Abstract
Purpose
The aim of this study was to investigate the efficacy and safety of conversion surgery for advanced hepatocellular carcinoma (HCC) after hepatic arterial infusion chemotherapy (HAIC).
Patients and Methods
Data from 172 HCC patients treated at Sun Yat-sen University Cancer Center between January 2016 and June 2021 with effective assessment of HAIC treatment response were retrospectively analyzed. Clinical pathological data, treatment process, survival, and occurrence of adverse events were recorded. Patients were grouped according to whether they achieved imaging remission after HAIC, underwent conversion surgery, and met the surgical resection criteria. Efficacy and safety were analyzed.
Results
The median progression-free survival (PFS) and overall survival (OS) in the imaging remission group were 8.6 months and 26.3 months, respectively, which were longer than the 4.6 months (P<0.05) and 15.6 months (P<0.05) in the nonremission group. Compared with 6.7 months and 18.9 months in the HAIC maintenance group, the median PFS and median OS in the conversion surgery group were 16.5 months (P<0.05) and 45.0 months (P<0.05), but there was a higher risk of treatment-related hemoglobin decrease, alanine aminotransferase increase, aspartate aminotransferase increase, and total bilirubin increase (P<0.05). The risk of biliary fistula, abdominal hemorrhage and ascites in the HAIC conversion surgery group was higher than that of the single surgery group (P<0.05). Compared with the conversion surgery group, the median PFS and median OS of patients in the HAIC maintenance group who met the resection criteria were shorter: 7.1 months (P<0.05) and 21.7 months (P<0.05), respectively. All adverse events during the study were less than moderate, and no toxicity-related deaths occurred during follow-up.
Conclusion
HAIC-based conversion therapy had acceptable toxic effects and could effectively stabilize intrahepatic lesions in advanced HCC, improve the survival benefit of patients, and provide some patients with the opportunity for conversion surgery to further improve prognosis.
Introduction
Liver cancer is the fourth most common malignant tumor and the second leading cause of tumor death in China,Citation1 posing a serious threat to the life and health of Chinese people. It is also the sixth most common malignant tumor and the third leading cause of tumor death in the world.Citation2,Citation3 The number of liver cancer patients in China accounts for half of the cases in the world, and more than 90% of these patients have hepatocellular carcinoma (HCC).Citation4 Surgical treatment is still the most effective radical method for liver cancer. Liver transplantation and liver resection complement each other. Due to the severe shortage of donor livers, liver resection is still the first choice for the long-term survival of liver cancer patients. However, because of strong insidiousness and lack of obvious symptoms in the early stage of the disease, more than 60% of liver cancer patients are in the middle and advanced stages at the initial diagnosis,Citation5 the median survival time is approximately 2 years,Citation6 and surgical removal is feasible in only 15% to 30% when the diagnosis is first made. Most patients have lost the opportunity for surgical eradication therapy,Citation7 and palliative systemic therapy is often the only remaining option for these patients. In the past few years, with the introduction and improvement of novel therapies, the treatment of advanced liver cancer has rapidly developed.
“Primary Liver Cancer Diagnosis and Treatment Standards” are based on the premise that various medical treatment methods, including interventional therapy, make it possible for cases of unresectable HCC in the middle and advanced stages to be converted into cases of surgical resection.Citation6 In the 1980s, in view of the limitations of local treatment methods at that time, transcatheter arterial chemoembolization (TACE) was widely used in the treatment of liver cancer after continuous development and improvement and is still one of the standard treatments for intermediate-stage liver cancer.Citation8,Citation9 However, long-term clinical experience shows that TACE still has certain limitations due to the abundant blood supply of HCC tumors; the post-TACE formation of new blood vessels and the establishment of collateral circulation renders TACE a means of palliative treatment only in most cases.Citation10,Citation11 The prognosis and surgery conversion rate after TACE is poor. Therefore, there is an urgent need for better treatment strategies for advanced HCC.
