Abstract
This overview provides an analysis of some of the immunotherapies currently in use and under investigation, with a special focus on the tumor microenvironment, which we believe is a major factor responsible for the general failure of immunotherapy to date. It is our expectation that combining immunotherapy with methods of altering the tumor microenvironment and targeting regulatory T cells and myeloid cells will yield favorable results.
Introduction
Metastasis occurs in the late stages of cancer development and partly represents the failure of both the innate and adaptive immune systems.Citation1 Several factors support this process; among these, angiogenesis and chronic peritumoral inflammation may be two of the most important.Citation1–Citation3 There are also clinical factors that must be considered and may be of equal importance, such as tumor volume,Citation4 prior treatment history, and the quality and quantity of that treatment,Citation5,Citation6 the struggle for nutrients,Citation7 and the presence or absence of neurotransmitters.Citation8 Despite this complexity, a clearer vision of the interactions between tumor and stroma/environment is possible, and its importance alongside immunotherapy is becoming more and more evident.Citation9,Citation10
Further, with the advent of new drugs (eg, ipilimumab), there has been a renewed clinical interest in cancer immunotherapy. Results from some of these newer immunologic drugs have suggested that active immunotherapy represents an important pathway to eliminating residual disease and obtaining durable and long-lasting responses in cancer patients.Citation10,Citation11
Within the tumor area, several cells are present at the same time. While some of these cells are normal residents, such as fibroblasts and cells of the immune system (leukocytes, lymphocytes, and macrophages), others are recruited. For example, several classes of cell types are recruited to the tumor environment specifically because of the hypoxic microenvironment.Citation11–Citation14 Resident cells initially try to continue their homeostatic existence controlling tumor growth but are eventually substituted by more immature cells – myeloid-derived suppressor cells (MDSCs) – recruited by bone marrow.Citation13,Citation14 Once MDSCs arrive into the tumor area, they are quickly differentiated into cells such as type 2 macrophages (M2s)Citation14 or N2-type neutrophils,Citation15 which are able to sustain this environment of angiogenesis and chronic inflammation.Citation3,Citation9 Ultimately, this results in the creation of an increasingly immunosuppressive environment,Citation16 with the invasion of MDSCs,Citation16 regulatory T cells (Tregs),Citation17 Indoleamine 2, 3-dioxygenase (IDO) cells,Citation18 and exosomes,Citation19 all of which present abundantly in the tumor microenvironment and merit comment as a supporting cast in this progression.Citation9
Cancer immunotherapy
The importance of immunotherapy in cancer treatment and its relationship with other therapies is becoming increasingly better defined. This is certainly the case with chemotherapy, radiotherapy, and hyperthermia. The principal aim of these therapies is to kill tumor cells, which results in the elicitation of the tumor cells’ antigens by way of presenting cells (dendritic cells [DCs] and macrophages). Thus, learning more about the effects of these treatments on the immune system is very important, and the effects are an important aspect of the interaction of these therapies with immune cells.Citation20–Citation22 The development of recombinant interleukin (IL)-2Citation23 and of adoptive immunity against melanoma and renal cell carcinoma, by Rosenberg,Citation24 has confirmed that immunity plays a fundamental role in tumor control and has opened a further therapeutic opportunity.
When the historical first attempts to use immunotherapy in the care of the cancer patient are discussed, mention must be made of Dr William Coley, the New York surgeon who developed what eventually became known as “Coley’s toxins.”Citation25,Citation26 These were used with a certain level of success, but the arrival of chemotherapy largely resulted in a universal abandonment of such therapies. However, as understanding of the role played by natural immunity and of toll-like receptors in cancer treatment has increased,Citation27 the use of microbial treatments utilizing specific bacteria and oncolytic viruses has regained a certain importance.Citation28,Citation29 Further, with our ever-increasing knowledge of cancer immunity, it has become clear that cytotoxic lymphocytes (CTLs) and natural killer (NK) cells play a pivotal role in the cascade of antitumor immunity. We also know that the cytotoxic cell-killing function of these effectors of immune surveillance can be significantly enhanced by various modifying factors. Adoptive immunotherapy of cancer includes cytokines, particularly IL-2 and interferons α and γ, as well as ex vivo activated lymphocytes, the so-called lymphokine-activated killer (LAK) cells. In addition, recently, a promising biotherapy approach involving the design of tumor vaccines based on antigen-presenting DCs has been extensively developed. These two approaches have great potential, and biotherapy clinical trials of them are ongoing in the world’s major cancer centers.Citation24,Citation30
The next part of this article briefly describes some aspects of adoptive immunotherapy and the modification of aspects of the tumor microenvironment, particularly Tregs, exosomes, and MDSCs.
