Abstract
Purpose
This study aimed to assess the effectiveness and safety of combining hepatic arterial infusion chemotherapy (HAIC) with lenvatinib (LEN) and PD-1 inhibitors in treating arterioportal shunt (APS) in hepatocellular carcinoma (HCC) patients with portal vein tumor thrombus (PVTT).
Patients and Methods
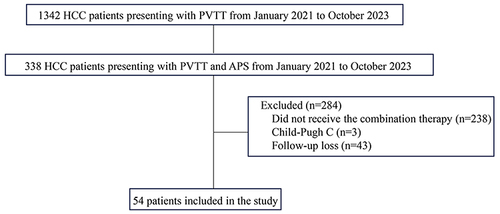
Conducted retrospectively, the study enrolled 54 HCC patients with APS and PVTT treated with HAIC, LEN, and PD-1 inhibitors at our center between January 2021 and October 2023. APS improvement, APS recanalization, tumor response, PVTT response rate, overall survival (OS), intrahepatic progression-free survival (InPFS), and adverse events were evaluated.
Results
APS improvement was observed in 42 patients (77.8%), with all improvement occurring within two treatment sessions. Complete APS occlusion was achieved in 40 patients (74.1%), and no recanalization occurred. The best objective response rate (ORR) and ORR after two HAIC sessions were 74.1% and 66.7%, respectively. The best PVTT response and PVTT response after two HAIC sessions were 98.1% and 94.4%, respectively. The median OS and InPFS were 10.0 months and 5.0 months, respectively. OS and InPFS were longer in patients with APS occlusion compared to those without (OS 12.1 vs 4.4 months, P<0.001, InPFS 6.2 vs 2.3 months, P=0.049). ALBI grade, extrahepatic spread, APS disappearance were potential prognostic factors for OS, while APS grade and extrahepatic spread being independently associated with InPFS. No treatment-related mortality occurred.
Conclusion
Combining HAIC with LEN and PD-1 inhibitors proves to be both effective and safe in managing APS in HCC with PVTT, potentially improving patient survival.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide.Citation1 In China, portal vein tumor thrombus (PVTT) is diagnosed in 44% to 62.2% of HCC patients.Citation2,Citation3 Arterioportal shunt (APS) occurs in 28.8%-63.2% of advanced HCC cases, often in conjunction with PVTT.Citation4,Citation5 APS is typically considered an adverse prognostic factor, as severe APS can exacerbate portal hypertension, leading to severe complications like ruptured gastro-esophageal varices, refractory ascites.Citation6–9 Moreover, HCC patients with moderate or severe APS are ineligible for transarterial chemoembolization (TACE). Therefore, the effective treatment of shunt tracts to alleviate portal pressure and facilitate subsequent tumor embolization is critical.
Transcatheter embolization is the common choice for APS in clinical practice. Various embolic agents have been used to occlude APS, such as polyvinyl alcohol (PVA), gelatin sponge, microsphere, absolute ethanol, coils, N-butyl-2-cyanoacrylate (NBCA).Citation10–17 However, each of these embolic agents has drawbacks. Gelatin sponge is typically reabsorbed within a few weeks,Citation18,Citation19 leading to a high recanalization rate. Coils are suitable for simple shunts but not for those caused by tumors. NBCA demands advanced operator skills with a risk of ectopic embolization. Additionally, fast blood flow in severe APS often limits precise embolization due to poor shunt visualization. Hence, there is an urgent need for a safe and effective treatment to address this current dilemma.
Recent evidence supports the survival benefits of hepatic arterial infusion chemotherapy (HAIC) with oxaliplatin, fluorouracil, and leucovorin for advanced HCC.Citation20,Citation21 The Barcelona Clinic Liver Cancer staging system (BCLC) proposes systemic therapy for HCC with PVTT.Citation22 Given the proven effectiveness of both HAIC and systemic therapy in advanced HCC companied with PVTT,Citation21,Citation23,Citation24 it’s reasonable to consider this combination for the treatment of APS in HCC patients with PVTT.
