Abstract
Purpose
Early recurrence (ER) is associated with poor prognosis in hepatocellular carcinoma (HCC). In this study, we developed and externally validated a nomogram based on the hemoglobin, albumin, lymphocytes, and platelets (HALP) score to predict ER for patients with BCLC stage 0/A HCC who underwent radical liver resection.
Patients and Methods
A total of 808 BCLC stage 0/A HCC patients from six hospitals were included in this study, and they were assigned to a training cohort (n = 500) and an external validation cohort (n = 308). We used univariate and multivariate Cox regression analysis to identify the independent risk factors for disease-free survival (DFS). We also established and externally validated a nomogram based on these risk predictors. The nomogram was evaluated using the area under the receiver operating characteristic curve (AUC), the concordance index (C-index), the calibration curve, decision curve analysis (DCA), and Kaplan‒Meier analysis.
Results
Multivariate COX regression showed that HBV DNA ≥10,000 IU/mL (P < 0.001), HALP score ≤38.20 (P < 0.001), tumor size (P = 0.003), clinically significant portal hypertension (P = 0.001), Edmondson-Steiner grade (III–IV) (P = 0.007), satellite nodules (P < 0.001), and MVI (P = 0.001) were independent risk factors for post-operative tumor recurrence. The AUC of our nomogram for predicting the 2-year and 5-year DFS was 0.756 and 0.750, respectively, in the training cohort and 0.764 and 0.705, respectively, in the external validation cohort. We divided the patients into low-, intermediate- and high-risk groups according to the risk score calculated by the nomogram. There were statistically significant differences in the DFS and overall survival (OS) among the three groups of patients (P < 0.001).
Conclusion
We developed and externally validated a new nomogram, which is accurate and can predict ER in BCLC stage 0/A HCC patients after curative liver resection.
Introduction
As of 2022, primary liver cancer was the sixth most diagnosed cancer and the third leading cause of cancer-related death in the world, with about 865,000 new cases and 757,948 deaths.Citation1 75–85% of primary liver cancer is hepatocellular carcinoma (HCC).Citation1 For patients with Barcelona Clinic Liver Cancer (BCLC) stage 0/A HCC, hepatectomy is the most effective treatment method.Citation2,Citation3 However, even after active surgical treatment, the prognosis of HCC patients remains poor due to high recurrence rates. The 5-year recurrence rate after radical hepatectomy is as high as 50–70%.Citation4,Citation5 Recurrence within two years of liver resection (LR) is defined as early recurrence (ER).Citation6 Compared with late recurrence, patients with ER have poor tumor biology and worse prognoses.Citation6,Citation7 Therefore, identifying the risk factors for HCC recurrence, especially ER, and providing early post-operative adjuvant therapy for high-risk patients will be beneficial in improving their prognoses. Currently, multiple nomograms exist to predict HCC recurrence. However, they encompass a broad spectrum of tumor stages, including advanced stages such as BCLC stage C and TNM stage IV, leading to imprecise prognostic predictions for patients with early-stage HCC.Citation8–11 Hence, there is a pressing need to develop a specialized prediction model specifically for patients with BCLC stage 0/A HCC.
The hemoglobin, albumin, lymphocyte, and platelet (HALP) score is a new composite biomarker index defined as hemoglobin × albumin × Lymphocytes / platelets, first proposed by Chen et al in 2015 to predict the prognosis of gastric carcinoma patients.Citation12 Since then, many researchers have proved that the HALP score is an effective predictor of the overall prognosis of various tumors, such as colorectal cancer,Citation13 bladder cancer,Citation14 and gastrointestinal stromal tumors.Citation15 It is often used to evaluate patients’ inflammatory responses and nutritional status. In general, a low HALP score before treatment is associated with a reduced survival rate of cancer patients.Citation16 Recently, Zhou et al found that a low preoperative HALP score indicates a poor prognosis in HCC patients who underwent LR,Citation17 suggesting that the HALP score can predict the postoperative recurrence of HCC.
In this study, we aimed to confirm the clinical significance of a low HALP score in predicting the postoperative recurrence of HCC. We then developed and externally validated a novel nomogram based on the HALP score to predict ER in BCLC stage 0/A HCC patients who underwent LR. The results of this study may help guide individualized treatments and improve patient outcomes.
