 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
We determined the efficacy and safety of chitosan (CS)-coated poly(lactic-co-glycolic) acid (PLGA) nanoparticles (NPs) as a delivery system for a vaccine to protect chickens against Newcastle disease virus (NDV). The newly constructed vaccine contained DNA (the F gene) of NDV. The Newcastle disease virus (NDV) F gene deoxyribonucleic acid (DNA) plasmid (pFDNA)-CS/PLGA-NPs were spherical (diameter =699.1±5.21 nm [mean ± standard deviation]) and smooth, with an encapsulation efficiency of 98.1% and a Zeta potential of +6.35 mV. An in vitro release assay indicated that CS controlled the burst release of plasmid DNA, such that up to 67.4% of the entire quantity of plasmid DNA was steadily released from the pFDNA-CS/PLGA-NPs. An in vitro expression assay indicated that the expression of nanoparticles (NPs) was maintained in the NPs. In an immunization test with specific pathogen-free chickens, the pFDNA-CS/PLGA-NPs induced stronger cellular, humoral, and mucosal immune responses than the plasmid DNA vaccine alone. The pFDNA-CS/PLGA-NPs did not harm 293T cells in an in vitro assay and did not harm chickens in an in vivo assay. Overall, the results indicated that CS-coated PLGA NPs can serve as an efficient and safe mucosal immune delivery system for NDV DNA vaccine.
Introduction
Virulent Newcastle disease virus (NDV) is the prototype of the paramixoviruses that causeNewcastle disease (ND). The virus, which causes high mortality among mature chickens and chicks,infects the respiratory tract, nerves, or intestines. NDV mainly expresseshemagglutinin-neuramindase and fusion (F) glycoproteins. In many countries, ND is a catastrophicproblem for the poultry industry.Citation1 Although thedisease is difficult to control, vaccines are available. The efficacy of NDV vaccines depends on theinduction of F glycoproteins.Citation2 Traditional NDVvaccines include two types: inactivated vaccines and attenuated live vaccines.Citation3 Both of these traditional types of vaccines have important limitations,including reversion to virulence and induction of respiratory pathological changes. In addition, thedifficulty in differentiating between vaccinated chickens and naturally infected chickenscomplicates diagnosis.
In research by Robinson et al the injection of chickens with plasmid DNA provided a new way toprotect chickens from lethal influenza viruses.Citation4 Inthe same year, Fynan et alCitation5 reported that theinclusion of gold-encapsulated plasmid particles in influenza virus vaccines reduced the requirementfor DNA in the vaccines to 0.4 pg, which was 1/250th of the amount reported formerly. Reducing theDNA content was important because DNA-based vaccines can induce long-term cellular and humoralimmune reactions in animals and humans.Citation6–Citation8
DNA vaccines have not been widely used for several other reasons. Some studies have shown thatthe vaccines, which are usually administered via intramuscular (IM) injection, can fail to reach theantigen-presenting cells and therefore fail to induce immune responses because of difficulty incrossing cell membranes.Citation9–Citation11 Sun et al reported that effective immunization oflarge animals required large amounts of DNA.Citation12Researchers have recently suggested several measures that could increase the efficacy of DNAvaccines. These measures include plasmid DNA optimization, improvement of delivery methods, thetargeting of the antigen-presenting cells, and the use of immunologic adjuvants.Citation13,Citation14
Recent research has indicated that polymeric nanoparticles (NPs) can be used as potent adjuvantsas part of a “nano” mucosal immune delivery system. NPs are biodegradable andbiocompatible, have low toxicity, and protect the antigen or DNA from damage.Citation15–Citation17 Amongall the polymers, polyesters based on polylactic acid, polyglycolic acid, and their copolymers,poly(lactic-co-glycolic) acids (PLGAs), have attracted the most attention and have been used ascarriers for a wide range of vaccines.Citation18–Citation21
PLGA is authorized by the US Food and Drug Administration (FDA), and PLGA NPs (microspheres) havebeen thoroughly studied as a protein or DNA vaccine mucosal delivery system that protects theencapsulated protein or DNA vaccine from enzyme digestion and that extends the release time of theprotein or DNA vaccine.Citation22,Citation23 A number of studies have reported improved antibody responses whenantigens are orally administered in PLGA particles.Citation24–Citation27 Nevertheless, PLGA NPs havelimited use in mucosal vaccination because of their poor mucoadhesivity and immunoenhancing ability.In recent years, chitosan (CS) has been used as a coating material for PLGA NPs because of itsbiological adhesive properties and ability to improve the immunological response to mucosalvaccination.Citation28,Citation29 By modifying the surface of PLGA NPs, CS provides the followingadvantages: 1) it decreases the burst release of the encapsulated protein or DNA; 2) it increasesthe stability of biological macromolecules; 3) it enhances the inversion of Zeta potential, andpromotes cellular adhesion and retention of the delivery system at the target site; and 4) it offersthe possibility of conjugating targeting ligands to free amino groups on its surface.Citation30 Budhian et al reported that coating PLGA NPs with CSreduced the burst release of haloperidol from 70% to 36%.Citation31 Tahara et al have also successfully developed gene delivery vectors using CS surfacemodification of PLGA NPs.Citation32,Citation33
In this study, we prepared CS-coated PLGA NPs containing the F gene plasmid DNA of NDV(pFDNA-CS/PLGA-NPs) and assessed the ability of the preparation to induce immune responses andprotect specific pathogen-free (SPF) chickens from ND after intranasal (IN) administration. We alsoassessed the bioactivity and safety of the pFDNA-CS/PLGA-NPs with in vitro expression and cellcytotoxicity assays.