Adoptive immunotherapy
NKs
NK cells were discovered in humans and mice in 1975, with specific functional criteria that correspond to their ability to lyse certain tumor cells in the absence of prior stimulation. NK cells are large granular lymphocytes that belong to the innate immune system. Unlike T or B lymphocytes of adaptive or antigen-specific immunity, NK cells do not rearrange T-cell receptor (TCR) or immunoglobulin genes from their germ-line configuration.Citation31–Citation37 Morphologically, NK cells are large granular lymphocytes that show (due to a large number of secreting granules) high functional activity. NK cells make up only 5%–20% of the total number of lymphocytes,Citation34 including those that express cluster of differentiation (CD) 16 and CD56 surface markers. NK cells are able to detect and lyse cells despite deficiency in the expression of major histocompatibility complex (MHC) class I molecules, improving our understanding of the function and the role of NK cells in the immune response.Citation36 NK cells have IL-2 receptors and can evidently be activated by this endogenous cytokine or its exogenous analogs. NK cells are thus effectors of innate immunity and, unlike T-killer cells, their function does not require a cascade of antigen-presentation reactions.Citation37
As with neutrophils, NK cells may be considered the first line of defense of immune surveillance, as they can cause lysis of a transformed cell after contacting it, without any additional stimuli. However, their triggering function relies on a complex balance between inhibitory and activating signals that requires not only deficient MHC class I expression on target cells but also the expression of inducible ligands of activating NK-cell receptors.Citation36,Citation37 Both of these points are crucial for antitumor immunity performance, since, in the course of transformation, tumor cells may shed MHC molecules, lose tissue-specific antigens, and, what is more, can acquire features of embryonic cells (low-differentiated embryo carcinomas), thereby “escaping” specific immunity. However, these particular malignant cells may become the target for NKs, which have the ability to recognize and destroy a wide range of abnormal cells (including tumor, virus-infected, antibody-bound, and allogeneic cells) and stressed cells without damaging healthy and normal “self ” cells.Citation34,Citation36,Citation37
LAK generation
IL-2 stimulation of lymphocytes leads to expression of the so-called LAK cells. LAKs are a heterogeneous population of cells consisting primarily of NK, NKT, and T cells, which are generated in vitro by culture of peripheral blood mononuclear cells in the presence of IL-2. The major effector subset in the LAK population is of NK cells, which are mechanistically equivalent to peripheral blood NK cells but are more cytotoxic against tumor cells, including NK-resistant targets.Citation38–Citation41
Adoptive IL-2/LAK therapy of cancer
The first true clinical progress in immunotherapy was seen after the introduction of recombinant DNA technology for the production of immune-stimulating cytokines. Since 1985, studies on combined IL-2 and LAK cell treatment have been published.Citation23,Citation24 Such clinical trials have shown that high-dose IL-2 alone or in combination with LAK cells mediates objective tumor regression in 17%–28% of patients with metastatic renal cancer or metastatic melanoma, while prolonged remission was even observed in some patients with metastatic cancers.Citation23,Citation24,Citation40
Some authors have reported on clinical trials of the systemic treatment with high-dose IL-2 and tumor-infiltrating lymphocytes (autologous lymphocytes can be isolated from tumor-infiltrating cells, which presumably express tumor-specific TCRs) of patients with advanced cancer. Such treatment resulted in a 34% objective response rate of patients with metastatic melanoma.Citation40 Although there was considerable clinical interest in LAKs for antitumor therapy by the end of the last century, LAK therapy has failed to obtain public support as a standard therapy for cancer patients. This was largely the result of limited responses to the immunotherapy when compared with those to chemotherapy or radiation therapy, and there were concerns about toxicity associated with the IL-2 infused simultaneously in order to maintain LAK activation. Another confounding factor was that most studies on immunotherapy used terminal-stage patients with virtually no remaining immune response capabilities, as they had failed to respond to previous conventional treatments.Citation41
More recently, a new, cell-based immunotherapy utilizing activated lymphocytes has been suggested as an adjuvant regimen to radical surgery of cancer patients. Kimura and YamaguchiCitation42 conducted a randomized trial of 174 patients with non-small-cell lung carcinoma comparing IL-2/LAK therapy in combination with chemotherapy versus chemotherapy alone. Patients had undergone curative resection of their lung carcinoma and received six to eight courses of IL-2/LAK therapy over 2 years. The authors reported an improvement in the 5- and 9-year survival rates of 21% and 28%, respectively.
Adjuvant treatment of solid tumors has also involved cytokine-induced killers (CIKs). CIK cells are a heterogeneous subset of ex vivo expanded T lymphocytes presenting a mixed T-NK phenotype and have unrestricted MHC antitumor activity.Citation43 In the setting of hepatocellular carcinoma and gastric cancers, adjuvant infusions of autologous CIK cells after surgical resection resulted in a significant increase in disease-free survival.Citation44–Citation46 To increase IL-2/LAK immunotherapy effectiveness, local and loco-regional infusions were performed, allowing for the effective concentration of activated killers at the site of the lesion. The most significant clinical effects were achieved with intra-cavity infusions of IL-2 and LAKs in patients with malignant effusions (pleuritis, ascites, and pericarditis). Malignant effusion regression was seen in 70%–95% of cases, showing good tolerance and effectiveness in chemotherapy-resistant cancer types.Citation47 One of the advantages of adjuvant loco-regional immunotherapy is that these low IL-2 immunostimulating doses cause no marked side effects, including immune- and/or myelosuppression, which are characteristic of high-dose cytokine therapy.
These LAK- and CIK-cell immunotherapy methods aim to stimulate the innate chain of antitumor immunity, which is a reasonable approach because most tumors express little to no MHC or tumor antigens. It is also necessary to consider the fact that T killers constitute an essential part of lymphoid cell populations and are responsible for a more specific mechanism of action – in these conditions, they are not involved in the antitumor defense function. Therefore, another promising approach in antitumor biotherapy is focusing on designing vaccines, in particular DC-based vaccines, to activate adaptive immunotherapy.