To the best of our knowledge, limited reports exist regarding HAIC combined with lenvatinib (LEN) and PD-1 inhibitors for this condition. Thus, we conducted a retrospective study to evaluate the effectiveness and safety of this integrated therapy for treating APS in HCC patients with PVTT.
Materials and Methods
Patient Selection
This study was approved by the Ethics Committee of our center (II2024-050-01). Informed consent was waived due to the retrospective study nature.
Between January 2021 and October 2023, we consecutively enrolled HCC patients accompanied by APS and PVTT who received the combination therapy comprising HAIC, LEN and PD-1 inhibitors. The primary inclusion criteria were as follows: (1) Diagnosis of HCC accompanied by PVTT with the diagnosis confirmed by histologic or clinical features according to the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver;Citation25,Citation26 (2) Confirmation of APS via digital subtraction angiography (DSA). In DSA images, APS manifests as early opacification of the portal vein during the hepatic arterial phase; (3) Initial treatment with HAIC, LEN and PD-1 inhibitors; (4) A Child-Pugh score of Class A or B; (5) An Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1 and (6) Age above 18 years old. The exclusion criteria were: (1) Coexistence with other malignancies; (2) Severe heart or lung dysfunction; (3) Loss to follow-up; or (4) Life expectancy of less than 3 months.
PVTT and APS Classification
The grading of PVTT followed the classification suggested by the Japan Society of Hepatology.Citation27 The evaluation of APS and its severity was conducted through DSA, and the APS grading system was derived from prior studies,Citation14,Citation15 as follows: Shunt extending to the subsegmental portal branch was designated as Grade 1, indicating mild APS. Shunt reaching the segmental portal branch was defined as Grade 2. Shunt reaching the main portal branch of the ipsilateral lobe was categorized as Grade 3. Both Grade 2 and 3 were considered moderate APS. Grade 4 was assigned to shunt reaching the main portal branch of the contralateral lobe and/or the main portal vein. Grade 5 was defined for cases where the shunt reached the main portal vein presenting with hepatofugal portal venous flow. Both Grade 4 and 5 were classified as severe APS ().
Table 1 APS Classification
Procedure
All patients underwent superior mesenteric angiography and hepatic angiography to assess the tumor and APS. These angiographies were performed using a high-pressure syringe at a rate of 4 mL per second for 3 seconds at a pressure of 300 psi. The degree of APS was assessed by DSA as described above. Subsequently, a microcatheter was superselectively positioned in the feeding arteries of the tumors, covering both intrahepatic and extrahepatic tumor feeding arteries. In cases where APS was not detectable in angiography, the artery associated with the tumor was embolized with drug-eluting beads TACE (DEB-TACE) or conventional TACE (cTACE). Then, the microcatheter was placed and fixed in feeding hepatic artery, in which APS could be observed in angiography, to deliver chemotherapy. This chemotherapy regimen included: oxaliplatin (85mg/m2 infusion for 2 h), leucovorin (400mg/m2 for 2 h), and 5-fluorouracil (400mg/m2 in bolus, and then 2400 mg/m2 continuous infusion 46 h). Repeated HAIC sessions were carried out at intervals of 3–4 weeks. If APS did not get improved after more than 4 sessions of HAIC, alternative treatment options were considered. DEB-TACE or cTACE could be used in combination with HAIC when the APS was absent after integrated therapy following a multidisciplinary consultation.
Lenvatinib and PD-1 Inhibitors
Concurrent LEN and PD-1 inhibitors were administrated at least three days before or after HAIC Procedure. Patients under 60 kg received a daily oral dose of 8 mg of LEN, and those over 60 kg received a daily oral dose of 12 mg of LEN. Simultaneously, all patients received intravenous PD-1 inhibitors every 3–4 weeks (200mg sintilimab, 200mg tislelizumab, 200mg camrelizumab, or 200mg pembrolizumab). Treatment was halted in the event of any unacceptable or severe adverse event (AE) (grade 3 or higher AE) or any intolerable grade 2 drug-related AE. Discontinuation also occurred upon the observation of disease progression.
