?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A novel amphiphilic triblock pH-sensitive poly(β-amino ester)-g-poly(ethylene glycol) methyl ether-cholesterol (PAE-g-MPEG-Chol) was designed and synthesized via the Michael-type step polymerization and esterification condensation method. The synthesized copolymer was determined with proton nuclear magnetic resonance and gel permeation chromatography. The grafting percentages of MPEG and cholesterol were determined as 10.93% and 62.02%, calculated from the area of the characteristic peaks, respectively. The amphiphilic copolymer was confirmed to self-assemble into core/shell micelles in aqueous solution at low concentrations. The critical micelle concentrations were 6.92 and 15.14 mg/L at pH of 7.4 and 6.0, respectively, obviously influenced by the changes of pH values. The solubility of pH-responsive PAE segment could be transformed depending on the different values of pH because of protonation–deprotonation of the amino groups, resulting in pH sensitivity of the copolymer. The average particle size of micelles increased from 125 nm to 165 nm with the pH decreasing, and the zeta potential was also significantly changed. Doxorubicin (DOX) was entrapped into the polymeric micelles with a high drug loading level. The in vitro DOX release from the micelles was distinctly enhanced with the pH decreasing from 7.4 to 6.0. Toxicity testing proved that the DOX-loaded micelles exhibited high cytotoxicity in HepG2 cells, whereas the copolymer showed low toxicity. The results demonstrated how pH-sensitive PAE-g-MPEG-Chol micelles were proved to be a potential vector in hydrophobic drug delivery for tumor therapy.
Introduction
In recent years, more and more attention has been paid to the biocompatibility of pH-sensitive polymers that are developed as drug delivery systems for cancer therapy.Citation1–Citation4 However, there are still some obstacles, such as stability, solubility of the drug, sensitivity, and toxicity.Citation5,Citation6 Recently, pH-response polymers, including the protonable group, such as polymeric micelles,Citation7–Citation11 nanoparticles,Citation12–Citation16 liquid emulsion,Citation17 and liposomes,Citation5,Citation18–Citation20 have been investigated to enhance antitumor efficiency with reduced side effects. Among these vectors, polymeric micelles have a core/shell architecture. Generally, the internal core is composed of the hydrophobic segments that provide the loading space for the small molecule hydrophobic drug. The shell is built by hydrophilic segments that could protect the drug-loaded system and affect the release behaviors in aqueous medium. In addition, the polymeric micelles are designed, synthesized, modified, and received easily.Citation21 Therefore, the pH stimulus-response polymeric micelles are investigated as smart drug delivery carriers for cancer therapy. For example, poly(β-amino ester) (PAE),Citation22–Citation24 containing tertiary amine, was first reported by Danusso and FerrutiCitation25 in 1970. Lynn and Langer,Citation26 Lynn et alCitation27 and Shenoy et alCitation28 synthesized PAE via Michael-type polymerization. Then, properties of these biodegradable and pH-sensitive materials were also investigated. In 2006, Ko et alCitation29 and Hwang et alCitation30 designed and synthesized a novel pH-response micelle self-assembled from diblock polymer poly(ethylene glycol)-β-PAE, which was used as an anticancer drug carrier, exhibiting controlled release behavior with change of pH. In previous studies, our team also synthesized amphiphilic block copolymer with random hydrophobic/pH-sensitive structure of poly(ethylene glycol) methyl ether-β-(polylactic acid-co-poly[β-amino esters]) (MPEG-β-[PLA-co-PAE]) and doxorubicin (DOX) selected as the model drug, and some results have been achieved.Citation31 However, there are still various challenges for the emerging vectors that prevent their further application.Citation2,Citation5 The increase of drug loading capacity, good controlled release behavior, and well biocompatibility are the pivotal parts in the process of development of novel effect carriers. The low drug loading capacity results in high drug cost and imperfect therapeutic efficacy. Poor release behavior (eg, burst release) caused shortened effective time of the drug and magnified side effects. Inferior biocompatibility enhanced the toxicity for normal issues and reduced the effect of the system. It is well known that extracellular pH in tumor cells is weakly acidic (around 6.5–7.0), and the lysosomal environment is more acidic (about 5.0), compared with the normal physiological environment of pH 7.4.Citation32–Citation34 Therefore, these are the preconditions for the pH stimulus-response system. The ideal carrier should be compact and keep the drug in the core in a normal pH environment and release them at the weakly acidic pH according to what is desired.
