Abstract
Systemic lupus erythematosus (SLE) is a classic antibody-mediated systemic autoimmune disease characterised by the development of autoantibodies to ubiquitous self-antigens (such as antinuclear antibodies and antidouble-stranded DNA antibodies) and widespread deposition of immune complexes in affected tissues. Deposition of immune complexes in the kidney results in glomerular damage and occurs in all forms of lupus nephritis. The development of nephritis carries a poor prognosis and high risk of developing end-stage renal failure despite recent therapeutic advances. Here we review the role of DNA-anti-DNA immune complexes in the pathogenesis of lupus nephritis and possible new treatment strategies aimed at their control.
Introduction
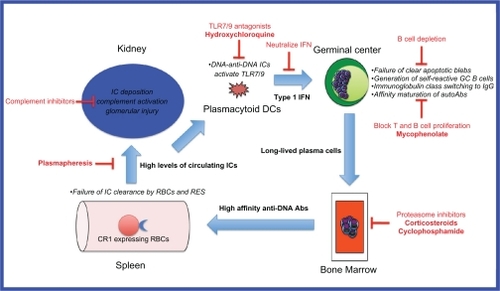
Systemic lupus erythematosus (SLE) is a complex, heterogeneous disease of multi-factorial etiology where multiple genetic, environmental and sex hormonal influences converge to break down B cell tolerance to self-antigens normally sequestered inside the cell nucleus.Citation1 Recent insights obtained from genetic mouse models and genome-wide association scans in large patient cohorts have enabled the identification of several key players in the multistep pathogenesis of lupus (). These studies reveal a positive feedback loop whereby inefficient clearance of apoptotic blebs by macrophages results in positive selection of germinal center B cells, which have self-reactivity against nuclear antigens exposed on these blebs. These self-reactive B cells undergo T cell-dependent affinity maturation and isotype switching,Citation2 and differentiate into long-lived plasma cells which reside in the bone marrow. The high affinity IgG anti-DNA antibodies secreted by these cells bind to the DNA to form immune complexes which activate plasmacytoid dendritic cells (pDCs) via toll-like receptor- (TLR-) 9 to produce inflammatory cytokines such as interferon-alpha. These cytokines augment the humoral immune response and lead to further autoantibody production. The high levels of circulating DNA-anti-DNA immune complexes overwhelm the capacity of the reticuloendothelial system (RES) to clear them, and they are deposited in various tissues including glomeruli where local complement activation results in glomerular injury.Citation3
Figure 1 Model of DNA-anti-DNA immune complex generation and glomerular damage in lupus nephritis and potential therapeutic targets.
Nephritis is a common complication of SLE, occurring in 14% to 55% of patients, with higher rates seen in Asian, African, and Hispanic populations.Citation4 Histological patterns of lupus nephritis have been classified by the World Health Organization and, more recently, by the International Society of Nephrology/Renal Pathology Society (ISN/RPS) ().Citation5 These histologic patterns are predictive of prognosisCitation6 and provide a basis for treatment guidelines to prevent end-organ damage and improve mortality and morbidity. Despite improvements in the long-term survival of patients with SLE,Citation7 patients who develop nephritis still have a worse prognosis with a 10-year survival of only 88% compared with 94% for patients without nephritis.Citation8
Table 1 Classification of lupus nephritis
The mainstay of treatment for lupus nephritis has been corticosteroids, azathioprine, cyclophosphamide and, more recently, mycophenolate. These drugs are toxic with significant side effects and, despite their use, up to 20% of patients with nephritis will still progress to end-stage renal failure and require renal replacement therapy. It is timely therefore to re-examine the role of immune complexes in the pathogenesis of lupus nephritis and update the current status of new therapeutic strategies that target immune complexes.