In recent years, hepatic artery infusion chemotherapy (HAIC) has gradually attracted attention, which is approved by the clinical guideline of the Chinese Society of Clinical Oncology (CSCO) in patients with unresectable hepatocellular carcinoma, especially whose tumors were larger than 7cm or TNM stage III/IV. HAIC of the FOLFOX regimen (oxaliplatin + folinate calcium + 5-fluorouracil) significantly improved overall survival and surgery conversion rate in patients with advanced HCC, especially in patients with unresectable large tumor or portal vein tumor thrombus (PVTT), compared with TACE.Citation12–15 Besides, HAIC combined with tyrosine kinase inhibitors (TKIs) and programmed cell death protein-1 (PD-1) inhibitors also could prolong the overall survival (OS) and progression-free survival (PFS) in HCC patients with large tumor burden or vascular invasion.Citation16,Citation17 Other studies also showed that for HCC whose tumor burden was mainly concentrated in the liver or combined with portal vein tumor thrombosis, FOLFOX-HAIC was more effective than drug-targeted therapy with a higher tumor remission rate.Citation18 Patients after HAIC treatment may substantially reduce tumor burden or considerable shrinkage of tumor thrombi in large blood vessel staining and, thereby, obtain the opportunity for conversion resection or ablation radical cure, which can improve the prognosis.Citation12 After unresectable liver cancer transformed into a resectable lesion, the 5-year survival rate after surgery is comparable to that of early liver cancer patients who receive radical resection.Citation13,Citation19
Compared with the traditional single conversion method, the oxaliplatin-based FOLFOX regimen significantly improves the tumor response rate, has a higher conversion resection rate and better safety and is simple to operate and easy to popularize.Citation20–23 However, the popularization and application of HAIC is not yet mature, and there are still different understandings in terms of indications and operating specifications. At the same time, the importance of conversion therapy is giving patients the opportunity to receive radical treatment, thereby allowing patients to obtain longer disease-free survival and overall survival, but conversion therapy still needs to be supported by evidence from controlled studies.
Although HAIC treatment can effectively prolong the overall survival of patients with advanced unresectable liver cancer,Citation9,Citation24 it also faces shortcomings in terms of efficacy and safety. Treatment also progresses within 1.0 to 1.5 years.Citation25 For patients with imaging remission, evidence regarding whether surgery is necessary is currently inconclusive. Although HAIC therapy has a higher tumor remission rate than TACE, sorafenib, etc,Citation26 multiple HAIC implementations may cause liver damage, which in turn affects the safety of conversion hepatectomy. Therefore, surgical safety has also become an important factor to be evaluated before conversion resection: these considerations not only require the evaluation of safety necessary for general hepatectomy but also require focusing on evaluating the potential impact of early conversion on the patient’s liver.Citation8
In view of the lack of related research reports, this study was designed to retrospectively analyze and explore prognosis in cases of conversion resection of advanced HCC after HAIC.
Materials and Methods
Patients
This study retrospectively analyzed pathologically diagnosed HCC patients at Sun Yat-sen University Cancer Center from January 2016 to June 2021 and collected clinical data that were used to effectively evaluate the efficacy of HAIC treatment. Inclusion criteria included the following: (1) the initial diagnosis met the diagnostic criteria for primary liver cancer, and the result of biopsy or postoperative pathological diagnosis was HCC; (2) with unresectable hepatocellular carcinoma which was confirmed by the multidisciplinary treatment (MDT); (3) Child-Pugh classification of liver function was A or B; (4) HAIC treatment involved more than 2 courses; (5) no other tumor diseases were present; (6) and no recent treatment for liver cancer had been performed. The exclusion criteria included the following: (1) secondary liver cancer; (2) Child-Pugh grade C liver function or accompanying jaundice and ascites; (3) combination with TACE, radiotherapy, immunotherapy, or targeted therapy; (4) severe cardiovascular, cerebrovascular, liver or kidney diseases; (5) contraindication to medication; (6) and incomplete medical information or patient lost to follow-up.
Treatment discontinuation was based on patient disease progression, death, severe drug toxicity, or a change in treatment regimen. The final follow-up occurred in December 2022. Contrast-enhanced CT or MRI was performed every 4–8 weeks with a routine follow-up interval of 2 to 4 months. All patients signed an informed consent form approved by the hospital ethics committee, and the study complied with medical ethics regulations.