Materials and Methods
Patients Included
We retrospectively selected the patients included in this study. Data for the training cohort originated from patients who underwent treatment at the Tongji Hospital, affiliated to Tongji Medical College of Huazhong University of Science and Technology, from February 2015 to September 2018. The external validation cohort data was from patients treated at the West China Hospital, Taihe Hospital, Huangshi Central Hospital, Jingzhou Central Hospital, and Xiangyang Central Hospital from July 2013 to June 2023. This study was approved by the Institutional Ethics Committees of all six hospitals. The identities of all included patients were anonymized before the analysis. Therefore, the requirement for informed consent was waived.
Data for all BCLC stage 0/A HCC patients who underwent LR in the above hospitals during the period were reviewed. The inclusion criteria were as follows: (1) age 14–75 years; (2) no history of other malignant diseases; (3) no previous treatment for HCC before liver resection; and (4) Child-Pugh class A or selected class B preoperative liver function. The exclusion criteria were as follows: (1) incomplete baseline data; (2) without R0 resection; and (3) combined HCC and intrahepatic cholangiocarcinoma (ICC). A total of 808 BCLC stage 0/A HCC patients met the inclusion criteria and were included in this study. Of the 808 patients, 500 were included in the training group, and 308 were in the external validation group. The flow chart of the patient selection process for the training and external validation groups is presented in .
Data Collected
We retrospectively collected the clinical and pathological data of all BCLC stage 0/A HCC patients included in this study. The variables collected included age, sex, HBV DNA load, HBsAg, alpha-fetoprotein (AFP) concentration, total bilirubin (TB), albumin (ALB), lymphocyte count, hemoglobin, platelet count, number of tumors, tumor size, surgical approach, resection method, estimated blood loss (EBL), operation time (OT), blood transfusion, cirrhosis, Edmondson-Steiner grade, microvascular invasion (MVI), and satellite nodules. All laboratory test results were obtained within one week leading up to the operation. In this study, clinically significant portal hypertension (CSPH) was defined as gastroesophageal varices detected by gastroscopy, or platelet count <10,000 cells/mL and splenomegaly (maximum diameter >12 cm based on CT scan).Citation18 The albumin-bilirubin (ALBI) score was calculated by 0.66 * Log10 [TBIL (μmol/L)] - 0.085 * [ALB (g/L)]. Patients with ALBI ≤ −2.60 were classified as ALBI grade 1, those with −2.60 < ALBI ≤ −1.39 were classified as ALBI grade 2, and those with ALBI > −1.39 were classified as ALBI grade 3.Citation19 We calculated the HALP score by hemoglobin(g/L) * albumin(g/L) * Lymphocyte(*10^9/L) / platelet(*10^9/L).Citation12 We used the R package survminer to calculate the optimal cut-off values.Citation20 The cut-off thresholds for HBV DNA and the HALP score were 10,000 IU/mL and 38.20, respectively.
Follow-Up
Follow-up visits were scheduled one month after discharge, every two months within the first year, and every three months thereafter. The follow-up included liver function tests, serum AFP levels, and abdominal ultrasound. Enhanced abdominal CT or enhanced abdominal MRI was performed every six months. Disease-free survival (DFS) was defined as the time interval between surgery and detection of tumor recurrence. All tumor relapses were diagnosed through CT or MRI. Early recurrence was defined as recurrence within two years after LR.
Statistical Analysis
Continuous variables were represented by means and standard deviations, or medians and interquartile ranges (IQR). Categorical variables were reported as counts and percentages. Independent risk factors were identified using multivariate Cox regression analyses. The nomogram was constructed from the independent risk factors of recurrence identified by the Cox regression analysis. The area under the receiver operating characteristic (ROC) curve and the concordance index (C-index) were used to assess the discrimination performance of the model in predicting DFS. The calibration plot and decision curve analysis (DCA) were used to evaluate the calibration of the nomogram and the net benefit, respectively. We used the X-tile software (version 3.6.1; Yale University, New Haven, CT, USA) to group the patients by risk. Afterwards, the patients were divided into the low-, intermediate- and high-risk groups based on their risk scores. Then, DFS and OS curves were plotted using the Kaplan‒Meier method and compared using the Log rank test.