Outcomes
We assessed several parameters in this study, including APS improvement, APS recanalization, tumor response, PVTT response rate, overall survival (OS), intrahepatic progression-free survival (InPFS), and AEs.
Improvement in APS was defined as a decrease in APS grade by at least two levels in subsequent angiograms. APS disappearance was characterized by the absence of APS in consecutive angiograms. APS recanalization indicated the reappearance of APS during follow-up. Therapeutic effectiveness was assessed using the modified Response Evaluation Criteria in Solid Tumors (mRECIST),Citation28 which categorizes the outcomes into complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The objective response rate (ORR) was determined as the combined total of CR and PR, while the disease control rate (DCR) was computed as the cumulative count of CR, PR, and SD. The mRECIST criteria consider PVTT as a non-target lesion, and the PVTT radiologic response was defined as CR, non-CR-non-PD and PD. The best response observed during treatment and response after two HAIC sessions were recorded. OS was calculated as the period between the start of treatment and either the date of death or the last follow-up visit. InPFS was defined as the time from commencement of treatment to intrahepatic tumor progression or death. According to the Common Terminology Criteria for Adverse Events version 5.0, AEs were assessed.
Data Collection
All patients underwent continuous follow-up until their demise or October 2023. These follow-up evaluations, which include observation of clinical symptoms and necessary examinations, were performed 4–6 weeks after the initial intervention. The investigations encompassed abdominal contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI), alpha-fetoprotein (AFP), routine blood measurements, hepatic function, kidney function, blood coagulation and blood ammonia.
Statistical Analysis
Unless otherwise specified, continuous variables were displayed as the median with interquartile range (IQR). Categorical data were expressed in frequencies. The outcomes of survival were evaluated by the Kaplan–Meier method and compared with the Log rank test. Hazard ratio (HR) was estimated with a Cox proportional hazards model. Covariates with P<0.10 from univariate Cox analyses were incorporated into the multivariate Cox analysis. Using the Cox proportional hazards model, multivariate analysis was conducted to ascertain the important prognostic factors. Statistical tests were two-sided, with P<0.05 indicating statistical significance. Statistical analyses in R (version 4.3.1; R Project for Statistical Computing, https://www.r-project.org/), and SPSS (version 26.0; IBM, Somers, NY) were applied.
Results
Patient Characteristics
A total of 54 patients were included in this study between January 2021 and October 2023 at our center (). The baseline characteristics of these patients are summarized in . These patients collectively underwent 190 HAIC treatments, with a median number of 3 sessions (IQR, 2–4). The majority of patients (50 [92.6%]) had a hepatitis B virus infection. The median tumor size was 12.3cm (IQR, 8.4–14.8). Most of the PVTT (52 [96.3%]) cases were categorized as Vp3-Vp4. Extrahepatic spread was observed in 17 patients (31.5%). Regarding APS severity, the majority of patients (44 [81.5%]) presented with severe APS, while 10 patients had moderate APS. There were no cases of grade 1 APS. The patients received PD-1 inhibitors from the following categories: sintilimab for 41 (75.9%), tislelizumab for 3 (5.6%), camrelizumab for 9 (16.7%) and pembrolizumab for 1 (1.9%).
Table 2 Baseline Characteristics
Outcome of APS
APS improvement was achieved in 42 (77.8%) patients (). Specifically, improvement was observed in 7 cases of moderate APS and in 35 cases of severe APS. It’s noteworthy that all cases of APS improvement occurred within the first two treatment sessions. During the last follow-up angiography, complete occlusion of APS () was observed in 40 (74.1%) patients. More specifically, APS disappearance was noted in 7 cases classified as moderate and in 33 cases categorized as severe. No cases of recanalization of previously disappeared APS were observed in the follow-up angiographies.