HAIC Procedure
All patients received more than 2 courses of HAIC treatment. The number of HAIC cycles were positively correlated with tumor size and clinical stage.Citation27 The maximum times of HAIC were 8 times. The FOLFOX regimen administration method was as follows: oxaliplatin 85 mg/m2 arterial infusion for 2 hours, leucovorin 400 mg/m2 arterial infusion for 1 hour, or oxaliplatin 130 mg/m2 arterial infusion after 3 hours of injection; calcium folinate 200 mg/m2 infusion for 2 hours, followed by 5-fluorouracil 400 mg/m2 arterial bolus injection, and 5-fluorouracil 2400 mg/m2 arterial infusion for 46 hours. The HAIC course was administered every 3 weeks, with the catheter removed at the end of the infusion. Imaging examinations (contrast-enhanced MRI was preferred; if not available, contrast-enhanced CT was performed) were performed every 4–8 weeks after each course of HAIC treatment, serum markers were measured 4–8 weeks after each course of HAIC treatment, and both findings were combined to evaluate the treatment reaction.
Surgery
After several senior surgeons comprehensively considered the possibility of surgical resection of the patient and determined that sufficient liver volume could remain after tumor resection, liver dynamic magnetic resonance imaging (MRI) and chest CT examination were performed to determine the location and volume of liver lesions. The size, peripheral vascular distribution and distant metastasis were assessed. The specific criteria for considering feasible surgical resection were summarized as follows: (1) CR/PR was determined by the efficacy evaluation; (2) the residual liver volume met the surgical requirements; (3) radical tumor resection or relatively radical resection could be achieved; and (4) surgical contraindications were excluded. Surgical resection was considered inapplicable for the following reasons: (1) invasion of the portal vein or inferior vena cava; (2) multiple lymph node metastases, distant metastases or intrahepatic lesions that could not be cured; (3) insufficient residual liver volume after radical resection; and (4) contraindications to surgery. Intraoperative ultrasound was used to reconfirm tumor size and surgical margins. All surgical procedures were performed by the same group of surgeons.
Collection of Clinical Data
Patient clinical data regarding the following were collected and recorded:(1) Serological tests, including routine blood and liver and kidney function tests and tumor marker levels, including white blood cell count, absolute neutrophil value, hemoglobin, platelet count, alanine aminotransferase, aspartate aminotransferase, serum bilirubin before and after treatment, serum albumin, serum creatinine, and alpha-fetoprotein; (2) Evaluation of the efficacy of HAIC: According to the RECIST 1.1 standard, complete response (CR) was defined as the disappearance of target lesions after treatment; partial response (PR) was defined as the reduction of the sum of the diameters of target lesions by ≥30%; progressive disease (PD) was defined as an increase in the sum of target lesion diameters by ≥20% or the appearance of new lesions; stable disease (SD) was defined as the reduction of target lesions without partial response or the increase of target lesions without progression; and objective response rate (ORR), defined as the proportion of patients with CR or PR; Besides, according to modified RECIST (mRECIST), modified CR (mCR), modified PR (mPR), modified SD (mSD), modified PD (mPD), and modified ORR (mORR) were also evaluated; (3) Adverse events after HAIC treatment/resection, including biliary fistula, abdominal cavity, postoperative bleeding, ascites, pneumonia, intestinal obstruction, fever, nausea, and vomiting. Adverse events were assessed according to Common Terminology Criteria Adverse Events Version 4.0; (4) Survival data: The survival data of patients from the start of treatment to the observation end point were collected and analyzed statistically, including PFS and OS. PFS was calculated as the time from receiving treatment until disease progression, relapse, or last follow-up. OS was calculated as the time from treatment until death or last follow-up.
Statistical Analyses
The measurement data that was confirmed to be a normal distribution was compared by the t-test while the data that was not confirmed to be a normal distribution was compared by the rank sum test. The rate comparison was performed by the chi-square test. The survival data were analyzed by the Log rank test (Kaplan-Meier survival analysis), and survival curves were plotted. P<0.05 was considered indicative of statistical significance.
