as fluorescent biological labelsScience19982815385201320169748157
- KimDParkSLeeJHJeongYYJonSAntibiofouling polymer-coated gold nanoparticles as a contrast agent for in vivo X-ray computed tomography imagingJ Am Chem Soc2007129247661766517530850
- HuangXEl-SayedIHQianWEl-SayedMACancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorodsJ Am Chem Soc200612862115212016464114
- HainfeldJFSlatkinDNSmilowitzHMThe use of gold nanoparticles to enhance radiotherapy in micePhys Med Biol20044918N309N31515509078
- ZhangXDWuDShenXSize-dependent radiosensitization of PEG-coated gold nanoparticles for cancer radiation therapyBiomaterials201233276408641922681980
- ZhangXDChenJLuoZEnhanced tumor accumulation of sub-2 nm gold nanoclusters for cancer radiation therapyAdv Healthc Mater20143113314123873780
- ZhangXDChenJMinYMetabolizable Bi2Se3 Nanoplates: Biodistribution, Toxicity, and Uses for Cancer Radiation Therapy and ImagingAdvanced Functional Materials2014241217181729
- LiuZCaiWHeLIn vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in miceNat Nanotechnol200721475218654207
- HongGLeeJCRobinsonJTMultifunctional in vivo vascular imaging using near-infrared II fluorescenceNat Med201218121841184623160236
- WuDZhangX-DLiuP-XZhangL-AFanF-YGuoM-LGold nanostructure: fabrication, surface modification, targeting imaging, and enhanced radiotherapyCurrent Nanoscience201171110118
- MurphyCJGoleAMStoneJWGold nanoparticles in biology: beyond toxicity to cellular imagingAcc Chem Res200841121721173018712884
- BoisselierEAstrucDGold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicityChem Soc Rev20093861759178219587967
- ChenJWangHLongWSex differences in the toxicity of polyethylene glycol-coated gold nanoparticles in miceInt J Nanomedicine201382409241923861586
- HudoklinSZupančičDMakovecDKreftMERomihRGold nanoparticles as physiological markers of urine internalization into urothelial cells in vivoInt J Nanomedicine201383945395324143099
- ZhangXDWuHYWuDToxicologic effects of gold nanoparticles in vivo by different administration routesInt J Nanomedicine2009577178121042423
- LewinskiNColvinVDrezekRCytotoxicity of nanoparticlesSmall200841264918165959
- PanYNeussSLeifertASize-dependent cytotoxicity of gold nanoparticlesSmall20073111941194917963284
- De JongWHHagensWIKrystekPBurgerMCSipsAJGeertsmaREParticle size-dependent organ distribution of gold nanoparticles after intravenous administrationBiomaterials200829121912191918242692
- ZhangGYangZLuWInfluence of anchoring ligands and particle size on the colloidal stability and in vivo biodistribution of polyethylene glycol-coated gold nanoparticles in tumor-xenografted miceBiomaterials200930101928193619131103
- LipkaJSemmler-BehnkeMSperlingRABiodistribution of PEG-modified gold nanoparticles following intratracheal instillation and intravenous injectionBiomaterials201031256574658120542560
- BalasubramanianSKJittiwatJManikandanJOngCNYuLEOngWYBiodistribution of gold nanoparticles and gene expression changes in the liver and spleen after intravenous administration in ratsBiomaterials20103182034204220044133
- MonopoliMPWalczykDCampbellAPhysical–chemical aspects of protein corona: relevance to in vitro and in vivo biological impacts of nanoparticlesJ Am Chem Soc201113382525253421288025
- CedervallTLynchILindmanSUnderstanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticlesProc Natl Acad Sci U S A200710472050205517267609
- LundqvistMStiglerJEliaGLynchICedervallTDawsonKANanoparticle size and surface properties determine the protein corona with possible implications for biological impactsProc Natl Acad Sci U S A200810538142651427018809927
- ZhangXDWuDShenXSize-dependent in vivo toxicity of PEG-coated gold nanoparticlesInt J Nanomedicine201162071208121976982
- SchlachterEKWidmerHRBregyAMetabolic pathway and distribution of superparamagnetic iron oxide nanoparticles: in vivo studyInt J Nanomedicine201161793180021980242