Table 3 Outcome of APS
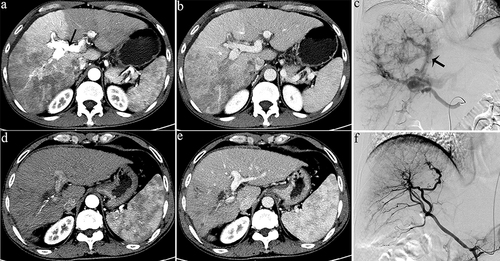
Figure 2 Representative radiographical images of a patient who achieved APS disappearance after treatment. Images of man aged 50 years with HCC and severe APS after receiving the combination therapy. Axial contrast-enhanced CT images in the arterial phase (a) and portal phase (b) before the initiation of treatment. CT images taken during the arterial phase (a) displayed early enhancement of portal vein branches (indicated by a black arrow), representing APS. Hepatic angiography (c) revealed severe APS (indicated by a black arrow). Subsequent axial contrast-enhanced CT images in the arterial (d) and portal (e) phase were taken after three sessions of HAIC, showing no apparent APS. Hepatic angiography in image (f) confirmed the absence of APS.
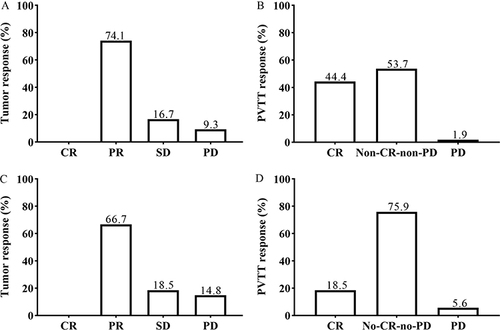
Tumor Response and PVTT Response
According to the mRECIST criteria,Citation28 both the best response observed during treatment and response after two HAIC sessions were recorded (). Concerning the best response during treatment, no CR was observed, while there were 40 cases (74.1%) with PR, 9 cases (16.7%) with SD, and 5 cases (9.3%) with PD. The DCR was 90.7% (49/54), and the ORR was 74.1% (40/54). At the same time, the best PVTT response was observed in 53 patients (CR: 24 [44.4%], non-CR-non-PD: 29 [53.7%]). Regarding the response after two HAIC sessions, no cases achieved CR, while 36 cases (66.7%) showed PR, 10 cases (18.5%) had SD, and 8 cases (14.8%) experienced PD. The DCR was 85.2% (46/54) and the ORR was 66.7% (36/54). The PVTT response after two HAIC sessions was observed in 51 patients (CR: 10 [18.5%], non-CR-non-PD: 41 [75.9%]).
Survival
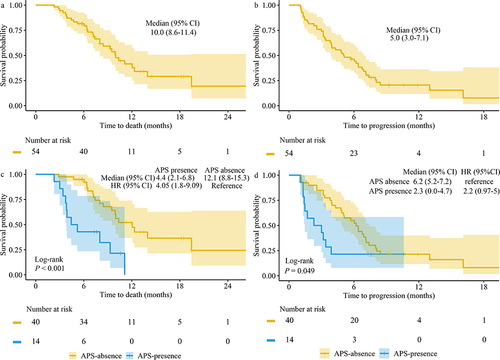
The follow-up period concluded in October 2023, with a median duration of 11.9 months. At the conclusion of the observation period, 30 (55.6%) patients had passed away. The median OS was 10.0 months (95% CI, 8.6 to 11.4) (). At the data cutoff, a total of 44 (81.5%) patients had experienced disease progression or death, and the median InPFS was 5.0 months (95% CI, 3.0 to 7.1) (). To investigate the influence of APS on Survival, we compared the survival outcomes between patients who presented no APS and those who presented APS during follow-up. Among these cases, APS disappeared in 40 (74.1%) patients, while APS persisted in 14 (25.9%) patients. There were notable differences between the two groups in both median OS and InPFS. Patients without APS had longer OS and InPFS compared to those with APS (OS 12.1 months [95% CI, 8.8 to 15.3] vs 4.4 months [2.1 to 6.8], P<0.001; InPFS 6.2 months [5.2 to 7.2] vs 2.3 months [0.0 to 4.7], P=0.049; and ).