Materials and methods
Preparation of the pFDNA-CS/PLGA-NPs
The eukaryotic expression plasmid pVAX I-opti F DNA, which contains the F gene of NDV, wasencapsulated in PLGA NPs (pFNDV-PLGA-NPs) by a water/oil/water double emulsion-solvent evaporationmethod.Citation34 Previous research indicated that theoptimal conditions for preparation of these NPs were 50 watts (w) for 30 seconds for the primaryemulsion, 50 w for 60 seconds for the secondary emulsion, a DNA:PLGA ratio of 0.5:100, andcombination of 40 mg/mL PLGA with 2.0% polyvinyl alcohol (PVA).Citation35 Accordingly, pFDNA-CS/PLGA-NPs were prepared in three main steps.First, 40 mg of PLGA was dissolved in 1 mL of methylene chloride (oil phase), and 800 μg ofthe DNA solution was added with the primary emulsion. Second, 2 mL of 2% PVA and the secondaryemulsion (W2) were added to the primary emulsion; the resulting compound emulsion(primary and secondary emulsions) was added in drops to the CS solutions, which contained 15 mL of0.5% PVA, and the preparation was shaken at 500 r/min for 5 hours. Third, the CS/PLGA NPs containingpFDNA were recovered by centrifugation (4,500 r/min, 10 minutes, 4°C), washed three timeswith sterilized deionized water, centrifuged, and freeze-dried. The resulting NPs were referred toas pFDNA-CS/PLGA-NPs.
Characterization of the pFDNA-CS/PLGA-NPs
We determined the effects of CS concentration (0, 0.2, 0.4, 0.6, 0.8, and 1.0 mg/mL) and CSvolume (0, 1.5, 3.0, 5.0, and 7.5 mL) on the characteristics of the pFDNA-CS/PLGA-NPs, respectively.Encapsulation efficiency (EE) was measured as previously described.Citation3 The morphological and surface characteristics of the pFDNA-CS/PLGA-NPswere examined by JEM-200EX transmission electron microscopy (Hitachi Ltd., Tokyo, Japan). Theparticle size and Zeta potentials were measured using a Zetasizer 2000 from Malvern Instruments(Malvern, UK).
In vitro release of the pFDNA-CS/PLGA-NPs
An in vitro release assay was carried out to determine the release of plasmid DNA from theNPs.Citation3 Briefly, samples were periodically collected(after 0, 6, 12, 24, 36, 48, 72, 96, 120, 144, 168, 192, 216, and 240 hours) and centrifuged at10,000 r/min for 10 minutes at 4°C. The collected pFDNA-CS/PLGA-NPs were counted. Allexperiments were performed five times.
In vitro transfection and western blot analysis of the pFDNA-CS/PLGA-NPs
293T cells were grown in poly-lysine-treated 6-well plates and cultured at 37°C in aCO2 incubator. An in vitro transfection experiment was carried out according to theinstructions from the Lipofectamine™ 2000 reagent kit (Invitrogen™; LifeTechnologies Corp, Carlsbad, CA, USA), using four groups: 1) the naked plasmid DNA group; 2) thepFDNA-CS/PLGA-NP transfected group; 3) the blank CS/PLGA-NP group; and 4) the negative cell controlgroup. Western blot analysis was carried out as previously described.Citation36 Briefly, after 72 hours of transfection, the 293T cells werecollected and disrupted using radioim-munoprecipitation assay solution (50 mmol/L Tris-HCl[pH 8.5], 5 mmol/L 2-hydroxy-1-ethanethiol, 100 mmo1/L KCl, 1 mmol/Lphenylmethanesulfonyl fluoride, and 1% Nonidet P-40 (octylphenoxypolyethoxyethanol). The lysate wascentrifuged at 14,000 r/min at 4°C, and the supernatant was subjected to sodium dodecylsulfate polyacrylamide gel electrophoresis. Proteins were transferred to a nitrocellulose membrane(Amersham; GE Healthcare, Little Chalfont, UK) using a BioRad semidry unit. The membrane was washedwith phosphate-buffered saline (PBS), blocked with 5% fat-free milk overnight, and then incubatedwith an NDV-positive serum at a 1:500 dilution for 1 hour. After the membrane was washed three timeswith PBS with Tween® (phosphate buffered saline with Tween-20), fluoresceinisothiocyanate labeled goat anti-chicken secondary antibody was added at a dilution of 1:5,000 for 1hour. The image was acquired with an Odyssey infrared imaging system (LI-COR Odyssey; LI-CORBiosciences Inc., Lincoln, NE, USA).
The safety of pFDNA-CS/PLGA-NPs
Cell Counting Kit-8 reagent was used to evaluate the in vitro cytotoxicity, and optical densityat 450 nm (OD450) was measured to determine the survival rate of 293T cells. For the invivo assay, 30 4-week-old SPF chickens from the Harbin Veterinary Research Institute LaboratoryAnimal Center were randomly assigned to two groups. The in vitro and in vivo cytotoxicity of thepFDNA-CS/PLGA-NPs were evaluated as previously described.Citation36 Chickens in one group were immunized, by IN route, with 0.2 mL of the pFDNA-CS/PLGA-NPs,which contained a total of 200 μg of plasmid DNA. Chickens in the other group wereimmunized, by IM route, with 0.2 mL of the naked plasmid DNA. The chickens were continuouslyobserved for 21 days, and any abnormalities were noted.
