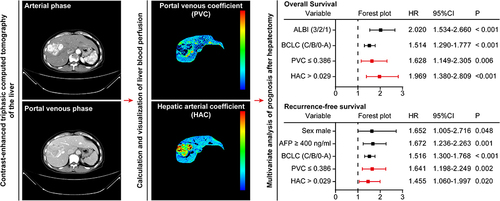
Abstract
Background
The dominant artery blood supply is a characteristic of hepatocellular carcinoma (HCC). However, it is not known whether the blood supply can predict the post-hepatectomy prognosis of patients with HCC. This retrospective study investigated the prognostic value of the portal venous and arterial blood supply estimated on triphasic liver CT (as a portal venous coefficient, PVC, and hepatic arterial coefficient, HAC, respectively) in patients with HCC following hepatectomy.
Methods
HCC patients who were tested by triphasic liver CT 2 weeks before hepatectomy and received R0 hepatectomy at the Second Affiliated Hospital, Kunming Medical University between January 1, 2016 and December 31, 2020, were retrospectively screened. Their PVC and HAC, and other variables were analyzed for the prediction of overall survival (OS) and recurrence-free survival (RFS) using the least absolute shrinkage and selection operator and Cox proportional hazard regression models.
Results
Four hundred and nineteen patients (53.2 ± 10.6 years of age and 370 men) were evaluated. A shorter OS was independently associated with higher blood albumin and total bilirubin grade [hazard ratio (HR) 2.020, 95% confidence interval (CI) 1.534–2.660], higher Barcelona Clinic Liver Cancer (BCLC) stage (HR 1.514, 95% CI 1.290–1.777), PVC ≤ 0.386 (HR 1.628, 95% CI 1.149–2.305), and HAC > 0.029 (HR 1.969, 95% CI 1.380–2.809). A shorter RFS was independently associated with male (HR 1.652, 95% CI 1.005–2.716), higher serum α-fetoprotein ≥ 400 ng/mL (HR 1.672, 95% CI 1.236–2.263), higher BCLC stage (HR 1.516, 95% CI 1.300–1.768), tumor PVC ≤ 0.386 (HR 1.641, 95% CI 1.198–2.249), and tumor HAC > 0.029 (HR 1.455, 95% CI 1.060–1.997).
Conclusion
Tumor PVC or HAC before hepatectomy is valuable for independently predicting postoperative survival of HCC patients.
Introduction
Hepatocellular carcinoma (HCC) is a common and extremely fatal cancer globally.Citation1,Citation2 Hepatectomy is a kind of curative treatment for HCC,Citation3 but the post-hepatectomy overall survival (OS) and recurrence-free survival (RFS) are far from satisfactory.Citation4 Predicting the probability of unfavorable outcomes in patients considering hepatectomy as a treatment option can facilitate appropriate redirection towards alternative treatment options or the implementation of neo-adjunctive before hepatectomy and/or adjunctive therapies. Hence, the discovery of new clinical measures for accurately predicting the OS and RFS of those patients will be of significance in the management of those patients.
There are two sources of blood supply to the healthy liver, including about 75–80% of blood supply through the portal vein and 20–25% of blood supply by the hepatic artery in humans.Citation5 The shifting of the primary blood supply from the portal vein to the hepatic artery is a characteristic of HCC.Citation6 However, it remains unknown whether measurement of blood supply to the liver by the portal vein and the hepatic artery can predict the post-hepatectomy OS and RFS of HCC patients. There is little information on the value of these measures in the prognosis of HCC.
Invasive measurements of blood flow to the liver include inert gas clearance techniques, radionuclide-labeled red blood cell dilution techniques, and electromagnetic flowmetry.Citation7 The non-invasive liver perfusion CT technique was developed in 1995.Citation8 But this method still has some problems, hindering its application in clinical settings: it typically requires a dedicated protocol and scanning the liver at 20–40 time points after contrast injection, leading to a high dose of radiation exposure in the recipients; and calculating liver blood supply is highly complicated, because of using multi-organ time attenuation curves and the dual-maximum-slope model.Citation8
The portal venous coefficient (PVC) and hepatic arterial coefficient (HAC) are newly developed methods for non-invasive estimation of the relative blood supply to the liver by the portal vein and the hepatic artery, respectively.Citation9 More specifically, PVC reflects the similarity in the enhancement patterns between the target liver tissue and the portal vein, while HAC reflects the similarity in the enhancement patterns between the target liver tissue and the abdominal aorta.Citation9 These indices can be measured using the routine triphasic liver CT enhanced by contrast in a non-invasive, lower-radiation, and easier way compared with the aforementioned methods.Citation9 PVC and HAC are useful for differentiating HCC from benign hepatic lesions,Citation9,Citation10 and they can predict the OS of HCC patients after transcatheter arterial chemoembolization (TACE).Citation11
This retrospective study analyzed the potential value of PVC and HAC for the prediction of post-hepatectomy OS and RFS in HCC patients to help select patients for hepatectomy.