Examination of Factors Influencing Survival
Univariate and multivariate analysis showed ALBI grade, extrahepatic spread, APS disappearance were significantly correlated with OS. Regarding InPFS, univariate analysis showed APS grade, PVTT classification, extrahepatic spread, APS disappearance were significantly correlated with InPFS. Multivariate survival analysis identified both APS grade and extrahepatic spread as potential prognostic factors for InPFS ( and ).
Table 4 Univariate and Multiple Analysis of Prognostic Factor for OS
Table 5 Univariate and Multiple Analysis of Prognostic Factor for InPFS
Safety
AEs associated with the treatment are presented in . Throughout the follow-up period, all patients experienced AEs of varying grades. In total, 11 patients (20.4%) encountered AEs of grades 3–4. The most frequently observed grade 3–4 adverse events were leukopenia, hypoalbuminemia, neutropenia. Adverse events leading to death did not occur.
Table 6 Adverse Events
Discussion
In the progression of HCC, tumors often infiltrate hepatic portal vein, creating direct communication between hepatic artery and portal vein, leading to the development of APS.Citation29 Severe APS exacerbates complications related to portal hypertension, such as intractable ascites and esophageal varices, and hinders chemoembolization.Citation6–9
The present study demonstrates the effectiveness and safety of combining HAIC, LEN, and PD-1 inhibitors in controlling APS. The results demonstrated substantial enhancement in the long-term effective control of APS, tumor response, and patient survival.
While various treatments for APS have been suggested, the majority of studies primarily concentrate on embolic material selection,Citation10–16 often overlooking the correlation between PVTT and APS.Citation4,Citation30 In our perspective, fistula formation and tumor thrombus mutually contribute to and strengthen each other. Our study demonstrates substantial APS improvement within two treatment sessions, with complete occlusion observed in the majority of cases, emphasizing the effectiveness of this combined therapy for APS management. Notably, our results showed no cases of APS recanalization during the follow-up period, indicating the sustained impact of this treatment approach for APS. This might be due to the positive tumor response and the successful management of PVTT.
Zhou and colleaguesCitation14 reported their experience with the use of ethanol-soaked gelatin sponge to embolize APS. They found an initial APS improvement rate of 97%, but the rate dropped to 54% at the first follow-up, indicating a high APS recanalization rate. Kim and co-authorsCitation15 observed that HCC patients with APS may experience advantages from PVA embolization, showing an APS improvement rate (80%) similar to our study. Nonetheless, it’s important to note that their study had a limited sample size, with only 19 patients included. In our clinical practice, we have observed that particles ranging from 350 to 560 μm are too small to effectively occlude large shunts. On the other hand, larger PVA particles with a diameter of 500 μm or more can be employed for embolization, effectively APS. However, there is a potential drawback as they may result in microcatheter blockage during the injection process and are incapable of concurrently occluding peripheral vessels of the tumor. DuanCitation12 employed NBCA for APS embolization, and the observed APS improvement rate (66.6%) was also noteworthy. NBCA can effectively and permanently block the shunt, however, it demands a high level of technical expertise from operators, as over-embolization can lead to ectopic embolization or catheter adhesion. Some researchers explored TACE combination with Tyrosine kinase inhibitors for patients with advanced HCC with APS.Citation31,Citation32 However, the severity and/or improvement of APS were not mentioned.
Many previous studies on HCC with APS either had limited sample sizes or did not assess the severity of APS. The APS grading method utilized by ZhouCitation14 aligned with the approach employed in this study, and their reported median OS was 12.7 months, which was superior to the results of our study. Nevertheless, the majority of APS in their study were classified as grade 3 or lower. Additionally, over half of the patients had tumors with a maximum diameter of less than 5 cm, and some were classified as non-BCLC stage C, contributing to an extended patient life cycle.
