Abstract
The purpose of this review is to summarize current information regarding the pathophysiology and management of vaginal atrophy (sometimes called “atrophic vaginitis”) and to identify barriers to its treatment with local (or “topical”) vaginal estrogen therapy. Relevant clinical trials, meta-analyses, and reviews were identified through the PubMed database. Local estrogen therapy is effective and safe for treatment of vaginal atrophy; however, barriers to treatment (eg, patient reluctance to discuss the condition, misinformation, incomplete understanding of the effectiveness and safety of available therapies) result in its underuse. Health care providers can help overcome barriers to effective treatment of vaginal atrophy by facilitating discussion with women about vaginal health. Discussions should occur at routine preventive health care examinations and during episodic visits when patients present with symptoms of vaginal atrophy. Education and counseling should include information on the importance of maintaining vaginal health and the benefits and risks of treatment, including the demonstrated effectiveness and safety profile of low-dose local estrogen therapy.
Introduction
Vaginal atrophy is a common condition, with related symptoms affecting up to about half (45%) of postmenopausal womenCitation1,Citation2 and almost two-thirds (61.5%) of postmenopausal breast cancer survivors.Citation3 It is characterized by vaginal dryness, dyspareunia, vaginal itching, discharge, and pain.Citation4 Urinary symptoms, including frequent urinary tract infection and incontinence, are also commonly present ().
Table 1 Symptoms and signs of estrogen deficiency and vaginal atrophyCitation4,Citation8,Citation10
For many women, vaginal atrophy interferes with normal sexual functioning.Citation5–Citation7 Vaginal atrophy may cause discomfort or pain during intercourse, difficulty in achieving orgasm, decreased sexual desire, embarrassing vaginal and urinary symptoms, and diminished perception of sexual attractiveness. These symptoms may reduce intercourse frequency, interfere with personal relationships, and decrease feelings of intimacy.Citation6
Despite the prevalence of vaginal atrophy among postmenopausal women, the topic of vaginal health is frequently absent in preventive health evaluations. Furthermore, many effective treatments for vaginal atrophy are available. Therefore, it is prudent to review the pathophysiology, current treatment recommendations, safety profile of low-dose local vaginal estrogen, and strategies to overcome barriers to the effective treatment of vaginal atrophy.
Causes of vaginal atrophy
Estrogen maintains vaginal health through interaction with estrogen receptors in the vagina.Citation8 The North American Menopause Society (NAMS) notes that vaginal atrophy is most commonly associated with the diminished estrogen levels associated with menopause and aging.Citation9 Low levels of estrogen in the postmenopausal state lead to gradual changes in the urogenital system, including reduced collagen content and thinning of epithelium, altered appearance and function of smooth muscle cells, increased density of connective tissue, fewer blood vessels, decreased enervation and hyperplasia of terminal nociceptor nerve fibers.Citation8,Citation10 These changes can result in reduced flexibility of the vaginal vault, reduced vaginal blood flow, altered sensation, and increased pH.
