Abstract
Background and Aims
High incidence of hepatocellular carcinoma (HCC) exists in patients with liver cirrhosis (LC), but the predictive accuracy of noninvasive scoring systems (NSSs) is yet to be elucidated. The present study aimed to evaluate the predictive ability of fibrosis-4 (FIB-4), aminotransferase-to-platelet ratio index (APRI), and gamma-glutamyl transpeptidase to platelet ratio (GPR) in patients with LC, and to establish a new model with more accuracy.
Methods
Data from 94 patients with compensated LC and 134 patients with decompensated cirrhosis (DC) were collected. The prediction accuracy of NSSs, including APRI, GPR, and FIB-4, was compared.
Results
During a median follow-up of 37.5 months, 9 patients in the compensated LC group and 38 in the DC group developed HCC. For 228 patients, the area under the receiver operating characteristic curve (AUROC) of APRI, GPR, and FIB-4 was 0.596, 0.625, and 0.654, respectively. Multivariable logistic analysis showed that age, gamma-glutamyl transpeptidase (GGT), and platelet (PLT) were independent risk factors for HCC development, and a new model encompassing age, GGT, and PLT was superior to NSSs (all P<0.05). With an optimal cutoff value of 0.216, Model (Age_GGT_PLT) achieved 68.09% sensitivity and 69.61% specificity.
Conclusion
NSSs, including APRI, GPR, and FIB-4, has a non-optimal accuracy in predicting HCC development in patients with HBV-related LC. Thus, the new model consisting of age, GGT, and PLT may be more accurate than NSSs.
Background
Hepatocellular carcinoma (HCC) accounts for 90% of liver cancers and hence, is a major health burden in Asia.Citation1,Citation2 Chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infection is the leading course of HCC development.Citation2 Although the risk of HCC can be reduced by antiviralsCitation3,Citation4 the incidence of HCC remains high in patients with liver cirrhosis (LC).Citation3,Citation5,Citation6
Liver fibrosis is a major risk factor for HCC development.Citation7,Citation8 Noninvasive scoring systems (NSSs), including fibrosis-4 (FIB-4), aminotransferase-to-platelet ratio index (APRI), and gamma-glutamyl transpeptidase to platelet ratio (GPR), have a moderate accuracy of liver fibrosis evaluation and adequately predict the long-term outcomes.Citation9,Citation10 FIB-4 is a valuable risk marker for HCC development after HCV eradication.Citation8,Citation11,Citation12 Data from a small sample-size study suggested that a combination of APRI and alpha-fetoprotein (AFP) can predict HCC development in patients with HCV-related decompensated cirrhosis (DC).Citation13
For patients with chronic hepatitis B (CHB), both FIB-4 and APRI were useful in predicting HCC development.Citation14,Citation15 FIB-4 at 1-year of treatment was superior to FIB-4 before treatment in predicting HCC.Citation16 However, another study showed that elevated FIB-4 was not reliable for HCC risk stratification in non-Asia CHB patients.Citation17 Data from West Africa showed that the sensitivity of APRI score >2 for diagnosis of cirrhosis was only 45.4%.Citation18 A high level of APRI was maintained in patients who developed HCC.Citation19 Nishikawa et al reported that FIB-4 rather than APRI could be a valuable predictor for HCC development in CHB patients undergoing entecavir treatment.Citation20 In addition, APRI combined with FIB-4 could stratify HCC in CHB patients with low-level viremia.Citation21 Zhu et al reported that GPR was better than FIB-4 in predicting HCC development for elderly CHB patients.Citation22 Notably, the NSSs in HBV-related DC was limited.
Regarding the patients with DC who are at high-risk for liver biopsy, whether NSSs can predict HCC development is yet to be elucidated. Herein, we investigated the predictive accuracy of FIB-4, APRI, and GPR in patients with HBV-related DC and long-term antiviral therapy, and a new model was established.