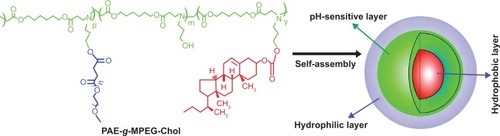
In the present study, the purpose was to design an improved pH-sensitive micelle for anticancer drug delivery depending on the value of pH. The micelle was formed by the novel synthesized amphiphilic triblock copolymer poly(β-amino ester)-g-poly(ethylene glycol) methyl ether-cholesterol (PAE-g-MPEG-Chol). The hydrophilic MPEG, composed of the shell and stretching on the surface of the particles, provided a tight protective shuck to maintain the stability of the drug-loaded system during the long-time circulation and increase the bloodstream half-life of the system because of the nonimmunogenic, nonantigenic, and nontoxic properties.Citation35,Citation36 PAE was selected as the pH-sensitive segment in order to control the drug release in different pH environments. With the purposes of high drug loading capacity and well biocompatibility of the carrier, the cholesterol, an important part of mammalian cell membranes, was introduced as the core in the micelle because of the high hydrophobicity of the sterol group and well biodegradability and pathogenicity.Citation37,Citation38 The hydrophilic PEG and hydrophobic cholesterol moieties were conjugated on the side of the PAE main chain, resulting in a rake-like shape. DOX was used as the model small molecule hydrophobic drug and was loaded into PAE-g-MPEG-Chol micelles by dialysis method.Citation39 shows the molecule structure and the schematic micelle of designed polymer in aqueous solution. In the normal environment, DOX was protected in the core and the system could keep a stable and compact structure. But in the tumor cell’s weakly acidic circumstances, the loaded DOX could be released with an improved rate because of the swollen micelle structure caused by the ionization of basic groups in the PAE motif. The physicochemical properties of polymer, such as particle size and zeta potential, were also examined by a variety of experimental techniques.
Materials and methods
Materials
Methoxypoly(ethylene glycol) (MPEG, Mn=2,000, Aldrich), succinic anhydride (SA, 99%, Alfa Aesar), 3-amino-1-propanol (AP, 99%, Alfa Aesar), hexane-1,6-dioldiacrylate (HDD, 99%), methylthiazoltetrazolium (MTT), and phosphate-buffered saline (PBS) were purchased from Sigma Chemical Co., and 1st BASE (Malaysia), respectively. N,N′-dicyclohexylcarbodiimide (DCC, 99%, Alfa Aesar), 4-(dimethyl amino)pyridine (DMAP, 99%, Alfa Aesar), cholesteryl chloroformate (Chol, 99%, Alfa Aesar), anhydrous dichloromethane (DCM), anhydrous triethylamine (TEA), 1,4-dioxane, and other materials were received and prepared as described in previous work.Citation31
Synthesis of PAE
This segment was synthesized by Michael-type step polymerization. In brief, HDD (1 mol) was dissolved in anhydrous DCM under nitrogen. The flask was immersed in an ice bath, and then AP (1 mol) was dropped slowly. The resulting heterogeneous mixture was stirred at 0°C for 2 hours, allowed to attain room temperature, and then stirred at 100°C for a further 5 hours. Lastly, the reaction was cooled to ambient temperature. The reaction mixture was solved in DCM and precipitated with n-hexane three times to obtain the solid. After being dried under vacuum for 24 hours, the designed PAE was received.
Synthesis of methoxypoly(ethylene glycol)-carboxyl (MPEG-COOH)
MPEG (1 mol), SA (1.5 mol), and DMAP (0.1 mol) were dissolved in anhydrous 1,4-dioxane, and then anhydrous TEA (1 mol) was added dropwise. The resulting solution was stirred at 25°C for 24 hours. The reaction solution was evaporated and then precipitated with n-hexane three times. After being dried under vacuum for 24 hours, the designed MPEG-COOH was received.
Synthesis of PAE-g-MPEG block copolymer
MPEG-COOH (1 mol), DMAP (1 mol), and DCC (1 mol) were dissolved in anhydrous DCM in a 50 mL flask, respectively. PAE (1 mol) was dissolved in anhydrous DCM and dropped slowly in the flask. The mixture was stirred at room temperature for 24 hours under nitrogen. After filtration, the received solution was precipitated with n-hexane three times. After being dried under vacuum for 24 hours, the designed block polymer was received.
Synthesis of PAE-g-MPEG-Chol block copolymer
PAE-g-MPEG (1 mol) was dissolved in anhydrous DCM, and then anhydrous TEA (15 mol) was added. Cholesterol (15 mol) was also dissolved and added dropwise in the flask. The reaction mixture was stirred at 25°C for 24 hours. After being dried under vacuum for 24 hours, the designed block copolymer was received.
Characterization of the polymer
Gel permeation chromatography (GPC) was executed by an Agilent 1200 series GPC system (liquid chromatogram quant pump, polar gel 5 mm 500 Å, 10,000 Å, and 100,000 Å columns in series, refractive index detector, mobile phase: high-performance liquid chromatography grade tetrahydrofuran, 1.0 mL/min, 30°C) to determine the average molecular weight (Mn) and polydispersity index (Mw/Mn).
Proton nuclear magnetic resonance (1H NMR) test was adopted on a spectrometer (AVANCE III 400, Bruker, 250 MHz, Switzerland) at 25°C. Deuterated chloroform (CDCl3-d) and tetramethylsilane were used as solvent and an internal standard, respectively.
Dynamic light scattering (Malvern Zetasizer Nano S, Malvern, UK) was carried out to detect the particle size (Dh) and distribution (polydispersity index). Briefly, after being purified by a 0.45 μm pore size filter, the received samples were measured in a 1.0 mL quartz cuvette using a diode laser of 800 nm at 25°C. The scattering angle was 90°.