Tumor-infiltrating lymphocytes (TILs) in cancer immunotherapy
TILs derived from patients with a variety of histological cancer types have demonstrated that cellular immune reactions against established malignancies exist in humans. TILs are heterogeneous populations of T cells, which contain not only CD4+ and CD8+ T lymphocytes (as previously reported),Citation30,Citation38,Citation40 but also a small and, in some cases, significant fraction of γδ T cells, with a prevalence of the Vδ1 subset.Citation48,Citation49
TILs that infiltrate melanoma could specifically recognize tumor-associated antigens.Citation30 Chemotherapy-induced lymphodepletion prior to adoptive cell infusion may lead to the dramatic enhancement of the persistence of the transferred cells and improved anticancer effects.Citation30 Early results in patients with metastatic melanoma treated with the adoptive transfer of autologous TILs selected for antitumor activity – expanded in vitro and then re-infused into patients along with IL-2, following a lymphodepleting preparative regimen – do exist.Citation30 In clinical trials with increasing lymphodepletion prior to infusion of autologous TILs, objective response rates between 49% and 72% were seen for patients with metastatic melanoma.Citation30
Limitations of TIL therapy, including the requirement for surgery to isolate the tumor and the need to consistently generate T cells with antitumor activity, have led to novel strategies for redirecting normal T cells to recognize tumor-associated antigens (eg, NY-ESO-1, carcinoembryonic antigen [CEA], anti-CD20) using genetically engineered tumor antigen-specific TCRs or chimeric antigen receptor genes. As an alternative to TIL therapy, highly avid TCRs can be cloned from naturally occurring T cells, and then gene transfer vectors can be used to introduce these into the patient’s lymphocytes. In this manner, large numbers of antigen-specific T cells can be rapidly generated, in comparison with the long-term expansion required for TILs. These highly reactive T-cell clones are able to recognize and effectively lyse target tumor cells.Citation30,Citation48
Recently, several clinical trials have reported the clinical efficacy and benefit of gene-modified T cells for the treatment of different cancers, including melanoma, colorectal and synovial cell cancers, neuroblastoma, and lymphoma. In patients with synovial cell cancer, the measurable response rate was 66%, while in melanoma patients this was 45%.Citation47,Citation50
Autologic vaccines based on DCs
DCs are the quintessential antigen-presenting cells (APC) and have the unique ability to induce a primary immune response. DCs both prime naive cytotoxic T cells and activate long-term memory cells. In addition to these essential functions in adaptive immunity, DCs can also activate B cells and NKs.Citation51
Methods of DC generation to produce antitumor vaccines
Mature DCs for antitumor vaccines are typically generated from CD14+ monocytes according to a well-known two-stage method. The initial stage is cultivation for 6–7 days in the presence of granulocyte macrophage colony-stimulating factor and IL-4 in macrophage-conditioned medium.Citation52
The second stage – DC maturation – may proceed in the presence of various factors, such as bacteria (live or dead), bacterial products, lipopolysaccharide, viruses, two-strand RNA or its analog poly-I:C, pro-inflammatory factors and their combinations (IL-1β, tumor necrosis factor-α, IL-6, prostaglandin E2 [PGE2]), and CD40 ligand (CD40L). Compared to mature Dendritic Cells, maturing DCs lose their ability for endocytosis and the processing of antigens.Citation51,Citation52
Early studies on the use of DCs involved only small groups of patients but reported potentially promising results.Citation53,Citation54 Today, we have access to the results of over 200 clinical trials that have assessed DC-based vaccines, yet their clinical effectiveness and expedience for use in cancer patients becomes more and more doubtful. Rosenberg et al argued that early optimism for DC vaccines was based on dubious surrogate end-points, which lacked robustness, rather than on proof of antitumor effects.Citation55–Citation57 One trial, conducted at the Surgery Branch of the National Cancer Institute on 440 patients, yielded an overall objective response rate of only 2.6%.Citation55 This was comparable to the 4.0% response rate reported in 40 other smaller studies on a combined total of 756 patients.Citation57 More recent studies have shown partial or complete regression rates of 4%–12% in patients with advanced cancer.Citation55,Citation57
When compared with IL-2/LAK therapy, the clinical effectiveness of DC-based therapy has not been reported to be more effective, and was sometimes less, than that of IL-2/LAK.Citation55,Citation57 These limited response rates may be due to the fact that DC-vaccine therapy results in stimulation of effector cells of innate immunity, where the antitumor effect is not specifically taught to T lymphocytes for long-lasting protection. Moreover, there are even data that suggest DC vaccination can have a detrimental effect and may even be associated with a worse outcome.Citation57
In recent years, there have been reports about the efficiency of a new cell-based immunotherapy for advanced prostate cancer called “sipuleucel-T.” Sipuleucel-T is an autologous active cellular immunotherapy consisting of peripheral blood mononuclear cells, including APCs. It is the first therapeutic cancer vaccine to have received US Food and Drug Administration approval. The treatment resulted in a prolonged median overall survival, but only in patients with no signs of disease progression.Citation58–Citation60 It is hypothesized that the activated APCs promote endogenous T cells to destroy prostatic acid phosphatase (PAP)-bearing prostate cancer cells, although the vaccine’s precise mechanism of action is not yet understood.Citation58–Citation60 However, there is a lack of data about the generation of adaptive immunity using this method, suggesting that its antitumor effect is achieved mainly by activating effectors of innate immunity as a result of stimulating factors secreted by DCs; that is to say, the DCs may be considered a cellular adjuvant to NK cells and other innate immunity cells.
Overall, DC-based vaccines have not demonstrated any significant clinical efficacy and the outcomes of clinical trials have largely been poor. A more efficient approach using this method might be to use it to prolong progression-free survival in cancer patients who have had maximal cytoreduction via surgery and/or chemotherapy.Citation61 DCs may also be used as a cellular adjuvant for other cancer immunotherapy strategies, in particular, in combination with LAK (CIK) therapy.Citation62,Citation63 Such an approach may improve the effectiveness of cell-based antitumor immunotherapy due to the simultaneous activation of both innate and adaptive immunity. Further, taking into account NK/DC interactions, such combination treatments may increase the effectiveness of LAK cells by DC stimulation and enhance the generation of CTLs in their presence.