Methods
Patients and the Primary Endpoint
A total of 307 patients with HBV-related LC were enrolled in the third People’s hospital of Changzhou from May 2010 to July 2020. The patients were divided into compensated LC and DC groups based on the criteria of Chinese guidelines for the prevention and treatment of CHB (2019 version).Citation23 LC was diagnosed according to the histological, ultrasonographic, or endoscopic evidence, while patients with ascites, hepatic encephalopathy, spontaneous bacterial peritonitis, variceal bleeding or hepatorenal syndrome, were diagnosed as DC. HCC was diagnosed according to the guidelines for the diagnosis and treatment of primary liver cancer in China (2019 edition).Citation24 All patients received nucleos(t)ide analogues (NAs) treatment and were followed up for at least 6 months. Patients who developed HCC during these 6 months of follow-up were excluded. Patients had malignant tumor or other hepatitis virus co-infection at admission, had detectable HBV DNA at the end of follow-up, or lost to follow-up were also excluded.
The protocol was approved by the Ethics Committee of the third People’s hospital of Changzhou according to the Declaration of Helsinki, 2013 (Approval No. 02A-A20210005), and written informed consent was obtained from all participants.
Score Systems
NSSs, including FIB-4,Citation12 APRI,Citation19 and GPR,Citation22 were evaluated, as described previously.
Statistical Analysis
Data were presented as median (interquartile range, IQR) for continuous variables and frequencies for categorical values. Mann–Whitney U-test and chi-square test were employed as required. Correlation analysis was performed using Pearson’s correlation analysis. Logistic regression analysis was performed to analyze the risk factors for HCC development. The predictive accuracy of NSSs was compared according to the area under the receiver operating characteristic curve (AUROC) using MedCalc version 15.2.2 software for Windows (Medcalc software, Mariakerke, Belgium). The data were analyzed using SPSS version 25.0 (Armonk, NY, USA), and P<0.05 was considered statistically significant.
Results
Characteristics of Patients
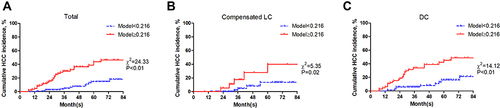
Until the last follow-up on May 4, 2022, data from 228 patients who achieved sustained virological response were analyzed. 94/228 patients were diagnosed with compensated LC, and 134/228 patients presented DC. During a median follow-up of 37.5 months, 9 patients in the compensated LC group and 38 patients in the DC group developed HCC (χ2=11.911, P<0.01).
As shown in , patients with HCC were older than those without HCC (Z=3.822, P<0.01). Moreover, patients with HCC had fewer platelets (PLTs, P=0.01) and higher GPR, APRI, and FIB-4 than those without HCC (Z=2.635, 2.024, and 3.257, respectively, all P<0.05). Strikingly, the follow-up was longer in patients without than those with HCC (P<0.01).
Table 1 Characteristics of Patients with and without HCC Development During Follow-Up
Development of a New Risk Model Incorporating Age, GGT, and PLT
As shown in , univariate analysis showed that age (P<0.01), GGT (P=0.09), PLT (P=0.01), and AFP (P=0.06) are associated with HCC development. Multivariable analysis showed that age (odds ratio (OR): 1.067, 95% confidence interval (CI): 1.029–1.106, P<0.01), GGT (OR: 1.005, 95% CI: 1.001–1.010, P=0.03), and PLT (OR: 0.993, 95% CI: 0.986–1.000, P=0.04) were the independent risk factors for HCC development (). Then, a new model was developed: Model (Age_GGT_PLT)(y=1). The AUROC of Model (Age_GGT_PLT) was 0.729, which was significantly higher than APRI, GPR, and FIB-4 (AUROC: 0.596, 0.625, and 0.654, respectively, all P<0.05) (). For patients with DC, Model (Age_GGT_PLT) had a higher AUROC (0.689) than APRI and FIB-4 (AUROC: 0.545, and 0.573, respectively; P=0.01 and 0.03, respectively) (), but not higher than GPR (AUROC: 0.608, P=0.16) ().
Table 2 Risk Factors for HCC Development in Patients with Liver Cirrhosis
Figure 1 Comparison of AUROC between Model (Age_GGT_PLT) and NSSs. (A) total population, (B) patients with compensated LC, (C) patients with DC.
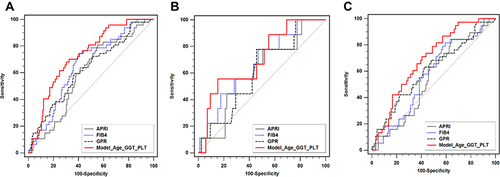
Moreover, for the 228 patients, FIB-4 had a higher AUROC than APRI (P<0.01) (). On the other hand, no significant difference was detected in AUROC between the three NSSs for both compensated LC and DC groups ( and ).