Univariate and multivariate Cox regression analyses were performed using SPSS 26.0 (IBM Corp., Armonk, New York, USA). The nomogram, ROC curves, C-index, calibration curve, DCA, and survival figures were prepared or performed using R software version 4.2.1 (R Project for Statistical Computing, Vienna, Austria). Bilateral P < 0.05 was considered to be statistically significant.
Results
Baseline Characteristics
A total of 808 BCLC stage 0/A HCC patients who received radical LR were included in this study according to the inclusion and exclusion criteria. Of these, 500 patients were in the training cohort, and 308 were in the external validation cohort. All patients in this study had R0 liver resection, and most had well-preserved liver function (Child-Pugh class A). Based on the follow-up data and diagnostic criteria, 171 (34.2%) patients in the training cohort and 92 (29.9%) patients in the external validation cohort experienced early recurrence. Male patients were predominant in both cohorts (85.6% and 86.7% respectively). The average ages were 53.23 and 54.09 in the training and external validation cohorts, respectively. Microvascular invasion was detected in 109 (21.8%) patients in the training cohort and 67 (21.8%) patients in the external validation cohort. The demographic, clinical, pathological, and imaging characteristics of the BCLC stage 0/A HCC patients in the training and external validation cohorts are shown in .
Table 1 Baseline Characteristics of BCLC Stage 0/A HCC Patients Who Underwent Hepatectomy in the Training and External Validation Cohorts
Risk Factors Selection
Univariate Cox regression analysis showed that gender, age, number of tumors, surgical approach, resection method, EBL, operation time, blood transfusion, and cirrhosis had no significant relationship with recurrence in patients with BCLC stage 0/A HCC (P > 0.05 for all variables, ). However, HBV DNA load ≥10,000 IU/mL (P < 0.001), positive HBsAg (P = 0.009), AFP level ≥400 ng/mL (P < 0.001), ALBI grade 2 (P = 0.002), HALP score ≤38.20 (P < 0.001), tumor size (P < 0.001), BCLC stage A (P = 0.001), CSPH (P = 0.002), Edmondson-Steiner grade (III–IV) (P < 0.001), satellite nodules (P < 0.001) and MVI (P < 0.001) were associated with relapse of HCC.
Table 2 Univariate and Multivariate Cox Regression Analysis of Independent Risk Factors Associated with Disease-Free Survival (DFS) in the Training Cohort
Multivariate Cox regression analysis showed that HBV DNA load ≥10,000 IU/mL [hazard ratio (HR) = 1.820; 95% confidence interval (CI), 1.357–2.440; P < 0.001], HALP score ≤38.20 (HR = 1.963; 95% CI, 1.414–2.726; P < 0.001), tumor size (HR = 1.080; 95% CI, 1.026–1.138; P = 0.003), CSPH (HR = 1.793; 95% CI, 1.274–2.524; P = 0.001), Edmondson-Steiner grade (III–IV) (HR = 1.457; 95% CI, 1.110–1.914; P = 0.007), satellite nodules (HR = 1.935; 95% CI, 1.349–2.777; P < 0.001) and MVI (HR = 1.642; 95% CI, 1.209–2.229; P = 0.001) were independent risk factors for post-operative recurrence of BCLC stage 0/A HCC patients ().
