Abstract
Prurigo nodularis is a chronic inflammatory skin disease consisting of severely pruritic nodules that can be very debilitating for patients. The basis of this skin condition is immunological dysregulation and neural amplification, driven by T-lymphocytes, mast cells, eosinophilic granulocytes, macrophages, and cytokines mediating itchy processes. Further complicating this already taxing diagnosis is the lack of approved treatment and consensus on management; although there are off-label treatments utilized as therapy. Immunomodulators are the cornerstone of treatment for PN, and additional novel therapies targeting key players in the immunological cascade are currently undergoing investigation. In this review, we will highlight targets of the immune cascade and explore current immunomodulating treatments as well as immunotherapies on the horizon for the management of prurigo nodularis.
Introduction
Prurigo nodularis (PN) is a chronic inflammatory condition of the skin. It classically presents as single or multiple, intensely pruritic, symmetrically distributed nodules on the trunk or extremities that are firm, flesh-to-red colored, and hyperkeratotic in appearance.Citation1–Citation3 This pruritic, recalcitrant condition potentiates a vicious itch-scratch cycle that defines and exacerbates the disease.Citation4 In fact, PN is amongst the itchiest of skin conditions.Citation5 PN is a distinct entity; however, it may also occur in the setting of other itchy conditions such atopic dermatitis (AD), which is the most frequent concurrent skin dermatitis, and other inflammatory skin disorders, pruritic systemic etiologies, neuropathic, and psychiatric disorders.Citation1,Citation6 PN profoundly negatively affects quality of life in patients, including a significant health, mental health, and economic burden.Citation7–Citation9
It has been estimated that there are approximately 87,634 cases of PN and 125,322 ambulatory care visits per year.Citation10,Citation11 Another estimate suggests that the prevalence of PN is approximately 36.7 to 148.53 per 100,000 persons.Citation12 Additionally, PN poses a significant health-care burden due to the increased prevalence of comorbidities and increased utilization of specialty care.Citation13
The exact pathophysiology of PN is not entirely understood. However, studies in the past have shown that the underlying mechanism of PN may be due to an interplay between immunological dysfunction and neural dysregulation.Citation14 Currently, there are no approved treatments for PN by the Food and Drug Administration (FDA).Citation2 However, the use of immunotargeting therapies may provide benefit for patients with PN given the current understanding of its inflammatory mechanism. Previously, the mainstay treatment for PN was immunosuppressants such as steroids; however, there are numerous other immunomodulating treatments that have been utilized to treat PN as of late.Citation1,Citation4 Additionally, treatment with novel therapies, including biologics and small molecules, that directly target key players in the PN immunologic cascade are currently being investigated.Citation15 In this review, we aim to discuss the pathogenesis of PN and examine the current and future systemic immune therapies.
Background
The pathobiological mechanism of PN is comprised of both a heightened inflammatory response and amplified neuronal circuitry.Citation1,Citation3,Citation14 Evaluation of the dermis and epidermis has revealed that there are numerous changes that occur within PN lesions. Nearly all skin cells are affected by PN, including keratinocytes, mast cells, dendritic cells, endothelial cells, eosinophils, collagen fibers, and nerve fibers.Citation16
Epidermal changes include hyperplasia, thickened orthohyperkeratosis, focal parakeratosis, and hypergranulosis.Citation1,Citation3,Citation14,Citation16 Within the dermis, histopathological studies show dense infiltrates consisting of T lymphocytes, mast cells, and eosinophilic granulocytes.Citation1,Citation3,Citation14,Citation16 In correspondence with the upregulated inflammatory cells, several pro-inflammatory and pruritic cytokines have also been identified in PN lesions. These include Th2-associated cytokines such as interleukin (IL) 31 and IL-4, both recognized as itchy cytokines thought to underly many pruritic conditions;Citation14 the IL-31 axis has been found to correlate with intense itch in PN patients.Citation17 IL-31 binds to heterodimeric IL-31 receptor A (IL-31RA) and oncostatin M (OSM) beta receptor found on keratinocytes, nerves, and eosinophils. The binding of IL-31 to its respective receptors mediates the activation of JAK 1, JAK 2, and STAT 3 predominantly.Citation18 The subsequent downstream effect increases the sensation of pruritus and scratching behavior. The number of dermal IL-31+ cells and dermal IL-31RA+ cells has been found to be increased in PN lesioned skin and correlates with intense itch in these patients.Citation18 The main dermal cells that express IL-31 in PN are T cells and macrophages, while the major cells expressing IL-31RA are mast cells and macrophages.Citation18
Another itch mediator associated with the Th2 inflammatory response is extracellular matrix protein, periostin; one study analyzed PN lesions and found that periostin was upregulated in the dermis as well as significantly correlated with itch intensity in these patients.Citation19 Additional cytokines implicated in the PN immunologic cascade include tryptase, eosinophil cationic protein, histamine, prostaglandins, and neuropeptides.Citation1,Citation3,Citation14,Citation16 Eosinophil cationic protein and neuropeptides are amongst the granules released by eosinophilic granulocytes, in addition to eosinophil derived neurotoxin, eosinophil protein X, and major basic protein.Citation1,Citation3,Citation14,Citation16 Neurotoxicity may be promoted by eosinophil cationic protein and eosinophil protein X, which are significantly increased in lesions of PN.Citation14