Control of the tumor microenvironment
The tumor microenvironment is a microcosm of cells and extracellular matrices that continuously interact and evolve. The support cells (ie, fibroblasts, adipocytes), the matrix, and the immunity cells that are partly comprised of normal residents and partly of recruited cells (Tregs, MDSCs, macrophages, and neutrophils) work in concert with the tumor cells to create an inflammatory microenvironment that permits their growth and metastasis.Citation11–Citation13 The inflammatory microenvironment is largely responsible for the failure of host immune surveillance.Citation64 Further, the excessive production of lipid mediators such as PGE2, immune regulator cytokines such as IL-10, and transforming growth factor-β (TGF-β) carries through to immunosuppression.Citation65,Citation66 The excessive production of these lipid mediators in this already inflamed microenvironment allows for control of Tregs and MDSCs,Citation65–Citation67 creating the perfect conditions for chronic disease.Citation65 The immunosuppressive activity of PGE2 has long been known, and we know it is continually produced by tumor cells and by their stroma.Citation68 Its excessive production can regulate Treg and MDSC recruitment.Citation65–Citation70 Cyclooxygenase-2 (COX2) inhibitors have been demonstrated to decrease PGE2, and thus the recruitment of Tregs in mouse mammary modelsCitation71 and lung models,Citation72 and are associated with a differentiation effect on MDSCsCitation70,Citation73 that has been demonstrated in rare tumors such as mesothelioma.Citation74
Leibovici et alCitation11,Citation12 have noted that the tumor microenvironment is a target for TGF-β action that stimulates tumor progression via pro-tumorigenic effects on vascular, immune, and fibroblastic cells. According to these authors,Citation11,Citation12 there are several preclinical types of TGF-β inhibitors, each of which must be used prudently. Another approach to controlling the excessive production of TGF-β in the tumor microenvironment is the use of oral proteolytic enzymes, as demonstrated by Desser et alCitation75 in patients with rheumatoid arthritis, osteomyelofibrosis, and herpes zoster. The mechanism of action of these enzymes seems to be linked to inactivation by α2 macroglobulin.Citation75 MDSCs are identified as CD11b+Gr1+ in mice and CD33+HLA–DR–Lin–in humans although numerous additional markers (eg S100A, etc) have been used to categorize MDSC subsets.Citation13,Citation76–Citation78 MDSCs are a heterogeneous group of mature and immature myeloid cellsCitation79 that demonstrate strong immunosuppressive activity, due to several factors triggered by the phosphorylation of signal transducer and activator of transcription 3 (STAT3).Citation80 Once STAT3 is phosphorylated, MDSCs are activated and produce various products such as PGE2, vascular endothelial growth factor, reactive oxygen species, IL-10 and IL-6, nitric oxide synthase, and low-molecular-weight cytoplasmic proteins called S100A8/9 (which is able to fix calcium).Citation80,Citation81 Due to their heterogeneity, MDSCs are not easily treated; nonetheless, currently, several clinical approaches are in development and trials are underway to this end.Citation13,Citation81–Citation83 Various approaches have been used to attempt to control the recruitment,Citation82 differentiation,Citation13,Citation81 and number – by appropriate chemotherapy – of MDSCs.Citation84 Recently, Ghiringhelli et al reported on a novel approach to controlling tumor immunity and inflammation via polyphenols such as resveratrol, curcumin, genistein, and epigallocatechin.Citation85 This novel treatment method is of particular interest at least because the side effects are minimal, and these natural molecules act simultaneously on a number of key control points (IL-10, TGF-β, PGE2, leukotrienes) and by increasing tumor cell death.Citation85 Some of the drugs used to modulate MDSCs in humans and their mechanisms of action are presented in Citation13,Citation17,Citation70–Citation92 and .
Figure 1 Innate and adaptive immunity in cancer and therapeutic interventions.
Abbreviations: ATRA, all-trans retinoic acid; CCL3, chemokine (C-C motif) ligand 3; CD, cluster of differentiation; COX2, cyclooxygenase-2; CTL, cytotoxic lymphocyte; CTLA-4, cytotoxic T-lymphocyte antigen 4; CXCL12, chemokine (C-X-C motif) ligand 12; DCs, dendritic cells; IDC, immature dendritic cells; IL, interleukin; PD-1, programmed cell death protein 1; PGE2, prostaglandin E2; ROS, reactive oxygen species; TGF-β, transforming growth factor beta; VEGF, vascular endothelial growth factor.
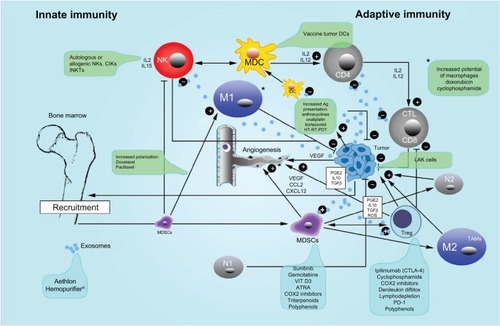
Table 1 Clinical drugs modulating human myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) and their mechanism(s) of action
Another group of cells present in the blood and the hypoxic tumor microenvironment that are able to suppress the host immune responseCitation93,Citation94 is the Tregs, classified as CD4+CD25+FOxp3. They contribute to angiogenesis and to poor survival in many solid tumors such as ovary, breast, colorectal, lung, and pancreatic cancers.Citation93–Citation95 Tregs can be depleted or modulated in five ways: (1) by depletion (gemcitabine,Citation93 metronomic cyclophosphamide,Citation96 denileukin diftitox),Citation97 (2) by irradiation,Citation98 (3) by inhibition of Treg function (CTLA-4 antibodies [ie, ipilimumab]),Citation99 (4) by blocking their migration to the tumor,Citation100 and (5) by modifying key molecules in tumor microenvironment (ie, COX2 inhibitors,Citation101 STAT3,Citation17 extracellular cyclic AMP and adenosine)Citation102 (Citation17,Citation93–Citation102 and ).
A molecule of special interest for controlling Treg is ipilimumab.Citation103–Citation105 Ipilimumab (Yervoy®, Bristol-Myers Squibb, New York, NY, USA) is a human immunoglobulin 1 monoclonal antibody able to bind to CTLA-4 receptors, thus blocking their interaction with protein B7.Citation104 Several clinical trials have been conducted with this drug, particularly on melanoma and castration-resistant prostate cancer.Citation104–Citation109 A survival benefit has been obtained using a dose of 3 mg/kg endovenous every 3 weeks for four doses,Citation104 with or without a melanoma vaccine polypeptide (glycoprotein 100).
