Abstract
Purpose
The differential diagnosis of atypical hepatocellular carcinoma (aHCC) and atypical benign focal hepatic lesions (aBFHL) usually depends on pathology. This study aimed to develop non-invasive approaches based on conventional blood indicators for the differential diagnosis of aHCC and aBFHL.
Patients and Methods
Hospitalized patients with pathologically confirmed focal hepatic lesions and their clinical data were retrospectively collected, in which patients with HCC with serum alpha-fetoprotein (AFP) levels of ≤200 ng/mL and atypical imaging features were designated as the aHCC group (n = 224), and patients with benign focal hepatic lesions without typical imaging features were designated as the aBFHL group (n = 178). The performance of indexes (both previously reported and newly constructed) derived from conventional blood indicators by four mathematical operations in distinguishing aHCC and aBFHL was evaluated using the receiver operating characteristic (ROC) curve and diagnostic validity metrics.
Results
Among ten previously reported derived indexes related to HCC, the index GPR, the ratio of γ-glutamyltransferase (GGT) to platelet (PLT), showed the best performance in distinguishing aHCC from aBFHL with the area under ROC curve (AUROC) of 0.853 (95% CI 0.814–0.892), but the other indexes were of little value (AUROCs from 0.531 to 0.700). A new derived index, sAGP [(standardized AFP + standardized GGT)/standardized PLT], was developed and exhibited AUROCs of 0.905, 0.894, 0.891, 0.925, and 0.862 in differentiating overall, BCLC stage 0/A, TNM stage I, small, and AFP-negative aHCC from aBFHL, respectively.
Conclusion
The sAGP index is an efficient, simple, and practical metric for the non-invasive differentiation of aHCC from aBFHL.
Introduction
Primary hepatic carcinoma (PHC) is one of the common malignancies, with an estimated 906,000 new cases and 830,000 deaths in 2020, ranking as the sixth most frequent cancer and the third leading cause of cancer-related death worldwide.Citation1 Due to difficulties in early diagnosis, 60% of patients with PHC have progressed to intermediate or advanced stages at the time of diagnosis, and the majority lose the opportunity for radical surgery, resulting in an overall 5-year survival rate of <10%.Citation2 In contrast, early diagnosis followed by effective treatment can significantly change the course of the disease and improve prognosis, resulting in an overall 5-year survival rate of >70%.Citation3 Therefore, early diagnosis of PHC is important.
Hepatocellular carcinoma (HCC) accounts for 90% of PHC. At present, the clinical diagnosis of HCC mainly depends on serum alpha-fetoprotein (AFP) and imaging examination. In the Asia-Pacific guidelines for the management of hepatocellular carcinoma, AFP ≥ 200 ng/mL is used as a criterion for HCC surveillance.Citation4 However, up to 30% of HCC patients do not present with elevated serum AFP levels.Citation5 In addition, approximately 40% of patients with hepatitis and 30% of patients with cirrhosis have elevated AFP levels, even exceeding 200ng/mL.Citation6
Imaging examination is an important tool in the diagnosis of HCC. The typical imaging features of HCC are hyper-enhancement during the arterial phase followed by washout during the portal and late phases. However, 5–41% of HCC do not have these typical features, while some benign focal hepatic lesions (BFHL) are radiologically difficult to distinguish from atypical HCC, which leads to the requirement for pathological examination to clarify the diagnosis.Citation7 Unfortunately, preoperative pathological examination requires liver biopsy to obtain tissue specimens. Liver biopsy, in addition to causing pain to the patient, may cause bleeding, infection, needle track tumor seeding, and injury (pneumothorax, hemothorax, and injury to the gallbladder, bowel, or kidney). Therefore, non-invasive diagnostic approaches are important for patients with focal hepatic lesions but whose imaging examinations are difficult to diagnose qualitatively. Here, we defined the HCC and BFHL with AFP < 200ng/mL and uncertain imaging diagnosis as atypical HCC (aHCC) and atypical BFHC (aBFHL), respectively.