There are a few changes that occur to nerves within PN lesions. Some of these changes are mediated by neuropeptides. The common neuropeptides found to be upregulated in PN lesions include nerve growth factor (NGF), substance P (SP), and calcitonin gene-related peptide. NGF binds to receptor, tyrosine receptor kinase A (TrkA). NGF may regulate nerve cell growth, survival, and differentiation, potentially explaining findings of increased papillary dermal nerve fibers in immunohistochemical studies.Citation20 The finding of increased dermal nerve fibers appears to play a large role in the pathogenesis of PN, epitomizing the coaction of immunomodulators and nerve plasticity.Citation1,Citation3,Citation14,Citation16 Interestingly, nerve density was diminished in epidermal evaluation of PN lesion, but displayed recovery in healing nodules, suggesting that perhaps mechanical irritation from the itch-scratch cycle may be the cause of epidermal nerve hypoplasia. SP, produced and released by neurons, binds to neurokinin-1 receptors (NK1R), some of which are found in the skin.Citation1 This reaction elicits a neural inflammatory response by propagating vasodilation, plasma extravasation, and degranulation of mast cells.Citation1
Another cellular pathway that is associated with PN is Th17. Although many allergic skin conditions favor Th1 and Th2 cellular pathways, it is emerging that Th17 also plays a role in different phenotypes of inflammatory skin conditions.Citation21 Cytokine IL-23 acts on Th17 cells to increase IL-17 release.Citation22 Expression of IL-17 plays a key role in psoriatic conditions and is associated with other itchy, inflammatory skin conditions such as atopic dermatitis, hidradenitis suppurativa, pemphigus, and systemic sclerosis.Citation22,Citation23 Wong et al reported that Th17 were upregulated in lesioned PN skin and that IL-17 induced keratinocyte expression of vasodilator and histamine-independent itch inducer, endothelin-1 (ET1).Citation24
Treatment of PN is difficult because there are no approved FDA treatments and lack of clear guidelines; it requires multimodal management considering the complexity and intractable nature of the disease.Citation25 The treatment modalities currently used for PN target the underlying immune and neural dysregulation, such as local agents, phototherapy, systemic neuromodulating treatments, and systemic immunomodulatory agents.Citation1,Citation4,Citation26 Topical and intralesional corticosteroids, anesthetics, calcineurin inhibitors (ie, pimecrolimus, tacrolimus), vitamin D derivatives (ie, calcipotriene), and capsaicin are amongst the regional therapies that are often first employed in the stepwise management of PN.Citation1,Citation4,Citation26 Narrowband ultraviolet B light (NBUVB) and psoralen plus ultraviolet A light (PUVA) are two types of phototherapies that can also be introduced as a first- or second-line choices of treatment. In PN cases that are refractory to these treatments, systemic therapies are the next step. Neuromodulating therapies include gabapentinoids, NK1R antagonists (ie, aprepitant, serlopitant), mu-opioid antagonists (ie, naltrexone), mu-opioid antagonist/kappa-opioid agonist (ie, butorphanol), selective serotonin reuptake inhibitors (SSRIs) (ie, paroxetine, fluvoxamine), serotonin and norepinephrine reuptake inhibitors (SNRIs) (ie, duloxetine), and thalidomide.Citation1,Citation4,Citation26 One randomized controlled trial evaluated the use of NK1R antagonist, serlopitant, in patients with PN and found that it reduced pruritus in refractory PN and overall well tolerated, with most common adverse effects including nasopharyngitis, diarrhea, and fatigue.Citation27 Lastly, systemic immunomodulating therapies include oral corticosteroids, methotrexate, cyclosporine, and azathioprine.Citation1,Citation4,Citation26 Currently, other promising systemic immunotherapies targeting key players in PN inflammation are being evaluated and undergoing Phase II and II trials, such as IL-4 inhibitors, IL-31 antagonists, anti-OSM beta receptors, receptor tyrosine kinase KIT inhibitors, and janus kinase (JAK) inhibitors ().Citation1,Citation4,Citation15,Citation26,Citation28,Citation29
Table 1 A Summary of the Existing Systemic Immunomodulating Therapies and the Investigational Systemic Immunomodulating Therapies
Current Immunotherapeutics Used for PN
Corticosteroids
As PN is a skin condition that is a result of aberrant immune processes, systemic immunosuppressants such as corticosteroids are a choice of treatment, especially in intractable cases.Citation30 Corticosteroids are immunomodulators primarily by altering signal transduction pathways of inflammatory processes via binding of the glucocorticoid receptor.Citation31,Citation32 This transrepression mechanism is thought to underly the anti-inflammatory efficacy of corticosteroids.Citation31,Citation32 For the treatment of PN, systemic corticosteroids are not preferred due to broad immunosuppression and adverse effects; however, they may be employed to curb severe acute flares of the disease when other treatments fail. Ultimately, the use of oral corticosteroids is limited by its significant side effect profile, consisting of osteoporosis, muscle atrophy, eye impairment, diabetes mellitus, and hypertension amongst others.Citation33 For the treatment of PN, alternative immunosuppressant agents such as cyclosporine, methotrexate, or emerging biologic/small molecule treatments are preferred.Citation1
Methotrexate
Methotrexate (MTX), a folic acid antagonist, has immunomodulatory properties which decrease pruritus through an unknown mechanism. It has proven efficacy and a good tolerance profile in other inflammatory dermatoses including atopic dermatitis, psoriasis, and bullous pemphigoid.Citation34 Two studies have highlighted its efficacy for use in PN as monotherapy.Citation35 One retrospective review of 13 patients with treatment resistant PN taking 7.5–20 mg MTX weekly for 6 months reported a ≥75% decrease in PN lesion involvement and pruritus severity in 10 of 13 patients.Citation36 Another study of 39 PN patients taking 5–25 mg weekly showed lesion improvement for 91% of patients and pruritus improvement in 89% of patients after 3 months.Citation34 When combined with alitretinoin, MTX demonstrated near-complete or complete remission of PN in 5 of 6 patients who were refractory to MTX alone, suggesting that MTX (10–20 mg per week) and alitretinoin (10–30 mg per day) could be a potential treatment option for patients with difficult-to-treat PN.Citation37