DNA-anti-DNA immune complexes in the pathogenesis of lupus nephritis
Raised serum levels of circulating immune complexes have long been described in lupus, and correlate with disease activity.Citation9 The role of anti-DNA antibodies in lupus nephritis is also well documented, and the evidence for the involvement of complexes containing these autoantibodies is summarized in . Despite the evidence linking DNA-anti-DNA immune complexes to lupus nephritis, the precise mechanism of renal damage is still unknown. In the prevailing hypothesis, nucleosomes released from apoptotic cells bind to autoanti-bodies and deposit in glomeruli, resulting in complement activation and thus tissue injury. An alternative hypothesis is that anti-DNA antibodies cross-react with non-DNA components in glomeruli, but this is thought to be less likely.Citation10
Table 2 Evidence for role of DNA-containing immune complexes in the pathogenesis of lupus nephritis
Doubts about the importance of DNA-anti-DNA immune complexes arise because not all patients with anti-DNA antibodies develop lupus nephritis. Furthermore, glomerular immune complex deposition may be seen without clinically overt renal disease,Citation11 suggesting that additional factors are necessary for the development of renal pathology. Particular characteristics of anti-DNA antibodies may make some more nephritogenic than others. For example, it has been postulated that the isotype and subclass of the antibody is important. In particular, the IgG isotypeCitation12 and specifically the IgG3Citation13,Citation14 or IgG2Citation14 subclasses present a higher risk of clinical nephritis. Although there is some evidence that avidity of anti-dsDNA antibodies may also play a role in vitro,Citation15,Citation16 their role in vivo has been questioned.Citation10,Citation17
The specificity of anti-DNA antibodies is another important factor in pathogenicity. A specificity for nucleosomes rather than DNA,Citation10 the presence of cationic moieties that bind to negatively charged glycosaminoglycans such as heparan sulfate,Citation18 and cross-reactivity of antibodies with alpha-actininCitation19 are linked to an increased likelihood of renal pathology. Consistent with the idea of immune complex-mediated damage being central to the pathogenesis of lupus nephritis, the availability of extra-cellular chromatinCitation17 has been identified as another factor linked to the development of nephritis. Abnormalities in DNA fragmentation as a result of reduced levels of the endonuclease DNase1 have been identified in mouse models of lupus nephritis, perhaps predisposing to the deposition of chromatin in glomeruli.Citation20
Once DNA-anti-dsDNA immune complexes have been formed, they are normally cleared by the RES but defects of some of the clearance mechanisms have been described in SLE, including aberrant interactions with Fcγ receptors (FcγRs), complement and complement receptors, and anti-C1q antibodies. With respect to the first of these interactions, a particular polymorphism in FcγRIIB is associated with SLE in Asian populations.Citation21 FcγRIIB has a cytoplasmic tail which mediates inhibitory functions. Therefore, FcγRIIB signaling is important in controlling the immune response, and deficiency may predispose to autoimmunity.Citation21 The activating FcγRs are also involved in the pathogenesis of lupus nephritis. Immune complex binding to FcγRI and FcγRIII trigger monocytes and macrophages to release proinflammatory mediators and chemokines which recruit immune effector cells that contribute to renal damage.Citation22–Citation24 Increased expression of FcγRI on monocytes has been found to correlate with the presence of active lupus nephritis.Citation23 Secondly, genetic variations in C4 can affect the handling of immune complexes. Deficiency in C4A relative to C4B is common in SLE, and has also been associated with the development of lupus nephritis. C4A prevents immune complex precipitation, and therefore deficiency could result in increased deposition. A number of other variants of C4 may promote or protect against immune complex damage.Citation25 Thirdly, immune complexes that bind and activate complement can also be cleared by high affinity complement binding receptor type 1 (CR1, CD35). SLE patients have reduced expression of CR1 on their erythrocytes, perhaps contributing to defective clearance of immune complexes.Citation26 Fourthly, the presence of anti-C1q antibodies can influence the handling of immune complexes. These autoantibodies have been associated with the presence and activity of lupus nephritis.Citation27,Citation28 Infusion of anti-C1q antibodies results in deposition in glomeruli in mice, which are not pathogenic unless C1q-fixing antiglomerular basement membrane antibodies (at subnephritogenic doses) are also present.Citation29 It is thought that anti-C1q antibodies amplify the complement cascade by themselves fixing and activating complement, recruiting further anti-C1q antibodies, and thus increasing the risk of renal damage.
Immune complexes containing DNA signal via TLR-9 and can activate plasmacytoid dendritic cells,Citation30 which then process and present chromatin-derived peptides to costimulate T helper cells. Chromatin-specific T cells interact with dsDNA-specific B cells, facilitating the secretion of anti-DNA antibodies. Renal damage results from a combination of complement activation and cellular inflammation. A Th1 response is associated with diffuse proliferative lupus nephritis, while a Th2 response is associated with the membranous form.Citation31 Thrombotic microangiopathy caused by antiphospholipid antibodies may also contribute to the final pathology.
Therapies of lupus nephritis targeting immune complex formation
The mainstays of treatment of lupus nephritis are corticosteroid therapy combined with cyclophosphamide or mycophenolate for induction therapy, and corticosteroids combined with azathioprine or mycophenolate for maintenance therapy. The evidence for the use of these agents is summarized in and . These agents are not specifically targeted at the reduction of DNA-anti-DNA immune complexes per se. However, a reduction in autoantibody formation and hence immune complex generation occurs after the broad immunosuppression caused by these agents, and a decrease in serum anti-dsDNA antibody levels accompanied clinical improvement in most studies listed.