Correlation Between APRI, GPR, FIB-4, and Model (Age_GGT_PLT)
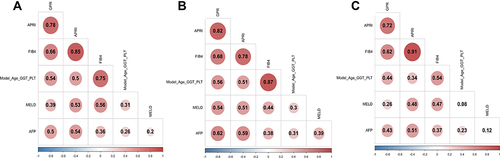
Model (Age_GGT_PLT) was positively correlated with APRI, GPR, and FIB-4 (all P<0.05). For patients with DC, Model (Age_GGT_PLT) was also positively correlated with MELD score and AFP (both P<0.05). In addition, APRI, GPR, and FIB-4 showed a positive correlation with MELD and AFP (all P<0.05) ().
Figure 2 Correlation analysis between Model (Age_GGT_PLT), NSSs, MELD, and AFP. (A) total population, (B) patients with compensated LC, (C) patients with DC.
Risk Stratification for Cumulative Incidence of HCC
With an optimal cutoff value of 0.216, Model (Age_GGT_PLT) achieved 68.09% sensitivity and 69.61% specificity (Youden index=0.377). Then, the patients were divided into two groups: low-risk (Model (Age_GGT_PLT) <0.216) and high-risk (Model (Age_GGT_PLT) ≥0.216). The patients were well stratified in both compensated LC and DC groups (both P<0.05) ().
Discussion
Herein, we evaluated the predictive accuracy of NSSs in patients with LC, which showed that APRI, GPR, and FIB-4 have a non-optimal ability to predict HCC development. Multivariate analysis showed that age, GGT, and PLT were independent risk factors for HCC development. A model encompassing age, GGT, and PLT was superior to NSSs in predicting HCC development, especially for patients with DC.
Although a sustainable virological response was achieved, the incidence of HCC remained high in patients with liver cirrhosis, especially in those with DC.Citation6 Recent studies reported that the risk of HCC differs extensively across etiologies, age, and sex in patients with cirrhosis.Citation25,Citation26 Thus, HCC surveillance in elderly patients with HBV or HCV-related LC is imperative. NSSs, including APRI, GPR, FIB-4, is a risk factor for HCC development in patients with CHB and fatty liver disease.Citation16,Citation27,Citation28 In the present study, APRI, GPR, and FIB-4 presented similar abilities in predicting HCC development in patients with DC, but neither was optimal according to AUROC (all <0.7). Thus, it is speculated that cirrhosis influences the predictive accuracy of NSSs.
The present study showed that age, GGT, and PLT are the independent risk factors for HCC development in patients with LC. Data from another study showed that serum GGT during antiviral treatment is associated with HCC development in non-cirrhotic elderly patients; however, the correlation was not significant in cirrhotic patients.Citation29 In the present study, the new model incorporating age, GGT, and PLT, was superior to NSSs in the total population and in patients with DC, but not in those with compensated LC, which could be attributed to the limited sample size in the compensated LC group. In addition, Kaplan–Meier analysis showed that the new model was valuable in both compensated LC and DC groups. Since a high risk of HCC exists in patients with DC, the new model is recommended in clinical practice.
Strikingly, the present study has several limitations. First, it is a single-center study with a limited sample size, thereby necessitating prospective multi-center study to substantiate these results. Second, HBV DNA at baseline, the genotypes, and mutations of HBV were not analyzed. Third, the family history of HCC was not collected in the present study.
Conclusions
NSSs, including APRI, GPR, and FIB-4, presents a non-optimal accuracy in predicting HCC development, and a new model consisting of age, GGT, and PLT provides an accurate prediction in patients with HBV-related DC and sustained virological response.
Abbreviations
HCC, hepatocellular carcinoma; LC, liver cirrhosis; DC, decompensated cirrhosis; CHB, chronic hepatitis B; HBV, hepatitis B virus; HCV, hepatitis C virus; NSS, noninvasive scoring system; FIB-4, fibrosis-4; APRI, aminotransferase-to-platelet ratio index; GPR, gamma-glutamyl transpeptidase to platelet ratio; MELD, model for end-stage liver disease; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBil, total bilirubin; AFP, alpha-fetoprotein; GGT, gamma-glutamyl transpeptidase; INR, international normalized ratio; PLT, platelet; AUROC, area under the receiver operating characteristic curve; Model (Age_GGT_PLT), a model consisting of age, gamma-glutamyl transpeptidase, and platelet.