Additionally, cancer treatments (surgery, pelvic radiation, chemotherapy, and endocrine therapy) and chemoprevention therapies may cause or contribute to vaginal atrophy by modifying endocrine activity (which particularly affects the vaginal epithelium), impairing vascular supply to the vaginal tissue or altering the anatomy of vaginal canal.Citation9 Symptoms of vaginal atrophy are commonly associated with endocrine breast cancer therapies, including tamoxifen (when used in premenopausal women) and aromatase inhibitors.Citation11 Symptoms may also arise in premenopausal women who undergo temporary induction of menopause for treatment of hormone-sensitive advanced breast cancer and in women at high risk for breast or ovarian cancer who undergo bilateral oophorectomy.Citation11
Treatment
Nonhormonal therapies (eg, vaginal moisturizers, lubricants, continued sexual activity) are considered first-line therapies for women with vaginal atrophy ().Citation9 However, while these therapies may provide symptom relief, they do not address the underlying condition (loss of vaginal integrity due to estrogen deficiency). NAMS therefore recommends that nonhormonal therapies should be followed by a discussion of hormonal therapies, according to the patient’s preference, with consultation from her oncologist.Citation9
Figure 1 Approach to treatment of vaginal atrophy.Citation9,Citation11
†Undiagnosed abnormal uterine bleeding, breast cancer (except in appropriately selected patients being treated for metastatic disease), estrogen-dependent neoplasia, deep vein thrombosis or pulmonary embolism, arterial thromboembolic disease within the past year, liver disease/dysfunction, pregnancy, or hypersensitivity to estrogen therapy.Citation35 ‡Concomitant progestogen therapy is recommended in women with an intact uterus and who are receiving systemic estrogen therapy to prevent endometrial proliferation and adenocarcinoma.Citation12
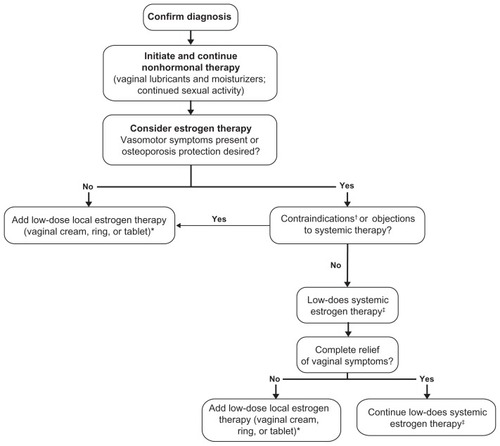
Estrogen therapy, delivered systemically or locally (to limit systemic absorption), may be considered for women in whom nonhormonal therapies are ineffective.Citation9 If vasomotor symptom relief or osteoporosis prevention is desired and the patient has no contraindications or objections to systemic estrogen therapy, systemic estrogen therapy may be considered as a treatment for vaginal atrophy.Citation12 These patients should be informed of the potential risks of therapy, including deep vein thrombosis, pulmonary embolism, coronary heart disease, and breast cancer. Low doses of orally administered conjugated equine estrogens (0.3 mg/day), ultra-low doses of continuous combined hormone-replacement therapy (estradiol 0.5 mg/day plus norethisterone acetate 0.1 to 0.25 mg/day), and ultra-low-dose transdermal estradiol (0.0125 to 0.014 mg/day) have shown safety and beneficial effects on both vasomotor symptoms and measures of vaginal atrophy (eg, pH, maturation index, vaginal symptoms) in studies of up to 2 years’ duration.Citation13–Citation19 However, oral estradiol is less commonly prescribed because it undergoes significant first-pass metabolism through the liver, which leads to a large reduction in bioavailable drug.Citation20 Generally, for women with an intact uterus, concomitant progestogen therapy is recommended when receiving systemic estrogen therapy, to prevent endometrial hyperplasia and adenocarcinoma.Citation12
In women with a history of hormone-dependent cancer, the potential risks associated with systemic estrogen therapy with or without progestogen may outweigh potential benefits; therefore, systemic therapy is best avoided in this subgroup of patients with vaginal atrophy. Also, not all women experience complete relief of vaginal symptoms with systemic estrogen therapy and additional local estrogen therapy may be required for persistent vaginal symptoms.Citation12
Local estrogen therapy is commonly recommended for patients with primarily vaginal symptoms and moderate-to-severe vaginal atrophy. Commercially available local estrogen therapies () include an estradiol vaginal cream,Citation21 a conjugated estrogen (CE) vaginal cream,Citation22 an estradiol vaginal ring,Citation23 and an estradiol vaginal tablet.Citation24 These various formulations have shown comparable effectivenessCitation25,Citation26 for the relief of vaginal atrophy symptoms, including vaginal dryness, itching, discomfort, and dyspareunia; thus, therapy selection for individual patients is largely driven by provider and patient preference.Citation9 The potential benefits and limitations of the available formulations are summarized in . Local estrogen therapy can provide sufficient estrogen to the vaginal tissues, with limited systemic absorption,Citation9 so low-dose vaginal estrogen may achieve an effect in the tissue that is similar to the response obtained from oral or transdermal dosing regimens.