Table 3 Trials of induction therapies for lupus nephritis
Table 4 Trials of maintenance therapies in lupus nephritis. All received glucocorticoids unless otherwise specified
Specific strategies for targeting immune complex formation include: reducing autoantibody production (targeting B cells), reducing the binding of autoantibodies, reducing the availability of nucleosomal material, increasing the clearance of immune complexes, and interfering in the feedback loop (). This is a theoretical framework, and while the mechanisms of action of some of the currently used treatments for lupus nephritis may fall into these categories, further research is necessary in each of these areas to understand their mechanisms and potential clinical efficacy.
Reducing autoantibody production (targeting B cells and plasma cells)
Theoretically, autoantibody production may be reduced by depletion of B cells (either by targeting B cell surface molecules or by removing factors required for B cell survival); interfering with the development or function of plasma cells; or by inducing B-cell tolerance.
B cell depletion
Anti-nucleosome and anti-dsDNA antibodies are modestly reduced by anti-CD20 mAbs such as rituximab, which effect B cell depletion. The reduction in these titers suggests that these autoantibodies are produced by a B cell population with more rapid turnover than cells that produce anti-ENA, anti-tetanus or antipneumococcal antibodies, which persist. However, their incomplete reduction may reflect the presence of longer-lived plasma cells which do not express CD20.Citation32 Trials of rituximab, however, have yielded conflicting results on clinical endpoints (see ). Uncontrolled, observational, and retrospective studies seemed to demonstrate benefit in lupus nephritis, but two major randomized trials failed to find benefit. The LUpus Nephritis Assessment with Rituximab (LUNAR) trial, which specifically included patients with proliferative lupus nephritis, did not demonstrate any difference in the proportion of patients obtaining a renal response to rituximab compared with placebo.Citation33 However, the use of mycophenolate rather than cyclophosphamide as background therapy in this trial has been criticized, as it is thought that the effects of rituximab may be enhanced, or synergistic, with cyclophosphamide.Citation34 Further, given that all participants were treated with mycophenolate, any effect of rituximab may have been masked. The other major trial, Rituximab in patients with Severe Systemic Lupus Erythematosus (EXPLORER),Citation35 excluded major organ threatening disease and thus <2% of the patients had renal involvement. This trial found no difference in the rituximab compared with placebo-treated groups, but given its patient characteristics, this finding cannot be applied to patients with lupus nephritis. Contrary to these findings, prospective follow-up of 31 patients with lupus nephritis from a cohort of 136 patients entered in the French Autoimmunity and Rituximab registry,Citation36 demonstrated renal response in 74% of patients, with complete response in 45%. Unfortunately, trials of another anti-CD20 agent, ocrelizumab (BELONG), for lupus nephritis have been halted due to concerns over serious and opportunistic infections.Citation37
A plethora of other anti-B cell therapies is on the horizon, targeting all aspects important for B cell existence and function, such as survival factors, differentiation factors, co-stimulatory factors, cell-signaling pathways, and homing factors.Citation38 Most of these studies are in preliminary phases, or have not been evaluated in human lupus nephritis. Belimumab, a fully human recombinant monoclonal antibody that binds to and inhibits B lymphocyte stimulator (BLyS, also known as B cell activating factor or BAFF) has been shown to reduce anti-dsDNA titers by 29%, but patients with lupus nephritis were excluded from early trials.Citation39,Citation40 More recently, the large phase III studies, BLISS-52 and BLISS-76, have shown promise with improvements in the SLE responder index, though more information on lupus nephritis and belimumab is awaited. The Food and Drug Administration has granted this drug a priority review designation as a potential treatment for SLE (GSK press release August 19, 2010). Epratuzumab, targets CD22, a surface molecule involved in regulating B cell receptor signaling, and modifies B cell function. A phase II study has had promising results, with improvements in BILAG scores despite lack of reduction in anti-dsDNA levels, although there were too few patients with lupus nephritis to draw any conclusions about efficacy in this domain.Citation41
Targeting plasma cells
If B cell depletion does indeed reduce immune complexes it may do so indirectly by killing the precursor germinal center B cells that give rise to antibody-secreting plasma cells. To reduce autoantibody production more effectively, agents targeting plasma cells specifically may be more useful. Indeed, corticosteroids may well exert their beneficial effect by this mechanism, among others. Proteasome inhibitors have been introduced into the therapeutic armamentarium for multiple myeloma due to their ability to cause apoptosis of plasma cells.Citation42 The use of proteasome inhibitors in SLE has been promising in mouse models, eliminating autoreactive plasma cells, reducing anti-dsDNA antibody levels, and preventing nephritis;Citation43 human trials are underway.