Development and Validation of a Novel Nomogram for Predicting the DFS
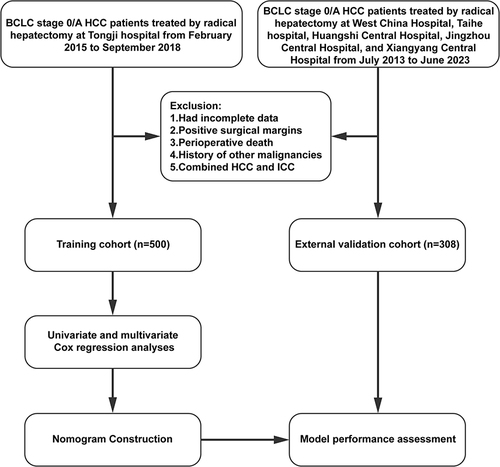
Based on the independent risk factors from the multivariate analysis, we constructed a nomogram for predicting the 2-year and 5-year DFS of BCLC stage 0/A HCC patients (). Higher total scores calculated using the nomogram were associated with poorer prognoses. The area under the ROC curve (AUC) values for our nomogram for predicting the 2- and 5-year DFS were 0.756 and 0.750 in the training cohort and 0.764 and 0.705 in the external validation cohort, respectively ( and ). The C-index of discrimination for predicting the training cohort’s 2- and 5-year DFS rates was 0.708 (95% CI: 0.677–0.740). For the external validation cohort, the C-index was 0.705 (95% CI: 0.657–0.752). The calibration curve showed that the predicted 2- and 5-year recurrence probabilities were similar to the actual probabilities in the training and external validation datasets (). The DCA for the nomogram showed high net benefits in the reasonable threshold probabilities in the training and external validation datasets ().
Figure 2 (A) The nomogram model for predicting 2-year and 5-year DFS. (B) The 2-year and 5-year receiver operating characteristic (ROC) curve of DFS in the training cohort. (C) The 2-year and 5-year ROC curves of DFS in the external validation cohort.
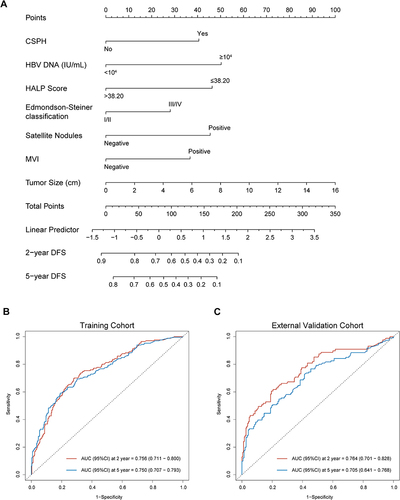
Figure 3 Calibration curve for predicting the 2-year DFS (A) and the 5-year DFS (B) in the training cohort and the 2-year DFS (C) and the 5-year DFS (D) in the external validation cohort.
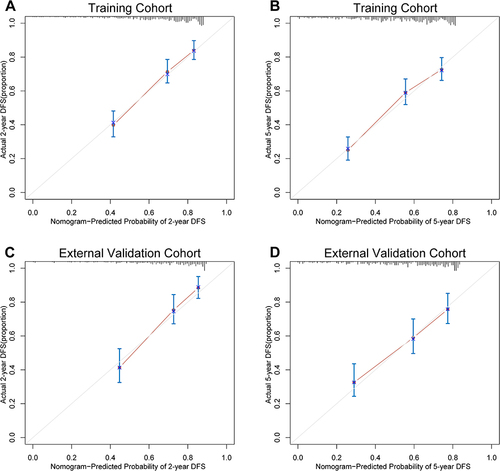
Figure 4 DCA plots for the nomogram in predicting the 2-year DFS (A) and the 5-year DFS (B) in the training cohort and the 2-year DFS (C) and the 5-year DFS (D) in the external validation cohort.
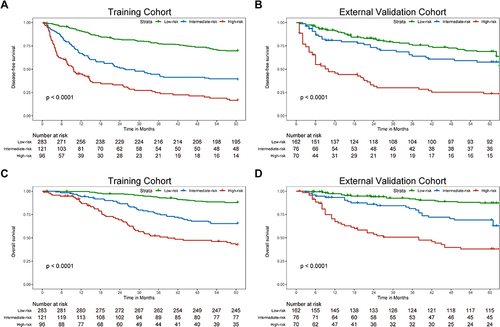
Furthermore, according to the total risk scores calculated from the nomogram prediction model, we used the X-tile software to divide the training and external validation cohorts into low-, intermediate- and high-risk groups. As shown in , patients in the low-risk groups had the best DFS and OS, while those in the high-risk groups had the worst DFS and OS. This indicated that our nomogram had excellent stratification ability.