Adverse effects of MTX occur as a result of its antifolate properties. Nausea and fatigue at treatment initiation are common. Other symptoms indicative of toxicity include mucositis, diarrhea, skin rashes, pancytopenia, transaminitis, and acute kidney injury.Citation38 Pulmonary fibrosis is one concern when using MTX; however, one systematic review found that it is unlikely that MTX causes pulmonary fibrosis and instead the finding is related to underlying disease.Citation39 Alternative studies suggest that pulmonary fibrosis is increased in patients treated with MTX;Citation40 therefore, more research is required for a definitive understanding of this pathology and it is advisable to monitor these symptoms nonetheless. Additionally, the use of MTX raises the concern of malignancy and many cancers such as stomach cancer, colorectal cancer, prostate cancer, ovarian cancer, malignant melanoma, and lung cancer have demonstrated an increased risk with MTX therapy.Citation41 Skin cancer such as squamous cell carcinoma was found to be increased in MTX-treated groups when compared to placebo as well.Citation42 Likewise, it is unclear if the relationship between MTX and carcinogenicity is causal but it should be considered when administering MTX.Citation40 Adverse events may be reduced with folic acid or folinic acid supplementation, though folinic acid may affect efficacy of treatment.Citation43 According to an expert panel consensus in 2020, methotrexate for difficult-to-treat PN should be given in doses of 7.5–15 mg orally weekly, starting at 7.5 mg for 2 weeks, then increasing by 2.5–5.0 mg weekly as needed.Citation25
Cyclosporine
Cyclosporine is a calcineurin inhibitor most likely improving pruritus through IL-2 signaling modulation.Citation35 Two studies have examined its effectiveness in PN. One was a clinical trial of 14 patients with refractory PN who took 3–5 mg/kg of oral cyclosporine daily.Citation44 Thirteen of 14 patients (92.9%) demonstrated significant improvement in itch with the maximal effect occurring after 2 weeks to 12 months. A retrospective chart review of 8 patients taking 2–4 mg/kg of cyclosporine revealed remission in 6 patients and partial improvement in 1 patient with an average improvement time of 3 weeks (the final patient was lost to follow-up).Citation45 The most frequent adverse effects are altered renal and hepatic functions, gingival hyperplasia, gastric upset, diarrhea, hypertrichosis, neuropathy, hypertension, temporary serum lipid elevation, and weight gain, though side effects are usually tolerable in PN cases.Citation44 More severe adverse effects such as immunosuppression and malignancy can also occur with cyclosporin treatment.Citation46 Cyclosporin is currently recommended as first-line systemic therapy for severe, chronic, refractory PN with a plan to transition to topical or phototherapy after 3–6 months.Citation45 According to an expert panel consensus in 2020, cyclosporine should be started at 3 mg/kg daily for 2–4 weeks, then increased by 0.5–1.0 mg/kg daily every 2–4 wk as tolerated.Citation25
Azathioprine
Azathioprine is a synthetic purine analog derived as a 6-mercaptopurine (6-MP) prodrug resistant to immediate catabolism. Azathioprine is cleaved into 6-MP and an imidazole derivative methylnitroimidazole by sulphyldryl-containing compounds and then enzymatically converted into active toxic purine analogs 6-thioguanine nucleotides and metabolites. It can then be incorporated into the replicating DNA and de novo pathway of purine synthesis, causing blockage of DNA and purine synthesis.Citation47 As lymphocytes lack a salvage pathway and rely on de novo synthesis of purines, they are particularly susceptible to azathioprine resulting in the inhibition of T and B cell proliferation.Citation48
Azathioprine has been shown to be efficacious at treating pruritus, including those with PN. In a retrospective review of 96 patients for azathioprine treatment in chronic intractable pruritus, azathioprine reduced itch from an average visual analog scale itch score of 9.25/10 to 1.625/10.Citation49 Lear et al reported 2 patients with severe PN were responsive to azathioprine 50 mg twice daily, with marked improvement in skin appearance and pruritus after 2–3 months of continuous usage. Remission was achieved regardless of whether there was a family background of atopy.Citation50 However, azathioprine use was not curative, as recurrence of PN appeared a few months after treatment termination.
Adverse side effects of azathioprine are common. For the treatment of chronic pruritus, 65% of patients experienced adverse effects and 33% underwent complete drug withdrawal. The most common adverse effects reported were transaminitis, gastrointestinal upset (nausea, diarrhea, epigastric pain), azathioprine hypersensitivity, myelosuppression, and infection. Transaminitis and myelosuppression were transient in some cases and normalized with a drug holiday or adjusted dose. The most concerning side effect was a relative increase in lymphoproliferative malignancy or nonmelanoma skin cancer, although the risk is low in individuals with limited risk factors.Citation49,Citation50 Additionally, patients with thiopurine methyl transferase deficiency are at increased risk of severe azathioprine toxicity and prior analysis for this genotype should be evaluated to reduce risk of adverse effects.Citation51
Current guidelines for treatment of PN with azathioprine is 50–200 mg of oral azathioprine daily. Patients may be initiated with 50 mg of oral azathioprine with gradual increase by 50 mg daily every 2–4 weeks as tolerated.Citation25
Novel Immunotherapy in PN
Anecdotal reports indicate monoclonal antibody and small molecules are effective in PN, and investigational studies proving the same are in progress. Namely, these therapies include IL-4 antagonist dupilumab, IL-31 inhibitor nemolizumab, anti-OSM beta receptor vixarelimab, tyrosine kinase KIT receptor inhibitor CDX-0159, and JAK inhibitor abrocitinib ().
Figure 1 A summary of the pathogenesis of prurigo nodularis and the targets of novel immunotherapies dupilumab, nemolizumab, vixarelimab, CDX-1059, and abrocitinib.