Immunization of SPF chickens
A total of 120 4-week-old SPF chickens were randomly assigned to six groups. Group 1 was treatedwith PBS buffer IM. Groups 2 and 3 were treated with blank CS/PLGA-NPs IM or IN, respectively. Group4 was treated with 200 μg of naked plasmid DNA (0.1 mL) IM. Groups 5 and 6 were treated with0.2 mL pFDNA-CS/PLGA-NPs (containing 200 μg of plasmid DNA) IM or IN, respectively. Alltreatments were repeated 14 days later.
Detection of immunoglobulin antibody in the serum of immunized chickens
Blood samples were collected from the wing veins of the six groups of chickens at 7, 14, 21, 28,35, and 42 days after the first treatment. The serum was obtained by centrifugation at 3,000 r/minfor 10 minutes at 4°C. Enzyme-linked immunosorbent assay (ELISA) was performed to assess thetiters of the NDV-specific immunoglobulin G (IgG) in the sera, using a NDV IgG ELISA Kit (RapidbioCo., Ltd., West Hills, CA, USA) according to the instruction manual.
Detection of IgA antibody in mucosa extracts of immunized chickens
Serum, tears, bile, and tracheal fluid were collected from two chickens from each of the sixgroups of treated (immunized) chickens, at 7, 14, 21, 28, 35, and 42 days after the first treatment.IgA antibody was evaluated with a NDV IgA ELISA Kit.
Lymphocyte proliferation in immunized chickens
To assess the cell-mediated immune responses of immunized chickens at 14, 28, and 42 days afterthe first treatment, we measured lymphocyte proliferation with the3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide colorimetric assay, as previouslydescribed.Citation37 This was done with ten chickens fromeach of the six groups. The stimulation index (SI) was determined using the following formula:Citation38(1)
where T is the mean value (number of OD at 570 nm) of the test group (pFDNA-CS/PLGA-NPs IN,pFDNA-CS/PLGA-NPs IM, pVAX I-opti F IM, blank CS/PLGA-NPs IN, or blank CS/PLGA-NPs IM), andOD570 C is the mean value of the control (PBS) group.
Protection against NDV strain F48E9
The ability of pFDNA-CS/PLGA-NPs to protect chickens against NDV strainF48E9 was determined. Strain F48E9 is highly virulent(mean death time ≤60 hours, intracerebral pathogenicity index >1.6). Protection wasdetermined as previously described.Citation3 Briefly, whenthe level of ND serum antibody of every immune group increased to 6.0 log 2, eight chickens wereselected at random from the six groups and infected by IM route with 0.1 mL of the highly virulentNDV strain F48E9, for challenge studies with a viral titer of 104.5egg infectious dose (EID)50/0.1 mL. Clinical signs of disease and mortality weremonitored on a daily basis, and continuously observed for 14 days. The infected chickens andcorresponding negative control chickens were euthanized, and the glandular stomach, duodenum, andbursa of Fabricius were collected for examination by histological staining.
Statistical analysis
Unless noted otherwise, all experiments were repeated three times, and each value was measured intriplicate. Data are presented as means ± standard deviation. Means were compared usingone-sided Student’s t-tests. Differences were considered to bestatistically significant at P<0.05.
Results
Preparation and characterization of the pFDNA-CS/PLGA-NPs
Based on EE, the optimal CS concentration and volume for preparation of pFDNA-CS/PLGA-NPs was 0.6mg/mL () and 1.5 mL (), respectively. With this concentration and volume, the mean EEwas about 98.7%. The pFDNA-CS/PLGA-NPs were spheres with smooth surfaces. They did not aggregate,and they did not suffer from subsidence damage (). The mean diameter was 699.1±5.21 nm, particle-size dispersity was 0.005 (), and Zeta potential was 6.35±2.75 mV(). Using the optimal CS concentration of0.6 mg/mL and volume of 1.5 mL, we prepared and assessed five independent batches ofpFDNA-CS/PLGA-NPs. The EE values did not significantly differ (P>0.05)among the five batches, indicating that the preparation procedure was reproducible and reliable.
Table 1 Encapsulation efficiency and diameter of the pFDNA-CS/PLGA-NPs as affected by chitosanconcentration
Table 2 Encapsulation efficiency of the pFDNA-CS/PLGA-NPs as affected by chitosan volume
Figure 1 Transmission electron micrographs of the pFDNA-CS/PLGA-NPs at (A) 1,000×magnification and (B) 3,000× magnification.
Abbreviation: pFDNA-CS/PLGA-NPs, chitosan-coated Newcastle disease virus F geneencapsulated in poly(lactic-co-glycolic) acid nanoparticles.
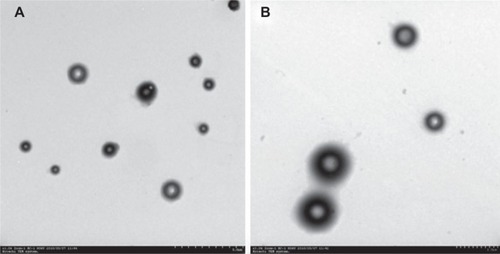
Figure 2 Size and Zeta potential of the pFDNA-CS/PLGA-NPs.
Notes: (A) Size distribution. The mean (± SD) diameter was699.1±5.21 nm. (B) The Zeta potential was 6.35±2.75 mV.