Results
Characteristics of the Total Study Cohort
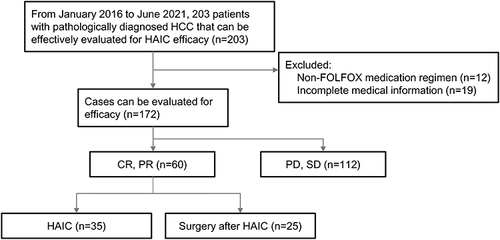
From January 2016 to June 2021, 203 patients were pathologically diagnosed with HCC and could be effectively evaluated for HAIC. After exclusion of 12 cases of HAIC with a non-FOLFOX medication regimen and 19 cases of incomplete data, a total of 172 patients were feasible for efficacy evaluation, a total of 172 patients who met the criteria were included, and they were assigned to the experimental group and the control group for comparison according to the following criteria (). Among them, there were 153 males and 19 females, aged 22–73 years, with a median age of 51 years. The baseline characteristics of the enrolled patients is shown in .
Table 1 Baseline Characteristics of the Enrolled Patients
Effect of HAIC Treatment on Imaging Remission
Imaging remission was defined as CR or PR after HAIC treatment, while nonremission was defined as PD or SD. According to the RECIST 1.1 criteria, patients were classified into an imaging remission group (n=60) and a nonremission group (n=112). The objective response rate after HAIC treatment in the enrolled patients was 34.9%. Besides, patients with mCR or mPR were 77, and the mORR after HAIC was 44.8%. And in the imaging remission group, 25 (41.7%) patients accepted the conversion surgery after HAIC treatment, while in the imaging nonremission group, 26 (23.2%) patients accepted the conversion surgery (p=0.012). The conversion surgery rate after HAIC was 29.7% in the enrolled patients. The baseline characteristics of the patients in the imaging remission and nonremission groups were shown in Supplementary Table 1.
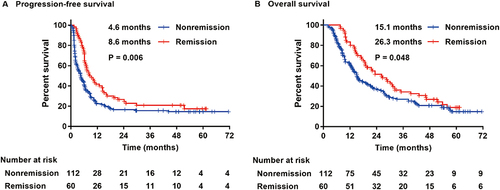
The median PFS and median OS in the imaging remission group were 8.6 (95% CI: 4.551–12.649) months and 26.3 (95% CI: 18.683–33.917) months, respectively, which were longer than the 4.6 (95% CI: 2.859–6.341) months and 15.1 (95% CI: 11.789–18.411) months in the non-remission group, and the difference between the two groups was statistically significant (P=0.006; P=0.048) ().
Figure 2 Kaplan-Meier plots of progression-free survival (A) and overall survival (B) in the imaging remission group and nonremission group.
The results of univariate and multivariate analysis showed that patients with ECOG 0, Child-Pugh class B, tumor size lower than 10 cm and no extrahepatic metastases were associated with longer PFS (), and patients with tumor size lower than 10 cm and without portal vein tumor thrombosis were associated with longer OS ().
Table 2 Univariate and Multivariate Analysis of Progression-Free Survival of the Enrolled Patients
Table 3 Univariate and Multivariate Analysis of Overall Survival of the Enrolled Patients
Patients with HCC who were female, with ECOG 0, tumor size lower than 10 cm, a single tumor, or multiple tumors located on one side and no extrahepatic metastases had a higher conversion surgery rate (P<0.05, ).
Table 4 Analysis of Conversion Surgery Rate of the Enrolled Patients
Efficacy and Safety of HAIC Maintenance Treatment and Conversion Surgery
The imaging remission subgroup was then divided into the HAIC maintenance group (n=35) and conversion surgery group (n=25) according to different treatment methods. HAIC maintenance was defined as receiving only HAIC during treatment without liver resection; conversion surgery was defined as undergoing liver resection after HAIC treatment. The baseline characteristics of the patients in the maintenance and conversion surgery groups were shown in Supplementary Table 2.
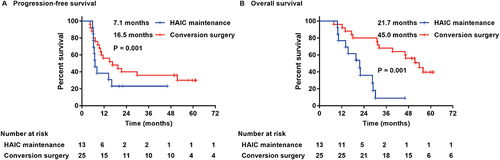
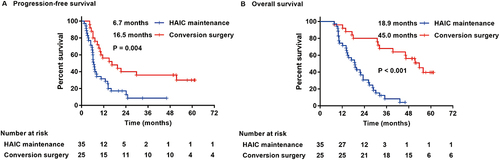
Compared with the HAIC maintenance group, the median PFS and median OS in the conversion surgery group were longer, reaching 16.5 (95% CI: 3.444–29.556) months and 45.0 (95% CI: 37.748–52.289) months, respectively. The values for the maintenance group were 6.7 (95% CI: 5.734–7.666) months and 18.9 (95% CI: 14.032–23.768) months, respectively, and the difference between the two groups was statistically significant (P=0.004; P<0.001) ().