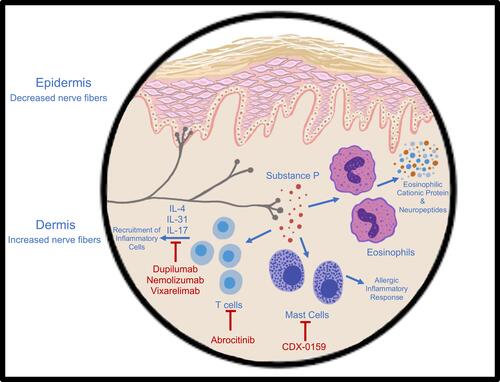
Dupilumab is now the systemic drug of choice for AD. Its primary mechanism of action is monoclonal antibody inhibition of the IL-4 receptor alpha subunit, which prevents binding of IL-4 and IL-13 and thus downregulates the pro-inflammatory and pruritic signaling pathway. As PN has demonstrated a type-2 inflammatory response involving these cytokines, as well as an overlap with AD, blockade of this receptor can decrease the progression of PN and curb symptoms such as severe itch. Current literature points to numerous successful cases that support the efficacy of dupilumab for PN.Citation52–Citation62 Importantly, the clinical response of itch reduction was efficacious in many cases.Citation63,Citation64 Many reports also endorse the prevention of new nodules and the reduction of current lesion size. One cohort study on 16 PN patients receiving dupilumab reported that 50% achieved complete resolution of pruritus and lesions, 41.7% had partial resolution, and only one patient demonstrated no response and subsequently discontinued treatment following 6 months.Citation65 At 12 months, the number of patients with complete resolution increased.
Dupilumab was reported to be efficacious for recalcitrant PN and PN-phenotypic AD in a few case reports as well.Citation66–Citation68 A retrospective cohort study evaluating dupilumab for refractory PN reported an improvement in Investigator Global Assessment (IGA) scores in 63.2% of patients following 16 weeks of treatment, and evidence of continued benefit for up to 52 weeks while remaining on treatment.Citation69 As of late, there are two Phase III clinical trials underway that will investigate dupilumab for PN in randomized, double-blind, placebo controlled studies.Citation70,Citation71 One of these studies has reported preliminary data consistent with significant reduction of itch and lesions in PN patients with long-term treatment of dupilumab.Citation72 Overall, dupilumab is well tolerated long term, with rare and mild adverse reactions, such as conjunctivitis, nasopharyngitis, injection site reactions, and skin infections.Citation73–Citation75 The current treatment guideline for the indication of PN is a 600 mg induction dose, followed by 300 mg maintenance dose every 2 weeks.Citation26
IL-31 is another cytokine associated with the Th2 immune cascade that is specifically correlated with intense pruritus in certain skin conditions, including PN.Citation18 Dampening the IL-31 axis may mitigate the itch-scratch cycle in PN patients and help reduce the inflammatory response.Citation76,Citation77 Therefore, it is thought that blockade of IL-31 receptors, IL-31RA and OSM beta receptor, can be beneficial for PN patients.
Nemolizumab is a monoclonal antibody that inhibits IL-31RA. One phase II randomized, double-blind clinical trial was conducted over a 12-week trial where nemolizumab was administered subcutaneously every 4 weeks in patients with moderate-to-severe PN and pruritus.Citation78 Results revealed that average itch numerical rating scores (NRS) were significantly reduced by about 53%. Additionally, a reduction in lesion severity was also reported. Adverse effects reported in this study were mild and uncommon, consisting of abdominal pain, diarrhea, and musculoskeletal symptoms. This initial evaluation of nemolizumab for the treatment of PN was promising in helping reduce pruritus in PN patients, with a rapid onset within 48 hours of the first dose.Citation79 One study evaluated the transcriptome of PN patients after treatment with nemolizumab and found that following 12 weeks, downstream inflammatory factors were decreased and reflective of transcriptome changes.Citation80 Longer and larger studies are necessary to better explore the use of the IL-31RA inhibitor in PN patients. Currently, there are 4 clinical trials that are assessing the efficacy and safety of nemolizumab for treatment of PN.Citation81–Citation84
Vixarelimab works by inhibiting the IL-31 signaling pathway by antagonizing the OSM beta receptor. This is an effective treatment due to the role of IL-31 in PN pathogenesis. One phase II clinical trial evaluating this mechanism of treatment reported that vixarelimab demonstrated improvement in PN signs and symptoms, with an average pruritus reduction of 70% by week 8 of treatment as well as significantly improved nodules as early as week 4.Citation85,Citation86
CDX-0159 is a human monoclonal antibody that inhibits tyrosine kinase KIT receptors. This receptor is pivotal in the treatment of PN because it is involved in mast cell regulation and the initiation of allergic inflammatory processes.Citation87 One of the upregulated cells in PN lesions is mast cells. CDX-0159 is being evaluated for efficacy and safety in treating PN in one clinical trial presently.Citation88 The janus kinase-signal transducer and activator (JAK STAT) pathway upregulates CD4 T cells as well as numerous inflammatory cytokines and has been recognized for immune pathogenesis in other skin conditions such as atopic dermatitis.Citation89 Although there is less available information on the use of tyrosine kinase inhibitors and JAK inhibitors, they may serve as good treatment choices for PN. One study evaluated the JAK STAT pathway in prurigo nodules to determine if this inflammatory pathway may be prevalent in the disease.Citation90 The results reported that STAT 2 and 3 were upregulated in PN lesions, as well as corresponding cytokines of Th2, Th17, and Th22. STATs are intracellular transcription factors that propagate extracellular signals from JAK receptors to the nucleus.Citation90 Numerous cytokines activate the JAK response. Th1, Th2/Th17/Th22, and Th2 cellular pathways are mediated by STAT 1, 3, and 6, respectively.Citation90 Therefore, the use of JAK inhibitors may be effective at decreasing disease progression. One case report demonstrates the efficacy of oral tofacitinib, a JAK 1 and JAK 3 inhibitor, in minimizing skin lesions by 50% and completely diminishing pruritus in a patient with intractable PN.Citation91 Another case series examining the treatment of topical tofacitinib showed a meaningful reduction in itch in 2 patients with PN.Citation92 Additionally, JAK inhibitors decrease pruritus in certain skin conditions.Citation93 One of these skin conditions include AD; AD-related clinical trials demonstrate that JAK inhibitor abrocitinib has a mild side effect profile.Citation94 At this moment, JAK 1 inhibitor abrocitinib and drug INCB054707 are small molecule treatments that are currently being studied for the treatment of PN in clinical trials.Citation95,Citation96 Although not yet explored, the implication of IL-17 in PN pathogenesis may lend itself to future therapies that can diminish Th17 inflammation.