Fourier transform infrared (FT-IR) spectrophotometer (Nicolet Nexus for Euro, USA) was used to confirm the structure of the synthesized polymer. The spectra were taken from 400 cm−1 to 4,000 cm−1.
Transmission electron microscopy (TEM, Hitachi H-7650, Japan) was used to detect the morphologies of polymeric micelles and DOX-loaded particles operating at 80 kV.
Phase transition pH values of PAE
PAE was dissolved in deionized water containing HCl, resulting in polymer solution (2 mg/mL, pH 4.0). The value of pH was gradually increased by dropped 0.1 M NaOH aqueous solution. The transmittance of the mixture was measured using an ultraviolet-visible spectrophotometer (UV-vis, 500 nm) at different pH values.
Potentiometric titration
The base dissociation constant (pKb) value of the PAE was determined by acid-base titrations. In brief, the polymer was dissolved (1 mg/mL, deionized water) equally, and then the value of the pH was adjusted to 3 by HCl solution. Finally, the solution was titrated by NaOH (0.1 mol/mL) at increments of 100 μL. The values of the pH were monitored with an automatic titration titrator (Hanon T-860, Jinan, People’s Republic of China) at 25°C.
Critical micelle concentration measurement
The critical micelle concentration (CMC) of amphiphilic polymer PAE-g-MPEG-Chol was estimated by the fluorescence probe technique using pyrene as a fluorescence probe as described in previous work.Citation6,Citation31,Citation40 In brief, after a certain concentration of pyrene acetone solution was confected, a series of concentrations of the samples (0.0001–0.1 mg/mL) containing pyrene were prepared. Before measurement, the received mixture was equilibrated in the dark for 1 day at 25°C. The sample was tested with a fluorescence spectrophotometer (F-4500, Hitachi, Japan) with an emission wavelength of 373 nm and a bandwidth of 0.2 nm and scanning at wavelengths 300–350 nm.
Preparation of blank and DOX-loaded micelles
The micelles were prepared by the dialysis method. Briefly, different amounts of DOX-HCl (0, 10, 20, and 40 mg) and the polymer (40 mg) were mixed and solved in dimethyl formamide (DMF) (40 mL). Overdoses of TEA (0.01 mL for per 10 mg of drug) were added in order to remove the hydrochloride for the DOX-loaded micelles. The received solution was dialyzed against 1 liter of deionized water for 48 hours at 25°C with molar weight cut-off of 3,500–4,000 Da. In the first 12 hours, the deionized water was replaced six times and then every 6 hours for the rest of the time. Subsequently, the polymeric micelles were received after lyophilization.
The prepared DOX loading content (LC) and entrapment efficiency (EE) in the polymeric micelles were investigated by a UV-vis spectrophotometer (UV-2450, Shimadzu, Japan) at 480 nm. In brief, DOX-loaded micelles (1 mg) were solved in DMF (10 mL), and then the drugs were out from the broken micelles and dissolved in DMF. The sample was tested and recorded to compare with a standard curve of pure DOX/DMF solution in order to calculate the concentration of the DOX. The LC and EE were calculated using the following equations:
In vitro release of DOX from PAE-g-MPEG-Chol micelles
An in vitro drug release test was performed at low drug concentration in order to acquire sink conditions. Briefly, the amount of DOX-loaded micelles (4 mg) was dissolved equally in PBS (4 mL), and the resulting solution was added in a cellulose dialysis sack (molar weight cut-off 3,500–4,000 Da). The sacks were placed in PBS with different pH (7.4 and 6.0) in the quantitative beakers. The experiments were carried out at 37°C with stirring at 110 rpm. The desired volume of the solution was taken to detect the concentration of DOX by UV-vis spectrophotometry, and the same volume of fresh PBS was added. The cumulative drug release percent (Er) can be worked out using EquationEquation 3(3)
where mDOX is the amount of drug loaded in the particle, V0 is the volume of the release media in the beaker, and Ci is the concentration of DOX in the ith sample.
Cytotoxicity test
The cytotoxicity of free DOX or DOX-loaded PAE-g-MPEG-Chol micelles was investigated against HepG2 cells by the standard MTT assay.Citation41–Citation44 The cell culture of HepG2 cells was described in previous work.Citation31 Various concentrations of free DOX and DOX-loaded micelles solution were prepared. After discarding the culture supernatants, fresh medium or sample solutions (200 μL) were added into every well and remained for 24 hours or 48 hours in the incubator, respectively. After the control and every experimental well was treated with fresh medium (100 μL) and MTT solution (20 μL, 5 mg/mL), respectively, the cells were incubated for another 4 hours. Finally, the used media were discarded and dimethyl sulfoxide (200 μL) was added in every hole. The absorbance of the plates was detected by a microplate reader (Multiskan Spectrum, Thermo Scientific, Finland) at 490 nm after 15 minutes of being shaken. The cell viability was defined as the ratio of the different absorbance of sample and blank and different absorbance of control and blank.