Figure 3 Kaplan-Meier plots of progression-free survival (A) and overall survival (B) in the HAIC maintenance group and conversion surgery group.
In terms of the incidence of adverse reactions, compared with the HAIC maintenance group, the more common treatment-related adverse events in the conversion surgery group included anemia, elevated alanine aminotransferase, elevated aspartate aminotransferase, and hyperbilirubinemia, and the differences were statistically significant (P=0.050; P=0.009; P=0.017; P=0.006) ().
Table 5 Comparison of Treatment-Related Adverse Events Between the HAIC Maintenance Group and Conversion Surgery Group
At the same time, we retrospectively analyzed the clinical data of surgery-related adverse reactions in patients with liver cancer who underwent direct surgical resection or surgery after HAIC at Sun Yat-sen University Cancer Center from January 2016 to December 2022 to further compare safety between direct surgery and surgery after HAIC. According to whether preoperative HAIC treatment was performed, the patients were assigned to the direct surgery group (n=213) or the post-HAIC surgery group (n=107). Compared with direct liver resection, patients undergoing conversion surgery after HAIC had higher risks of postoperative biliary fistula, abdominal hemorrhage, and ascites, and the differences were statistically significant (P=0.006; P=0.002; P=0.003). No toxicity-related deaths occurred during follow-up. The detailed comparison between the two groups is shown in .
Table 6 Comparison of Treatment-Related Adverse Events Between the Hepatectomy Group and Hepatectomy After HAIC Group
Effects of HAIC Maintenance Therapy and Conversion Surgery in Patients Who Met the Criteria for Surgical Resection
Patients who met the surgical criteria after HAIC but no surgery was performed (n=13) were compared with the patients who underwent conversion surgery (n=25). Among them, the median PFS and median OS of the conversion surgery group were longer, reaching 16.5 (95% CI: 3.444–29.556) months and 45.0 (95% CI: 37.748–52.289) months, respectively, which were 7.1 (95% CI: 5.456–8.744) months and 21.7 (95% CI: 11.392–32.008) months in the non-surgery group, and the difference between the two groups was statistically significant (P=0.001; P=0.001) ().
Discussion
HAIC has the advantages of being minimally invasive, safe, efficient, and highly reproducible and has now become an optional treatment for advanced liver cancer, especially in East Asian countries such as China and Japan.Citation19,Citation28–30 Compared with standard treatment regimens for liver cancer, both negative and positive results of HAIC on prognosis have been reported, and the conclusions of different studies are not consistent.Citation9,Citation26 However, reassuringly, the ease of operation, safety, and survival benefit of HAIC in the treatment of HCC have been initially demonstrated. The results of studies showed that HAIC provided a relatively high response rate and acceptable toxicity in the treatment of HCC, which to a certain extent reflected the safety and efficacy of HAIC in the treatment of liver cancer;Citation12–18,Citation21,Citation22,Citation27 in addition, HAIC still provided substantial benefit in some patients, eg, in patients with portal vein thrombosis or high intrahepatic tumor burden.Citation18,Citation31 When combined with PD-1 inhibitors or TKIs, HAIC could also improve the prognosis of patients.Citation18,Citation32–34 However, even so, the evidence for an overall survival benefit of HAIC compared with standard care is insufficient. A recent clinical trial showed that HAIC treatment with sorafenib combined with the FOLFOX regimen can improve the objective response rate of HCC patients with portal vein invasion compared with sorafenib alone, but the survival benefit of patients is not satisfactory. Effective remission of HCC by HAIC did not translate into an overall survival benefit.Citation18
In this study, the median PFS and median OS of patients who achieved Imaging remission after HAIC were longer than those of patients without Imaging remission, indicating that HAIC had a certain remission rate in the treatment of HCC patients and effectively improved their prognosis. HAIC treatment, as a conversion therapy, helps to improve the survival and prognosis of patients with advanced HCC, but there is still the disadvantage of a lower objective remission rate with monotherapy. Clinically, HAIC has been combined with a variety of treatment modalities, including radiotherapy, targeted therapy and immunotherapy, to enhance its therapeutic effect; although the mechanism of action of the combined new therapy is not yet clear, studies have shown that combined therapy is significantly better.Citation18,Citation34 Although studies of HAIC on survival outcomes, especially OS benefit, are inconclusive, most combination therapies show clinical signs of increased objective response rates compared with HAIC alone. The results of a randomized clinical trial conducted by Shi Ming’s team from Sun Yat-Sen University Cancer Center proved that compared with sorafenib alone, HAIC treatment with sorafenib combined with FOLFOX can prolong the overall survival of patients with HCC invading the portal vein by 6.24 months.Citation18