Figure 5 The Kaplan-Meier survival curves for low-, intermediate- and high-risk groups of BCLC stage 0/A HCC patients after radical liver resection based on our nomogram calculated risk scores. The disease-free survival Kaplan-Meier survival curves in the training cohort (A) and the external validation cohort (B). The overall survival Kaplan-Meier survival curves in the training cohort (C) and the external validation cohort (D).
Discussion
Radical liver resection significantly improves the survival of HCC patients. However, high postoperative recurrence rates (reaching 50%-70%) remain a major limiting factor for long-term survival in HCC patients.Citation4,Citation21 Therefore, implementing adjuvant therapy for individuals at high risk of postoperative recurrence has become crucial in enhancing the long-term survival of these patients.Citation22 The first Phase 3 study reporting positive Results for adjuvant treatment of HCC demonstrated improved recurrence-free survival in patients receiving atezolizumab plus bevacizumab compared to active surveillance in those at high risk of recurrence after liver resection or ablation.Citation23 Additionally, preliminary results from an ongoing study at our center on adjuvant therapy after radical liver resection indicate that the 2-year disease-free survival rates for the adjuvant anti-PD-1 and adjuvant TACE groups are 60.3% and 42.6%, respectively. These results suggest that postoperative adjuvant therapy can delay recurrence and extend overall survival for a considerable number of patients. Therefore, it is essential to identify the patients with high recurrence risk in advance to enable the implementation of effective preventive measures.
Patients with BCLC stage 0/A HCC are the primary candidates for liver resection.Citation24 However, due to the varying criteria for patient selection, existing prognostic models often encompass a wide range of tumor stages, including advanced stages such as BCLC stage C and TNM stage IV, which has made it challenging to use a single model to accurately evaluate the prognosis of patients with early-stage HCC.Citation9,Citation11,Citation25 Therefore, developing a specialized model to predict the prognosis of BCLC stage 0/A HCC patients is of great significance.
Currently, the commonly used staging systems like BCLC stage and Tumor-Node-Metastasis (TNM) staging system of the American Joint Committee on Cancer (AJCC) primarily consider factors such as tumor size, number, and MVI.Citation24,Citation26 Because the postoperative recurrence of HCC is influenced by numerous factors, including nutritional and immune status, portal hypertension, HBV DNA load, Edmondson-Steiner grade, satellite nodules, etc;Citation18,Citation27–30 the ability to perform individualized assessments of patients using the common staging systems is limited.
Previous prognostic models mainly focused on tumor-related variables and did not consider other conditions, such as the nutritional and immune status of the patients or liver cirrhosis. The HALP score is a new comprehensive marker of the nutritional and immune status that is an important risk biomarker for a variety of digestive system cancers, including gastric cancer,Citation12,Citation31 esophageal squamous cell carcinoma (ESCC),Citation32,Citation33 colorectal cancer,Citation34,Citation35 and intrahepatic cholangiocarcinoma.Citation36,Citation37 However, since the HALP score was only developed in 2015, original studies exploring the utility of this novel index in HCC are still lacking.Citation12,Citation16 Recently, Zhou et alCitation17 and Toshida et alCitation30 reported that a low HALP score (<54.132; ≤45.6) is an independent predictor of poor OS in HCC patients (HR = 1.708; HR = 1.66). In this study, multivariate COX regression analysis also showed that a low HALP score (≤38.20) is an independent risk factor for recurrence in BCLC stage 0/A HCC patients after surgery (HR = 1.85). Here, we developed and externally validated a novel nomogram that includes the HALP score that effectively predicted ER in postoperative BCLC stage 0/A HCC patients.