Abbreviations: pFDNA-CS/PLGA-NPs, chitosan-coated Newcastle disease virus F geneencapsulated in poly(lactic-co-glycolic) acid nanoparticles; SD, standard deviation.
In vitro release of the pFDNA-CS/PLGA-NPs
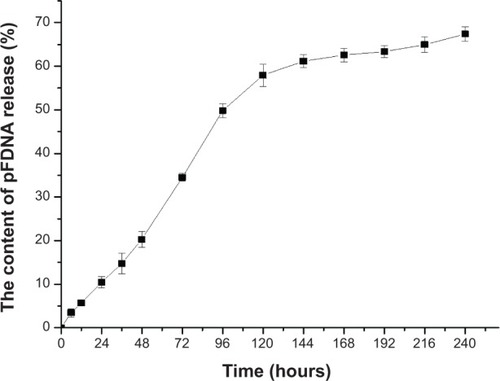
The plasmid DNA encapsulated in the CS/PLGA-NPs was gradually released throughout the assay,although the release was faster during the first 120 hours than during the subsequent 120 hours(). The rate of release was relatively slowand sustained, apparently because the CS reduced desorption and diffusion.
Figure 3 In vitro release profiles of the plasmid DNA pVAX I-opti F from pFDNA-CS/PLGA-NPs.
Note: Values are expressed as means ± SD (n=3).
Abbreviations: pFDNA-CS/PLGA-NPs, chitosan-coated Newcastle disease virus F geneencapsulated in poly(lactic-co-glycolic) acid nanoparticles; SD, standard deviation; n, number oftest; pVAX I-optiF, eukaryotic expression plasmids.
In vitro expression of the pFDNA-CS/PLGA-NPs
Specific fluorescence was detected in the naked plasmid DNA group () and the pFDNA-CS/PLGA-NP transfected group () but not in the blank CS/PLGA-NPs group () or in the negative cell control group (). Expression of the antigen was furtherdemonstrated by western blot (), whichindicated that the expected 58 kDa antigen was expressed in 293T cells transfected withpFDNA-CS/PLGA-NPs and naked plasmid DNA but not in cells treated with the blank CS/PLGA-NPs or inthe negative cell control group. These results proved that the pFDNA-CS/PLGA-NPs could express andprotect the antigen in vitro.
Figure 4 In vitro expression of the pFDNA-CS/PLGA-NPs in 293T cells as indicated by indirectimmunofluorescence analysis at ×40 (A–D) and westernblotting (E).
Notes: (A) The naked plasmid DNA group; (B)pFDNA-CS/PLGA-NP-transfected group; (C) blank CS/PLGA-NP group; and (D)293T cell group as the negative control. (E): From left to right are lanes: 1, M, 2, 3,4. Lane 1: naked plasmid DNA group. M: protein marker. Lane 2: 293T cells as the negative control.Lane 3: pFDNA-CS/PLGA-NP-transfected group. Lane 4: blank CS/PLGA-NP group.
Abbreviations: CS/PLGA-NP, chitosan-coated poly(lactic-co-glycolic) acidnanoparticle; pFDNA-CS/PLGA-NP, chitosan-coated Newcastle disease virus F gene encapsulated inpoly(lactic-co-glycolic) acid nanoparticle.
Safety of the pFDNA-CS/PLGA-NPs
The survival rate of 293T cells was 85.14%±8.13% when treated with pFDNA-CS/PLGA-NPs andwas 84.72%±6.04% for nontreated control cells; the difference was not statisticallysignificant (P>0.05). Cell morphology was similar for cells in thepFDNA-CS/PLGA-NPs and control groups. Chickens immunized with either the pFDNA-CS/PLGA-NPs or thenaked plasmid DNA did not exhibit nervous signs, clinical symptoms, or necropsy lesions within the 3weeks following treatment. These in vitro and in vivo results showed that the pFDNA-CS/PLGA-NPs weresafe.
Immunization of SPF chickens with pFDNA-CS/PLGA-NPs
IgG antibody in serum
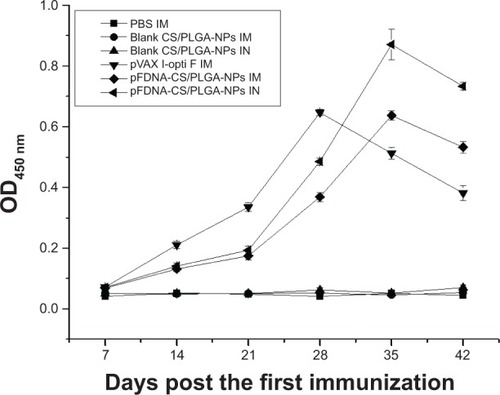
IgG antibody titers in sera peaked on day 28 for chickens immunized IM with naked plasmid DNA butpeaked on day 35 for chickens immunized IM or IN with pFDNA-CS/PLGA-NPs; the titer on day 35 and day42 was greater (P<0.05) with pFDNA-CS/PLGA-NPs than with naked plasmid DNA(). IgG antibody titer did not increase inchickens treated with PBS alone or with blank CS/PLGA-NPs.
Figure 5 IgG antibody titers in the serum of SPF chickens immunized with PBS (IM), blank CS/PLGA-NPs (IM),blank CS/PLGA-NPs (IN), pVAX I-opti F (the naked plasmid DNA) (IM), pFDNA-CS/PLGA-NPs (IM), andpFDNA-CS/PLGA-NPs (IN).