Conclusion
Prurigo nodularis is a challenging complex inflammatory skin condition. Studies on PN have disclosed that the inflammatory response is mostly Th2 mediated and comprised of T lymphocytes, mast cells, macrophages and eosinophilic granulocytes, in addition to inflammatory and pruritic cytokines such as IL-4, IL-13, and IL-31.Citation1,Citation3,Citation14,Citation16 Current immunotherapeutic treatments for PN aim to decrease this immune response, including systemic corticosteroids, MTX, cyclosporine, and azathioprine. Promising new drugs include biologic monoclonal antibody treatments and small molecule therapies for the treatment of this extremely bothersome condition. These include IL-4 antagonists, IL-31 inhibitors, tyrosine kinase KIT receptor antagonists, and JAK inhibitors. The treatment of PN requires a multifaceted approach and the use of systemic immunomodulators will play a large role.
Disclosure
Dr. Gil Yosipovitch reports grants and personal fees from Galderma, PFIZER, Sanofi Regeneron, Kiniksa, Celldex, Novartis, Eli Lilly, and Bellus, personal fees from Cerave, Aslan, and Trevi, and personal fees and non-financial support from Arcutis, outside the submitted work; has been an investigator and consultant to Galderma, Pfizer, Sanofi Regeneron, Eli Lilly, Bellus, Kiniksa, Leo, Trevi, and Celldex; and reports no other potential conflicts of interest for this work. Angelina Labib, Ashley Vander Does, and Teresa Ju state no conflicts of interest for this work.
References
- Zeidler C, Yosipovitch G, Ständer S. Prurigo nodularis and its management. Dermatol Clin. 2018;36(3):189–197. doi:10.1016/j.det.2018.02.003
- Williams KA, Roh YS, Brown I, et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14(1):67–77. doi:10.1080/17512433.2021.1852080
- Mullins TB, Sharma P, Riley CA, Sonthalia S. Prurigo Nodularis. In: StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
- Leis M, Fleming P, Lynde CW. Prurigo Nodularis: review and emerging treatments. Skin Therapy Lett. 2021;26(3):5–8.
- Mollanazar NK, Sethi M, Rodriguez RV, et al. Retrospective analysis of data from an itch center: integrating validated tools in the electronic health record. J Am Acad Dermatol. 2016;75(4):842–844. doi:10.1016/j.jaad.2016.05.035
- Iking A, Grundmann S, Chatzigeorgakidis E, Phan NQ, Klein D, Ständer S. Prurigo as a symptom of atopic and non-atopic diseases: aetiological survey in a consecutive cohort of 108 patients. J Eur Acad Dermatol Venereol. 2013;27(5):550–557. doi:10.1111/j.1468-3083.2012.04481.x
- Janmohamed SR, Gwillim EC, Yousaf M, Patel KR, Silverberg JI. The impact of prurigo nodularis on quality of life: a systematic review and meta-analysis. Arch Dermatol Res. 2021;313(8):669–677. doi:10.1007/s00403-020-02148-0
- Silverberg JI, Hinami K, Trick WE, Cella D. Itch in the general internal medicine setting: a Cross-Sectional Study of prevalence and quality-of-life effects. Am J Clin Dermatol. 2016;17(6):681–690. doi:10.1007/s40257-016-0215-3
- Whang KA, Le TK, Khanna R, et al. Health-related quality of life and economic burden of prurigo nodularis. J Am Acad Dermatol. 2021;85(3):AB38. doi:10.1016/j.jaad.2021.06.176
- Whang KA, Mahadevan V, Bakhshi PR, et al. Prevalence of prurigo nodularis in the United States. J Allergy Clin Immunol Pract. 2020;8(9):3240–3241. doi:10.1016/j.jaip.2020.05.051
- Huang AH, Canner JK, Khanna R, Kang S, Kwatra SG. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Invest Dermatol. 2020;140(2):480–483.e484. doi:10.1016/j.jid.2019.07.697
- Ständer S, Augustin M, Berger T, et al. Prevalence of prurigo nodularis in the United States of America: a retrospective database analysis. JAAD Int. 2021;2:28–30. doi:10.1016/j.jdin.2020.10.009
- Wongvibulsin S, Sutaria N, Williams KA, et al. A nationwide study of prurigo nodularis: disease burden and healthcare utilization in the United States. J Invest Dermatol. 2021;141(10):2530–2533.e2531. doi:10.1016/j.jid.2021.02.756
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83(6):1559–1565. doi:10.1016/j.jaad.2020.04.183
- Müller S, Bieber T, Ständer S. Therapeutic potential of biologics in prurigo nodularis. Expert Opin Biol Ther. 2021;22(1):1–12.