In recent years, some studies have found that routine laboratory indicators alone or in combination are of certain value in the clinical practice of HCC.Citation8,Citation9 Given the limited value of single routine laboratory indicators, a number of indexes based on a combination of routine laboratory indicators have been derived by four arithmetic operations, and their diagnostic and prognostic performances for HCC have been evaluated, including inflammation-related derived indexes, such as neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic immune inflammatory index (SII), lymphocyte-to-monocyte ratio (LMR),Citation10–12 and liver function-related derived indexes, such as γ-glutamyltransferase to aspartate aminotransferase ratio (GGT/AST), γ-glutamyltransferase to alanine aminotransferase ratio (GGT/ALT), γ-glutamyltransferase to alkaline phosphatase ratio (GGT/ALP), γ-glutamyltransferase-to-platelet ratio (GPR), fibrinogen-to-prealbumin ratio (FPR), and fibrinogen-to-albumin ratio (FAR).Citation13–16 These derived indexes are potentially valuable for the diagnosis and prognosis of HCC.Citation17
The differential diagnostic value of these derived indexes for aHCC and aBFHL is unclear. We speculate that they may be of good value in distinguishing aHCC from aBFHL, as the above findings suggest that conventional clinical indicators contain important clinical information about HCC. Therefore, in the present study, we evaluated the performance of the above reported derived indexes for differentiating aHCC from aBFHL and, more importantly, tried to derive new indexes with better performance to distinguish aHCC from aBFHL.
Materials and Methods
Patients and Data Collection
Patients with focal hepatic lesions (FHL) who were hospitalized at the First Affiliated Hospital of Nanchang University from January 2015 to December 2020 and had a pathological diagnosis were retrospectively collected. The clinical and pathological data of each patient were obtained from the electronic medical records, including demographics data, results of first laboratory tests and imaging examinations before treatment after admission to hospital, and pathological results of FHL. Patients with the following conditions were excluded: (1) serum AFP levels >200 ng/mL; (2) typical imaging features on CT scans allowing for a definite diagnosis (3) concomitant medical conditions that may affect the results of laboratory blood tests; (4) received therapies that may affect the results of laboratory blood tests; (5) missing necessary data, including AFP, GGT, and contrast-enhanced CT/MRI scan. shows the flowchart of patient selection.
Data Processing and Statistical Analysis
Patients with aHCC were staged according to the Barcelona Clinic Liver Cancer (BCLC) staging system 2022Citation18 and the eighth version of the Tumor, Node, Metastases (TNM) staging system.Citation19 The reported derived indexes were calculated according to the algorithms in the literature: NLR, PLR, LMR, SII, GGT/AST, GGT/ALT, GGT/ALP, FPR, FAR, GPR, as well as Child-Pugh score and grade. The best reported derived indexes were used to derive new indexes with better diagnostic performance. In this process, the indicators were standardized using the upper limit of their normal range (ie, the ratio of the original value to the upper limit) and marked with the prefix “s” in order to improve the generalization ability of the new indexes in applications.
Measurement variables were presented as the mean ± standard deviation (SD) if normally distributed or median (M) (interquartile range, IQR) if non-normally distributed and compared between the two groups using Student’s t-test or Mann–Whitney U-test. Categorical variables were presented as count and percentage and compared between the two groups using Pearson’s Chi-squared test. P < 0.05 was considered statistically significant. The diagnostic performance of indexes was evaluated using the area under the receiver operating characteristic (ROC) curve (AUROC) and sensitivity, specificity, accuracy, positive predictive value, negative predictive value, positive likelihood ratio, and negative likelihood ratio. Statistical software SPSS 25.0 (IBM, Chi, USA) and MedCalc 22.0 (MedCalc Software Ltd, Ostend, BE) were used for data analyses.
Results
Demographic and Clinical Data of Patients
A total of 402 patients were enrolled in this study, including 224 cases of aHCC and 178 cases of aBFHL. The demographic and clinical characteristics of patients are shown in . Clinical tumor staging data were available in 212 aHCC patients, of which 23 (10.8%), 164 (77.4%), 11 (5.2%), and 14 (6.6%) cases were in BCLC stages 0, A, B, and C, respectively, and 180 (84.9%), 10 (4.7%), 16 (7.5%), and 6 (2.8%) cases were in TNM stages I, II, III, and IV, respectively. The majority of aHCC were hepatitis B virus-associated HCC, with an 84.4% (189/224) positive rate of hepatitis B virus serum markers. Patients with aBFHL included 109 cases of cavernous hemangioma, 19 cases of angiomyolipoma, 21 cases of focal nodular hyperplasia, and 29 cases of other diseases.