Induction of B cell tolerance
Induction of tolerance would be the ultimate way to reduce anti-dsDNA antibody concentrations. Although murine models have provided hope, human trials have again been unimpressive. Regular injections of nucleosomal peptide autoepitopes in lupus-prone mice reduced autoantibody levels and delayed the onset of nephritis by the induction of TGF-producing regulatory T cells.Citation44 However, abetimus, a conjugate composed of 4 identical strands of dsDNA, did not show any benefit in reducing renal flares in human SLE. Interestingly, abetimus did reduce the level of anti-dsDNA antibodies, possibly due to the formation of soluble complexes that were rapidly eliminated and, possibly, by tolerizing B cells and reducing autoantibody production.Citation45
Reducing the binding of autoantibodies
The mechanism of action of the antimalarials chloroquine and hydroxychloroquine in SLE has recently been revisited, because of the recognition of their inhibition of TLR-9 binding to DNA, by preventing acidification of the lysosome. However, hydroxychloroquine, as one of its many mechanisms of action, also affects the affinity of binding of antibodies to their targets. Hydroxychloroquine interferes with the binding of antiphospholipid antibodies in vitro, and causes a reduction in the levels of these autoantibodies as measured by commercially available ELISAs.Citation46 We recently demonstrated that the binding of anti-dsDNA antibodies as measured by the modified Farr assay is reduced by the addition of hydroxychloroquine in vitro.Citation47 This effect is likely to be due to the high protein-binding capacity of hydroxychloroquine,Citation48 and intercalation of DNA (if sharing this property with chloroquine),Citation49,Citation50 potentially modifying critical autoepitopes. Whether this affects the pathogenesis of human lupus nephritis is unknown.
Reducing the availability of DNA and nucleosomal material
Material for anti-dsDNA and antinucleosome antibodies to bind may originate from tissue damage in the kidneys, resulting in situ formation of complexes or, alternatively, from damage remotely, resulting in the formation of circulating immune complexes, which then deposit in glomeruli (reviewed by Fismen et alCitation51). A phase Ib trial of recombinant human DNase I (rhDNase) to hydrolyze extracellular DNA in patients with lupus did not reduce anti-dsDNA levels, the concentrations of circulating immune complexes, nor change other serological markers.Citation52 No further studies of rhDNase have been published.
Increasing the clearance of immune complexes
Plasmapheresis is able to lower the titer of anti-dsDNA antibodies but does not necessarily result in sustained clinical remission once withdrawn, possibly due to compensatory increased production by pathogenic B cell clones (rebound effect).Citation53 Removal of pathogenic anti-dsDNA antibodies physically by plasmapheresis may improve outcomes for those receiving intravenous (IV) cyclophosphamide. A combination of plasmapheresis and IV cyclophosphamide results in higher rates of complete renal remission than IV cyclophosphamide alone.Citation54,Citation55 However, not all trials have found benefit, and larger randomized trials are required to confirm these findings. Other small studies and case reports have also demonstrated benefit with immunoadsorption plasmapheresis,Citation56,Citation57 but further investigation is required to clarify the role of this treatment.
Breaking the feedback (amplification) loop
Signaling of immune complexes containing RNA and DNA via TLRs 7 and 9, respectively, activates plasmacytoid dendritic cells to produce large amounts of type I interferon. Type I interferons activate B cells and enhance antibody responses to soluble proteins, thereby completing a feedback loop resulting in the increased production of immune complexes.Citation58 One of the mechanisms of action for antimalarial drugs such as hydroxychloroquine in lupus is thought to be the inhibition of nucleic acid interaction with intracellular TLRs 7 and 9, possibly as a result of an increase in pH in microsomal compartments.Citation59 Novel treatments aimed at blocking TLR7 and TLR9 are being developed.Citation60 A phase I study of an anti-interferon-α monoclonal antibody has been completed,Citation61 and a phase II study is underway.
Conclusion
Immune complexes containing IgG anti-dsDNA antibodies and DNA play a significant role in the complex pathogenesis of lupus nephritis. Although strategies specifically aimed at reducing immune complexes in SLE are mostly novel, they provide a fertile area for further research. Disappointments with early trials of new therapeutics strengthen the argument that a combination of strategies aimed at different pathogenic mechanisms is likely to be necessary to improve the prognosis of this disease.
Disclosure
The authors report no conflicts of interest.