- Zeidler C, Ständer S. The pathogenesis of Prurigo nodularis–’Super-Itch’ in exploration. Eur J Pain. 2016;20(1):37–40. doi:10.1002/ejp.767
- Hashimoto T, Nattkemper LA, Kim HS, et al. Itch intensity in prurigo nodularis is closely related to dermal interleukin-31, oncostatin M, IL-31 receptor alpha and oncostatin M receptor beta. Exp Dermatol. 2021;30(6):804–810. doi:10.1111/exd.14279
- Furue M, Furue M. Interleukin-31 and pruritic skin. J Clin Med. 2021;10(9):1906. doi:10.3390/jcm10091906
- Hashimoto T, Nattkemper LA, Kim HS, et al. Dermal periostin: a new player in itch of Prurigo Nodularis. Acta Derm Venereol. 2021;101(1):adv00375. doi:10.2340/00015555-3702
- Rocco ML, Soligo M, Manni L, Aloe L. Nerve growth factor: early studies and recent clinical trials. Curr Neuropharmacol. 2018;16(10):1455–1465. doi:10.2174/1570159X16666180412092859
- Topal FA, Zuberbier T, Makris MP, Hofmann M. The role of IL-17, IL-23 and IL-31, IL-33 in allergic skin diseases. Curr Opin Allergy Clin Immunol. 2020;20(4):367–373. doi:10.1097/ACI.0000000000000658
- Liu T, Li S, Ying S, et al. The IL-23/IL-17 pathway in inflammatory skin diseases: from bench to bedside. Front Immunol. 2020;11:594735. doi:10.3389/fimmu.2020.594735
- Czarnecka-Operacz M, Polańska A, Klimańska M, et al. Itching sensation in psoriatic patients and its relation to body mass index and IL-17 and IL-31 concentrations. Postepy Dermatol Alergol. 2015;32(6):426–430. doi:10.5114/pdia.2015.56097
- Wong LS, Yen YT, Lin SH, Lee CH. IL-17A induces endothelin-1 expression through p38 pathway in Prurigo Nodularis. J Invest Dermatol. 2020;140(3):702–706.e702. doi:10.1016/j.jid.2019.08.438
- Elmariah S, Kim B, Berger T, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol. 2021;84(3):747–760. doi:10.1016/j.jaad.2020.07.025
- Williams KA, Huang AH, Belzberg M, Kwatra SG. Prurigo nodularis: pathogenesis and management. J Am Acad Dermatol. 2020;83(6):1567–1575. doi:10.1016/j.jaad.2020.04.182
- Ständer S, Kwon P, Hirman J, et al. Serlopitant reduced pruritus in patients with prurigo nodularis in a Phase 2, randomized, placebo-controlled trial. J Am Acad Dermatol. 2019;80(5):1395–1402. doi:10.1016/j.jaad.2019.01.052
- Erickson S, Heul AV, Kim BS. New and emerging treatments for inflammatory itch. Ann Allergy Asthma Immunol. 2021;126(1):13–20. doi:10.1016/j.anai.2020.05.028
- Pereira MP, Ständer S. Itch management: treatments under development. Curr Probl Dermatol. 2016;50:71–76.
- Nakashima C, Tanizaki H, Otsuka A, Miyachi Y, Kabashima K. Intractable prurigo nodularis successfully treated with combination therapy with a newly developed excimer laser and topical steroids. Dermatol Online J. 2014;20(6). doi:10.5070/D3206022873
- Cato AC, Wade E. Molecular mechanisms of anti-inflammatory action of glucocorticoids. Bioessays. 1996;18(5):371–378. doi:10.1002/bies.950180507
- Barnes PJ. Molecular mechanisms and cellular effects of glucocorticosteroids. Immunol Allergy Clin North Am. 2005;25(3):451–468. doi:10.1016/j.iac.2005.05.003
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96(1):23–43. doi:10.1016/S0163-7258(02)00297-8
- Klejtman T, Beylot-Barry M, Joly P, et al. Treatment of prurigo with methotrexate: a multicentre retrospective study of 39 cases. J Eur Acad Dermatol Venereol. 2018;32(3):437–440. doi:10.1111/jdv.14646
- Qureshi AA, Abate LE, Yosipovitch G, Friedman AJ. A systematic review of evidence-based treatments for prurigo nodularis. J Am Acad Dermatol. 2019;80(3):756–764. doi:10.1016/j.jaad.2018.09.020
- Spring P, Gschwind I, Gilliet M. Prurigo nodularis: retrospective study of 13 cases managed with methotrexate. Clin Exp Dermatol. 2014;39(4):468–473. doi:10.1111/ced.12365
- Bergqvist C, Fiani C, Simantov A, et al. Combined methotrexate and alitretinoin for the treatment of difficult-to-treat generalized prurigo nodularis: a case series. J Eur Acad Dermatol Venereol. 2021;35(8):e516–e519. doi:10.1111/jdv.17262
- Misra DP, Gasparyan AY, Zimba O. Benefits and adverse effects of hydroxychloroquine, methotrexate and colchicine: searching for repurposable drug candidates. Rheumatol Int. 2020;40(11):1741–1751. doi:10.1007/s00296-020-04694-2
- Quah E, Amoasii C, Mudawi T, Dawson J. Systematic literature review investigating whether methotrexate causes chronic pulmonary fibrosis. Future Healthc J. 2019;6(Suppl 2):4. doi:10.7861/futurehealth.6-2-s4
- Wang W, Zhou H, Liu L. Side effects of methotrexate therapy for rheumatoid arthritis: a systematic review. Eur J Med Chem. 2018;158:502–516. doi:10.1016/j.ejmech.2018.09.027
- Inose R, Hashimoto N, Hosomi K, Yokoyama S, Takada M. Association between malignancy and methotrexate and biological disease-modifying antirheumatic drugs in patients with rheumatoid arthritis. Int J Clin Pharmacol Ther. 2020;58(3):131–138. doi:10.5414/CP203604
- Vanni KMM, Berliner N, Paynter NP, et al. Adverse effects of low-dose methotrexate in a randomized double-blind placebo-controlled trial: adjudicated hematologic and skin cancer outcomes in the cardiovascular inflammation reduction trial. ACR Open Rheumatol. 2020;2(12):697–704. doi:10.1002/acr2.11187
- Cline A, Jorizzo JL. Does daily folic acid supplementation reduce methotrexate efficacy? Dermatol Online J. 2017;23:11. doi:10.5070/D32311037244
- Siepmann D, Luger TA, Ständer S. Antipruritic effect of cyclosporine microemulsion in prurigo nodularis: results of a case series. J Dtsch Dermatol Ges. 2008;6(11):941–946. doi:10.1111/j.1610-0387.2008.06745.x
- Wiznia LE, Callahan SW, Cohen DE, Orlow SJ. Rapid improvement of prurigo nodularis with cyclosporine treatment. J Am Acad Dermatol. 2018;78(6):1209–1211. doi:10.1016/j.jaad.2018.02.024
- Amber T, Tabassum S. Cyclosporin in dermatology: a practical compendium. Dermatol Ther. 2020;33(6):e13934. doi:10.1111/dth.13934
- Maltzman JS, Koretzky GA. Azathioprine: old drug, new actions. J Clin Invest. 2003;111(8):1122–1124. doi:10.1172/JCI200318384
- Patel AA, Swerlick RA, McCall CO. Azathioprine in dermatology: the past, the present, and the future. J Am Acad Dermatol. 2006;55(3):369–389. doi:10.1016/j.jaad.2005.07.059
- Maley A, Swerlick RA. Azathioprine treatment of intractable pruritus: a retrospective review. J Am Acad Dermatol. 2015;73(3):439–443.