- MaPLuoQChenJIntraperitoneal injection of magnetic Fe3O4-nanoparticle induces hepatic and renal tissue injury via oxidative stress in miceInt J Nanomedicine201274809481822973100
- GorthDJRandDMWebsterTJSilver nanoparticle toxicity in Drosophila: size does matterInt J Nanomedicine2011634335021383859
- PuvanakrishnanPParkJChatterjeeDKrishnanSTunnellJWIn vivo tumor targeting of gold nanoparticles: effect of particle type and dosing strategyInt J Nanomedicine201271251125822419872
- ChoiHSLiuWMisraPRenal clearance of quantum dotsNat Biotechnol200725101165117017891134
- FischerHCLiuLPangKSChanWCPharmacokinetics of nanoscale quantum dots: in vivo distribution, sequestration, and clearance in the ratAdvanced Functional Materials2006161012991305
- XieJXuCKohlerNHouYSunSControlled PEGylation of Monodisperse Fe3O4 Nanoparticles for Reduced Non-Specific Uptake by Macrophage CellsAdvanced Materials2007192031633166
- NiidomeTYamagataMOkamotoYPEG-modified gold nanorods with a stealth character for in vivo applicationsJ Control Release2006114334334716876898
- ZhouCLongMQinYSunXZhengJLuminescent gold nanoparticles with efficient renal clearanceAngewandte Chemie International Edition20111231432263230
- ChoiCHJZuckermanJEWebsterPDavisMETargeting kidney mesangium by nanoparticles of defined sizeProc Natl Acad Sci U S A2011108166656666121464325
- YangDDaiYLiuJUltra-small BaGdF5-based upconversion nanoparticles as drug carriers and multimodal imaging probesBiomaterials20143562011202324314558
- ZhangXDWuDShenXLiuPXFanFYFanSJIn vivo renal clearance, biodistribution, toxicity of gold nanoclustersBiomaterials201233184628463822459191
- SimpsonCASallengKJCliffelDEFeldheimDLIn vivo toxicity, biodistribution, and clearance of glutathione-coated gold nanoparticlesNanomedicine20139225726322772047
- AlricCMiladiIKryzaDThe biodistribution of gold nanoparticles designed for renal clearanceNanoscale20135135930593923702968
- HuangXZhangFZhuLEffect of Injection Routes on the Biodistribution, Clearance, and Tumor Uptake of Carbon DotsACS Nano2013775684569323731122
- FangJChandrasekharanPLiuXLManipulating the surface coating of ultra-small Gd2O3 nanoparticles for improved T1-weighted MR imagingBiomaterials20143551636164224290697
- GuYPCuiRZhangZLXieZXPangDWUltrasmall near-infrared Ag2Se quantum dots with tunable fluorescence for in vivo imagingJ Am Chem Soc20111341798222148738
- ChouLYZagorovskyKChanWCDNA assembly of nanoparticle superstructures for controlled biological delivery and eliminationNature Nanotechnology201491148155
- QianHZhuYJinRAtomically precise gold nanocrystal molecules with surface plasmon resonanceProc Natl Acad Sci U S A2012109369670022215587
- LuoZYuanXYuYFrom aggregation-induced emission of Au (I)–thiolate complexes to ultrabright Au (0)@ Au (I)–thiolate core–shell nanoclustersJ Am Chem Soc201213440166621667022998450
- XieJZhengYYingJYProtein-directed synthesis of highly fluorescent gold nanoclustersJ Am Chem Soc2009131388888919123810
- DongCQianHFangNRenJStudy of fluorescence quenching and dialysis process of CdTe quantum dots, using ensemble techniques and fluorescence correlation spectroscopyJ Phys Chem B200611023110691107516771367
- KlimovVMikhailovskyAXuSOptical gain and stimulated emission in nanocrystal quantum dotsScience2000290549031431711030645
- CognetLTsyboulskiDARochaJ-DRDoyleCDTourJMWeismanRBStepwise quenching of exciton fluorescence in carbon nanotubes by single-molecule reactionsScience200731658301465146817556581
- PanDZhangJLiZWuMHydrothermal Route for Cutting Graphene Sheets into Blue-Luminescent Graphene Quantum DotsAdvanced Materials201022673473820217780
- HanMYÖzyilmazBZhangYKimPEnergy band-gap engineering of graphene nanoribbonsPhys Rev Lett2007982020680517677729
- WangXOuyangYLiXWangHGuoJDaiHRoom-temperature all-semiconducting sub-10-nm graphene nanoribbon field-effect transistorsPhys Rev Lett20081002020680318518566