An ipilimumab study by Hodi et al showed a median overall improvement in survival for stage III and IV melanoma patients from 6 to 10 months, and the drug’s efficacy was unaffected by the presence of glycoprotein 100 vaccine.Citation106 A study by Robert et al on ipilimumab and dacarbazine for previously untreated metastatic melanoma, demonstrated an improvement in survival time, but increased side effects when compared with dacarbazine alone.Citation107 Melanoma patients with brain metastasesCitation108 as well as cases of uveal melanomaCitation109 have improved survival with Yervoy. Ipilimumab use has also been suggested for castration-resistant prostate cancer,Citation110 as already mentioned, and lung tumors.Citation111
However, important side effects from Yervoy have been reported, including tiredness, diarrhea, itching, rash, hemolytic anemia, infection, and death.Citation112 The seriousness of some of these side effects has led Bakacs et al to consider ipilimumab a “catastrophe” and they have suggested a critical reassessment of immune checkpoint blockade methodology.Citation113 It is our opinion that the effects of Yervoy on circulating Treg are certain, but their action on Tregs in the tumor microenvironment is uncertain and even doubtful. It has been suggested that monoclonal antibodies (mAbs) do not easily reach the targeted tumor mass.Citation114 Reuben et al used another CTLA-4 inhibitor, ticilimumab (now called “tremelimumab”), in melanoma, and achieved results similar to those obtained with ipilimumab.Citation115
Another class of immune checkpoint protein blockers is aimed to act as mAbs to programmed death receptor-1 (PD-1)Citation116 and its ligand, PD-L1. These receptors are not only expressed on MDSC cells and TregCitation116 but are also overexpressed in a variety of human cancers including lung, ovarian, skin, colon, esophagus, renal, stomach, and breast.Citation116,Citation117 Another humanized mAb, BMS-936558 (immunoglobulin 4; Bristol-Myers Squibb), has generated interesting, albeit preliminary, results.Citation118,Citation119 The most striking of these is that melanoma and renal-cell cancers are the most responsive tumors – second only to those which immunohistochemical analysis shows are positive for PD-1 receptors on their surface.Citation119–Citation121 Side effects have been less severe than with Yervoy according to Tang and Heng,Citation121 who assert that PD-L1 is selectively expressed on many tumors and within the tumor microenvironment when compared with CTLA-4. The most common side effects reported with use of the anti-PD-L1 antibody BMS-936559 and anti-PD-1 antibodies (eg, BMS-936558) have been fatigue, rash, pruritus, arthralgia, and nausea (listed in order of appearance).Citation121 As some authors have outlined, a combination of PD-1 and CTLA-4 antibodies seems possible and may be potentially synergistic; however, some caution must be exercised when using this combination in humans.Citation122
The last group of immunosuppressive molecules released by tumors that we address here is exosomes. Exosomes are microparticles 30–100 μm in size containing a variety of different molecules from signal peptides to mRNA, micro RNA, and lipids. They are either released into the extracellular fluid or may enter circulation, resulting in an increase in Treg numbers,Citation123 tumor progression,Citation124 and tumor immune evasion.Citation125 Recently, an interesting approach to removing these particles has been used that merits some attention. Using an ADAPT™ device (a “Hemopurifier®”; Aethlon Medical, San Diego, CA, USA), Marleau et alCitation19 were able to remove exosomes containing human epidermal growth factor receptor 2 oncoproteins in patients with breast cancer overexpressing human epidermal growth factor receptor 2 receptors. This method is not altogether new, but the first attempts by LentzCitation126 used ultrapheresis in the treatment of solid tumors. This newer Hemopurifier approach uses the same cartridges used in standard dialysis units, thus does not require the purchase of a new device, making this a novel and easily incorporated technique.
Conclusion
Cancer is a complex system that learns to adapt to its environment, slowly recruiting its host for its own selfish growth and maintenance needs and to evade the immune system. Our rapidly developing understanding of the immune system and the tumor microenvironment is allowing researchers and clinicians to better target treatments against cancer. Understanding that the tumor microenvironment is hypoxic and in a state of chronic inflammation allows us to change variables to make the stroma less tumor promoting. Stimulation of the innate immune system may lead to short-term benefits, to have a long-term benefic it must be followed by DC, IL-2/LAK or similar cytotoxic cell infusion. There are many exciting mAbs and drugs developed against various immune-related receptors such as Ipilimumab or PD-1, and for controlling Treg cells and MDSCs. Such immune system treatments hold a lot of promise. By harnessing our understanding of the immune system, we will be better able to work with this incredible system. By combining treatments aimed at both immunity and tumor microenvironment, it is our belief that a new benchmark in metastatic cancer therapy will be achieved.
Disclosure
The authors report no conflicts of interest in this work.