Materials and Methods
This study was performed, according to the Helsinki Declaration and approved by the Committee of Ethics at the Second Affiliated Hospital, Kunming Medical University (Shen-PJ-Ke-2022-60). The informed consent waiver was conferred by the Committee of Ethics as its retrospective collection.
Patients
We retrospectively screened all patients undergoing their first hepatectomy for pathology-confirmed primary HCC at the Second Affiliated Hospital, Kunming Medical University between January 1, 2016 and December 31, 2020. Patients were included only if contrast-enhanced triphasic CT of the liver was conducted within 2 weeks before hepatectomy, and R0 margin was confirmed based on pathological analysis of surgical samples.
Patients were excluded if: (1) the hepatectomy was preceded by any kind of neoadjuvant treatment, such as TACE, transcatheter arterial embolization, transcatheter arterial infusion, hepatic arterial infusion chemotherapy, targeted and/or immunotherapy; (2) preoperative CT displayed that the portal venous enhancement early peaked in the arterial phase, a hallmark of arteriovenous fistula that affected the accuracy of PVC and HAC measurements; (3) complete occlusion of the portal venous main trunk that affected the accuracy of PVC and HAC measurements; (4) the maximum diameter in the tumor was ≤ 1 cm; (5) they had life-threatening comorbidities, such as cardiovascular disease, chronic kidney disease, infection with human immunodeficiency virus, or another malignancy; or (6) their survival status was unknown at the last follow-up.
CT Protocol
A triphasic CT with contrast was conducted in the liver using a CT scanner with 256 slices (Brilliance iCT, Philips Healthcare, Cleveland, OH, USA). After non-contrast images were obtained, 70–90 mL of iopromide (370 mg iodine/mL, Ultravist®, Bayer Schering Pharma AG, Berlin, Germany) was delivered via intravenous injection at a perfusion speed of 4–5 mL/s, and then 45 mL of saline was infused in the same manner. Following the contrast injection, the liver was scanned in three phases: the late arterial phase (30–35 s), the portal venous phase (60–70 s), and the delayed phase (3 min). The technical parameters for triphasic CT included tube voltage, 120 kVp; tube current-time product, 220 mAs; section collimation, 128 × 0.625 mm; helical pitch, 0.915; and scan time per spiral, 0.4 s. A soft tissue kernel and 2.5-mm thickness of slices were used to construct images.Citation9
Calculation of PVC and HAC
Tumor PVC and HAC were determined, based on the largest tumor in each patient using an Image Viewer workstation (Centricity PACS, GE HealthCare, Barrington, IL, USA) as previously described.Citation9,Citation11,Citation12 Elliptical regions of interest (ROIs) with a minor axis of ≥ 5 mm in length were drawn around each of the following areas: the largest tumor, the aorta close to the celiac trunk root, and the main portal vein trunk in the vicinity of its branching. The tumor ROI was designed to cover the tumor, but avoided necrotic tissues and large vessels as much as possible. The same ROIs were used for the unenhanced, late arterial, and portal venous phases. The mean attenuation in all ROIs was measured in Hounsfield units.
PVC and HAC were also determined from background liver tissue in the same lobe as the largest tumor. These ROIs were designed to avoid the tumor and large vessels. Other procedures were the same as those of tumor PVC and HAC.
The HAC and PVC were calculated, based on a perfusion model assuming (1) no delay between when enhancement was observed in the supplying vessel and when it was observed in the tissue being fed, and (2) negligible vascular permeability to contrast.Citation9 In this model, the portal venous branches have the same enhancement as the portal venous trunk, and the hepatic arterial branches have the same enhancement as the aorta.Citation9 HCC or the liver has both the portal venous and hepatic arterial branches, so that the enhancement curves of each point in the HCC or liver parenchyma were linearly combined from the portal venous and hepatic arterial (aortal) enhancement curves.Citation9 For each point, the enhancement was described using the equation
where vi refers to the portal venous enhancement, ai refers to the hepatic arterial (aortal) enhancement, and i refers to the imaging phase (1 = arterial, 2 = portal venous). PVC and HAC can take values between −1 and 1. The value of 1 indicates complete similarity in the enhancement patterns between the target liver tissue and the portal vein or the hepatic artery (aorta), while values of −1 indicate complete dissimilarity. Solving the above equations for each coefficient yields
A calculator and visualization program for these liver perfusion indies was developed using C# (https://dotnet.microsoft.com/zh-cn/languages/csharp, Data Sharing Statement) by ourselves for simplifying the calculation and visualization of the coefficients. The running environment of the program: Windows 10 or 11 and Settings-System-Display-Scale = 100%. The coefficients were independently evaluated by Y.K.L. and W.H.Z., and discrepancies were resolved through discussion with Y.K. (2, 3, and 10 years of experience in abdominal CT, respectively).