In this study, although the surgical conversion rate after single HAIC was only 14.5%, the median OS and PFS after conversion surgery were 45.0 months and 16.5 months, respectively, which were higher than those in the maintenance HAIC treatment group, indicating that the survival benefit of postconversion resection was significantly better than that of palliative maintenance HAIC. It must be acknowledged that surgical resection allows patients to minimize tumor burden before drug-resistance-induced tumor progression, thereby increasing the chance of tumor cure and prolonging survival. Therefore, for patients with intermediate-to-advanced liver cancer, successful conversion surgery will greatly improve their survival prognosis, which fully reflects the effectiveness of conversion surgery. Studies have also shown that patients with initially unresectable liver cancer can be converted to resection with systemic or local therapy, and has shown promising survival benefits.Citation7,Citation35,Citation36
In addition, there were some patients in this study who met the criteria for surgical resection after receiving several courses of HAIC treatment and could be converted to surgery. Due to the difficulty of surgery, the high risk of serious postoperative complications, insufficient remaining liver volume, family financial difficulties, lack of willingness and other reasons, these patients did not undergo conversion surgery. Patients in the HAIC maintenance treatment that met surgical criteria showed poor OS or PFS compared with the cohort in the conversion surgery group. The reason is that although a patient’s tumor has a tendency to downstage after HAIC treatment, the tumor continues to progress after the emergence of drug resistance, and the tumor burden does not drop but rises, suggesting that subsequent conversion surgery is still the key to further improving survival and prognosis.
FOLFOX’s HAIC treatment regimen has begun to emerge in the conversion treatment of HCC, and various combination treatment regimens with it as the core have also produced preliminary results,Citation18,Citation34 which can be seen in the findings of this study. However, current scholars are continuing to explore combining HAIC, immunotherapy, targeted therapy drugs and other treatment methods in efforts to find the optimal combination.Citation23 Although considerable research results have shown that HAIC-based conversion therapy can effectively prolong the survival, there is still a lack of large randomized controlled trials to provide strong evidence. In recent years, Li QJ et alCitation13 have shown that HAIC provides a higher tumor response rate than TACE for patients with large unresectable liver cancer and can significantly prolong survival time. At the same time, for patients with advanced HCC, lenvatinib combined with PD1 and HAIC can significantly improve the RFS rate (80.6%) of patients and can also prolong the OS (mOS, not reached) and PFS (10.5 months) of patients.Citation34 However, not all unresectable liver cancers can be transformed. In our study we found that patients with HCC who were female, with ECOG 0, tumor size lower than 10 cm, a single tumor, or multiple tumors located on one side, and no extrahepatic metastases had a higher conversion surgery rate.
There are also certain limitations to our study. First, because this study was a retrospective study conducted in a single center, selection bias was inevitable In addition, the relatively small number of cases is another factor that restricts the further expansion of the conclusions of this study. What’s more, due to the heterogeneity of HAIC implementation in terms of patient selection, chemotherapy regimen and dosing, and HAIC implementers, these heterogeneities may lead to differences in treatment effects, thereby increasing the difficulty of interpreting trial results.
Conclusion
In summary, FOLFOX HAIC was accompanied by acceptable toxic effects and could effectively stabilize intrahepatic lesions in advanced HCC, improving the survival benefit of patients. Once surgical criteria are met after HAIC, conversion surgery is encouraged since further improvement of the prognosis may be obtained under the premise of ensuring surgical safety.