In the follow-up data from the IMbrave150 study,Citation33 the median OS and progression-free survival (PFS) for the high-risk subgroup (defined as patients with Vp4 type PVTT, bile duct invasion, or liver infiltration >50%) who received Atezolizumab + Bevacizumab treatment were 7.6 months and 5.4 months, respectively. In this study, most of the included patients had a maximum tumor diameter greater than 10 cm, and most PVTT cases were of the Vp4 type. Therefore, the majority of the population in this study belonged to the high-risk group in the IMbrave150 study. The overall median OS in this study (10.0 months) was superior to the median OS of the high-risk subgroup in the IMbrave150 study. This indicates that the combination therapy has greater advantages for treating high-risk advanced liver cancer patients.
APS disappearance emerged as an independent risk factor for OS. This may be due to the relief of portal pressure and the disappearance of the APS creating favorable conditions for subsequent effective TACE. ALBI grade and extrahepatic spread were other potential factors for OS. These findings are consistent with those reported in previous studies.Citation34,Citation35 In multivariate analysis, APS grade and extrahepatic spread became independent predictors of InPFS. Patients in higher APS grades may experience more severe complications that could impact survival outcomes. In this study, patients with extrahepatic dissemination had shorter InPFS. This has also been reported in previous studies.Citation36
In terms of AEs, all patients experienced at least one side effect, primarily related to drug-related adverse reactions. However, the combination therapy did not result in any unexpected toxicities, and no additional adverse reactions were observed in this study. Compared to the LEAP002 study,Citation37 our research observed relatively fewer types of adverse events related to lenvatinib and PD-1 inhibitors. However, the incidence rates of some adverse events were comparable. We believe that the combination therapy is unlikely to reduce the incidence of adverse events. The potential reasons for this could be selection bias due to the patient selection process and bias resulting from a relatively small sample size. In addition, since this is a retrospective study, the accuracy of recording adverse reactions is certainly not as reliable as in prospective studies.
Our study possesses several limitations that merit acknowledgment. First, its retrospective design and single-institution setting with a relatively small patient population may introduce selection bias. Second, the absence of a control group limits the strength of the evidence. However, in clinical practice, few advanced HCC patients in China receive only systemic therapy, making it challenging to collect suitable control cases. Third, the variability in PD-1 inhibitors used in our patient population may have limited the extent to which we can attribute the impact on APS to PD-1 inhibitors.
Conclusion
The combined treatment approach of HAIC with LEN and PD-1 inhibitors demonstrates effective and durable control of APS in HCC patients with PVTT, contributing to the prolonged survival of patients.
Abbreviations
PVTT, Portal vein tumor thrombosis; APS, Arterioportal shunt; TAE, Transcatheter arterial embolization; TACE, Transcatheter arterial chemoembolization; FOLFOX-HAIC, Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin; LEN, Lenvatinib; PFS, Progression-free survival; ORR, Objective response rate; mRECIST, Modified Response Evaluation Criteria in Solid Tumors; anti-VEGF, Anti-vascular endothelial growth factor; TKIs, Tyrosine kinase inhibitors; ICIs Immunotherapy checkpoint inhibitors; DSA, Digital subtraction angiography; ECOG PS, Eastern Cooperative Oncology Group performance status; DEB-TACE, Drug-eluting beads transcatheter arterial chemoembolization; cTACE, Conventional transcatheter arterial chemoembolization; AEs, adverse events; AFP, alpha fetoprotein; CT, Computed tomography; MRI, Magnetic resonance; OS, Overall survival; CR, Complete remission; PR, Partial remission; SD, stable disease; PD, progressive disease; DCR, Disease control rate; IQR, Interquartile range.