References
- CrociDOSalatinoMTumor immune escape mechanisms that operate during metastasisCurr Pharm Biotechnol201112111923193621470132
- MareelMConstantinoSEcosystems of invasion and metastasis in mammary morphogenesis and cancerInt J Dev Biol2011557–967168422161824
- MacDonaldNChronic inflammatory states: their relationship to cancer prognosis and symptomsJ R Coll Physicians Edinb201141324625321949925
- GermainRNTumor immunologyBenaceraffBUnanueERTextbook of ImmunologyBaltimore, MDWilliams & Wilkins1979196217
- BarrettAJSavaniBNDoes chemotherapy modify the immune surveillance of hematological malignancies?Leukemia2009231535818830260
- ShurinMRNaiditchHGutkinDWUmanskyVShurinGVChemoImmunoModulation: immune regulation by the antineoplastic chemotherapeutic agentsCurr Med Chem201219121792180322414087
- WensveenFMvan GisbergenKPElderingEThe fourth dimension in immunological space: how the struggle for nutrients selects high-affinity lymphocytesImmunol Rev201224918410322889217
- VossMJEntschladenFTumor interactions with soluble factors and the nervous systemCell Commun Signal201082120822525
- ShiaoSLGanesanAPRugoHSCoussensLMImmune microenvironments in solid tumors: new targets for therapyGenes Dev201125242559257222190457
- MellmanICoukosGDranoffGCancer immunotherapy comes of ageNature2011480737848048922193102
- LeiboviciJItzhakiOHuszarMSinaiJThe tumor microenvironment: part 1Immunotherapy20113111367138422053887
- LeiboviciJItzhakiOHuszarMSinaiJTargeting the tumor microenvironment by immunotherapy: part 2Immunotherapy20113111385140822053888
- MartinFApetohLGhiringhelliFRole of myeloid-derived suppressor cells in tumor immunotherapyImmunotherapy201241435722150000
- HaoNBLüMHFanYHCaoYLZhangZRYangSMMacrophages in tumor microenvironments and the progression of tumorsClin Dev Immunol2012201294809822778768
- FridlenderZGSunJKimSPolarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TANCancer Cell200916318319419732719
- BianchiGBorgonovoGPistoiaVRaffaghelloLImmunosuppressive cells and tumour microenvironment: focus on mesenchymal stem cells and myeloid derived suppressor cellsHistol Histopathol201126794195121630223
- ByrneWLMillsKHLedererJAO’SullivanGCTargeting regulatory T cells in cancerCancer Res201171226915692022068034
- MunnDHBlocking IDO activity to enhance anti-tumor immunityFront Biosci (Elite Ed)2012473474522201909
- MarleauAMChenCSJoyceJATullisRHExosome removal as a therapeutic adjuvant in cancerJ Transl Med20121013422738135
- MaYConfortiRAymericLL. How to improve the immunogenicity of chemotherapy and radiotherapyCancer Metastasis Rev2011301718221298323
- RosentalBAppelMYYossefRHadadUBrusilovskyMPorgadorAThe effect of chemotherapy/radiotherapy on cancerous pattern recognition by NK cellsCurr Med Chem201219121780179122414084
- BaronzioGGramagliaAFiorentiniGHyperthermia and immunity. A brief overviewIn Vivo2006206A68969517203747
- ChangAERosenbergSAOverview of interleukin-2 as an immunotherapeutic agentSemin Surg Oncol1989563853902688029
- RosenbergSAThe development of new immunotherapies for the treatment of cancer using interleukin-2. A reviewAnn Surg198820821211353041925
- TsungKNortonJALessons from Coley’s ToxinSurg Oncol2006151252816814541
- WiemannBStarnesCOColey’s toxins, tumor necrosis factor and cancer research: a historical perspectivePharmacol Ther19946435295647724661
- Rakoff-NahoumSMedzhitovRToll-like receptors and cancerNat Rev Cancer200991576319052556
- LawsonKAMorrisDGOncolytic virotherapy for renal cell carcinoma: a novel treatment paradigm?Expert Opin Biol Ther201212789190322564154
- PatyarSJoshiRByravDSPrakashAMedhiBDasBKBacteria in cancer therapy: a novel experimental strategyJ Biomed Sci20101712120331869
- RestifoNPDudleyMERosenbergSAAdoptive immunotherapy for cancer: harnessing the T cell responseNat Rev Immunol201212426928122437939
- KiesslingRKleinEWigzellH“Natural” killer cells in the mouse. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotypeEur J Immunol1975521121171234049
- CaligiuriMAHuman natural killer cellsBlood2008112346146918650461
- BironCAvan den ElsenPTuttMMMedveczkyPKumarVTerhorstCMurine natural killer cells stimulated in vivo do not express the T cell receptor alpha, beta, gamma, T3 delta, or T3 epsilon genesJ Immunol19871395170417103497976
- RobertsonMJRitzJBiology and clinical relevance of human natural killer cellsBlood19907612242124382265240
- KärreKLjunggrenHGPiontekGKiesslingRSelective rejection of H-2-deficient lymphoma variants suggests alternative immune defense strategyNature198631960556756783951539
- LanierLLNatural killer cells: from no receptors to too manyImmunity1997643713789133416
- LanierLLMissing self, NK cells, and the white albumJ Immunol200517411656515905491
- GrimmEAMazumderAZhangHZRosenbergSALymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin-2 activated autologous human peripheral blood lymphocytesJ Exp Med19821556182318416176669
- RosenbergSALotzeMTYangJCProspective randomized trial of high-dose interleukin-2 alone or in conjunction with lymphokine-activated killer cells for the treatment of patients with advanced cancerJ Natl Cancer Inst19938586226328468720
- KammulaUSMarincolaFMCancer immunotherapy: is there real progress at last?BioDrugs199911424926018031135
- RosenbergSAImmunotherapy of patients with advanced cancer using interleukin-2 alone or in combination with lymphokine activated killer cellsImportant Adv Oncol19882172573042605
- KimuraHYamaguchiYAA phase III randomized study of interleukin-2 lymphokine-activated killer cell immunotherapy combined with chemotherapy or radiotherapy after curative or noncurative resection of primary lung carcinomaCancer199780142499210707
- SangioloDCytokine induced killer cells as promising immunotherapy for solid tumorsJ Cancer2011236336821716717