Ethics Approval and Informed Consent
This study was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki. This research was approved by the institutional review board of Sun Yat-sen University Cancer Center (Ethical review no. B2022-238-01). The study used retrospective anonymous clinical data that were obtained after each patient agreed to treatment.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; drafted, revised or critically reviewed the article; agreed on the journal to which the article will be submitted; gave final approval of the version to be published; agreed to take responsibility and be accountable for the contents of the article.
Disclosure
The authors report no conflicts of interest in this work.
Data Sharing Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Additional information
Funding
References
- Zheng R, Zhang S, Zeng H, et al. Cancer incidence and mortality in China, 2016. J Nat Cancer Center. 2022;2(1):1–9. doi:10.1016/j.jncc.2022.02.002
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. Cancer J Clinicians. 2019;69(1):7–34. doi:10.3322/caac.21551
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clinicians. 2018;68(6):394–424. doi:10.3322/caac.21492
- Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35(9):2155–2166. doi:10.1111/liv.12818
- Zhou J, Sun H, Wang Z, et al. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer. 2020;9(6):682–720. doi:10.1159/000509424
- Zhong JH, Ke Y, Gong WF, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260(2):329–340. doi:10.1097/SLA.0000000000000236
- Zhao HT, Cai JQ. Chinese expert consensus on neoadjuvant and conversion therapies for hepatocellular carcinoma. World J Gastroenterol. 2021;27(47):8069–8080. doi:10.3748/wjg.v27.i47.8069
- Lencioni R, De Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64(1):106–116. doi:10.1002/hep.28453
- Chen X, Zhang B, Yin X, Ren Z, Qiu S, Zhou J. Lipiodolized transarterial chemoembolization in hepatocellular carcinoma patients after curative resection. J Cancer Res Clin Oncol. 2013;139(5):773–781. doi:10.1007/s00432-012-1343-7
- Jiang C, Cheng G, Liao M, Huang J. Individual or combined transcatheter arterial chemoembolization and radiofrequency ablation for hepatocellular carcinoma: a time-to-event meta-analysis. World J Surg Oncol. 2021;19(1):81. doi:10.1186/s12957-021-02188-4
- He MK, Le Y, Li QJ, et al. Hepatic artery infusion chemotherapy using mFOLFOX versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: a prospective non-randomized study. Chinese J Cancer. 2017;36(1):83. doi:10.1186/s40880-017-0251-2
- Li QJ, He MK, Chen HW, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: a randomized Phase III trial. J Clin Oncol. 2022;40(2):150–160. doi:10.1200/JCO.21.00608
- Deng M, Cai H, He B, Guan R, Lee C, Guo R. Hepatic arterial infusion chemotherapy versus transarterial chemoembolization, potential conversion therapies for single huge hepatocellular carcinoma: a retrospective comparison study. Int J Surg. 2023;109(11):3303–3311. doi:10.1097/JS9.0000000000000654
- Si T, Huang Z, Khorsandi SE, Ma Y, Heaton N. Hepatic arterial infusion chemotherapy versus transarterial chemoembolization for unresectable hepatocellular carcinoma: a systematic review with meta-analysis. Front Bioeng Biotechnol. 2022;10:1010824. doi:10.3389/fbioe.2022.1010824
- Long T, Yang Z, Zeng H, et al. Comparable clinical outcomes between transarterial chemoembolization or hepatic arterial infusion chemotherapy combined with tyrosine kinase inhibitors and PD-1 inhibitors in unresectable hepatocellular carcinoma. J Hepatocell Carcinoma. 2023;10:1849–1859. doi:10.2147/JHC.S436211
- Lin Z, Chen D, Hu X, et al. Clinical efficacy of HAIC (FOLFOX) combined with lenvatinib plus PD-1 inhibitors vs. TACE combined with lenvatinib plus PD-1 inhibitors in the treatment of advanced hepatocellular carcinoma with portal vein tumor thrombus and arterioportal fistulas. Am J Cancer Res. 2023;13(11):5455–5465.