Table 2 Commercially available local estrogen therapies
Safety profile of low-dose local vaginal estrogen
Several large studies have provided evidence for the endometrial safety of various low-dose local vaginal estrogen formulations.Citation27–Citation29 The lowest commercially available formulation, the 10 mcg estradiol biweekly vaginal tablet, was associated with no increased risk for endometrial hyperplasia or carcinoma in postmenopausal women treated for 52 weeks (0.52% incidence of endometrial hyperplasia or carcinoma in 386 evaluable biopsy samples, compared with 0% to 1% background incidence).Citation27 In another study, the vaginal ring, which releases 7.5 mcg estradiol per day, was associated with endometrial thickening to >7 mm in two of 126 women at 48 weeks, but evidence of proliferation was absent at biopsy.Citation28 Additionally, low-dose regimens of the CE cream (0.3 mg CE once daily [21 days on/7 days off] or twice weekly) did not result in endometrial hyperplasia or carcinoma in 155 biopsies evaluated at 52 weeks.Citation29 The studies mentioned and othersCitation25 show that it is probable that low-dose local vaginal estrogen poses a minimal risk for endometrial hyperplasia and carcinoma. Therefore, NAMS advises that progestogen therapy is generally not needed when low-dose estrogen is administered locally for the treatment of vaginal atrophy.Citation12
Women living with breast cancer and breast cancer survivors comprise a population of particular interest with regard to the safety of vaginal estrogen preparations. Their numbers are growing because the overall incidence of breast cancer is increasing, while improved cancer treatments are improving survival times.Citation30 The risks associated with vaginal estrogen use in women with a history of breast cancer have been addressed in the literature. Pruthi et alCitation31 concluded that safety and risk of recurrence is undefined in this population; however, the 10 mcg estradiol vaginal tablet or estradiol vaginal ring can be considered after appropriate disclosure to the patient. Dew et alCitation32 evaluated the risk for breast cancer recurrence with the use of low-dose vaginal estrogen and were unable to show an increased risk. However, a small study in which six women were using aromatase inhibitors and vaginal estrogen (25 mcg estradiol vaginal tablet, which has since been replaced with a 10 mcg estradiol vaginal tablet) showed a short-term increase in serum estradiol that varied by individual.Citation33 In two of the six women, serum estradiol levels did not return to baseline. Based on these results, Kendall et alCitation33 cautioned against using vaginal estrogen in patients using aromatase inhibitors because systemic absorption of the former could increase serum estradiol, thereby negating the effectiveness of the latter, which work by near-total suppression of estrogenic stimulation.Citation33
Health care providers (HCPs) should counsel women who have a history of breast cancer regarding the risks and benefits of local vaginal estrogen for the treatment of vaginal atrophy. Quality of life issues, including sexual health, should also be addressed to help women make an informed decision regarding this therapy. Clinicians should also be aware that using local vaginal estrogen for women who have a history of breast cancer is considered off-label because of the black-box warning included in the package insert, which describes the increased risk for breast cancer associated with estrogen plus progestin therapy found in the Women’s Health Initiative study.Citation24
Overcoming barriers to the use of local estrogen therapy
Multiple barriers prevent the treatment of vaginal atrophy with local estrogen therapy in women who may benefit from treatment (). First, many women are uncomfortable discussing their vaginal health. Results of one international survey indicated that the majority of women with vaginal discomfort (≥70%) had not discussed it with their general practitioner or gynecologist.Citation2 HCPs can address vaginal atrophy by initiating a discussion about vaginal health during preventive health evaluations. This discussion can be individually adapted to each patient’s learning preferences and should reinforce that vaginal atrophy is common among peri- and postmenopausal women. In addition, it should include an open conversation regarding improvement or exacerbation of symptoms with over-the-counter products. If a patient reports vaginal discomfort, with or without self-treatment, a physical examination should be performed. If evidence of vaginal atrophy is noted, a discussion about current treatment recommendations should follow.