Statistical analysis
The experimental data were presented with average values expressed as the mean ± standard deviation. Statistical significance was determined by Student’s t-test (Excel, 2007) and considered to be significant when the P-values were less than 0.05 (P<0.05).
Results and discussion
Synthesis and characterization of the PAE-g-MPEG-Chol block copolymer
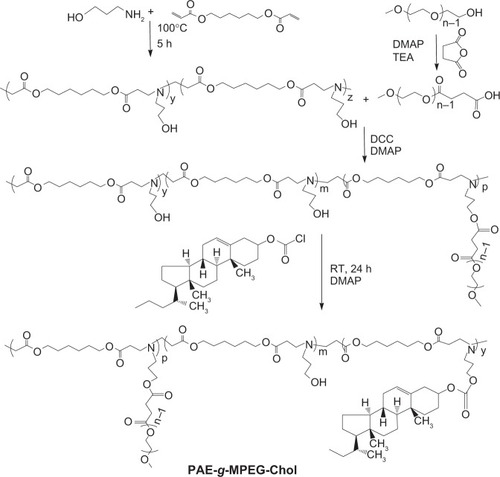
PAE-g-MPEG-Chol was synthesized via a Michael-type step polymerization and esterification reaction. As shown in , MPEG-OH was catalyzed with succinic anhydride by DMAP and TEA to synthesize MPEG-COOH. Subsequently, AP and HDD were used to synthesize PAE monomer, and then PAE was grafted with MPEG by esterification reaction using DCC and DMAP as coupling agent and catalyst, respectively. Lastly, PAE-g-MPEG was conjugated with cholesterol segment. PAE, PAE-g-MPEG, and PAE-g-MPEG-Chol were synthesized and detected, as shown in . In the present study, MPEG was grafted as the water-soluble part by conjugating it to the PAE segment that exhibited pH sensitivity caused by the tertiary amines in the main chain. The polymer was also modified by cholesterol on the side chain introduced in order to increase the drug loading space and the biocompatibility.
Table 1 GPC and 1H NMR data of PAE-g-MPEG-Chol and its precursors
Figure 2 The synthetic route of poly(β-amino ester)-g-poly(ethylene glycol) methyl ether-cholesterol (PAE-g-MPEG-Chol).
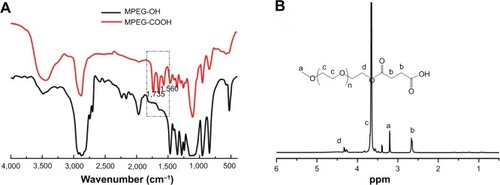
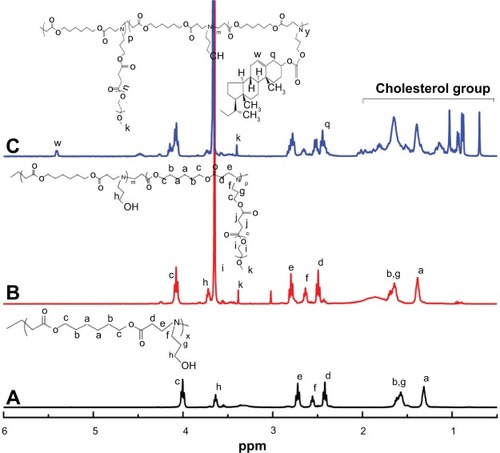
The chemical structures of the obtained MPEG-COOH, PAE, and PAE-g-MPEG-Chol block copolymer were confirmed by FT-IR and 1H NMR. As shown in the FT-IR spectra (), the absorption peaks of C=O and –COO– with stretching and antisymmetric stretching vibration in MPEG-COOH (red line) occurred at 1,735 cm−1 and 1,560 cm−1, respectively, which were not present in MPEG-OH (black line). With regard to 1H NMR spectra (), the characteristic MPEG peaks at 3.65 ppm (c) due to –OCH2– CH2– protons could be found obviously. The signal at 3.25 ppm (a) was ascribed to CH3–O– of MPEG-COOH unit. The signal at 4.35 ppm (d) was due to the –CH2–O–C=O unit. The characteristic MPEG-COOH peak at 2.63 ppm (b) was due to –C-H2–CH2–COOH. All these results demonstrated that MPEG-COOH was synthesized successfully.
Figure 3 Fourier transform infrared spectra (A) of MPEG-OH and MPEG-COOH and proton nuclear magnetic resonance spectrum (B) of MPEG-COOH.