- WengDSZhouJZhouQMMinimally invasive treatment combined with cytokine-induced killer cells therapy lower the short-term recurrence rates of hepatocellular carcinomasJ Immunother2008311637118157013
- WuCJiangJShiLXuNProspective study of chemotherapy in combination with cytokine-induced killer cells in patients suffering from advanced non-small cell lung cancerAnticancer Res2008286B3997400219192663
- ShiLZhouQWuJEfficacy of adjuvant immunotherapy with cytokine-induced killer cells in patients with locally advanced gastric cancerCancer Immunol Immunother201261122251225922674056
- ShubinaIZhBliumenbergAGVolkovSMDemidovLVKiselevskiĭMVAdoptive immunotherapy of malignanciesVestn Ross Akad Med Nauk200711915 Russian18080522
- MorganRADudleyMEWunderlichJRCancer regression in patients after transfer of genetically engineered lymphocytesScience2006314579612612916946036
- DoniaMEllebaekEAndersenMHStratenPSvaneIMAnalysis of Vδ1 T cells in clinical grade melanoma-infiltrating lymphocytesOncoimmunol20121812971304
- ShiHLiuLWangZImproving the efficacy and safety of engineered T cell therapy for cancerCancer Lett2013328219119723022475
- KellerRDendritic cells:their significance in health and diseaseImmunol Lett20017811312211578684
- SallustoFNicoloCDe MariaRCorintiSTestiRCeramide inhibits antigen uptake and presentation by dendritic cellsJ Exp Med19961846241124168976196
- HsuFJBenikeCFagnoniFVaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cellsNat Med19962152588564842
- NestleFOAlijagicSGillietMVaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cellsNat Med1998433283329500607
- RosenbergSAYangJCRestifoNPCancer immunotherapy: moving beyond current vaccinesNat Med200410990991515340416
- OshitaCTakikawaMKumeADendritic cell-based vaccination in metastatic melanoma patients: phase II clinical trialOncol Rep20122841131113822895835
- EggermontAMTherapeutic vaccines in solid tumours: can they be harmful?Eur J Cancer200945122087209019477117
- ChrvalaCAThe changing landscape of treatment options for metastatic castrate-resistant prostate cancer: challenges and solutions for physicians and patientsP T201237845346323091338
- PloskerGLSipuleucel-T: in metastatic castration-resistant prostate cancerDrugs201171110110821175243
- Di LorenzoGFerroMBuonerbaCSipuleucel-T (Provenge®) for castration-resistant prostate cancerBJU Int20121102 Pt 2E99E10422177289
- CathelinDNicolasABouchotAFraszczakJLabbéJBonnotteBDendritic cell-tumor cell hybrids and immunotherapy: what’s next?Cytotherapy201113777478521299362
- LiHWangCYuJDendritic cell-activated cytokine-induced killer cells enhance the anti-tumor effect of chemotherapy on non-small cell lung cancer in patients after surgeryCytotherapy20091181076108319929470
- WestEJScottKJJenningsVAMelcherAAImmune activation by combination human lymphokine-activated killer and dendritic cell therapyBr J Cancer2011105678779521847125
- AllenMLouise JonesJJekyll and Hyde: the role of the microenvironment on the progression of cancerJ Pathol2011223216217621125673
- KhatamiMUnresolved inflammation: ‘immune tsunami’ or erosion of integrity in immune-privileged and immune-responsive tissues and acute and chronic inflammatory diseases or cancerExpert Opin Biol Ther201111111419143221663532
- Ben-BaruchAInflammation-associated immune suppression in cancer: the roles played by cytokines, chemokines and additional mediatorsSemin Cancer Biol2006161385216139507
- KalinskiPRegulation of immune responses by prostaglandin E2J Immunol20121881212822187483
- Le BitouxMAStamenkovicITumor-host interactions: the role of inflammationHistochem Cell Biol200813061079109018953558
- HariziHGualdeNPivotal role of PGE2 and IL-10 in the cross-regulation of dendritic cell-derived inflammatory mediatorsCell Mol Immunol20063427127716978535
- SevkoAUmanskyVMyeloid-derived suppressor cells interact with tumors in terms of myelopoiesis, tumorigenesis and immunosuppression: thick as thievesJ Cancer20134131123386900
- KaravitisJHixLMShiYHSchultzRFKhazaieKZhangMRegulation of COX2 expression in mouse mammary tumor cells controls bone metastasis and PGE2-induction of regulatory T cell migrationPLoS One201279e4634223029485
- SharmaSYangSCZhuLTumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancerCancer Res200565125211522015958566
- ObermajerNMuthuswamyRLesnockJEdwardsRPKalinskiPPositive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cellsBlood2011118205498550521972293
- VeltmanJDLambersMEvan NimwegenMCOX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma. Celecoxib influences MDSC functionBMC Cancer20101046420804550
- DesserLHolomanovaDZavadovaEPavelkaKMohrTHerbacekIOral therapy with proteolytic enzymes decreases excessive TGF-beta levels in human bloodCancer Chemother Pharmacol200147SupplS10S1511561866
- SrivastavaMKAnderssonÅZhuLMyeloid suppressor cells and immune modulation in lung cancerImmunotherapy20124329130422401635
- PastułaAMarcinkiewiczJMyeloid-derived suppressor cells: a double-edged sword?Int J Exp Pathol2011922737821314739
- NagarajSGabrilovichDIMyeloid-derived suppressor cells in human cancerCancer J201016434835320693846
- GretenTFMannsMPKorangyFMyeloid derived suppressor cells in human diseasesInt Immunopharmacol201111780280721237299
- ChalminFMignotGGhiringhelliFMyeloid-derived suppressor cells: a key player in cancerMed Sci (Paris)2010266–757657920619155
- FilipazziPHuberVRivoltiniLPhenotype, function and clinical implications of myeloid-derived suppressor cells in cancer patientsCancer Immunol Immunother201261225526322120756
- KaoJKoECEisensteinSTargeting immune suppressing myeloid-derived suppressor cells in oncologyCrit Rev Oncol Hematol2011771121920304669
- NaiditchHShurinMRShurinGVTargeting myeloid regulatory cells in cancer by chemotherapeutic agentsImmunol Res2011502–327628521717082
- DjeuJWeiSChemoimmunomodulation of MDSCs as a novel strategy for cancer therapyOncoimmunology20121112112222720231