References
- RahmanAIsenbergDAMechanisms of disease: systemic lupus erythematosusN Engl J Med200835892993918305268
- RadicMMarionTMonestierMNucleosomes are exposed at the cell surface in apoptosisJ Immunol20041726692670015153485
- BieseckerGKatzSKofflerDRenal localization of the membrane attack complex in systemic lupus erythematosus nephritisJ Exp Med1981154177917947033435
- OrtegaLMSchultzDRLenzOLupus nephritis: pathologic features, epidemiology, and a guide to therapeutic decisionsLupus20101955757420089610
- WeeningJJD’AgatiVDSchwartzMMThe classification of glomerulonephritis in systemic lupus erythematosus revisitedKidney Int20046552153014717922
- NajafiCCKorbetSMLewisEJSignificance of histologic patterns of glomerular injury upon long-term prognosis in severe lupus glomerulonephritisKidney Int2001592156216311380817
- BorchersATKeenCLShoenfeldYSurviving the butterfly and the wolf: mortality trends in systemic lupus erythematosusAutoimmun Rev2004342345315351310
- CerveraRKhamashtaMAFontJMorbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patientsMedicine (Baltimore)20038229930814530779
- LevinskyRJCameronJSSoothillJFSerum immune complexes and disease activity in lupus nephritisLancet1977156456765659
- MortensenESRekvigOPNephritogenic potential of anti-DNA antibodies against necrotic nucleosomesJ Am Soc Nephrol20092069670419329762
- CruchaudAChenaisFFournieGJImmune complex deposits in systemic lupus erythematosus kidney without histological or functional alterationsEur J Clin Invest197519752973091149788
- ForgerFMatthiasTOppermannMClinical significance of anti-dsDNA antibody isotypes: IgG/IgM ratio of anti-dsDNA antibodies as a prognostic marker for lupus nephritisLupus200413364414870916
- AmouraZKoutouzovSChabreHAntinucleosome antibodies of the IgG3 subclass are markers of renal pathogenicity in systemic lupus erythematosusArthritis Rheum200043768410643702
- BijlMDijstelbloemHMOostWWIgG subclass distribution of autoantibodies differs between renal and extra-renal relapses in patients with systemic lupus erythematosusRheumatology (Oxford)200241626711792881
- VillaltaDRomelliPBSavinaCAnti-dsDNA antibody avidity determination by a simple reliable ELISA method for SLE diagnosis and monitoringLupus200312313612587824
- JaekelH-PTrabandtAGrobeNAnti-dsDNA antibody subtypes and anti-C1q antibodies: toward a more reliable diagnosis and monitoring of systemic lupus erythematosus and lupus nephritisLupus20061533534516830879
- FentonKATommerasBMarionTNPure anti-dsDNA mAbs need chromatin structures to promote glomerular mesangial deposits in BALB/c miceAutoimmunity20104317918819835488
- Kohro-KawataJWangPKawataYHighly cationic anti-DNA antibodies in patients with lupus nephritis analyzed by two-dimensional electrophoresis and immunoblottingElectrophoresis199819151115159694304
- MostoslavskyGFischelRYachimovichNLupus anti-DNA autoantibodies cross-react with a glomerular structural protein: a case for tissue injury by molecular mimicryEur J Immunol2001311221122711298348
- ZykovaSNSeredkinaNBenjaminsenJReduced fragmentation of apoptotic chromatin is associated with nephritis in lupus-prone (NZB x NZW)F(1) miceArthritis Rheum20085881382518311834
- LeeYHJiJDSongGGFcgamma receptor IIB and IIIB polymorphisms and susceptibility to systemic lupus erythematosus and lupus nephritis: a meta-analysisLupus200918872773419502269
- BergtoldAGavhaneAD’AgatiVFcR-bearing myeloid cells are responsible for triggering murine lupus nephritisJ Immunol20061777287729517082647
- LiYLeePYSobelESIncreased expression of FcγRI/CD64 on circulating monocytes parallels ongoing inflammation and nephritis in lupusArthritis Res Ther200911R619144150
- ClynesRCalvaniNCrokerBPModulation of immune response in pristane-induced lupus by expression of activation and inhibitory Fc receptorsClin Exp Immunol200514123023715996187
- KarpDRComplement and systemic lupus erythematosusCurr Opin Rheumatol20051753854216093830
- KavaiMImmune complex clearance by complement receptor type 1 in SLEAutoimmun Rev2008816016418602499
- SinicoRAIkehataMGiammarresiGAnti-C1q autoantibodies in lupus nephritis: prevalence and clinical significanceAnn N Y Acad Sci2005105019320016014534
- TrendelenburgMLopez-TrascasaMPotlukovaEHigh prevalence of anti-C1q antibodies in biopsy-proven active lupus nephritisNephrol Dial Transplant2006213115312116877491
- TrouwLAGroeneveldTWLSeelenMAAnti-C1q autoantibodies desposit in glomeruli but are only pathogenic in combination with glomerular C1q-containing immune complexesJ Clin Invest200411467968815343386
- BouleMWBroughtonCMackayFToll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexesJ Exp Med20041991631164015197227
- KaveriSVMouthonLBayryJBasophils and nephritis in lupusN Engl J Med20103631080108220825323
- CambridgeGLeandroMJTeodorescuMB cell depletion therapy in systemic lupus erythematosus: effect on autoantibody and antimicrobial antibody profilesArthritis Rheum2006543612362217075806
- FurieRLooneyRRovinBEfficacy and safety of ritux-imab in subjects with active proliferative lupus nephritis (LN): results from the randomized, double-blind phase III LUNAR studyAmerican College of Rheumatology National Meeting 2009Philadelphia (PA)2009 Oct 17–21 Abstract 1149.