Follow-Up
In line with standard practice at our medical center, patients were monitored every one to two months during the first six months following hepatectomy, then every three to four months until two years post hepatectomy, and every six months thereafter.Citation13 The last follow-up was conducted in February 2023.
For each time of follow-up, patients underwent a complete physical examination, assay of serum α fetoprotein level, chest CT or X-ray, and at least one of the following types of abdominal imaging: ultrasonography, contrast-enhanced triphasic CT, and/or magnetic resonance imaging (MRI).Citation13 Patients with a HCC lesion in any imaging modality were diagnosed with recurrence,Citation13 and treated, as appropriate, by a liver transplantation, repeat hepatectomy, radiofrequency ablation, TACE, or systemic therapy.Citation14
The date of death was determined based on records at the vital statistics branch of the provincial government. RFS and OS were calculated from the time of hepatectomy.
Data Collection
The following data at admission were retrospectively analyzed in the present study: sex; age, which was stratified into ≤ 65 or > 65 years old;Citation15,Citation16 albumin and total bilirubin (ALBI) score, which is a well-established model for assessing liver function in patients with HCC and is calculated using the following formula: ;Citation17 hepatitis B and/or C viral infection by detecting serum surface antigen of hepatitis B virus and detecting serum antibodies against hepatitis C virus;Citation18 liver cirrhosis, diagnosed by ultrasonography, triphasic liver CT, and/or MRI as described;Citation19 a serum α fetoprotein level of < 400 ng/mL or ≥ 400 ng/mL;Citation20 and Barcelona Clinic Liver Cancer (BCLC) stage.Citation14
The pathological characteristics of surgical samples were retrospectively analyzed: including tumor differentiation; microvascular invasion (MVI) at M0, M1, or M2;Citation20 satellite nodules; and lymph node metastasis. Tumor differentiation was graded as good, equivalent to Edmondson-Steiner grades I or I–II; moderate, equivalent to Edmondson-Steiner grades II or II–III; or poor, equivalent to Edmondson-Steiner grades III, IV, or III–IV.Citation21
Statistics
Data were analyzed statistically with SPSS 26.0 (SPSS, Chicago, IL, USA), X-tile 3.6.1 (https://medicine.yale.edu/lab/rimm/research/software/),Citation22 and the glmnet package in R-4.2.2 (https://cran.r-project.org/bin/windows/base/old/).Citation23 Normally distributed continuous data are expressed as mean ± standard deviation (SD) while the skewed data as median and inter-quartile range (IQR). Categorical data are presented as number and percentage. The inter-group difference was tested by Student’s t test, Mann–Whitney’s U-test, and chi-squared test where applicable.
PVC and HAC were categorized as “low” or “high” levels based on cutoff values that stratified patients into those with good or poor OS and the highest chi-squared value in the Log rank test within the X-tile.Citation22 Cohen’s kappa coefficient was applied to determine inter-rater agreement for the categorization of PVC and HAC.
Variables associated with survival were preliminarily screened using regression with the least absolute shrinkage selection operator (LASSO), in which lambda was selected via 10-fold cross-validation, based on the minimum criterion. The resulting variables were evaluated using Cox’s proportional hazard regression with a forward stepwise likelihood ratio selection. The final results were reported as hazards ratio (HR) and 95% confidence interval (CI). The sample size was to ensure at least 10 events for each predictor parameter. A P-value of < 0.05 was considered statistically significant.
Results
Patient Characteristics
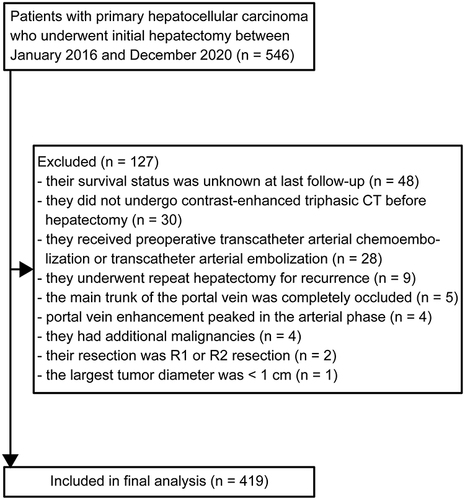
Of the 546 patients undergoing hepatectomy during the observation period, 127 were excluded from the final analysis for the following reasons: their survival status was unknown at the last follow-up (n = 48), they did not undergo a triphasic CT on the liver before hepatectomy (n = 30), they received preoperative TACE or transcatheter arterial embolization (n = 24), they underwent repeat hepatectomy for recurrent HCC (n = 9), the main trunk of the portal vein was completely occluded (n = 5), portal venous enhancement peaked in the arterial phase to suggest arteriovenous fistula (n = 4), they had additional malignancies (n = 4), their resection was determined to be R1 or R2 (n = 2), or the largest tumor diameter was < 1 cm (n = 1) (). The data from remaining 419 patients were analyzed, and their demographic and clinical characteristics are shown in .