Note: Values are expressed as mean ± SD (n=5).
Abbreviations: CS/PLGA-NPs, chitosan-coated poly(lactic-co-glycolic) acidnanoparticles; Ig, immunoglobulin; IM, intramuscular; IN, intranasal; OD450, opticaldensity at 450 nm; PBS, phosphate buffered saline; pFDNA-CS/PLGA-NPs, chitosan-coated Newcastledisease virus F gene encapsulated in poly(lactic-co-glycolic) acid nanoparticles; SPF, specificpathogen-free; SD, standard deviation; pVAX I-optiF, eukaryotic expression plasmids.
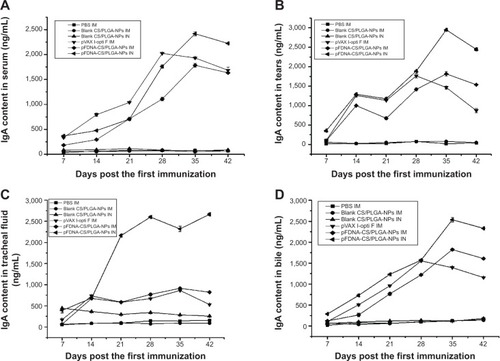
IgA antibody in mucosa extracts
The IgA antibody content was significantly higher (P<0.05) in thepFDNA-CS/PLGA-NPs IN group than in the other groups, for tears (), tracheal fluid (), and bile (). In serum,the IgA antibody content did not differ between the naked plasmid DNA IM group and thepFDNA-CS/PLGA-NPs IN group during day 7 to day 28 () but was higher in the latter group on day 35 and day 42. These results indicated that thepFDNA-CS/PLGA-NPs induced a stronger and more sustained mucosal immune response than the nakedplasmid DNA.
Figure 6 IgA antibody content in serum (A), tears (B), tracheal fluid(C), and bile (D) of SPF chickens immunized with PBS (IM), blankCS/PLGA-NPs (IM), blank CS/PLGA-NPs (IN), pVAX I-opti F (the naked plasmid DNA) (IM),pFDNA-CS/PLGA-NPs (IM), and pFDNA-CS/PLGA-NPs (IN).
Note: Values are expressed as mean ± SD (n=5).
Abbreviations: CS/PLGA-NPs, chitosan-coated poly(lactic-co-glycolic) acidnanoparticles; Ig, immunoglobulin; IM, intramuscular; IN, intranasal; PBS, phosphate bufferedsaline; pFDNA-CS/PLGA-NPs, chitosan-coated Newcastle disease virus F gene encapsulated inpoly(lactic-co-glycolic) acid nanoparticles; SPF, specific pathogen-free; SD, standard deviation; n,number of test; pVAX I-optiF, eukaryotic expression plasmids.
Lymphocyte proliferation
At 42 days after the first treatment, the SI was significantly higher(P<0.01) for chickens immunized with pFDNA-CS/PLGA-NPs (either IM or IN)than with the plasmid DNA or with blank CS/PLGA-NPs. The SI was significantly higher(P<0.05) with IN than with IM application of pFDNA-CS/PLGA-NPs. The resultsshowed that the pFDNA-CS/PLGA-NPs significantly enhanced the immune function of T lymphocytes ().
Table 3 The stimulation index of T lymphocyte proliferation in SPF chickens after immunization
Protection against NDV strain F48E9
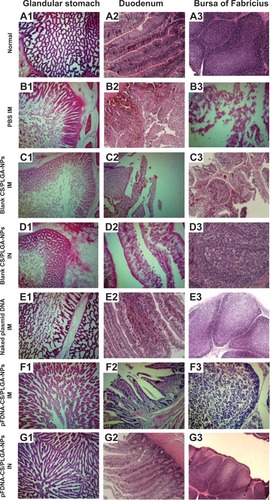
Within 2 to 5 days, the highly virulent NDV strain F48E9 had killed allchickens treated with PBS or blank CS/PLGA-NPs (). In contrast, strain F48E9 killed only 12.5%, 0%, and 50% of thechickens treated with pFDNA-CS/PLGA-NPs IM, pFDNA-CS/PLGA-NPs IN, and the naked plasmid DNA,respectively (). Feeding, drinking, andother aspects of behavior were normal for chickens treated with pFDNA-CS/PLGA-NPs IM and IN but weresomewhat abnormal for chickens treated with the naked plasmid DNA. Pathological andhistopathological changes were not evident in chickens treated with pFDNA-CS/PLGA-NPs IN (), but small histopathological changes wereevident in chickens treated with pFDNA-CS/PLGA-NPs IM () and naked plasmid DNA (). Histopathological changes typical of ND were evident in chickens treated with PBS andblank CS/PLGA-NPs (). Thesefindings demonstrated that treatment with the pFDNA-CS/PLGA-NPs IN induces an effective mucosalimmune response and protects chickens against ND.
Table 4 Protection of the immunized SPF chickens after challenge with the highly virulent NDV strainF48E9
Figure 7 Histopathology of the glandular stomach, duodenum, and bursa of Fabricius of normal chickens(A) and of chickens challenged with the highly virulent NDV strainF48E9 after treatment with PBS IM (B); blank CS/PLGA-NPs IM(C); blank CS/PLGA-NPs IN (D); the naked plasmid DNA IM (E);pFDNA-CS/PLGA-NPs IM (F); or pFDNA-CS/PLGA-NPs IN (G).