Abbreviations: MPEG-OH, methoxypoly(ethylene glycol)-hydroxyl; MPEG-COOH, methoxypoly(ethylene glycol)-carboxyl.
shows the 1H NMR spectra of PAE, PAE-g-MPEG, and PAE-g-MPEG-Chol polymers. As exhibited in (PAE segment), the signals at 1.40 ppm (a), 1.60 ppm (b), 4.00 ppm (c), 2.46 ppm (d), and 2.75 ppm (e) were, respectively, ascribed to the –COOCH2CH2CH2CH2CH2 CH2COO–, –COOCH2CH2CH2CH2CH2CH2COO–, –COOCH2CH2CH2CH2CH2CH2COO–, –COOCH2CH2N, and COOCH2CH2N of the HDD unit. The peaks of 2.55 ppm (f), 3.70 ppm (h), and 1.55 ppm (g) were caused by –NCH2CH2CH2 OH, –NCH2CH2CH2OH, and –NCH2CH2CH2OH of the AP unit. After MPEG was grafted (), the characteristic peaks of MPEG, as described, 3.45 ppm (k) and 3.65 ppm (i) were due to the CH3–O– and –OCH2–CH2–. The percent grafting of MPEG was determined as 10.93% by being calculated from the area of the characteristic peaks. With regard to the cholesterol-modified PAE-g-MPEG polymer (), the signals at 0.69–2.00 ppm were the characteristic cholesterol peaks, and it also had two peaks at 2.4 ppm (q) and 5.52 ppm (w). The percent grafting of cholesterol was received as 62.02%. Lastly, the segment ratios of block copolymers were confirmed, as shown in .
Figure 4 Proton nuclear magnetic resonance spectra of PAE (A), PAE-g-MPEG (B), and PAE-g-MPEG-Chol (C) in deuterated chloroform.
Abbreviations: PAE, poly(β-amino ester); PAE-g-MPEG, poly(β-amino ester)-g-poly(ethylene glycol) methyl ether; PAE-g-MPEG-Chol, poly(β-amino ester)-g-poly(ethylene glycol) methyl ether-cholesterol.
Characterization of pH-sensitive PAE segment
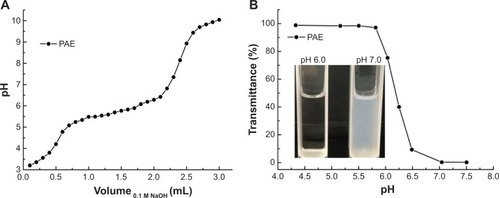
The sensitivity of the PAE was evaluated by an acid-base titration () and by monitoring the dispersion in water (). As seen in , beginning with NaOH solution addition, the value of the pH increased rapidly and then reached a plateau (5.5–6.5), attributed to the tertiary amine groups of ionized PAE. Then the pH increased quickly again with adding of NaOH solution. This proved that the pH-sensitive range of PAE was about 5.5–6.5, indicating the amine groups of PAE units could protonate in this range. The pKb was about 6.3 when the pKb value of the polymer was defined as the solution pH at 50% neutralization of tertiary amine groups.Citation24 With regard to transmittance test (), at pH <5.5, PAE could be dissolved in water and formed a clear solution. As the solution pH increased, the transmittance decreased significantly and the solution suddenly became turbid (pH from 5.5 to 7.5). When the pH increased to 7.0, the transmittance was almost zero (as shown bottom left). All these results demonstrate that the pH sensitivity of the PAE was around 6.5.Citation26
Micelle formation
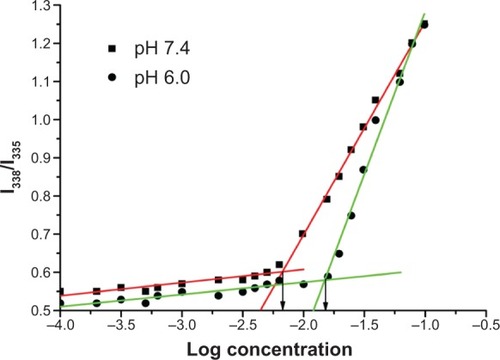
The formation of the micelles from PAE-g-MPEG-Chol polymer was proved by studying the CMCs at different pH values (7.4 and 6.0). As the PAE-g-MPEG-Chol concentrations increased, the intensity increased gradually. It can be observed that the third peak of the lowest concentration was at 335 nm, and this peak of the highest concentration shifted to 338 nm. The I338/I335 ratios were plotted against the logarithm of polymer concentrations. As shown in , the CMC values of PAE-g-MPEG-Chol were about 6.92 mg/L and 15.14 mg/L, respectively, at pH of 7.4 and 6.0. This change was due to the conversion of ionized pH-sensitive segment to hydrophilic ones by protonation of amine groups in the PAE segment, leading to enhanced repulsive forces among longer hydrophilic segments, which indicated more difficulty with micelle formation.