- Lear JT, English JS, Smith AG. Nodular prurigo responsive to azathioprine. Br J Dermatol. 1996;134(6):1151. doi:10.1111/j.1365-2133.1996.tb07964.x
- Black AJ, McLeod HL, Capell HA, et al. Thiopurine methyltransferase genotype predicts therapy-limiting severe toxicity from azathioprine. Ann Intern Med. 1998;129(9):716–718. doi:10.7326/0003-4819-129-9-199811010-00007
- Holm JG, Agner T, Sand C, Thomsen SF. Dupilumab for prurigo nodularis: case series and review of the literature. Dermatol Ther. 2020;33(2):e13222. doi:10.1111/dth.13222
- Husein-ElAhmed H, Steinhoff M. Dupilumab in prurigo nodularis: a systematic review of current evidence and analysis of predictive factors to response. J Dermatolog Treat. 2020;1–7. doi:10.1080/09546634.2020.1853024
- Tanis R, Ferenczi K, Payette M. Dupilumab treatment for prurigo nodularis and pruritis. J Drugs Dermatol. 2019;18(9):940–942.
- Beck KM, Yang EJ, Sekhon S, Bhutani T, Liao W. Dupilumab treatment for generalized prurigo nodularis. JAMA Dermatol. 2019;155(1):118–120. doi:10.1001/jamadermatol.2018.3912
- Toffoli L, Farinazzo E, Zelin E, et al. Dupilumab as promising treatment for prurigo nodularis: current evidences. J Dermatolog Treat;2021. 1–6. doi:10.1080/09546634.2021.1886232
- Fachler T, Maria Faitataziadou S, Molho-Pessach V. Dupilumab for pediatric prurigo nodularis: a case report. Pediatr Dermatol. 2021;38(1):334–335. doi:10.1111/pde.14464
- Romano C. Safety and effectiveness of dupilumab in Prurigo Nodularis. J Investig Allergol Clin Immunol. 2021;31(2):162–163. doi:10.18176/jiaci.0550
- Giura MT, Viola R, Fierro MT, Ribero S, Ortoncelli M. Efficacy of dupilumab in prurigo nodularis in elderly patient. Dermatol Ther. 2020;33(1):e13201. doi:10.1111/dth.13201
- Criado PR, Pincelli TP, Criado RFJ. Dupilumab as a useful treatment option for prurigo nodularis in an elderly patient with atopic diathesis. Int J Dermatol. 2020;59(10):e358–e361. doi:10.1111/ijd.14994
- Calugareanu A, Jachiet M, Lepelletier C, et al. Dramatic improvement of generalized prurigo nodularis with dupilumab. J Eur Acad Dermatol Venereol. 2019;33(8):e303–e304. doi:10.1111/jdv.15584
- Liu T, Bai J, Wang S, et al. Effectiveness of dupilumab for an elderly patient with prurigo nodularis who was refractory and contradicted to traditional therapy. J Asthma Allergy. 2021;14:175–178. doi:10.2147/JAA.S300975
- Mollanazar NK, Elgash M, Weaver L, Valdes-Rodriguez R, Hsu S. Reduced itch associated with dupilumab treatment in 4 patients with prurigo nodularis. JAMA Dermatol. 2019;155(1):121–122. doi:10.1001/jamadermatol.2018.3906
- Zhai LL, Savage KT, Qiu CC, Jin A, Valdes-Rodriguez R, Mollanazar NK. Chronic pruritus responding to dupilumab-a case series. Medicines. 2019;6(3). doi:10.3390/medicines6030072
- Calugareanu A, Jachiet M, Tauber M, et al. Effectiveness and safety of dupilumab for the treatment of prurigo nodularis in a French multicenter adult cohort of 16 patients. J Eur Acad Dermatol Venereol. 2020;34(2):e74–e76. doi:10.1111/jdv.15957
- Ferrucci S, Tavecchio S, Berti E, Angileri L. Dupilumab and prurigo nodularis-like phenotype in atopic dermatitis: our experience of efficacy. J Dermatolog Treat. 2021;32(4):453–454. doi:10.1080/09546634.2019.1659479
- Wieser JK, Mercurio MG, Somers K. Resolution of treatment-refractory prurigo nodularis with dupilumab: a case series. Cureus. 2020;12(6):e8737. doi:10.7759/cureus.8737
- Rambhia PH, Levitt JO. Recalcitrant prurigo nodularis treated successfully with dupilumab. JAAD Case Rep. 2019;5(5):471–473. doi:10.1016/j.jdcr.2019.03.016
- Georgakopoulos JR, Croitoru D, Felfeli T, et al. Long-term dupilumab treatment for chronic refractory generalized prurigo nodularis: a retrospective cohort study. J Am Acad Dermatol. 2021;85(4):1049–1051. doi:10.1016/j.jaad.2021.02.038
- Study of dupilumab for the treatment of patients with prurigo nodularis, inadequately controlled on topical prescription therapies or when those therapies are not advisable (PRIME2). Available from: https://ClinicalTrials.gov/show/NCT04202679. Accessed April 11, 2022.