Table 1 The Demographic and Clinical Characteristics of the Cohort
They had a mean age of 53.2 ± 10.6 years and the majority (88.3%) of them were men. Most patients had a history of chronic diseases, such as chronic hepatitis B and/or C (334, 79.7%) or liver cirrhosis (283, 67.5%). There were 229 (54.7%), 66 (15.8%), and 124 (29.6%) patients with HCC at the BCLC stages of 0-A, B, and C, respectively. There were 318 (75.9%) of patients with poor differentiation HCC, and 220 (52.5%) of HCC patients had MVI, including 134 (32.0%) of them with M1 grade and 86 (20.5%) of cases with M2 grade.
Those patients were followed up for a median of 4.4 years (IQR 3.3 to 5.5 years). During the follow-up period, 209 (49.9%) patients experienced HCC recurrence and 184 (43.9%) died.
PVC and HAC in Tumor and Background Liver Tissues
A preoperative CT scan of the liver for those 419 patients indicated a median tumor PVC of 0.313 (IQR 0.230 to 0.412), and the optimal cutoff was 0.386, based on OS (). The median PVC for background non-tumor liver tissue was 0.356 (IQR 0.269 to 0.459) with an optimal cutoff of 0.271. The median PVC for background non-tumor liver tissue was significantly greater than the median tumor PVC across all patients (P < 0.001) and those with HCC at BCLC stages 0-A (0.326, IQR 0.246 to 0.435, P = 0.035; ) or B-C (0.302, IQR 0.214 to 0.383, P < 0.001). The median tumor PVC was significantly greater in patients with HCC at BCLC stages 0-A than those with HCC at BCLC stages B-C (P = 0.007).
Figure 2 (A) Portal venous coefficient (PVC) and (B) hepatic arterial coefficient (HAC). Coefficients were evaluated for background non-tumor liver across all patients, and for the largest tumor in patients with Barcelona Clinic Liver Cancer stages 0-A or B-C.
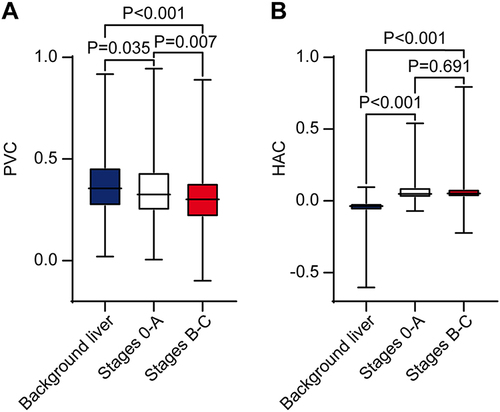
The median tumor HAC was 0.050 (IQR 0.025 to 0.093) with an optimal cutoff of 0.029 (). The median HAC for background non-tumor liver tissue was −0.037 (IQR −0.066 to −0.015) with an optimal cutoff was −0.018. The median HAC for background non-tumor liver tissue was significantly smaller than the median tumor HAC across all patients (P < 0.001) and those patients with HCC at BCLC stages 0-A (0.049, IQR 0.022 to 0.095, P < 0.001) or B-C (0.052, IQR 0.026 to 0.085, P < 0.001; ). There was no significant difference in median tumor HAC values between patients with HCC at BCLC stages 0-A and B-C (P = 0.691).
Cohen’s kappa coefficient was 0.741, 0.781, 0.777, and 0.720 for the tumor PVC, tumor HAC, liver PVC, and liver HAC, respectively.