Table 1 Demographic and Clinical Characteristics of the Patients
Diagnostic Performance of Reported Derived Indexes for aHCC
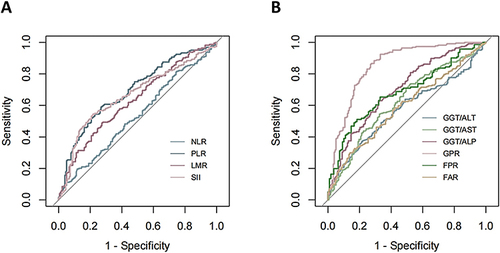
Previously reported derived indexes that were related to HCC included the inflammation-related (NLR, PLR, LMR, SII) and the liver function-related (GGT/AST, GGT/ALT, GGT/ALP, GPR, FPR, FAR). The diagnostic performance of these derived indexes for aHCC was analyzed using the ROC curve (), and the results showed that GPR had the best diagnostic performance, with an AUROC of 0.853 (95% CI: 0.814–0.892), whereas the AUROCs of other derived indexes were below 0.8 ().
Table 2 Reported Derived Indexes for the Diagnosis of Atypical Hepatocellular Carcinoma
Figure 2 Receiver operating characteristic curves of derived indexes reported previously for the diagnosis of atypical hepatocellular carcinoma. (A) Inflammation-related derived indexes; (B) Liver function-related derived indexes.
A New Derived Index and Its Diagnostic Performance for aHCC
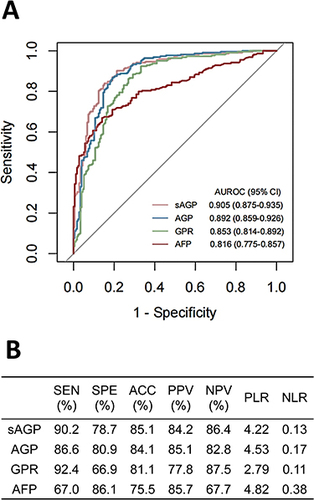
Among the reported derived indexes, GPR showed the greatest AUROC for the diagnosis of aHCC. Therefore, we combined the indicators of GPR with other indicators to construct new derived indexes to outperform GPR in terms of diagnostic performance, and the results showed that the AUROC of AGP [(AFP+GGT)/PLT] was the greatest among the new derived indexes and higher than those of GPR and AFP (). For better generalization of AGP, the three indicators in AGP (ie, AFP, GGT, and PLT) were standardized using the upper limit of normal range, and a standardized AGP, sAGP [(standardized AFP + standardized GGT) to standardized PLT ratio], was obtained, which further improved the diagnostic performance (). Based on the ROC curve analysis, the optimal cutoff values for sAGP, GPR, and AFP to diagnose aHCC were 1.92, 0.13, and 4.37, respectively. According to these cutoff values, the metrics of diagnostic validity for aHCC were calculated and are shown in .
Figure 3 Diagnostic performance of sAGP, AGP, GPR, and AFP in differentiating atypical hepatocellular carcinoma from atypical benign focal hepatic lesion. (A) Receiver operating characteristic curves; (B) Diagnostic validity metrics.
Diagnostic Performances of sAGP for Early, Small, and AFP-Negative aHCC
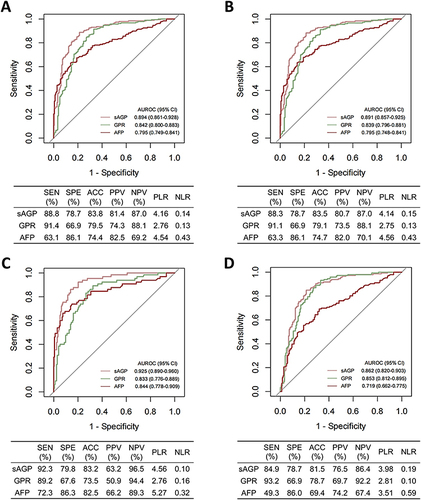
The AUROCs and diagnostic validity metrics of sAGP were calculated for aHCC subtypes, including early aHCC (BCLC stage 0/A, n = 187, and TNM stage I, n = 180), small aHCC (tumor diameter <3 cm, n = 65), and AFP-negative (AFP < 20 ng/mL, n = 146) aHCC, and the results showed that sAGP performed well in diagnosing various subtypes of aHCC and better than GPR and AFP ().