Table 3 Potential solutions to common barriers to identification and treatment of vaginal atrophy
Some women may be hesitant to use estrogen therapy, even if they have significant symptoms. Early termination of the Women’s Health Initiative randomized controlled trialCitation34 and subsequent media coverage raised public awareness of the potential risks of standard-dose systemic hormone therapy. Adverse effects, such as uterine bleeding, breast tenderness, nausea, abdominal bloating, and fluid retention,Citation35 may further hinder patient acceptance of systemic estrogen or estrogen plus progestogen therapy. Education regarding the risks and benefits of estrogen therapy, including the improved risk–benefit profiles of less-than-standard-dose systemic therapiesCitation36 and the endometrial safety of low-dose local therapies,Citation27–Citation29 can help facilitate acceptance among women for whom estrogen therapy is appropriate. It may be helpful to mention to a patient that the amount of estradiol administered locally (eg, by vaginal tablet or vaginal ring) in a low dose is much less than that administered in a standard oral dose. While a standard oral dose of estradiol is considered 1 mg/day, the vaginal tablet regimen (one 10 mcg tablet daily for 2 weeks followed by one tablet twice weekly thereafterCitation24) exposes a patient to about 1.14 mg estradiol (ie, roughly the same amount as the standard oral dose) over an entire year of treatment. For further comparison, patients using the ring are exposed to about 2.74 mg estradiol per year, while patients using the creams are generally exposed to more estrogen, depending on the regimen. Closely following these total doses, systemic absorption of estrogen remains lowest with the vaginal tablet,Citation37 followed by the ringCitation38 and creams.Citation39,Citation40
Barriers related to the use of local vaginal estrogen in particular might include its lack of effect on bone loss and vasomotor symptoms, the need for vaginal insertion, irregular treatment intervals, and concerns regarding the potential for improper dosing. Patients may also be apprehensive about potential discomforts associated with use of a vaginal medication (eg, leakage with creams). Additionally, patients may be uncertain regarding the uterine safety of local vaginal estrogen; therefore, HCPs should counsel women effectively regarding the safety data of various formulations. Counseling should also include advising women that use of the lowest effective dose of local vaginal estrogen is recommended to minimize systemic absorption and associated risks.Citation9
Counseling intervention
The decision for a woman to use a hormone medication depends on many individualized factors. A patient’s biological and psychological response to midlife and aging, tolerance for symptoms and side effects of the medication, and risk/benefit profile will influence both the HCP’s recommendation for intervention and the woman’s decision to implement the intervention. The Counseling and Behavioral Interventions Work Group of the United States Preventive Services Task Force recommends the “5 A’s” construct as a clinical counseling strategyCitation41:
Assess
Advise
Agree
Assist
Arrange
Counseling using the 5 A’s may be useful for overcoming the communication barriers between patients and their HCPs. Routine assessment of vaginal health during preventive health evaluations should facilitate timely identification of vaginal atrophy. HCPs can then provide personalized advice regarding vaginal atrophy and help the patient understand the goals, benefits, and risks of treatment and agree to undergo treatment. Additional counseling about proper treatment administration and what to expect from therapy assists the patient in achieving her goals, while arranging appropriate follow-up appointments should facilitate long-term management of this chronic condition.