Notes: Tissues are indicated above the three columns of micrographs, and treatmentsare indicated on the left side of each row of micrographs. (A1–A3) normal tissues of theglandular stomach, duodenum, and bursa of Fabricius; (B1, C1, D1, E1, F1 and G1) tissues of theglandular stomach PBS (IM), blank CS/PLGA (IM), blank CS/PLGA (IN), and the naked plasmid DNA (IM),pFDNA-CS/PLGAs (IM), pFDNA-CS/PLGAs (IM); (B2, C2, D2, E2, F2 and G2) tissues of the duodenum PBS(IM), blank CS/PLGA (IM), blank CS/PLGA (IN), and the naked plasmid DNA (IM), pFDNA-CS/PLGA (IM),pFDNA-CS/PLGA (IN); (B3, C3, D3, E3, F3 and G3) tissues of the bursa of Fabricius PBS (IM), blankCS/PLGA (IM), blank CS/PLGA (IN), and the naked plasmid DNA (IM), pFDNA-CS/PLGA (IM), pFDNA-CS/PLGA(IN).
Abbreviations: CS/PLGA-NPs, chitosan-coated poly(lactic-co-glycolic) acidnanoparticles; IM, intramuscular; IN, intranasal; PBS, phosphate-buffered saline; pFDNA-CS/PLGA-NPs,chitosan-coated Newcastle disease virus F gene encapsulated in poly(lactic-co-glycolic) acidnanoparticles; NDV, Newcastle disease virus.
Discussion
Polymeric NPs have been widely used to deliver drugs because they can increase drug stability,are easily absorbed by cells, have good adhesive properties, are able to target specific organs, andcan bind ligands on their surfaces.Citation39 Inparticular, biodegradable NPs are available that deliver medicines to target sites and that are thendegraded after passing the target site.Citation40 Wetherefore determined whether biodegradable polymers could be used to deliver newly developed DNAvaccines. Zhao et al previously reported that pFNDV-PLGA-NPs that were prepared with awater/oil/water double emulsion-solvent evaporation method did not change the encapsulated plasmidDNA but promoted the sustained release of the plasmid DNA and induced stronger mucosal immuneresponses than for nonencapsulated plasmid DNA.Citation35Because other researchers showed that CS NPs could sustain release and stabilize the plasmidDNA,Citation41 in the current study, we investigatedwhether CS could improve the performance of PLGA-NPs.
In our first experiments, we found that the EE was highest when NPs were prepared with a CSvolume of 1.5 mL and a CS concentration of 0.6 mg/mL. The EE of NPs determines the effectiveness ofthe gene delivery and subsequent expression of encoded genes in vitro and in vivo.Citation42 In this study, the use of 1.5 mL of CS at 0.6 mg/mLof CS produced an EE of 98.7%, which was higher than the previously reported EE for thepFNDV-PLGA-NPs without CS.Citation35 When CS was used atthis volume and concentration, the resulting pFD-NA-CS/PLGA-NPs had an appropriate size andmaintained the biological activity of the encapsulated plasmid DNA. In addition to not aggregating,the pFDNA-CS/PLGA-NPs had a core–shell structure, a diameter of 699.1±5.21 nm, goodstability, a Zeta potential of 6.35±2.75 mV, and a polydispersity index of 0.005.
We also conducted an in vitro assay of the release of the DNA encapsulated in pFDNA-CS/PLGA-NPs.Hu et alCitation43 showed that pH could influence therelease of plasmid DNA from NPs and that NPs are more swollen at pH 7.0–7.4 than at pH 4.0.Thus, our assay used pFDNA-CS/PLGA-NPs that were swollen in PBS at pH 7.4. The results of the assayrevealed a gradual and sustained release of DNA from the pFDNA-CS/PLGA-NPs, suggesting a strongphysical interaction between the drug and polysaccharide layer on the NPs’ surface. The CSslightly reduced the DNA burst release effect in the first 24 hours, indicating that the CS layersurrounding the PLGA-NPs acts as a physical barrier that slows the release of DNA from thePLGA-NPs.
The cellular uptake of the NPs was visualized by indirect immunofluorescence. The in vitro celltoxicity and expression studies indicated that the pFDNA-CS/PLGA-NPs did not damage cells andmaintained DNA bioactivity. The survival rate was higher than the previously reported 80.14% forpFNDV-PLGA-NPs without CS.Citation35 In vivo cytotoxicityassay showed that the pFDNA-CS/PLGA-NPs did not have pathological effects on chickens. Overall, theresults showed that the DNA in the NPs was not altered and that the NPs containing DNA and coatedwith CS would not harm chickens.
Analyses of IgG and IgA antibody responses revealed that IN immunization with pFDNA-CS/PLGA-NPsinduced stronger responses than immunization with naked plasmid DNA. The IgA antibody contents ofgroup pFDNA-CS/PLGA-NPs IN were much higher than the previously reported 800–1,000 ng/mL(P<0.05).Citation35 INimmunization was very effective in eliciting mucosal and systemic immune responses.Citation44,Citation45Our findings suggest that the nasal administration of pFDNA-CS/PLGA-NPs is effective in inducing theimmune response against ND, probably because the CS coating changes the surface charge of thePLGA-NPs and prolongs the antigen contact time with mucosal surfaces.Citation46
In summary, our comparison of the pFDNA-CS/PLGA-NPs and naked plasmid DNA showed that theimmunogenicity and protective immunity can be improved by encapsulating the plasmid DNA intoCS-coated PLGA NPs.