Figure 4 Receiver operating characteristic curves and diagnostic validity metrics of sAGP in the diagnosis of aHCC subgroups. (A) Early BCLC stage (0/A) aHCC vs aBFHL; (B) Early TNM stage (I) aHCC vs aBFHL; (C) Small aHCC (tumor diameter < 3 cm) vs aBFHL; (D) AFP-negative aHCC (AFP < 20 ng/mL) vs aBFHL.
Discussion
In this study, we investigated indexes derived from conventional laboratory blood indicators for the differential diagnosis of HCC and BFHL that are atypical on imaging. We evaluated the diagnostic value of previously reported ten derived indexes and found that only GPR had an AUROC greater than 0.8 in differentiating aHCC from aBFHL. In order to improve the diagnostic performance, the new index sAGP was derived by the combination of AFP with the two indicators (GGT and PLT) in GPR and showed good diagnostic performance for aHCC (AUROC = 0.905). More importantly, sAGP also performed well in diagnosing early-stage, small, and AFP-negative aHCC. These results suggest that sAGP is a valuable index for the clinical diagnosis of aHCC.
To our knowledge, this study is the first time to differentiate aHCC and BFHL based on conventional laboratory indicators. Similar studies previously reported are mainly based on imaging methods, particularly ultrasound. A machine learning model based on ultrasonography features for discriminating aHCC from hepatic focal nodular hyperplasia showed an AUROC of 0.86, sensitivity of 76.6%, and specificity of 80.5%.Citation20 Based on contrast-enhanced ultrasound images, a computer-aided diagnostic approach that extracted spatio-temporal semantics of the images achieved better performance in distinguishing aHCC from focal nodular hyperplasia, with an average accuracy 94.40%, specificity 93.62%, and sensitivity 94.76%.Citation21 Compared to these imaging-based methods, index sAGP derived from AFP, GGT, and PLT has outstanding advantages in terms of cost, convenience, and practicality, making it easy to apply at all levels of hospitals.
The index GPR, the ratio of GGT to PLT, showed the best diagnostic performance among the ten derived indexes. When HCC occurs, cancer cells synthesize large amounts of GGT, and intrahepatic bile duct obstruction also induces large amounts of GGT production by hepatocytes; meanwhile, inflammation around cancerous tissue contributes to increased permeability of hepatocyte membranes, making serum GGT levels elevated.Citation22 PLT is often reduced in HCC patients due to decreased production of hormone thrombopoietin (TPO) in the damaged liver and increased platelet destruction through phagocytosis in the enlarged spleen. Thrombocytopenia can also affect the growth and metastasis of HCC.Citation23,Citation24 GPR integrates both GGT and PLT by ratio to widen the difference between HCC and control, thus providing better diagnostic performance for HCC. Huang et alCitation15 found that the AUROCs of GPR were 0.884, 0.914, and 0.859 for discriminating AFP-negative HCC, AFP-negative HCC with tumor size <3 cm, AFP-negative HCC with BCLC-A stage from healthy controls, respectively. GPR can also be used for risk assessment and prognostic evaluation of HCC.Citation25,Citation26
When AFP was integrated into the index GRP, the new index sAGP was developed and showed good diagnostic performance for aHCC (AUROC = 0.905). AFP is the most widely used tumor marker for the diagnosis of HCC, but it is only elevated in 60–70% of HCC patients. Serum AFP levels are often low or normal in patients with small and high-differentiated HCC, which poses a problem for the clinical diagnosis of HCC without typical image features.Citation27 When the cut-off value was 200 ng/mL, the sensitivity and specificity of AFP in diagnosing HCC were 22.4% and 99.4%, respectively.Citation28 However, in the present study, AFP was valuable to some extent in differentiating aHCC from aBFHL, with an AUROC of 0.816 for the diagnosis of aHCC and a sensitivity of 67.0% and a specificity of 86.1% at the optimal cut-off value of 4.37 ng/mL. The good diagnostic performance of sAGP also indicates the importance of AFP in discriminating of aHCC from aBFHL. In fact, this importance of AFP was easy to understand, since AFP levels were significantly higher in aHCC than in aBFHL (median: 8.6 vs 2.4 ng/mL, p < 0.001), although AFP levels were low or even normal in aHCC.