Conclusion
HCPs such as gynecologists, primary care physicians, nurses, and nurse practitioners can play an essential role in reducing the barriers associated with discussion and treatment of vaginal atrophy. A discussion of the pathophysiology of vaginal atrophy, treatment options (including risks and benefits of treatment), and overall sexual health is pertinent to preventive health care. Additionally, a brief review of the safety profile of local vaginal estrogen may help women make informed decisions regarding their treatment. Counseling intervention using the 5 A’s can empower women to manage their vaginal symptoms effectively in the long-term, potentially improving patient quality of life.
Acknowledgments
Editorial assistance was provided by Tamara Rahim Grow, PhD, ETHOS Health Communications, Newtown, Pennsylvania, with financial assistance from Novo Nordisk, Inc, Princeton, New Jersey, in compliance with international guidelines on good publication practice.
Disclosure
LAC participated in an advisory board in October 2011 for Novo Nordisk; this program was completely independent of this article. She received no remuneration of any kind for the development of this article and declares no other conflicts of interest in this work.
References
- SantoroNKomiJPrevalence and impact of vaginal symptoms among postmenopausal womenJ Sex Med2009682133214219493278
- NappiREKokot-KierepaMWomen’s voices in the menopause: results from an international survey on vaginal atrophyMaturitas201067323323820828948
- CrandallCPetersenLGanzPAGreendaleGAAssociation of breast cancer and its therapy with menopause-related symptomsMenopause200411551953015356404
- SturdeeDWPanayNInternational Menopause Society Writing GroupRecommendations for the management of postmenopausal vaginal atrophyClimacteric201013650952220883118
- LevineKBWilliamsREHartmannKEVulvovaginal atrophy is strongly associated with female sexual dysfunction among sexually active postmenopausal womenMenopause2008154 Pt 166166618698279
- KrychmanMImpact of vaginal atrophy on quality of life and sexualityOBG Manage20102211S14S19
- KingsbergSKelloggSKrychmanMTreating dyspareunia caused by vaginal atrophy: a review of treatment options using vaginal estrogen therapyInt J Womens Health2010110511121072280
- ReimerAJohnsonLAtrophic vaginitis: signs, symptoms, and better outcomesNurse Pract2011361222821150811
- DenizGAntoineCLiebensFCarlyBPastijnARozenbergSTreatment of premature menopause in breast cancer patientsActa Chir Belg2007107326326617685250
- LaraLAUsecheBFerrianiRAThe effects of hypoestrogenism on the vaginal wall: interference with the normal sexual responseJ Sex Med200961303919170834
- Mac BrideMBRhodesDJShusterLTVulvovaginal atrophyMayo Clin Proc2010851879420042564
- North American Menopause SocietyThe 2012 hormone therapy position statement of: The North American Menopause SocietyMenopause201219325727122367731
- UtianWHShoupeDBachmannGPinkertonJVPickarJHRelief of vasomotor symptoms and vaginal atrophy with lower doses of conjugated equine estrogens and medroxyprogesterone acetateFertil Steril20017561065107911384629
- BachmannGASchaefersMUddinAUtianWHLowest effective transdermal 17beta-estradiol dose for relief of hot flushes in postmenopausal women: a randomized controlled trialObstet Gynecol2007110477177917906008
- BachmannGASchaefersMUddinAUtianWHMicrodose transdermal estrogen therapy for relief of vulvovaginal symptoms in postmenopausal womenMenopause200916587788219458560
- GuptaPOzelBStanczykFZFelixJCMishellDRJrThe effect of transdermal and vaginal estrogen therapy on markers of postmenopausal estrogen statusMenopause2008151949717882008
- SimonJABouchardCWaldbaumAUtianWZborowskiJSnabesMCLow dose of transdermal estradiol gel for treatment of symptomatic postmenopausal women: a randomized controlled trialObstet Gynecol2007109358859617329509