Although this study demonstrates the potential of CS-modified PLGA NPs as an efficient deliverysystem for NDV DNA vaccine in mucosal immunization, a number of challenges must be addressed. First,the trace amounts of initiator, toxic organics, and other impurities in the polymer must be removedbefore a vaccine is commercialized. Toxic solvent remaining in the natural polymer during NPpreparation must also be removed. Second, the cost of preparation must be reduced. Finally, thecontrolled and targeted release of NPs must be improved. We suspect that these challenges can be metby technological advancements in the biomedical and material sciences.
Acknowledgments
We gratefully acknowledge the Key Laboratory of Functional Inorganic Material Chemistry(Heilongjiang University), the Ministry of Education and Engineering Research Center of AgriculturalMicrobiology Technology, and the Ministry of Education for providing the facilities to carry outthis work. This work was supported, in part, by the National Natural Science Foundation of thePeople’s Republic of China (grant number 31072119), the Key Project of the Chinese Ministryof Education (grant number 212048), the Program for New Century Excellent Talents in University(grant number NCET-12-0707), the Innovative Research Team for Agricultural Microbiology FermentationTechnology at Heilongjiang Provincial University (grant number 2012td009), the Changjiang ScholarCandidates Program for Provincial Universities in Heilongjiang (grant number 2014CJHB005), theScientific and Technological Key Project of Heilongjiang Province (GC13B403), the Early Research andDevelopment Cultivation Project of Scientific and Technological Achievements Industrialization forProvincial Universities in Hei-longjiang (grant number 1253CGZH10), and the Innovation Foundation ofHarbin (grant number 2013RFQXJ030).
Disclosure
The authors report no conflicts of interest in this work.
References
- MeulemansGGonzeMCarlierMCPetitPBurnyALongLProtective effects of HN and F glycoprotein-specific monoclonalantibodies on experimental Newcastle diseaseAvianPathol198615476176818766577
- ArifinMAMelMAbdul KarimMIIderisAProduction of Newcastle disease virus by Vero cells grown on cytodex 1microcarriers in a 2-litre stirred tank bioreactorJ BiomedBiotechnol2010201058636320625497
- ZhaoKZhangYZhangXPreparation and efficacy of Newcastle disease virus DNA vaccineencapsulated in chitosan nanoparticlesInt JNanomedicine2014938940224426783
- RobinsonHLHuntLAWebsterRGProtection against a lethal influenza virus challenge by immunizationwith a haemagglutinin-expressing plasmidDNAVaccine19931199579608212843
- FynanEFWebsterRGFullerDHHaynesJRSantoroJCRobinsonHLDNA vaccines: protective immunizations by parenteral, mucosal, andgene-gun inoculationsProc Natl Acad Sci U SA1993902411478114828265577
- DonnellyJJWahrenBLiuMADNA vaccines: progress and challengesJImmunol2005175263363916002657
- HallengärdDHallerBKPeterssonSIncreased expression and immunogenicity of HIV-1 protease followinginactivation of the enzymaticactivityVaccine201129483984821109032
- XuKLingZYSunLBroad humoral and cellular immunity elicited by a bivalent DNA vaccineencoding HA and NP genes from an H5N1 virusViralImmunol2011241455621319978
- PachukCJMcCallusDEWeinerDBSatishchandranCDNA vaccines – challenges indeliveryCurr Opin MolTher20002218819811249641
- RobertsonJSGriffithsEAssuring the quality, safety, and efficacy of DNAvaccinesMolBiotechnol200117214314911395863
- WuHDennisVAPillaiSRSinghSRRSV fusion (F) protein DNA vaccine provides partial protection againstviral infectionVirusRes20091451394719540885
- SunJHouJLiDEnhancement of HIV-1 DNA vaccine immunoge-nicity by BCG-PSN, a noveladjuvantVaccine201331347247923174201
- ManojSBabiukLAvan Drunen Littel-van den HurkSApproaches to enhance the efficacy of DNAvaccinesCrit Rev Clin LabSci200441113915077722
- SunJLiDHaoYPosttranscriptional regulatory elements enhance antigen expression andDNA vaccine efficacyDNA CellBiol200928523324019388846
- CsabaNGarcia-FuentesMAlonsoMJThe performance of nanocarriers for transmucosal drugdeliveryExpert Opin DrugDeliv20063446347816822222
- MoghimiSMKisselTParticulate nanomedicinesAdv Drug DelivRev200658141451145517081650
- LiangMTDaviesNMBlanchfieldJTTothIParticulate systems as adjuvants and carriers for peptide and proteinantigensCurr DrugDeliv20063437938817076640
- HsuSHChanSHChiangCMChenCCJiangCFPeripheral nerve regeneration using a microporous polylactic acidasymmetric conduit in a rabbit long-gap sciatic nerve transectionmodelBiomaterials201132153764377521396706
- SantoVEDuarteARCGomesMEManoJFReisRLHybrid 3D structure of poly(D,L-lactic acid) loaded withchitosan/chondroitin sulfate nanoparticles to be used as carriers for biomacromolecules in tissueengineeringJ SupercritFluids2010543320327