However, there are some limitations in this study. First, this study is retrospective and some data is missing, which may introduce bias. Prospective studies are needed to confirm the findings. Second, this is a single-center study, and the results should be validated in an external patient cohort. Third, the patients in this study are all pathologically confirmed and thus mainly surgical patients, resulting in a small proportion of advanced HCC in the cohort, which differs from the distribution of HCC cases in the real world.
In conclusion, we derived the index sAGP from three blood indicators (AFP, GGT, and PLT) conventionally used in clinical practice using four arithmetic operations. This index exhibited good performance in the differentiation of aHCC (including early-stage, small, and AFP-negative aHCC) from aBFHL, outperforming previously reported derived indexes and AFP. Due to its low cost, convenience, and practicality, sAGP has great potential for clinical applications. However, multicenter and prospective studies are needed to further confirm the diagnostic value of sAGP for aHCC.
Patient Consent Statement
The Ethics Committee of the First Affiliated Hospital of Nanchang University waived the requirement for informed consent because this study was a retrospective investigation that did not require additional patient participation, and the medical records can be used for medical research without informed consent while ensuring that patients’ personal information is not disclosed. We confirm that the patient’s personal information has not been disclosed and will be kept confidential.
Ethical Statement
This study was approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University and conducted in accordance with the requirements of the Declaration of Helsinki.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
- Lok AS, Sterling RK, Everhart JE, et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138(2):493–502. doi:10.1053/j.gastro.2009.10.031
- Tsuchiya N, Sawada Y, Endo I, Saito K, Uemura Y, Nakatsura T. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2015;21(37):10573–10583. doi:10.3748/wjg.v21.i37.10573
- Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–370. doi:10.1007/s12072-017-9799-9
- Fu Y, Chen J, Li C, Chen L, Zhang Z, Huang Z. Effectiveness of ultrasound-guided percutaneous transhepatic puncture for the diagnosis of low-level alpha-fetoprotein liver cancer patients. Transl Cancer Res. 2021;10(6):2985–2990. doi:10.21037/tcr-21-701
- Liu D, Luo Y, Chen L, et al. Diagnostic value of 5 serum biomarkers for hepatocellular carcinoma with different epidemiological backgrounds: a large-scale, retrospective study. Cancer Biol Med. 2021;18(1):256–270. doi:10.20892/j.issn.2095-3941.2020.0207
- Li W, Wang W, Liu GJ, et al. Differentiation of atypical hepatocellular carcinoma from focal nodular hyperplasia: diagnostic performance of contrast-enhanced US and microflow imaging. Radiology. 2015;275(3):870–879. doi:10.1148/radiol.14140911
- Jing W, Peng R, Zhu M, et al. Differential expression and diagnostic significance of pre-albumin, fibrinogen combined with D-dimer in AFP-negative hepatocellular carcinoma. Pathol Oncol Res. 2020;26(3):1669–1676. doi:10.1007/s12253-019-00752-8
- Ou Y, Huang J, Yang L. The prognostic significance of pretreatment serum γ-glutamyltranspeptidase in primary liver cancer: a meta-analysis and systematic review. Biosci Rep. 2018;38(6):BSR20181058. doi:10.1042/BSR20181058
- Johnson PJ, Dhanaraj S, Berhane S, Bonnett L, Ma YT. The prognostic and diagnostic significance of the neutrophil-to-lymphocyte ratio in hepatocellular carcinoma: a prospective controlled study. Br J Cancer. 2021;125(5):714–716. doi:10.1038/s41416-021-01445-3