- Study of dupilumab for the treatment of patients with prurigo nodularis, inadequately controlled on topical prescription therapies or when those therapies are not advisable (LIBERTY-PN PRIME). Available from: https://ClinicalTrials.gov/show/NCT04183335. Accessed April 11, 2022.
- Dupixent (dupilumab) is the first biologic to significantly reduce itch and skin lesions in Phase 3 trial for prurigo nodularis, demonstrating the role of type 2 inflammation in this disease [press release]. Sanofi; 2021. Available from: https://www.sanofi.com/en/media-room/press-releases/2021/2021-10-22-07-00-00-2318876.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a Phase 3 randomized clinical trial. JAMA Dermatol. 2020;156(1):44–56. doi:10.1001/jamadermatol.2019.3336
- Deleuran M, Thaçi D, Beck LA, et al. Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study. J Am Acad Dermatol. 2020;82(2):377–388.
- Beck LA, Thaçi D, Deleuran M, et al. Dupilumab provides favorable safety and sustained efficacy for up to 3 years in an open-label study of adults with moderate-to-severe atopic dermatitis. Am J Clin Dermatol. 2020;21(4):567–577. doi:10.1007/s40257-020-00527-x
- Kabashima K, Irie H. Interleukin-31 as a clinical target for pruritus treatment. Front Med. 2021;8:638325. doi:10.3389/fmed.2021.638325
- Kwatra SG. Breaking the itch-scratch cycle in Prurigo Nodularis. N Engl J Med. 2020;382(8):757–758. doi:10.1056/NEJMe1916733
- Ständer S, Yosipovitch G, Legat FJ, et al. Trial of nemolizumab in moderate-to-severe prurigo nodularis. N Engl J Med. 2020;382(8):706–716. doi:10.1056/NEJMoa1908316
- Ständer S, Lacour J-P, Legat FJ, et al. Nemolizumab rapidly relieves itch and sleep disturbances in patients with prurigo nodularis. EMJ Dermatol. 2021;9(1):63-64.
- Tsoi LC, Hacini-Rachinel F, Fogel P, et al. Transcriptomic characterization of prurigo nodularis and the therapeutic response to nemolizumab. J Allergy Clin Immunol. 2021;149(4):1329–1339.
- A study to evaluate the durability of response and safety of nemolizumab for 24 weeks in participants with prurigo nodularis. Available from: https://ClinicalTrials.gov/show/NCT05052983. Accessed April 11, 2022.
- An efficacy and safety study of nemolizumab (CD14152) in participants with prurigo nodularis. Available from: https://ClinicalTrials.gov/show/NCT04501666. Accessed April 11, 2022.
- A study to assess the efficacy and safety of nemolizumab (CD14152) in participants with Prurigo Nodularis (PN). Available from: https://ClinicalTrials.gov/show/NCT04501679. Accessed April 11, 2022.
- A long-term study of nemolizumab (CD14152) in participants with Prurigo Nodularis (PN). Available from: https://ClinicalTrials.gov/show/NCT04204616. Accessed April 11, 2022.
- Study to assess the efficacy, safety, and tolerability of vixarelimab in reducing pruritus in Prurigo Nodularis. Available from: https://ClinicalTrials.gov/show/NCT03816891. Accessed April 11, 2022.
- Howard Sofen RB, Yosipovitch G, Silverberg J, et al. Vixarelimab reduced pruritus, improved nodules, and was well-tolerated in patients with Prurigo Nodularis in a Phase 2a, randomized, double-blind, placebo-controlled study. Abstract presented at: EADV Virtual Congress; October 29-31; 2020.
- Gilfillan AM, Rivera J. The tyrosine kinase network regulating mast cell activation. Immunol Rev. 2009;228(1):149–169. doi:10.1111/j.1600-065X.2008.00742.x
- A study of CDX-0159 in patients with Prurigo Nodularis. Available from: https://ClinicalTrials.gov/show/NCT04944862. Accessed April 11, 2022.
- Li H, Zhang Z, Zhang H, Guo Y, Yao Z. Update on the pathogenesis and therapy of atopic dermatitis. Clin Rev Allergy Immunol. 2021;61(3):324–338. doi:10.1007/s12016-021-08880-3
- Agrawal D, Sardana K, Mathachan SR, Bhardwaj M, Ahuja A, Jain S. A prospective study examining the expression of STAT 1, 3, 6 in prurigo nodularis lesions with its immunopathogenic and therapeutic implications. J Cosmet Dermatol. 2021. doi:10.1111/jocd.14709
- Molloy OE, Kearney N, Byrne N, Kirby B. Successful treatment of recalcitrant nodular prurigo with tofacitinib. Clin Exp Dermatol. 2020;45(7):918–920. doi:10.1111/ced.14320
- Ju T, Labib A, Does AV, Yosipovitch G. Topical JAK-STAT inhibitor tofacitinib is effective in reducing non-atopic dermatitis chronic itch: a case series. J Am Acad Dermatol. 2022. doi:10.1016/j.jaad.2022.03.012
- Erickson S, Nahmias Z, Rosman IS, Kim BS. Immunomodulating agents as antipruritics. Dermatol Clin. 2018;36(3):325–334. doi:10.1016/j.det.2018.02.014
- Gooderham MJ, Forman SB, Bissonnette R, et al. Efficacy and safety of oral janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a Phase 2 randomized clinical trial. JAMA Dermatol. 2019;155(12):1371–1379. doi:10.1001/jamadermatol.2019.2855
- Efficacy of abrocitinib for reducing pruritus in adults with Prurigo Nodularis and chronic pruritus of unknown origin. Available from: https://ClinicalTrials.gov/show/NCT05038982. Accessed April 11, 2022.
- A study to evaluate the efficacy and safety of INCB054707 in participants with Prurigo Nodularis. Available from: https://ClinicalTrials.gov/show/NCT05061693. Accessed April 11, 2022.