Predicting Post-Hepatectomy OS
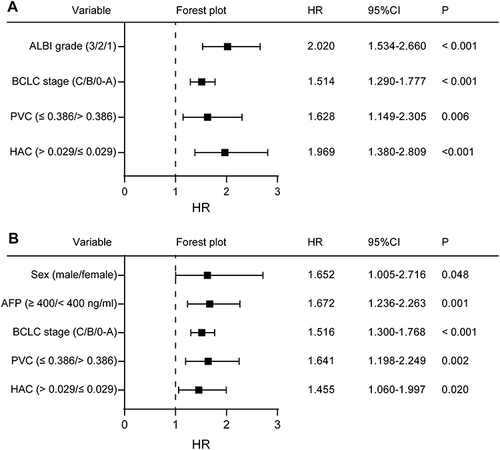
LASSO regression of 11 preoperative variables () unveiled that sex, ALBI grade, infection with hepatitis B and/or C virus, liver cirrhosis, serum α fetoprotein level, BCLC stage, tumor PVC, tumor HAC, and background liver tissue HAC were significantly associated with post-hepatectomy OS of HCC patients in this population ( and ). Multivariate Cox regression analysis of the nine variables revealed that a higher ALBI grade (HR 2.020, 95% CI 1.534 to 2.660, P < 0.001), higher BCLC stage (HR 1.514, 95% CI 1.290 to 1.777, P < 0.001), tumor PVC ≤ 0.386 (HR 1.628, 95% CI 1.149 to 2.305, P = 0.006), and tumor HAC > 0.029 (HR 1.969, 95% CI 1.308 to 2.809, P < 0.001) were independent risk factors for the worse post-hepatectomy OS of HCC patients ().
Figure 3 The least absolute shrinkage and selection operator (LASSO) regression was utilized to screen potential risk factors of the worse post-hepatectomy overall survival or recurrence-free survival of HCC patients. (A) Partial likelihood deviance plot. Vertical dotted lines indicate the optimal values, based on the minimum criterion (left line) or the criterion “1 - standard error” (right line). (B) LASSO coefficient profile plot. In each curve, the LASSO profile of a feature was plotted against the log (lambda) sequence.
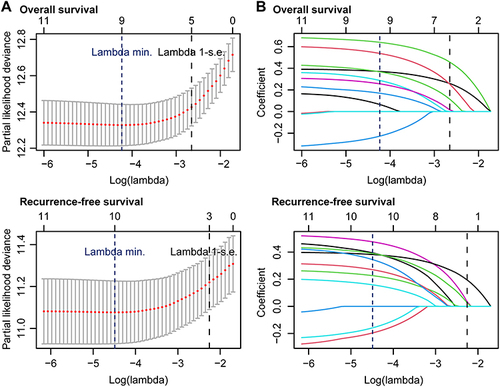
Figure 4 Forest plot of variables that emerged from multivariate regression analyses as independent predictors of (A) overall survival or (B) recurrence-free survival after hepatectomy.
Predicting Post-Hepatectomy RFS
LASSO regression of 11 preoperative variables () indicated that sex, age, ALBI grade, liver cirrhosis, serum α fetoprotein level, BCLC stage, tumor PVC, tumor HAC, background liver tissue PVC, and background liver tissue HAC were significantly associated with post-hepatectomy RFS of those patients ( and ). Multivariate Cox regression of the 10 variables revealed that the male sex (HR 1.652, 95% CI 1.005 to 2.716, P = 0.048), α fetoprotein ≥ 400 ng/mL (HR 1.672, 95% CI 1.236 to 2.263, P = 0.001), higher BCLC stage (HR 1.516, 95% CI 1.300 to 1.768, P < 0.001), tumor PVC ≤ 0.386 (HR 1.641, 95% CI 1.198 to 2.249, P = 0.002), and tumor HAC > 0.029 (HR 1.455, 95% CI 1.060 to 1.997, P = 0.020) were independent risk factors for the worse post-hepatectomy RFS of HCC patients ().
Predicting Clinicopathological Characteristics from Imaging
Stratification analyses revealed that HCC patients with tumor PVC ≤ 0.386 were more likely to have a higher ALBI grade, α fetoprotein ≥ 400 ng/mL, BCLC stages B-C, worse tumor cell differentiation, and MVI, compared with those with tumor PVC > 0.386 (Supplementary Table 1). In contrast, there was no significant difference in any of the clinicopathological characteristics that we examined between HCC patients with a tumor HAC of ≥ 0.029 and those with a tumor HAC of < 0.029 (Supplementary Table 2).
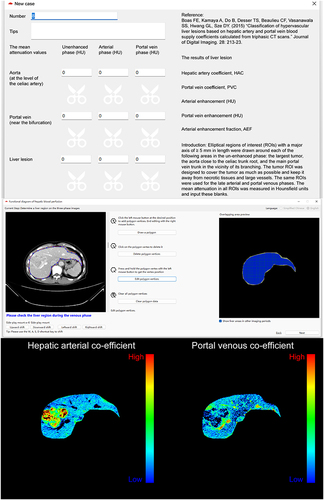
Development of a Liver Perfusion Software
A calculator and visualization software for the measurement of liver perfusion, named CALVIS LIVER PERFUSION, was developed (). PVC and HAC of HCC or background liver can be easily calculated by inputting the mean attenuation values of the portal vein, aorta, and HCC or the background liver in the unenhanced, arterial, and portal venous phases using the CALVIS LIVER PERFUSION. Furthermore, the calculated PVC and HAC for each pixel in the liver can be clearly expressed as color images by the software, in which, a bright red color means a high PVC or HAC value, and a bright blue color means a low PVC or HAC value. A hyper-vascular HCC has typically low PVC and high HAC values.