- LooneyRJB cell-targeted therapies for systemic lupus erythematosus: an update on clinical trial dataDrugs20107052954020297867
- MerrillJTNeuweltCMWallaceDJEfficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trialArthritis Rheum6222223320039413
- TerrierBAmouraZRavaudPSafety and efficacy of rituximab in systemic lupus erythematosus: results from 136 patients from the French AutoImmunity and Rituximab registryArthritis Rheum622458246620506527
- Roche and Biogen Idec decide to suspend ocrelizumab treatment – rheumatoid arthritis development programme on holdBiogen Idec2010 http://www.biogenidec.com/PRESS_RELEASE_DETAILS.aspx?ID=5981&ReqId=1400446. Accessed 2010 Oct 12.
- SanzILeeFEB cells as therapeutic targets in SLENat Rev Rheumatol2010632633720520647
- WallaceDJStohlWFurieRAA phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosusArthritis Care Res20096111681178
- JacobiAMHuangWWangTEffect of long-term belimumab treatment on B cells in systemic lupus erythematosus: extension of a phase II, double-blind, placebo-controlled, dose-ranging studyArthritis Rheum20106220121020039404
- DornerTKaufmannJWegenerWAInitial clinical trial of epratuzumab (humanized anti-CD22 antibody) for immunotherapy of systemic lupus erythematosusArthritis Res Ther20068R7416630358
- DingliDRajkumarSVHow best to use new therapies in multiple myelomaBlood Rev2010249110020359801
- NeubertKMeisterSMoserKThe proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritisNat Med20081474875518542049
- KangH-KMichaelsMABernerBRVery low-dose tolerance with nucleosomal peptides controls lupus and induces regulatory T cell subsetsJ Immunol20051743247325515749855
- CardielMHTumlinJAFurieRAAbetimus sodium for renal flare in systemic lupus erythematosus: results of a randomized, controlled phase III trialArthritis Rheum2008582470248018668592
- RandJHWuX-XQuinnASHydroxychloroquine directly reduces the binding of antiphospholipid antibody-β2-glycoprotein I complexes to phospholipid bilayersBlood20081121687169518577708
- ToongCWienholtLAdelsteinSHydroxychloroquine reduces binding of anti-double stranded DNA antibodies in vitroIntern Med J201040Suppl 417
- McLachlanAJCutlerDJTettSEPlasma protein binding of the enantiomers of hydroxychloroquine and metabolitesEur J Clin Pharmacol1993444814848359187
- ScariaPVCraigJCShaferRHDifferential binding of the enantiomers of chloroquine and quinacrine to polynucleotides: implications for stereoselective metabolismBiopolymers1993338878958318663
- Kwakye-BerkoFMeshnickSSequence preference of chloroquine binding to DNA and prevention of Z-DNA formationMol Biochem Paristol199039275278
- FismenSMortensenESRekvigOPNuclease deficiencies promote end-stage lupus nephritis but not nephritogenic autoimmunity in (NZBxNZW) F1 miceImmunol Cell Biol2010615
- DavisJCJrManziSYarboroCRecombinant human Dnase I (rhDNase) in patients with lupus nephritisLupus19998687610025601
- Mistry-BurchardiNSchonermarkUSamtlebenWApheresis in lupus nephritisTher Apher2001516117011467751
- YamajiKKimYJTsudaHLong-term clinical outcomes of synchronized therapy with plasmapheresis and intravenous cyclophosphamide pulse therapy in the treatment of steroid-resistant lupus nephritisTher Apher Dial200812429830518789117
- DanieliMGPalmieriCSalviASynchronised therapy and high-dose cyclophosphamide in proliferative lupus nephritisJ Clin Apher200217727712210709
- SugimotoKYamajiKYangKSImmunoadsorption plasmapheresis using a phenylalanine column as an effective treatment for lupus nephritisTher Apher Dial20061018719216684222
- TakahashiSWadaNHaradaKImmunoadsorbent apheresis eliminates pathogenic IgG in childhood lupus nephritisPediatr Int20074981782118045278
- PascualVFarkasLBanchereauJSystemic lupus erythematosus: all roads lead to type I interferonsCurr Opin Immunol20061867668217011763