Ethics Approval and Consent to Participate
The study was approved by the Ethics Committee of the Third People’s Hospital of Changzhou. All methods were carried out according to the Declaration of Helsinki, 2013, and written informed consent was obtained from all participants.
Consent for Publication
Consent for publication was obtained from all participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Su GL, Altayar O, O’Shea R, et al. AGA clinical practice guideline on systemic therapy for hepatocellular carcinoma. Gastroenterology. 2022;162(3):920–934. doi:10.1053/j.gastro.2021.12.276
- Zhang CH, Cheng Y, Zhang S, Fan J, Gao Q. Changing epidemiology of hepatocellular carcinoma in Asia. Liver Int. 2022;42:2029–2041. doi:10.1111/liv.15251
- Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153(4):996–1005 e1001. doi:10.1053/j.gastro.2017.06.012
- Kim BS, Seo YS, Kim YS, et al. Reduced risk of hepatocellular carcinoma by achieving a subcirrhotic liver stiffness through antiviral agents in hepatitis B virus-related advanced fibrosis or cirrhosis. J Gastroenterol Hepatol. 2018;33(2):503–510. doi:10.1111/jgh.13854
- Kim SU, Seo YS, Lee HA, et al. Hepatocellular carcinoma risk steadily persists over time despite long-term antiviral therapy for hepatitis b: a multicenter study. Cancer Epidemiol Biomarkers Prev. 2020;29(4):832–837. doi:10.1158/1055-9965.EPI-19-0614
- Zhu DM, Xie J, Ye CY, Qian MY, Xue Y. Risk of hepatocellular carcinoma remains high in patients with HBV-related decompensated cirrhosis and long-term antiviral therapy. Can J Gastroenterol Hepatol. 2020;2020:8871024. doi:10.1155/2020/8871024
- Nakai M, Yamamoto Y, Baba M, et al. Prediction of hepatocellular carcinoma using age and liver stiffness on transient elastography after hepatitis C virus eradication. Sci Rep. 2022;12(1):1449. doi:10.1038/s41598-022-05492-5
- Ioannou GN, Beste LA, Green PK, et al. Increased risk for hepatocellular carcinoma persists up to 10 years after HCV eradication in patients with baseline cirrhosis or high FIB-4 scores. Gastroenterology. 2019;157(5):1264–1278 e1264. doi:10.1053/j.gastro.2019.07.033
- Huang TH, Lin MT, Wang JH, et al. Clinical and novel application of FibroScan, FIB-4 and aspartate aminotransferase-to-platelet ratio index in liver fibrosis evaluation in patients with hepatocellular carcinoma and their roles in oesophageal variceal prediction. Int J Clin Pract. 2021;75(4):e13945. doi:10.1111/ijcp.13945
- Younes R, Caviglia GP, Govaere O, et al. Long-term outcomes and predictive ability of non-invasive scoring systems in patients with non-alcoholic fatty liver disease. J Hepatol. 2021;75(4):786–794. doi:10.1016/j.jhep.2021.05.008
- Kawaguchi T, Ide T, Amano K, et al. Enhanced liver fibrosis score as a predictive marker for hepatocellular carcinoma development after hepatitis C virus eradication. Mol Clin Oncol. 2021;15(4):215. doi:10.3892/mco.2021.2377
- Tamaki N, Kurosaki M, Yasui Y, et al. Change in fibrosis 4 index as predictor of high risk of incident hepatocellular carcinoma after eradication of hepatitis c virus. Clin Infect Dis. 2021;73(9):e3349–e3354. doi:10.1093/cid/ciaa1307
- Ji F, Zhou R, Wang W, et al. High post-treatment alpha-fetoprotein levels and aspartate aminotransferase-to-platelet ratio index predict hepatocellular carcinoma in hepatitis c virus decompensated cirrhotic patients with sustained virological response after antiviral therapy. J Interferon Cytokine Res. 2017;37(8):362–368. doi:10.1089/jir.2017.0040
- Kim JH, Kim JW, Seo JW, Choe WH, Kwon SY. Noninvasive tests for fibrosis predict 5-year mortality and hepatocellular carcinoma in patients with chronic hepatitis B. J Clin Gastroenterol. 2016;50(10):882–888. doi:10.1097/MCG.0000000000000574