- He M, Li Q, Zou R, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5(7):953–960. doi:10.1001/jamaoncol.2019.0250
- Tang ZY, Uy YQ, Zhou XD, et al. Cytoreduction and sequential resection for surgically verified unresectable hepatocellular carcinoma: evaluation with analysis of 72 patients. World J Surg. 1995;19(6):784–789. doi:10.1007/BF00299771
- Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31(28):3501–3508. doi:10.1200/JCO.2012.44.5643
- Lyu N, Lin Y, Kong Y, et al. FOXAI: a Phase II trial evaluating the efficacy and safety of hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin for advanced hepatocellular carcinoma. Gut. 2018;67(2):395–396. doi:10.1136/gutjnl-2017-314138
- Shao YY, Huang CC, Liang PC, Lin ZZ. Hepatic arterial infusion of chemotherapy for advanced hepatocellular carcinoma. Asia-Pac J Clin Oncol. 2010;6(2):80–88. doi:10.1111/j.1743-7563.2010.01287.x
- Chen CT, Liu TH, Shao YY, Liu KL, Liang PC, Lin ZZ. Revisiting hepatic artery infusion chemotherapy in the treatment of advanced hepatocellular carcinoma. Int J Mol Sci. 2021;22(23):12880. doi:10.3390/ijms222312880
- Nouso K, Miyahara K, Uchida D, et al. Effect of hepatic arterial infusion chemotherapy of 5-fluorouracil and cisplatin for advanced hepatocellular carcinoma in the nationwide survey of primary liver cancer in Japan. Br J Cancer. 2013;109(7):1904–1907. doi:10.1038/bjc.2013.542
- Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38(26):2960–2970. doi:10.1200/JCO.20.00808
- Choi JH, Chung WJ, Bae SH, et al. Randomized, prospective, comparative study on the effects and safety of sorafenib vs. hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma with portal vein tumor thrombosis. Cancer Chemother Pharmacol. 2018;82(3):469–478. doi:10.1007/s00280-018-3638-0
- Wang J, Zheng Z, Wu T, et al. Hepatic arterial infusion chemotherapy as a timing strategy for conversion surgery to treat hepatocellular carcinoma: a single-center real-world study. J Hepatocell Carcinoma. 2022;9:999–1010. doi:10.2147/JHC.S379326
- Shao YY, Wang SY, Lin SM. Management consensus guideline for hepatocellular carcinoma: 2020 update on surveillance, diagnosis, and systemic treatment by the Taiwan liver cancer association and the gastroenterological Society of Taiwan. JFormos Med Assoc. 2021;120(4):1051–1060. doi:10.1016/j.jfma.2020.10.031
- Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobil Surg Nutrit. 2020;9(4):452–463. doi:10.21037/hbsn-20-480
- Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: new trends. J Hepatol. 2020;72(2):250–261. doi:10.1016/j.jhep.2019.08.025
- Lin CC, Hung CF, Chen WT, Lin SM. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein thrombosis: impact of early response to 4 weeks of treatment. Liver Cancer. 2015;4(4):228–240. doi:10.1159/000367737
- Mei J, Tang YH, Wei W, et al. Hepatic arterial infusion chemotherapy combined with PD-1 inhibitors plus lenvatinib versus PD-1 inhibitors plus lenvatinib for advanced hepatocellular carcinoma. Front Oncol. 2021;11:618206. doi:10.3389/fonc.2021.618206
- Lyu N, Kong Y, Mu L, et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2018;69(1):60–69. doi:10.1016/j.jhep.2018.02.008
- He MK, Liang RB, Zhao Y, et al. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma. Therapeut Adv Med Oncol. 2021;13:17588359211002720. doi:10.1177/17588359211002720
- Lau WY, Ho SK, Yu SC, Lai EC, Liew CT, Leung TW. Salvage surgery following downstaging of unresectable hepatocellular carcinoma. Ann Surg. 2004;240(2):299–305. doi:10.1097/01.sla.0000133123.11932.19
- Zhang Y, Huang G, Wang Y, et al. Is salvage liver resection necessary for initially unresectable hepatocellular carcinoma patients downstaged by transarterial chemoembolization? Ten years of experience. oncologist. 2016;21(12):1442–1449. doi:10.1634/theoncologist.2016-0094