Figure 5 The calculator and visualization software for these liver perfusion indies. Upper panel, the interface of the calculator section. Middle panel, one of the interfaces of the visualization section. Downer panel, a typical HCC lesion displayed low PVC value and high HAC value. The software is available for free upon reasonable request from the corresponding author, Yang Ke.
Discussion
The dominant artery blood supply is a characteristic of hepatocellular carcinoma (HCC). However, it is not known whether the blood supply can predict the post-hepatectomy prognosis of patients with HCC. The key findings in the current study were that preoperative tumor PVC and HAC values effectively predicted the post-hepatectomy OS and RFS of HCC patients, even after adjustment for ALBI grade, liver cirrhosis, and BCLC stage. More specifically, a lower tumor PVC or higher tumor HAC was associated with worse post-hepatectomy OS and RFS of HCC patients. These results extended a previous finding that these two coefficients can predict OS of HCC patients after TACE,Citation11 and suggest that tumor PVC and HAC measures may be valuable for the prognosis and selection of HCC patients undergoing hepatectomy.
Our analysis revealed that a lower tumor PVC was linked to tumor characteristics and systemic parameters that have previously been associated with worse survival, including higher ALBI grade, α fetoprotein ≥ 400 ng/mL, BCLC stages B-C, worse tumor differentiation, and MVI. Interestingly, a higher HAC was significantly associated with worse post-hepatectomy OS and RFS of HCC patients, but this measure was not significantly associated with other clinical measures that are related to worse survival of HCC patients in this population. One possible explanation for this negative finding is that a higher tumor HAC is associated with the increased risk of HCC recurrence, but not with the growth of the primary HCC.
While preoperative PVC and HAC of tumors independently predicted the post-hepatectomy OS and RFS of HCC patients in our cohort, the corresponding coefficients for background non-tumor liver tissues did not. This contrasts with a previous observation that a lower PVC or higher HAC for background non-tumor liver tissues is associated with worse OS of HCC patients after TACE.Citation11 Possibly, HCC patients, who undergo hepatectomy, have generally stronger residual liver function than those who undergo TACE, which might overshadow an association between the background non-tumor liver tissue coefficients and survival.
Consistently, we found that several demographic and clinical characteristics of HCC patients were significantly linked to their worse prognosis. For example, male HCC patients are prone to early recurrence of HCC in a large international study,Citation24 possibly reflecting the adverse impact of the male sex hormones on the risk of recurrence.Citation25 Similarly, elevated serum α fetoprotein increases the risk of recurrence because it may correlate with a large population of cancer stem cells.Citation26 Furthermore, a higher ALBI grade is related to a worse survival of HCC patients regardless of whether they undergo tumor resection, liver transplantation, radiofrequency ablation, microwave ablation, TACE, radioembolization, and/or systematic therapies.Citation27
In our cohort, tumor PVC was lower than that in the background non-tumor liver, while tumor HAC was higher than that in background non-tumor liver, similar to a previous report.Citation9 We also found in our cohort that tumor PVC was significantly lower in patients with HCC at BCLC stages B-C than those with HCC at BCLC stages 0-A, but there was no significant difference in tumor HAC between these subgroups. This observation may be because HCC-related alterations in portal venous blood supply occur in both early and late disease processes, whereas alterations in hepatic arterial blood supply usually happen in the early, but not the late stage of disease process. These considerations contrast to the suggestion that during the HCC progression, portal venous blood flow continually decreases and arterial blood supply continually increases.Citation6 This apparent discrepancy between our finding and those of previous observation may be attributed to the fact that those patients in the previous studies may generally have been in an earlier stage of disease because patients with portal thrombus were excluded.Citation6 In addition, the previous study adopted a potentially more operator-dependent approach to semi-quantifying attenuation of lesions on CT images as hyper-, iso-, or hypo-attenuation by visually checking each lesion against the background non-tumor liver tissue.Citation6 Furthermore, in any case, we consider it unlikely that the volume of arterial blood (mL) per minute per milliliter of tumor tissue exhibits a continuous increase over time because the core of HCC tumors generally exhibits ischemia and hypoxia-induced necrosis as HCC progression.Citation28
We can use these imaging biomarkers to optimize our decision-making in a clinical setting. For instance, for a patient with initially resectable HCC, a high tumor HAC > 0.029, and/or a low tumor PVC ≤ 0.386, the patient may have a high probability of developing HCC recurrence and worse OS after hepatectomy. Rather, the patient may benefit from neoadjuvant TACE before hepatectomy,Citation29,Citation30 anatomic resection and wide resection margin in hepatectomy,Citation31 and/or adjuvant TACE and closely monitoring after hepatectomy.Citation29,Citation30 The patient can be recommended with these individualized treatments. Therefore, our findings may help in the selection of HCC patients for hepatectomy and in the management of HCC patients in clinical practice.