Characterization of blank and DOX-loaded micelles
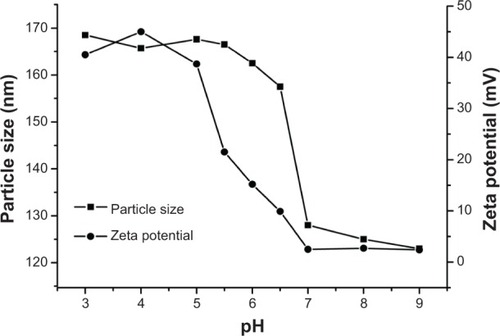
The properties of blank and drug-loaded micelles were further detected by some other methods. The results were collected and are shown in , , and . As expected, in , when the pH value decreased from 9.0 to 7.0, the particle sizes of the block copolymer were almost unchanged (about 125 nm). The reason may be that hardly any quantity of PAE protonated, leading to a compressed structure and isolated nanoparticle. With the pH values decreasing continuously (6.5–5.5), the micelle sizes increased obviously, from 125 nm to 165 nm. This could be explained by the tertiary amine groups of the PAE segment in the main chain being fully protonated, resulting in the transformation of hydrophobic to hydrophilic, which led to a loose and swollen structure. When the values of pH decreased continuously, the particle sizes almost reached a platform. With regard to zeta potential, and display the zeta potentials of the blank and DOX-loaded micelles as positive. Beginning with pH decreasing from 9.0 to 7.0, zeta potential was almost unchanged and low (<5 mV), resulting from absorption of hydroxyl on the surface. When pH dropped sequentially, zeta potential increased rapidly (around 40 mV), attributed to the full protonation of tertiary diamine groups. In addition, the increased high positive caused enhanced repulsive forces among outstretched hydrophilic chain, suggesting higher particle sizes. These results also demonstrate that drugs should be loaded above a pH of 7.0 in order to receive high LC. DOX was loaded into the polymeric micelles by membrane dialysis using deionized water. As the feed amount of DOX increased, the LC and EE also increased. The actual loading levels of DOX in the polymer were 9.5%, 28.3%, and 30.7%, depending on the different ratios of DOX/polymer, respectively. When the ratio of DOX/polymer was 20 mg/40 mg, the EE was the maximum (60%). When the ratio of DOX/polymer was 40/40 mg, the LC was slightly higher and the EE was moderately lower because of the limited drug loading space and aggregation of the drugs. The DOX-loaded micelles exhibited slightly larger than the blank ones, caused by the entrapped DOX in the micellar core and absorbed on the surface of the micelle. The other characteristic properties are showed in .
Table 2 Characteristic properties of DOX-loaded PAE-g-MPEG-Chol micelles
Figure 8 Transmission electron microscopy micrographs of poly(β-amino ester)-g-poly(ethylene glycol) methyl ether-cholesterol micelles (pH=7.4).
Note: The right image is the magnified result of the left image.
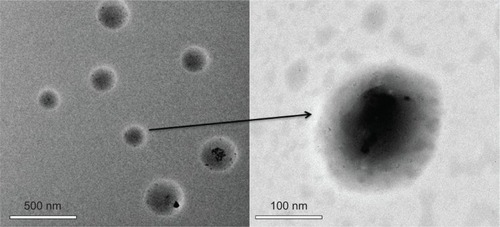
presents the TEM images of the blank PAE-g-MPEG-Chol micelles in the buffer solution (PBS, pH 7.4). It could be seen that the micelles showed a spherical morphology. Furthermore, the nanoparticles displayed a dark center and light periphery, indicating an obvious core/shell structure.
In vitro release of DOX from micelles
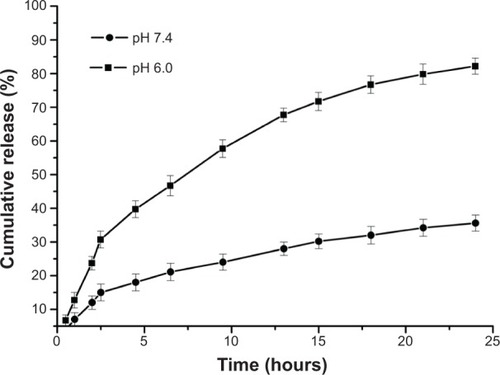
The PAE-g-MPEG-Chol micelles exhibited a pH-responsive property. In vitro drug release performance of the micelles was finished under physiological conditions (PBS, pH 7.4) and in a slightly acidic environment (PBS, pH 6.0), as shown in . It can be found obviously that the DOX release rates from the particles were significantly changed at different pH values. At the pH of 7.4, the PAE-g-MPEG-Chol micelle was compact and the DOX release rate was slow. After 24 hours, the cumulative release was about 33% for the DOX/polymer system, which indicated that the drug was still in the micellar core. With regard to pH of 6.0, the DOX release rate was enhanced markedly, and the cumulative release was 35% after 3 hours and almost 95% after 24 hours for the drug-loaded systems, respectively, demonstrating controlled release depending on pH change and without obvious burst release. The reason could be that the looser micelle structure was caused by the stronger protonation of amino groups in PAE moieties at lower pH conditions. In addition, the stronger electrostatic repulsion between PAE units, because of increased charge density of the micelle surface, led to the loose micelle structure. This phenomenon was consistent with the aforementioned characteristic results. In brief, the polymeric micelle can compress and protect DOX at a pH of 7.4 and release it at a pH of 6.0, indicating a well pH-controlled release behavior.