Ethics Approval and Informed Consent
The study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University (II2024-050-01). Given that this was a retrospective study of patient clinical data, a waiver of written informed consent was approved. All patient data were strictly confidential and used solely for the purpose of this study. During the data usage process, patient personal information was anonymized, in compliance with relevant privacy protection regulations.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA. 2021;71(1):7–33. doi:10.3322/caac.21654
- Llovet JM, Zucman rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nature Reviews Disease Primers. 2016;2(1):16018. doi:10.1038/nrdp.2016.18
- Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156(2):477–491. doi:10.1053/j.gastro.2018.08.065
- Ngan H, Peh W. Arteriovenous shunting in hepatocellular carcinoma: its prevalence and clinical significance. Clin. Radiol. 1997;52(1):36–40. doi:10.1016/S0009-9260(97)80303-0
- Okuda K, Musha H, Yamasaki T, et al. Angiographic demonstration of intrahepatic arterio-portal anastomoses in hepatocellular carcinoma. Radiology. 1977;122(1):53–58. doi:10.1148/122.1.53
- Kim JH, Sinn DH, Kim K, et al. Primary prophylaxis for variceal bleeding and the improved survival of patients with newly diagnosed hepatocellular carcinoma. Dig Dis Sci. 2016;61(11):3354–3362. doi:10.1007/s10620-016-4255-6
- Hsu CY, Lee YH, Huang YH, et al. Ascites in patients with hepatocellular carcinoma: prevalence, associated factors, prognostic impact, and staging strategy. Hepatol Internat. 2013;7(1):188–198. doi:10.1007/s12072-011-9338-z
- Zheng YW, Wang KP, Zhou JJ, et al. Portal hypertension predicts short-term and long-term outcomes after hepatectomy in hepatocellular carcinoma patients. Scand J Gastroenterol. 2018;53(12):1562–1568. doi:10.1080/00365521.2018.1538386
- Angeli P, Bernardi M, Villanueva C, et al. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406–460. doi:10.1016/j.jhep.2018.03.024
- Liu QS, Mei QL, Li YH. Polyvinyl alcohol terminal chemoembolization for hepatocellular carcinoma with hepatic arteriovenous shunts: safety, efficacy, and prognostic factors. Eur. J. Radiol. 2017;89:277–283. doi:10.1016/j.ejrad.2016.04.016
- Shi HB, Yang ZQ, Liu S, et al. Transarterial embolization with cyanoacrylate for severe arterioportal shunt complicated by hepatocellular carcinoma. CardioVasc Interventional Radiol. 2013;36(2):412–421. doi:10.1007/s00270-012-0410-4
- Duan F, Bai YH, Cui L, Li XH, Yan JY, Zhu HY. Transarterial embolization with N-butyl 2-cyanoacrylate for the treatment of arterioportal shunts in patients with hepatocellular carcinoma. J Cancer Res Ther. 2017;13(4):631–635. doi:10.4103/jcrt.JCRT_286_17
- Cai L, Li HL, Guo J, et al. Treatment efficacy and safety of drug-eluting beads transarterial chemoembolization versus conventional transarterial chemoembolization in hepatocellular carcinoma patients with arterioportal fistula. Cancer Biol Ther. 2022;23(1):89–95. doi:10.1080/15384047.2021.2020059
- Zhou WZ, Shi HB, Liu S, et al. Arterioportal shunts in patients with hepatocellular carcinoma treated using ethanol-soaked gelatin sponge: therapeutic effects and prognostic factors. J Vasc Interv Radiol. 2015;26(2):223–230. doi:10.1016/j.jvir.2014.11.002
- Kim YJ, Lee HG, Park JM, et al. Polyvinyl alcohol embolization adjuvant to oily chemoembolization in advanced hepatocellular carcinoma with arterioportal shunts. Korean J Radiol. 2007;8(4):311–319. doi:10.3348/kjr.2007.8.4.311
- Chan W, Poon W, Cho D, Chiu S, Luk S. Transcatheter embolisation of intrahepatic arteriovenous shunts in patients with hepatocellular carcinoma. Hong Kong Med J. 2010;16(1):48–55.