- JohnsonSREttingerBMacerJLEnsrudKEQuanJGradyDUterine and vaginal effects of unopposed ultralow-dose transdermal estradiolObstet Gynecol2005105477978715802405
- PanayNYlikorkalaOArcherDFGutRLangEUltra-low-dose estradiol and norethisterone acetate: effective menopausal symptom reliefClimacteric200710212013117453860
- BoothbyLADoeringPLKipersztokSBioidentical hormone therapy: a reviewMenopause200411335636715167316
- BachmannGSantenRJClinical manifestations and diagnosis of vaginal atrophyBasowDSUpToDateWaltham, MAUpToDate2011 Available from: http://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-vaginal-atrophy?source=search_result&search=Clinical+manifestations+and+diagnosis+of+vaginal+atrophy&selectedTitle=1-45Accessed August 28, 2012
- BondSHortonLSManagement of postmenopausal vaginal symptoms in womenJ Gerontol Nurs201036737
- RoySCaillouetteJCRoyTFadenJSVaginal pH is similar to follicle-stimulating hormone for menopause diagnosisAm J Obstet Gynecol200419051272127715167829
- BrunnerRLAragakiABarnabeiVMenopausal symptom experience before and after stopping estrogen therapy in the Women’s Health Initiative randomized, placebo-controlled trialMenopause201017594695420505547
- SucklingJLethabyAKennedyRLocal oestrogen for vaginal atrophy in postmenopausal womenCochrane Database Syst Rev20064CD00150017054136
- SimonJNachtigallLGutRLangEArcherDFUtianWEffective treatment of vaginal atrophy with an ultra-low-dose estradiol vaginal tabletObstet Gynecol200811251053106018978105
- SimonJNachtigallLUlrichLGEugster-HausmannMGutREndometrial safety of ultra-low-dose estradiol vaginal tabletsObstet Gynecol2010116487688320859151
- WeisbergEAytonRDarlingGEndometrial and vaginal effects of low-dose estradiol delivered by vaginal ring or vaginal tabletClimacteric200581839215804736
- BachmannGBouchardCHoppeDEfficacy and safety of low-dose regimens of conjugated estrogens cream administered vaginallyMenopause200916471972719436223
- PonzoneRBigliaNJacomuzziMEMaggiorottoFMarianiLSismondiPVaginal oestrogen therapy after breast cancer: is it safe?Eur J Cancer200541172673268116239103
- PruthiSSimonJAEarlyAPCurrent overview of the management of urogenital atrophy in women with breast cancerBreast J201117440340821645165
- DewJEWrenBGEdenJAA cohort study of topical vaginal estrogen therapy in women previously treated for breast cancerClimacteric200361455212725664
- KendallADowsettMFolkerdESmithICaution: Vaginal estradiol appears to be contraindicated in postmenopausal women on adjuvant aromatase inhibitorsAnn Oncol200617458458716443612
- RossouwJEAndersonGLPrenticeRLWriting Group for the Women’s Health Initiative InvestigatorsRisks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trialJAMA2002288332133312117397
- North American Menopause SocietyMenopause Practice: A Clinician’s Guide4th edMayfield Heights, OHThe North American Menopause Society2010
- LangerRDEfficacy, safety, and tolerability of low-dose hormone therapy in managing menopausal symptomsJ Am Board Fam Med200922556357319734403
- Eugster-HausmannMWaitzingerJLehnickDMinimized estradiol absorption with ultra-low-dose 10 microg 17beta-estradiol vaginal tabletsClimacteric201013321922720423242
- BachmannGThe estradiol vaginal ring – a study of existing clinical dataMaturitas199522SupplS21S298775773
- MartinPLYenSSBurnierAMHermannHSystemic absorption and sustained effects of vaginal estrogen creamsJAMA19792422426992700228093
- LabrieFCusanLGomezJLEffect of one-week treatment with vaginal estrogen preparations on serum estrogen levels in postmenopausal womenMenopause2009161303618820592
- WhitlockEPOrleansCTPenderNAllanJEvaluating primary care behavioral counseling interventions: an evidence-based approachAm J Prev Med200222426728411988383