- GhiringhelliFRebeCHichamiADelmasDImmunomodulation and anti-inflammatory roles of polyphenols as anticancer agentsAnticancer Agents Med Chem201212885287322292769
- KoJSZeaAHRiniBISunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patientsClin Cancer Res20091562148215719276286
- TonguMHarashimaNMonmaHMetronomic chemotherapy with low-dose cyclophosphamide plus gemcitabine can induce anti-tumor T cell immunity in vivoCancer Immunol Immunother201362238339122926062
- LathersDMClarkJIAchilleNJYoungMRPhase IB study of 25-hydroxyvitamin D(3) treatment to diminish suppressor cells in head and neck cancer patientsHum Immunol200162111282129311704292
- MirzaNFishmanMFrickeIAll-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patientsCancer Res200666189299930716982775
- ApetohLVégranFLadoireSGhiringhelliFRestoration of antitumor immunity through selective inhibition of myeloid derived suppressor cells by anticancer therapiesCurr Mol Med201111536537221568934
- van CruijsenHHoekmanKStamAGDefective differentiation of myeloid and plasmacytoid dendritic cells in advanced cancer patients is not normalized by tyrosine kinase inhibition of the vascular endothelial growth factor receptorClin Dev Immunol200720071731518320010
- UgelSDelpozzoFDesantisGTherapeutic targeting of myeloid-derived suppressor cellsCurr Opin Pharmacol20099447048119616475
- FacciabeneAMotzGTCoukosGT-regulatory cells: key players in tumor immune escape and angiogenesisCancer Res20127292162217122549946
- WilkeCMWuKZhaoEWangGZouWPrognostic significance of regulatory T cells in tumorInt J Cancer2010127474875820473951
- ZouWRegulatory T cells, tumour immunity and immunotherapyNat Rev Immunol20066429530716557261
- GhiringhelliFMenardCPuigPEMetronomic cyclophosphamide regimen electively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patientsCancer Immunol Immunother200756564164816960692
- CoolsNPonsaertsPVan TendelooVFBernemanZNRegulatory T cells and human diseaseClin Dev Immunol200720078919518317534
- MuranskiPBoniAWrzesinskiCIncreased intensity lymphodepletion and adoptive immunotherapy – how far can we go?Nat Clin Pract Oncol200631266868117139318
- GrazianiGTentoriLNavarraPIpilimumab: a novel immunostimulatory monoclonal antibody for the treatment of cancerPharmacol Res201265192221930211
- PereHTanchotCBayryJComprehensive analysis of current approaches to inhibit regulatory T cells in cancerOncoimmunology20121332633322737608
- YaqubSHenjumKMahicMRegulatory T cells in colorectal cancer patients suppress anti-tumor immune activity in a COX-2 dependent mannerCancer Immunol Immunother200857681382117962941
- MandapathilMSzczepanskiMJSzajnikMAdenosine and prostaglandin E2 cooperate in the suppression of immune responses mediated by adaptive regulatory T cellsJ Biol Chem201028536275712758020558731
- DienstmannRMarkmanBTaberneroJApplication of monoclonal antibodies as cancer therapy in solid tumorsCurr Clin Pharmacol20127213714522432839
- LipsonEJDrakeCGIpilimumab: an anti-CTLA-4 antibody for metastatic melanomaClin Cancer Res201117226958696221900389
- SinghNMadanRAGulleyJLIpilimumab in prostate cancerExpert Opin Biol Ther201313230331323265575
- HodiFSO’DaySJMcDermottDFImproved survival with ipilimumab in patients with metastatic melanomaN Engl J Med2010363871172320525992
- RobertCThomasLBondarenkoIIpilimumab plus dacarbazine for previously untreated metastatic melanomaN Engl J Med2011364262517252621639810
- MargolinKIpilimumab in a Phase II trial of melanoma patients with brain metastasesOncoimmunology2012171197119923170278
- KhattakMAFisherRHughesPGoreMLarkinJIpilimumab activity in advanced uveal melanomaMelanoma Res2013231798123211837
- GerritsenWRThe evolving role of immunotherapy in prostate cancerAnn Oncol201223Suppl 8viii22viii2722918924
- ReckMBondarenkoILuftAIpilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trialAnn Oncol2013241758322858559
- FellnerCIpilimumab (Yervoy) Ipilimumab (yervoy) prolongs survival in advanced melanoma: serious side effects and a hefty price tag may limit its useP T201237950351123066344
- BakacsTMehrishiJNMossRWIpilimumab (Yervoy) and the TGN1412 catastropheImmunobiology2012217658358921821307
- ThurberGMSchmidtMMWittrupKDFactors determining antibody distribution in tumorsTrends Pharmacol Sci2008292576118179828
- ReubenJMLeeBNLiCBiologic and immunomodulatory events after CTLA-4 blockade with ticilimumab in patients with advanced malignant melanomaCancer2006106112437244416615096
- TopalianSLDrakeCGPardollDMTargeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunityCurr Opin Immunol201224220721222236695
- OkazakiTHonjoTPD-1 and PD-1 ligands: from discovery to clinical applicationInt Immunol200719781382417606980
- ZitvogelLKroemerGTargeting PD-1/PD-L1 interactions for cancer immunotherapyOncoimmunology2012181223122523243584
- Ménétrier-CauxCCurielTFagetJManuelMCauxCZouWTargeting regulatory T cellsTarget Oncol201271152822327882
- TopalianSLHodiFSBrahmerJRSafety, activity, and immune correlates of anti-PD-1 antibody in cancerN Engl J Med2012366262443245422658127
- TangPAHengDYProgrammed Death 1 Pathway inhibition in Metastatic Renal Cell Cancer and Prostate CancerCurr Oncol Rep20131529810423263823
- CurranMAMontalvoWYagitaHAllisonJPPD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumorsProc Natl Acad Sci USA201010794275428020160101
- ZhangHGGrizzleWEExosomes and cancer: a newly described pathway of immune suppressionClin Cancer Res201117595996421224375
- CamussiGDeregibusMCBrunoSGrangeCFonsatoVTettaCExosome/microvesicle-mediated epigenetic reprogramming of cellsAm J Cancer Res2011119811021969178
- IeroMValentiRHuberVTumour-released exosomes and their implications in cancer immunityCell Death Differ2008151808817932500
- LentzMRContinuous whole blood UltraPheresis procedure in patients with metastatic cancerJ Biol Response Mod1989855115272795094