- LafyatisRYorkMMarshak-RothsteinAAntimalarial agents: closing the gate on Toll-like receptors?Arthritis Rheum2006543068307017009223
- FulmerTSparing steroids in lupusSciBX2010312
- YaoYRichmanLHiggsBWNeutralizaiton of interferon-α/β-inducible genes and downstream effect in a phase I trial of an anti-inteferon-α monoclonal antibody in systemic lupus erythematosusArthritis Rheum2009601785179619479852
- MadaioMPCarlsonJCataldoJMurine monoclonal anti-DNA antibodies bind directly to glomerular antigens and form immune depositsJ Immunol1987138288328893553329
- ChanTMLeungJKHoSKMesangial cell-binding anti-DNA antibodies in patients with systemic lupus erythematosusJ Am Soc Nephrol2002131219122911961009
- DuHChenMZhangYCross-reaction of anti-DNA autoantibodies with membrane proteins of human glomerular mesangial cells in sera from patients with lupus nephritisClin Exp Immunol2006145212716792669
- KramersCHylkemaMNvan BruggenMCJAnti-nucleosome antibodies complexed to nucleosomal antigens show anti-DNA reactivity and bind to rat glomerular basement membrane in vivoJ Clin Invest1994945685778040312
- MoriokaTFujigakiYBatsfordSRAnti-DNA antibody derived from a systemic lupus erythematosus (SLE) patient forms histone-DNA-anti-DNA complexes that bind to rat glomeruli in vivoClin Exp Immunol199610492968603540
- TsaoBPOhnishiKCheroutreHFailed self-tolerance and autoimmunity in IgG anti-DNA transgenic miceJ Immunol19921493503581607661
- RazEBrezisMRosenmannEAnti-DNA antibodies bind directly to renal antigens and induce kidney dysfunction in the isolated perfused rat kidneyJ Immunol1989142307630822785132
- EhrensteinMRKatzDRGriffithsMHHuman IgG anti-DNA antibodies deposit in kidneys and induce proteinuria in SCID miceKidney Int1995487057117474655
- RavirajanCTRahmanMAPapadakiLGenetic, structural and functional properties of an IgG DNA-binding monoclonal antibody from a lupus patient with nephritisEur J Immunol1998283393509485213
- KalaajiMFentonKAMortensenESGlomerular apoptotic nucleosomes are central target structures for nephritogenic antibodies in human SLE nephritisKidney Int20077166467217332738
- CasalsSPFriouGJMyersLLSignificance of antibody to DNA in systemic lupus erythematosusArthritis Rheum1964737939014202576
- CerveraRVinasORamos-CasalsMAnti-chromatin antibodies in systemic lupus erythematosus: a useful marker for lupus nephropathyAnn Rheum Dis20036243143412695155
- ter BorgEJHorstGHummelEJMeasurement of increases in anti-double-stranded DNA antibody levels as a predictor of disease exacerbation in systemic lupus erythematosus: A long-term, prospective studyArthritis Rheum19903356346432346519
- AdlerMKBaumgartenAHechtBPrognostic significance of DNA-binding capacity patterns in patients with lupus nephritisAnn Rheum Dis1975344444501221925
- HorowitzDMFurieRAAbetimus sodium: a medication for the prevention of lupus nephritis flaresExpert Opin Pharmacother2009101501150719505217
- DonadioJVJHolleyKEFergusonRHTreatment of diffuse proliferative lupus nephritis with prednisone and combined prednisone and cyclophosphamideN Engl J Med197829911511155309095
- HoussiauFAVasconcelosCD’CruzDImmunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamideArthritis Rheum2002462121213112209517
- HoussiauFAVasconcelosCD’CruzDThe 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose and high-dose intravenous cyclophosphamideAnn Rheum Dis201069616419155235
- PetriMBrodskyRAJonesRJHigh-dose cyclophosphamide versus monthly intravenous cyclophosphamide for systemic lupus erythematosus: a prospective randomized trialArthritis Rheum2010621487149320131296
- YeeCSGordonCDostalCEULAR randomised controlled trial of pulse cyclophosphamide and methylprednisolone versus continuous cyclophosphamide and prednisolone followed by azathioprine and prednisolone in lupus nephritisAnn Rheum Dis20036352552915082482