Cytotoxicity test
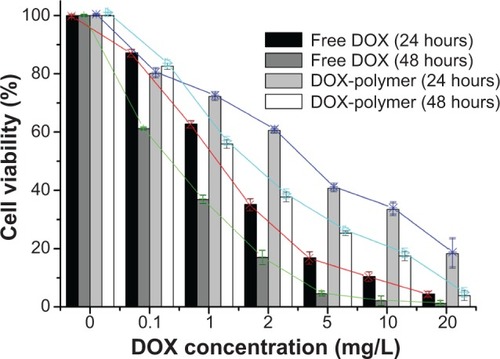
Cytotoxicity of novel polymer, free DOX, or DOX-loaded PAE-g-MPEG-Chol micelles was studied against HepG2 cells by MTT assay. The novel synthesized polymer display and there was almost no cytotoxicity in HepG2 cells (data not shown). presents the results of cytotoxicity tests treated with free DOX and DOX-loaded polymeric micelles for 24 hours and 48 hours, respectively. The values of IC50, defined as the DOX concentration when 50% of cells were killed, were 1.20 mg/L (24 hours), 0.45 mg/L (48 hours), 2.65 mg/L (24 hours), and 1.70 mg/L (48 hours) for free DOX and DOX-loaded micelles, respectively. The DOX-loaded micelles displayed lower cytotoxic effect than free DOX. As shown in the results, with regard to the highest concentration, the cell viability that was treated DOX-loaded micelles for 48 hours revealed similar (but still slightly less) killing capacity as free DOX treatment. This analysis indicated the drug could be controlled released according to the pH values, which was consistent with the results of the in vitro experiment.
Conclusion
The designed amphiphilic triblock pH-sensitive copolymer PAE-g-MPEG-Chol with MPEG and cholesterol segments modified structure was synthesized and applied for hydrophobic drug delivery (eg, DOX). The low CMC value of the copolymer in a normal environment could markedly improve micellar stability and extend the range of applications of micelles in controlled drug delivery. The transmittance, particle size, and zeta potential changed regularly with the values of pH, demonstrating that this novel copolymer displayed pH sensitivity as well as the results of the potentiometric titration. The DOX loading capacity was enhanced by being modified with cholesterol. DOX release rates were controlled by pH value. The release of DOX from the micelles was significantly accelerated with pH decreasing from 7.4 to 6.0. Almost all loaded drug could be released in a weakly acidic environment. The cytotoxic effect of DOX-loaded micelles was similar to that of free DOX. These experimental results demonstrate that PAE-g-MPEG-Chol micelles could be a potential carrier in tumor therapy.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (21176090), Team Project of Natural Science Foundation of Guangdong Province, China (S2011030001366), Specialized Research Fund for the Doctoral Program of Higher Education of China (20130172110009), Science and Technology Foundation of Guangdong Province (2012B050600010), and Fundamental Research Funds for the Central Universities (2013ZP0010, 2014ZP0020).
Disclosure
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
References
- TangSYinQZhangZCo-delivery of doxorubicin and RNA using pH-sensitive poly (β-amino ester) nanoparticles for reversal of multidrug resistance of breast cancerBiomaterials201435236047605924797883
- ZhouZLiLYangYXuXHuangYTumor targeting by pH-sensitive, biodegradable, cross-linked N-(2-hydroxypropyl) methacrylamide copolymer micellesBiomaterials201435246622663524814427
- FelberAEDufresneMHLerouxJCpH-sensitive vesicles, polymeric micelles, and nanospheres prepared with polycarboxylatesAdv Drug Deliv Rev2012641197999221996056
- SchlossbauerADohmenCSchaffertDWagnerEBeinTpH-responsive release of acetal-linked melittin from SBA-15 mesoporous silicaAngew Chem Int Edit2011503068286830
- HusseiniGAPittWGMicelles and nanoparticles for ultrasonic drug and gene deliveryAdv Drug Deliv Rev200860101137115218486269
- GuoXDZhangLJChenYQianYCore/shell pH-sensitive micelles self-assembled from cholesterol conjugated oligopeptides for anticancer drug deliveryAIChE J201056719221931
- CaoJSuTZhangLPolymeric micelles with citraconic amide as pH-sensitive bond in backbone for anticancer drug deliveryInt J Pharm20144711–2283624836667
- ZhangQVakiliMRLiXFLavasanifarALeXCPolymeric micelles for GSH-triggered delivery of arsenic species to cancer cellsBiomaterials201435257088710024840615
- LeeJEAhnEBakJMPolymeric micelles based on photocleavable linkers tethered with a model drugPolymer201455614361442
- GaoGHLiYLeeDSEnvironmental pH-sensitive polymeric micelles for cancer diagnosis and targeted therapyJ Control Release2013169318018423195533
- DesaleSSCohenSMZhaoYKabanovAVBronichTKBiodegradable hybrid polymer micelles for combination drug therapy in ovarian cancerJ Control Release2013171333934823665258
- ChatterjeeKSarkarSJagajjanani RaoKPariaSCore/shell nanoparticles in biomedical applicationsAdv Colloid Interfac2014209839
- MitragotriSLahannJPhysical approaches to biomaterial designNat Mater200981152319096389
- GantaSDevalapallyHShahiwalaAAmijiMA review of stimuli-responsive nanocarriers for drug and gene deliveryJ Control Release2008126318720418261822