- Yang Y, Wang MC, Tian T, et al. A high preoperative platelet-lymphocyte ratio is a negative predictor of survival after liver resection for hepatitis B virus-related hepatocellular carcinoma: a retrospective study. Front Oncol. 2020;10:576205. doi:10.3389/fonc.2020.576205
- Wang C, He W, Yuan Y, et al. Comparison of the prognostic value of inflammation-based scores in early recurrent hepatocellular carcinoma after hepatectomy. Liver Int. 2020;40(1):229–239. doi:10.1111/liv.14281
- Li J, Tao H, Zhang E, Huang Z. Diagnostic value of gamma-glutamyl transpeptidase to alkaline phosphatase ratio combined with gamma-glutamyl transpeptidase to aspartate aminotransferase ratio and alanine aminotransferase to aspartate aminotransferase ratio in alpha-fetoprotein-negative hepatocellular carcinoma. Cancer Med. 2021;10(14):4844–4854. doi:10.1002/cam4.4057
- Wang G, Lu X, Du Q, et al. Diagnostic value of the γ-glutamyltransferase and alanine transaminase ratio, alpha-fetoprotein, and protein induced by vitamin K absence or antagonist II in hepatitis B virus-related hepatocellular carcinoma. Sci Rep. 2020;10(1):13519. doi:10.1038/s41598-020-70241-5
- Huang L, Mo Z, Hu Z, et al. Diagnostic value of fibrinogen to prealbumin ratio and gamma-glutamyl transpeptidase to platelet ratio in the progression of AFP-negative hepatocellular carcinoma. Cancer Cell Int. 2020;20:77. doi:10.1186/s12935-020-1161-y
- Xu Q, Yan Y, Gu S, et al. A novel inflammation-based prognostic score: the fibrinogen/albumin ratio predicts prognoses of patients after curative resection for hepatocellular carcinoma. J Immunol Res. 2018;2018:4925498. doi:10.1155/2018/4925498
- Luo QQ, Wang T, Zhang KH. New indexes derived from routine blood tests and their clinical application in hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2022;46(10):102043. doi:10.1016/j.clinre.2022.102043
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
- Lee CW, Tsai HI, Yu MC, et al. A proposal for T1 subclassification in hepatocellular carcinoma: reappraisal of the AJCC. Hepatol Int. 2022:16(6);1353–1367. doi:10.1007/s12072-022-10422-8
- Li W, Lv XZ, Zheng X, et al. Machine learning-based ultrasomics improves the diagnostic performance in differentiating focal nodular hyperplasia and atypical hepatocellular carcinoma. Front Oncol. 2021;11:544979. doi:10.3389/fonc.2021.544979
- Huang Q, Pan F, Li W, et al. Differential diagnosis of atypical hepatocellular carcinoma in contrast-enhanced ultrasound using spatio-temporal diagnostic semantics. IEEE J Biomed Health Inform. 2020;24(10):2860–2869. doi:10.1109/JBHI.2020.2977937
- Moreira AJ, Rodrigues GR, Bona S, et al. Ductular reaction, cytokeratin 7 positivity, and gamma-glutamyl transferase in multistage hepatocarcinogenesis in rats. Protoplasma. 2017;254(2):911–920. doi:10.1007/s00709-016-1000-0
- Kurokawa T, Ohkohchi N. Platelets in liver disease, cancer and regeneration. World J Gastroenterol. 2017;23(18):3228–3239. doi:10.3748/wjg.v23.i18.3228
- Bihari C, Rastogi A, Shasthry SM, et al. Platelets contribute to growth and metastasis in hepatocellular carcinoma. APMIS. 2016;124(9):776–786. doi:10.1111/apm.12574
- Park YE, Kim BK, Park JY, et al. Gamma-glutamyl transpeptidase-to-platelet ratio is an independent predictor of hepatitis B virus-related liver cancer. J Gastroenterol Hepatol. 2017;32(6):1221–1229. doi:10.1111/jgh.13653
- Yang D, Wu H, Nong W, et al. A new model based on gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts prognostic outcome after curative resection of solitary hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2021;45(5):101509. doi:10.1016/j.clinre.2020.07.014
- She S, Xiang Y, Yang M, et al. C-reactive protein is a biomarker of AFP-negative HBV-related hepatocellular carcinoma. Int J Oncol. 2015;47(2):543–554. doi:10.3892/ijo.2015.3042
- Trevisani F, D’Intino PE, Morselli-Labate AM, et al. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol. 2001;34(4):570–575. doi:10.1016/s0168-8278(00)00053-2