- Xiao L, Liu Q, Zhao W, et al. Chemoembolisation with polyvinyl alcohol for advanced hepatocellular carcinoma with portal vein tumour thrombosis and arterioportal shunts: efficacy and prognostic factors. Clin. Radiol. 2018;73(12):1056.e17–1056.e22. doi:10.1016/j.crad.2018.08.002
- Coldwell DM, Stokes KR, Yakes WF. Embolotherapy: agents, clinical applications, and techniques. RadioGraphics. 1994;14(3):623–643. doi:10.1148/radiographics.14.3.8066276
- Siskin GP, Englander M, Stainken BF, Ahn J, Dowling K, Dolen EG. Embolic agents used for uterine fibroid embolization. Am J Roentgenol. 2000;175(3):767–773. doi:10.2214/ajr.175.3.1750767
- Lyu N, Kong Y, Mu L, et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2018;69(1):60–69. doi:10.1016/j.jhep.2018.02.008
- He M, Li Q, Zou R, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5(7):953–960. doi:10.1001/jamaoncol.2019.0250
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
- Zheng K, Zhu X, Fu S, et al. Sorafenib plus hepatic arterial infusion chemotherapy versus sorafenib for hepatocellular carcinoma with major portal vein tumor thrombosis: a randomized trial. Radiology. 2022;303(2):455–464. doi:10.1148/radiol.211545
- Fu Y, Peng W, Zhang W, et al. Induction therapy with hepatic arterial infusion chemotherapy enhances the efficacy of lenvatinib and pd1 inhibitors in treating hepatocellular carcinoma patients with portal vein tumor thrombosis. J Gastroenterol. 2023;58(4):413–424. doi:10.1007/s00535-023-01976-x
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
- Liver EAFTSOT. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
- Kudo M, Kawamura Y, Hasegawa K, et al. Management of hepatocellular carcinoma in japan: jsh consensus statements and recommendations 2021 update. Liver Cancer. 2021;10(3):181–223. doi:10.1159/000514174
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(01):052–060. doi:10.1055/s-0030-1247132
- Cao B, Tian K, Zhou H, Li C, Liu D, Tan Y. Hepatic arterioportal fistulas: a retrospective analysis of 97 cases. J Clin Transl Hepatol. 2022;10(4):620–626. doi:10.14218/jcth.2021.00100
- Wu H, Zhao W, Zhang J, Han J, Liu S. Clinical characteristics of hepatic Arterioportal shunts associated with hepatocellular carcinoma. BMC Gastroenterol. 2018;18(1):174. doi:10.1186/s12876-018-0899-3
- Sun T, Ren YQ, Kan XF, et al. Advanced hepatocellular carcinoma with hepatic arterioportal shunts: combination treatment of transarterial chemoembolization with apatinib. Orig Res Front Mol Biosci. 2020;7. doi:10.3389/fmolb.2020.607520
- Chen PK, Chiu SH, Tsai MT, et al. Combination therapy of sorafenib and drug-eluting bead transarterial chemoembolization for advanced hepatocellular carcinoma with and without hepatic arteriovenous shunt. J Chin Med Assoc. 2022;85(4):491–499. doi:10.1097/jcma.0000000000000696
- Cheng A-L, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–873. doi:10.1016/j.jhep.2021.11.030
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of Liver Function in Patients With Hepatocellular Carcinoma: a New Evidence-Based Approach—The ALBI Grade. J Clin Oncol. 2015;33(6):550–558. doi:10.1200/jco.2014.57.9151
- Bruix J, Cheng A-L, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two Phase III studies. J Hepatol. 2017;67(5):999–1008. doi:10.1016/j.jhep.2017.06.026
- Fulgenzi CAM, Cheon J, D’Alessio A, et al. Reproducible safety and efficacy of atezolizumab plus bevacizumab for HCC in clinical practice: results of the AB-real study. Eur. J. Cancer. 2022;175:204–213. doi:10.1016/j.ejca.2022.08.024
- Llovet JM, Kudo M, Merle P, et al. Lenvatinib plus pembrolizumab versus lenvatinib plus placebo for advanced hepatocellular carcinoma (LEAP-002): a randomised, double-blind, Phase 3 trial. Lancet Oncol. 2023;24(12):1399–1410. doi:10.1016/S1470-2045(23)00469-2