- Lee HW, Cho YY, Lee H, et al. Impact of tenofovir alafenamide vs. entecavir on hepatocellular carcinoma risk in patients with chronic hepatitis B. Hepatol Int. 2021;15(5):1083–1092. doi:10.1007/s12072-021-10234-2
- Tseng TC, Choi J, Nguyen MH, et al. One-year Fibrosis-4 index helps identify minimal HCC risk in non-cirrhotic chronic hepatitis B patients with antiviral treatment. Hepatol Int. 2021;15(1):105–113. doi:10.1007/s12072-020-10124-z
- Demir M, Grunewald F, Lang S, et al. Elevated liver fibrosis index FIB-4 is not reliable for HCC risk stratification in predominantly non-Asian CHB patients. Medicine. 2016;95(38):e4602. doi:10.1097/MD.0000000000004602
- Nartey YA, Awuku YA, Agyei-Nkansah A, et al. Ambulatory end-stage liver disease in Ghana; patient profile and utility of alpha fetoprotein and aspartate aminotransferase: platelet ratio index. BMC Gastroenterol. 2020;20(1):428. doi:10.1186/s12876-020-01581-9
- Hann HW, Wan S, Lai Y, et al. Aspartate aminotransferase to platelet ratio index as a prospective predictor of hepatocellular carcinoma risk in patients with chronic hepatitis B virus infection. J Gastroenterol Hepatol. 2015;30(1):131–138. doi:10.1111/jgh.12664
- Nishikawa H, Nishijima N, Enomoto H, et al. Comparison of FIB-4 index and aspartate aminotransferase to platelet ratio index on carcinogenesis in chronic hepatitis B treated with entecavir. J Cancer. 2017;8(2):152–161. doi:10.7150/jca.16523
- Paik N, Sinn DH, Lee JH, et al. Non-invasive tests for liver disease severity and the hepatocellular carcinoma risk in chronic hepatitis B patients with low-level viremia. Liver Int. 2018;38(1):68–75. doi:10.1111/liv.13489
- Zhu YF, Tan YF, Xu X, et al. Gamma-glutamyl transpeptidase-to-platelet ratio and the fibrosis-4 index in predicting hepatitis B virus-related hepatocellular carcinoma development in elderly chronic hepatitis B patients in China: a single-center retrospective study. Medicine. 2019;98(50):e18319. doi:10.1097/MD.0000000000018319
- Jia JD, Hou JL, Wei L, Zhuang H. [Highlights of the guidelines of prevention and treatment for chronic hepatitis B (2019 version)]. Zhonghua Gan Zang Bing Za Zhi. 2020;28(1):21–23. Chinese. doi:10.3760/cma.j.issn.1007-3418.2020.01.006
- Department of Medical Administration, NH, Health Commission of the People’s Republic of, C. [Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)]. Zhonghua Gan Zang Bing Za Zhi. 2020;28(2):112–128. Chinese. doi:10.3760/cma.j.issn.1007-3418.2020.02.004
- Bengtsson B, Widman L, Wahlin S, Stal P, Bjorkstrom NK, Hagstrom H. The risk of hepatocellular carcinoma in cirrhosis differs by etiology, age and sex: a Swedish nationwide population-based cohort study. United European Gastroenterol J. 2022;10:465–476. doi:10.1002/ueg2.12238
- Duberg AS, Lybeck C, Falt A, Montgomery S, Aleman S. Chronic hepatitis B virus infection and the risk of hepatocellular carcinoma by age and country of origin in people living in Sweden: a national register study. Hepatol Commun. 2022;6:2418–2430. doi:10.1002/hep4.1974
- Kim M, Lee Y, Yoon JS, et al. The FIB-4 index is a useful predictor for the development of hepatocellular carcinoma in patients with coexisting nonalcoholic fatty liver disease and chronic hepatitis B. Cancers. 2021;13(10). doi:10.3390/cancers13102301
- Liang LY, Lee HW, Wong VW, et al. Serum fibrosis index-based risk score predicts hepatocellular carcinoma in untreated patients with chronic hepatitis B. Clin Mol Hepatol. 2021;27(3):499–509. doi:10.3350/cmh.2020.0333
- Huang CF, Jang TY, Jun DW, et al. On-treatment gamma-glutamyl transferase predicts the development of hepatocellular carcinoma in chronic hepatitis B patients. Liver Int. 2022;42(1):59–68. doi:10.1111/liv.15085