- MahmoudifarNDoranPMChondrogenic differentiation of human adipose-derived stem cells inpolyglycolic acid mesh scaffolds under dynamic cultureconditionsBiomaterials201031143858386720153043
- ThevenotPTNairAMShenJLotfiPKoCYTangLThe effect of incorporation of SDF-1alpha into PLGA scaffolds on stemcell recruitment and the inflammatoryresponseBiomaterials201031143997400820185171
- SmithDJKingWFBarnesLATrantoloDWiseDLTaubmanMAFacilitated intranasal induction of mucosal and systemic immunity tomutans streptococcal glucosyltransferase peptide vaccinesInfectImmun20016984767477311447149
- PeacockZSBarnesLAKingWFInfluence of microparticle formulation on immunogenicity of SYI, asynthetic peptide derived from Streptococcus mutans GbpBOral MicrobiolImmunol2005201606415612949
- WierzbickiAKiszkaIKanekoHImmunization strategies to augment oral vaccination with DNA and viralvectors expressing HIV envelopeglycoproteinVaccine2002209–101295130711818148
- GarinotMFiévezVPourcelleVPEGylated PLGA-based nanoparticles targeting M cells for oralvaccinationJ ControlRelease2007120319520417586081
- Allaoui-AttarkiKFattalEPecquetSMucosal immunogenicity elicited in mice by oral vaccination withphosphorylcholine encapsulated in poly(D,L-lactide-co-glycolide)microspheresVaccine19981676856919562687
- RajkannanRDhanarajuMDGopinathDSelvarajDJayakumarRDevelopment of hepatitis B oral vaccine using B-cell epitope loadedPLGmicroparticlesVaccine200624245149515716713035
- Jabbal-GillIFisherANRappuoliRDavisSSIllumLStimulation of mucosal and systemic antibody responses againstBordetella pertussis filamentous haemagglutinin and recombinant pertussis toxin after nasaladministration with chitosan inmiceVaccine19981620203920469796062
- KawashimaYYamamotoHTakeuchiHKunoYMucoadhesive DL-lactide/glycolide copolymer nanospheres coated withchitosan to improve oral delivery of elcatoninPharm DevTechnol200051778510669921
- ChronopoulouLMassimiMGiardiMFChitosan-coated PLGA nanoparticles: a sustained drug release strategyfor cell culturesColloids Surf BBiointerfaces201310331031723261553
- BudhianASiegelSJWineyKIControlling the in vitro release profiles for a system ofhaloperidol-loaded PLGA nanoparticlesInt JPharm20083461–215115917681683
- TaharaKYamamotoHKawashimaYCellular uptake mechanisms and intracellular distributions ofpolysorbate 80-modified poly(D,L-lactide-co-glycolide) nanospheres for genedeliveryEur J PharmBiopharm201075221822420332026
- TaharaKSakaiTYamamotoHTakeuchiHHirashimaNKawashimaYImprovements in transfection efficiency with chitosan modifiedpoly(DL-lactide-co-glycolide) nanospheres prepared by the emulsion solvent diffusion method, forgene deliveryChem Pharm Bull(Tokyo)201159329830121372409
- ZhaoKLiGXJinYYPreparation and immunological effectiveness of a Swine influenza DNAvaccine encapsulated in PLGA microspheresJMicroencapsul201027217818620121488
- ZhaoKLiWHuangTPreparation and efficacy of Newcastle disease virus DNA vaccineencapsulated in PLGA nanoparticlesPLoSOne2013812e8264824386106
- ZhaoKShiXZhaoYPreparation and immunological effectiveness of a swine influenza DNAvaccine encapsulated in chitosannanoparticlesVaccine201129478549855621945253
- ZengWWangYShiXOptimization of codon usage of F gene enhanced efficacy of Newcastledisease virus DNA vaccineChin J of Anim InfectDis2009172816
- ZhaoFWuYZhangXEnhanced immune response and protective efficacy of a Treponemapallidum Tp92 DNA vaccine vectored by chitosan nanoparticles and adjuvanted withIL-2HumVaccin20117101083108921941092
- LöbenbergRAraujoLKreuterJBody distribution of azidothymidine bound to nanoparticles after oraladministrationEur J PharmBiopharm1997442127132
- BelbellaAVauthierCFessiHDevissaguetJPPuisieuxFIn vitro degradation of nanospheres from poly(D,L-lactides) ofdifferent molecular weights and polydispersitiesInt JPharm19961291–295102
- KawashimaYHandaTKasaiATakenakaHLinSYAndoYNovel method for the preparation of controlled-release theophyllinegranules coated with a polyelectrolyte complex of sodiumpolyphosphate-chitosanJ PharmSci19857432642684009432
- BoyogluSVigKPillaiSEnhanced delivery and expression of a nanoencapsulated DNA vaccinevector for respiratory syncytialvirusNanomedicine20095446347219341819
- HuYJiangXDingYGeHYuanYYangCSynthesis and characterization of chitosan-poly(acrylic acid)nanoparticlesBiomaterials200223153193320112102191
- XuJDaiWWangZChenBLiZFanXIntranasal vaccination with chitosan-DNA nanoparticles expressingpneumococcal surface antigen a protects mice against nasopharyngeal colonization by StreptococcuspneumoniaeClin VaccineImmunol2011181758121047997
- CrippsAWKydJMComparison of mucosal and parenteral immunisation in two animal modelsof pneumococcal infection: otitis media and acutepneumoniaVaccine200725132471247717110001
- YamamotoHKunoYSugimotoSTakeuchiHKawashimaYSurface-modified PLGA nanosphere with chitosan improved pulmonarydelivery of calcitonin by mucoadhesion and opening of the intercellular tightjunctionsJ ControlRelease2005102237338115653158