To generalize the use of PVC and HAC in clinical practice in decision-making, we developed a software CALVIS LIVER PERFUSION. It was different from the inaugural one made by Boas (http:// www.claripacs.com/calc/liver.html), which used information from the arterial, portal venous, and delayed phases.Citation9 In this study, we tried to use information from the delayed phase, leading to inaccurate PVC and HAC, which might be attributed to the long scanning interval between the portal venous and delayed phases and inevitable patient movement during the interval.
While our results come from a relatively large population, interpretations of our results should be careful because we retrospectively analyzed patients at a single institution. Further work should validate our findings in multiple patient populations. In addition, we measured tomographic enhancement on single axial slices, which might be less accurate than using the entire tumor volume because of intratumor heterogeneity.Citation32 Moreover, given that the role of PVC and HAC in the prognosis of hepatectomy and TACE has been explored, further prospective studies with a bigger population are necessary to validate our findings and extend to explore their role in predicting the outcome of targeted and immunotherapy for HCC.Citation11
Conclusions
A preoperative lower tumor PVC or higher tumor HAC was independently associated with worse post-hepatectomy OS and RFS of HCC patients. This non-invasive prognostic approach, if validated in other patient populations, may help surgeons precisely recommend hepatectomy to individuals, who are likely to benefit most and optimize their management.
Abbreviations
ALBI grade, albumin, and total bilirubin grade; BCLC stage, Barcelona Clinic Liver Cancer stage; CI, confident interval; CT, computed tomography; HAC, hepatic arterial coefficient; HCC, hepatocellular carcinoma; HR, hazards ratio; IQR, inter-quartile range; LASSO, least absolute shrinkage selection operator; PVC, portal venous coefficient; OS, overall survival; RFS, recurrence-free survival; ROI, regions of interest; TACE, transcatheter arterial chemoembolization.
Ethics Approval and Informed Consent
This study was approved by the Committee of Ethics at the Second Affiliated Hospital, Kunming Medical University (Shen-PJ-Ke-2022-60). Written informed consent was waived by the Committee of Ethics as its retrospective collection.
Disclosure
All authors declare that they have no competing interests in this work.
Acknowledgments
The current affiliation of Dr. Yu-Shan Wu is Department of Gastroenterology, West China Hospital, Sichuan University, Chengdu 610041, SC, China. The current affiliation of Dr. Wei-Hu Zhang is Department of Radiology, Northeast Yunnan Regional Central Hospital, Zhaotong 657000, YN, China.
Data Sharing Statement
The datasets used and/or analyzed during the current study and the CALVIS LIVER PERFUSION software are available from the corresponding author Y.K. upon reasonable request.
Additional information
Funding
References
- Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021;149(4):778–789. doi:10.1002/ijc.33588
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
- Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi:10.1056/NEJMra1713263
- Li C, Wang H, Chen R, et al. Outcomes and recurrence patterns following curative hepatectomy for hepatocellular carcinoma patients with different China liver cancer staging. Am J Cancer Res. 2022;12(2):907–921.