- DohmenCEdingerDFrohlichTNanosized multifunctional polyplexes for receptor-mediated siRNA deliveryACS Nano2012665198520822646997
- MicklerFMMocklLRuthardtNOgrisMWagnerEBräuchleCTuning nanoparticle uptake: live-cell imaging reveals two distinct endocytosis mechanisms mediated by natural and artificial EGFR targeting ligandNano Lett20121273417342322632479
- SarkerDKEngineering of nanoemulsions for drug deliveryCurr Drug Deliv20052429731016305433
- ChiangYTLoCLpH-responsive polymer-liposomes for intracellular drug delivery and tumor extracellular matrix switched-on targeted cancer therapyBiomaterials201435205414542424709521
- NinomiyaKYamashitaTKawabataSShimizuNTargeted and ultrasound-triggered drug delivery using liposomes co-modified with cancer cell-targeting aptamers and a thermosensitive polymerUltrason Sonochem20142141482148824418100
- LiXYZhaoYSunMGMultifunctional liposomes loaded with paclitaxel and artemether for treatment of invasive brain gliomaBiomaterials201435215591560424726749
- KedarUPhutanePShidhayeSKadamVAdvances in polymeric micelles for drug delivery and tumor targetingNanomedicine20106671472920542144
- KeeneyMOngSGPadillaADevelopment of Poly(β-amino ester)-based biodegradable nanoparticles for nonviral delivery of minicircle DNAACS Nano2013787241725023837668
- KozielskiKLTzengSYGreenJJA bioreducible linear poly(β-amino ester) for siRNA deliveryChem Commun2013494653195321
- ShenYTangHZhanYVan KirkEAMurdochWJDegradable poly(β-amino ester) nanoparticles for cancer cytoplasmic drug deliveryNanomedicine20095219220119223244
- DanussoFFerrutiPSynthesis of tertiary amine polymersPolymer197011288113
- LynnDMLangerRDegradable poly(β-amino esters): synthesis, characterization, and self-assembly with plasmid DNAJ Am Chem Soc2000122441076110768
- LynnDMAmijiMMLangerRpH-responsive polymer microspheres: rapid release of encapsulated material within the range of intracellular pHAngew Chem Int Edit200140917071710
- ShenoyDLittleSLangerRAmijiMPoly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs. 1. In vitro evaluationsMol Pharm20052535736616196488
- KoJParkKKimYSTumoral acidic extracellular pH targeting of pH-responsive MPEG-poly(beta-amino ester) block copolymer micelles for cancer therapyJ Control Release2007123210911517894942
- HwangSKimMHanJpH-sensitivity control of PEG-poly(β-amino ester) block copolymer micelleMacromol Res2007155437442
- ZhangCYYangYQHuangTXSelf-assembled pH-responsive MPEG-b-(PLA-co-PAE) block copolymer micelles for anticancer drug deliveryBiomaterials201233266273628322695069
- SchmidSLFuchsRMalePMellmanITwo distinct subpopulations of endosomes involved in membrane recycling and transport to lysosomesCell198852173833345561
- EnginKLeeperDBCaterJRThistlethwaiteAJTupchongLMcfarlaneJDExtracellular pH distribution in human tumoursInt J Hyperthermia19951122112167790735
- SchmidSFuchsRKielianMHeleniusAMellmanIAcidification of endosome subpopulations in wild-type Chinese hamster ovary cells and temperature-sensitive acidification-defective mutantsJ Cell Biol19891084129113002925786
- QinZChenYZhouWHeXBaiFWanMSynthesis and properties of polymer brushes composed of poly(diphenylacetylene) main chain and poly(ethylene glycol) side chainsEur Polym J2008441137323740
- HussainHMyaKYHeCSelf-assembly of brush-like poly[poly(ethylene glycol) methyl ether methacrylate] synthesized via aqueous atom transfer radical polymerizationLangmuir20082423132791328618986178
- IkonenECellular cholesterol trafficking and compartmentalizationNat Rev Mol Cell Biol20089212513818216769
- Hosta-RigauLZhangYTeoBMPostmaAStädlerBCholesterol – a biological compound as a building block in bionanotechnologyNanoscale2013518910923172231
- WangYZhangXYuPLiCGlycopolymer micelles with reducible ionic cores for hepatocytes-targeting delivery of DOXInt J Pharm20134411–217018023237873
- YangYQGuoXDLinWJZhangLJZhangCYQianYAmphiphilic copolymer brush with random pH-sensitive/hydrophobic structure: synthesis and self-assembled micelles for sustained drug deliverySoft Mater201282454464
- WangYIbrahimNLJiangJGaoSErathodiyilNYingJYConstruction of block copolymers for the coordinated delivery of doxorubicin and magnetite nanocubesJ Control Release2013169321121923499717
- XuLZhangHWuYDendrimer advances for the central nervous system delivery of therapeuticsACS Chem Neurosci20145121324274162
- ChenZZhangPCheethamAGControlled release of free doxorubicin from peptide–drug conjugates by drug loadingJ Control Release201419112313024892976
- MalarvizhiGLRetnakumariAPNairSKoyakuttyMTransferrin targeted core-shell nanomedicine for combinatorial delivery of doxorubicin and sorafenib against hepatocellular carcinomaNanomedicine201414S1549S9634