- IlleiGGYarboroCHKuroiwaTLong-term effects of combination treatment with fludarabine and low-dose pulse cyclophosphamide in patients with lupus nephritisRheumatology (Oxford)20074695295617317716
- GinzlerEMDooleyMAAranowCMycophenolate mofetil or intravenous cyclophosphamide for lupus nephritisN Engl J Med20053532219222816306519
- AppelGBContrerasGDooleyMAMycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritisJ Am Soc Nephrol2009201103111219369404
- RadhakrishnanJMoutzourisD-AGinzlerEMMycophenolate mofetil and intravenous cyclophosphamide are similar as induction therapy for class V lupus nephritisKidney Int20107715216019890271
- OngLMHooiLSLimTORandomized controlled trial of pulse intravenous cyclophosphamide versus mycophenolate mofetil in the induction therapy of proliferative lupus nephritisNephrology20051050451016221103
- ZhuBChenNLinYMycophenolate mofetil in induction and maintenance therapy of severe lupus nephritis: a meta-analysis of randomized controlled trialsNephrol Dial Transplant2007221933194217405792
- LanataCMMahmoodTFineDMCombination therapy of mycophenolate mofetil and tacrolimus in lupus nephritisLupus20101993594020388722
- GrootscholtenCLigtenbergGHagenECAzathioprine/methylprednisolone versus cyclophosphamide in proliferative lupus nephritis. A randomized controlled trialKidney Int20067073274216820790
- GrootscholtenCBajemaIMFlorquinSTreatment with cyclophosphamide delays the progression of chronic lesions more effectively than does treatment with azathioprine plus methylprednisolone in patients with proliferative lupus nephritisArthritis Rheum20075692493717328070
- ChanTMHistologic deterioration and more flares: the case against azathioprine plus methylprednisolone in the treatment of proliferative lupus nephritisArthritis Rheum20075670270417328038
- ZavadaJPesickovaSRysavaRCyclosporine A or intravenous cyclophosphamide for lupus nephritis: the Cyclofa-Lune studyLupus2010191281128920605876
- Ramos-CasalsMSotoMJCuadradoMJRituximab in systemic lupus erythematosus: A systematic review of off-label use in 188 casesLupus20091876777619578100
- LiEKTamLSZhuTYIs combination rituximab with cyclophosphamide better than rituximab alone in the treatment of lupus nephritis?Rheumatology (Oxford)20094889289819478041
- LateefALahiriMTengGGUse of rituximab in the treatment of refractory systemic lupus erythematosus: Singapore experienceLupus20101976577020118162
- Garcia-CarrascoMMendoza-PintoCSandoval-CruzMAnti-CD20 therapy in patients with refractory systemic lupus erythematosus: a longitudinal analysis of 52 Hispanic patientsLupus20101921321919965944
- JonsdottirTGunnarssonIMouraoAFClinical improvements in proliferative vs membranous lupus nephritis following B-cell depletion: pooled data from two cohortsRheumatology (Oxford)2010491502150420427294
- ContrerasGPardoVLeclercqBSequential therapies for proliferative lupus nephritisN Engl J Med200435097198014999109
- ContrerasGTozmanENaharNMaintenance therapies for proliferative lupus nephritis: mycophenolate mofetil, azathioprine and intravenous cyclophosphamideLupus200514s33s3815803929
- SahinGMSahinSKiziltasSMycophenolate mofetil versus azathioprine in the maintenance therapy of lupus nephritisRenal Failure20083086586918925525
- HoussiauFD’CruzDSangleSthe MAINTAIN Nephritis Trial GroupAzathioprine versus mycophenolate mofetil for long-term immunosuppression in lupus nephritis: results from the MAINTAIN Nephritis TrialAnn Rheum Dis2010692083208920833738
- WofsyDAppelGBDooleyMAAspreva Lupus Management Study maintenance resultsLupus201019Suppl27 (CS12.6).19933722
- MoroniGDoriaAMoscaMA randomized pilot trial comparing cyclosporine and azathioprine for maintenance therapy in diffuse lupus nephritis over four yearsClin J Am Soc Nephrol2006192593217699309