- Horvatits T, Drolz A, Trauner M, Fuhrmann V. Liver injury and failure in critical illness. Hepatology. 2019;70(6):2204–2215. doi:10.1002/hep.30824
- Tajima T, Honda H, Taguchi K, et al. Sequential hemodynamic change in hepatocellular carcinoma and dysplastic nodules: CT angiography and pathologic correlation. AJR Am J Roentgenol. 2002;178(4):885–897. doi:10.2214/ajr.178.4.1780885
- Thorne J, Bennett R, Shields R, Johnson J, Taylor I. A comparison of xenon-133 clearance with electromagnetic flowmeters and an indicator dilution method for the measurement of liver blood flow. Br J Surg. 1979;66(6):385–388. doi:10.1002/bjs.1800660604
- Blomley MJ, Coulden R, Dawson P, et al. Liver perfusion studied with ultrafast CT. J Comput Assist Tomogr. 1995;19(3):424–433. doi:10.1097/00004728-199505000-00016
- Boas FE, Kamaya A, Do B, et al. Classification of hypervascular liver lesions based on hepatic artery and portal vein blood supply coefficients calculated from triphasic CT scans. J Digit Imaging. 2015;28(2):213–223. doi:10.1007/s10278-014-9725-9
- Pang G, Duan Z, Shao C, Zhao F, Zhong H, Shao G. Heterogeneity analysis of triphasic CT scan perfusion parameters in differential diagnosis of hepatocellular carcinoma and hemangioma. Medicine. 2018;97(38):e12512. doi:10.1097/md.0000000000012512
- Borgheresi A, Gonzalez-Aguirre A, Brown KT, et al. Does enhancement or perfusion on preprocedure CT predict outcomes after embolization of hepatocellular carcinoma? Acad Radiol. 2018;25(12):1588–1594. doi:10.1016/j.acra.2018.02.027
- Boas FE, Brody LA, Erinjeri JP, et al. Quantitative measurements of enhancement on preprocedure triphasic CT can predict response of colorectal liver metastases to radioembolization. AJR Am J Roentgenol. 2016;207(3):671–675. doi:10.2214/ajr.15.15767
- Ke Y, Ma L, You XM, et al. Antiviral therapy for hepatitis B virus-related hepatocellular carcinoma after radical hepatectomy. Cancer Biol Med. 2013;10(3):158–164. doi:10.7497/j.issn.2095-3941.2013.03.006
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
- Pan X, Wu SJ, Tang Y, Zhou YF, Luo JW, Fang ZT. Safety and efficacy of transarterial chemoembolization combined with tyrosine kinase inhibitor and immune checkpoint inhibitors for unresectable hepatocellular carcinoma: a single center experience. J Hepatocell Carcinoma. 2023;10:883–892. doi:10.2147/jhc.S404500
- Zanuso V, Pirozzi A, Balsano R, Pressiani T, Rimassa L. Safety and efficacy of atezolizumab and bevacizumab combination as a first line treatment of advanced hepatocellular carcinoma. J Hepatocell Carcinoma. 2023;10:1689–1708. doi:10.2147/jhc.S347932
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-The ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi:10.1200/JCO.2014.57.9151
- Zhong JH, Ke Y, Gong WF, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260(2):329–340. doi:10.1097/SLA.0000000000000236
- Yoshiji H, Nagoshi S, Akahane T, et al. Evidence-based clinical practice guidelines for Liver Cirrhosis 2020. J Gastroenterol. 2021;56(7):593–619. doi:10.1007/s00535-021-01788-x
- Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 Edition). Liver Cancer. 2020;9(6):682–720. doi:10.1159/000509424
- Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7(3):462–503. doi:10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e
- Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. doi:10.1158/1078-0432.CCR-04-0713
- Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1–22.
- Chan AWH, Zhong J, Berhane S, et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol. 2018;69(6):1284–1293. doi:10.1016/j.jhep.2018.08.027
- Kim SH, Kamaya A, Willmann JK. CT perfusion of the liver: principles and applications in oncology. Radiology. 2014;272(2):322–344. doi:10.1148/radiol.14130091
- Kui X, Wu L, Ke Y, et al. The gene mutation profile in HCC patients with different AFP levels at diagnosis. Cancer Res. 2021;81(13_Supplement):2660. doi:10.1158/1538-7445.AM2021-2660
- Demirtas CO, D’Alessio A, Rimassa L, Sharma R, Pinato DJ. ALBI grade: evidence for an improved model for liver functional estimation in patients with hepatocellular carcinoma. JHEP Rep. 2021;3(5):100347. doi:10.1016/j.jhepr.2021.100347
- Wei T, Zhang XF, Bagante F, et al. Tumor necrosis impacts prognosis of patients undergoing curative-intent hepatocellular carcinoma. Ann Surg Oncol. 2021;28(2):797–805. doi:10.1245/s10434-020-09390-w
- Podlasek A, Abdulla M, Broering D, Bzeizi K. Recent advances in locoregional therapy of hepatocellular carcinoma. Cancers. 2023;15(13):3347. doi:10.3390/cancers15133347
- Nevola R, Delle Femine A, Rosato V, et al. Neoadjuvant and adjuvant systemic therapies in loco-regional treatments for hepatocellular carcinoma: are we at the dawn of a new era? Cancers. 2023;15(11):2950. doi:10.3390/cancers15112950
- Shi C, Zhao Q, Liao B, et al. Anatomic resection and wide resection margin play an important role in hepatectomy for hepatocellular carcinoma with peritumoural micrometastasis. ANZ J Surg. 2019;89(11):E482–E486. doi:10.1111/ans.15396
- Brenet Defour L, Mule S, Tenenhaus A, et al. Hepatocellular carcinoma: CT texture analysis as a predictor of survival after surgical resection. Eur Radiol. 2019;29(3):1231–1239. doi:10.1007/s00330-018-5679-5