Although a low HALP score is an unfavorable prognostic factor for HCC, the specific mechanism by which it affects the prognosis of HCC remains unclear. We have tried to explain this phenomenon by analyzing the four components of the HALP score. Clinical studies suggest that low hemoglobin levels are associated with poor survival in HCC patients.Citation38 This is likely due to tumor hypoxia caused by anemia, which might increase the invasiveness of cancer. For example, hypoxia-inducible factor (HIF) can activate vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF), which play a key role in tumor angiogenesis.Citation39 Albumin usually reflects the body’s nutritional status and inflammation level.Citation17 Studies have shown that the reduction of albumin promotes the migration and invasion of HCC cells by increasing urokinase plasminogen activator surface receptor (uPAR) and matrix metalloproteinase 2 (MMP2) and 9 (MMP9).Citation40 Lymphocytes have powerful anti-tumor immune functions and can inhibit tumor development. Elevated lymphocyte levels are associated with a good tumor prognosis.Citation41 In addition, clinical studies have found that inflammatory markers composed of lymphocytes can predict the prognosis of patients with HCC.Citation42–44 Cancer cell-platelet interactions are an important component of cancer metastasis.Citation45 Midorikawa et alCitation46 believed that high platelet counts are associated with poor prognoses in patients with hepatocellular carcinoma without cirrhosis. From the roles of the individual components of the score described above, it makes logical sense that lower HALP scores should be associated with worse oncological outcomes, which is consistent with the current literature.
Cirrhosis plays a crucial role in the occurrence of HCC, with approximately 70–90% of HCC patients having cirrhosis.Citation47 CSPH is an important prognostic indicator in patients with cirrhosis, and it results from increased intrahepatic vascular resistance caused by the dysregulation of liver sinusoidal endothelial cells (LSECs) and hepatic stellate cells (HSCs).Citation48,Citation49 There is still controversy over whether preoperative CSPH affects the surgical outcomes of HCC patients with compensated liver cirrhosis.Citation50 Cortese et alCitation51 analyzed HCC patients with Child-Pugh class A liver function who underwent liver resection and found no significant difference in OS and DFS between the CSPH and non-CSPH groups. Lopez et alCitation52 also reported no statistically significant difference in the perioperative and long-term prognosis between the CSPH and non-CSPH groups. However, Xia et alCitation18 found that in patients with BCLC stage A HCC, the CSPH group had worse OS and recurrence-free survival (RFS) than the non-CSPH group (HR = 2.340; HR = 2.577). A systematic review and meta-analysis found that CSPH negatively impacted the long-term prognosis of HCC patients after partial hepatectomy.Citation53 Another systematic review and meta-analysis evaluated the impact of CSPH on the prognosis of HCC patients with compensated liver cirrhosis who underwent surgical treatment and found that CSPH significantly increased the 3- and 5-year postoperative mortality.Citation50 Qin et alCitation54 investigated the predictive ability of ALBI grade plus CSPH (ALBI-P score) in patients with HCC after LR. Multivariate analysis showed that the ALBI-P score was an independent risk factor for postoperative recurrence (HR = 1.441) and death (HR = 1.332). These studies highlight the potential value of CSPH in predicting the postoperative recurrence of HCC. In this study, there were 72 cases (14.4%) with preoperative CSPH in the training group and 58 cases (18.8%) with preoperative CSPH in the external validation group. Multivariate Cox regression analysis showed that CSPH (HR = 1.793) is an independent risk factor for relapse after LR in patients with BCLC stage 0/A HCC.
Consistent with previous reports,Citation22,Citation27–29 the results of multivariate Cox regression analysis in this study showed that HBV DNA load ≥10,000 IU/mL, tumor size, Edmondson-Steiner grade (III–IV), satellite nodules, and MVI were independent risk factors for postoperative recurrence in patients with BCLC stage 0/A HCC. HBV DNA load reflects the level of viral replication, which is a key driver of liver injury and HCC development, and higher levels negatively impact the prognosis.Citation55 MVI refers to the presence of micrometastatic HCC emboli within hepatic vessels, detectable only under a microscope, and is a critical factor for early HCC recurrence and poor prognosis.Citation56 Edmondson-Steiner grade is a globally recognized and long-established histological classification standard for HCC. Generally, the poorer the differentiation, the stronger the tumor’s proliferation ability and angiogenic activity.Citation57 Satellite nodules are macroscopic or microscopic tumor cell nests located around or near the main tumor, sharing similar histological characteristics with the primary tumor. They are significant predictors of postoperative recurrence.Citation58 The impact of tumor size on prognosis is primarily due to the increased invasiveness of larger tumors. Studies have shown that when the tumor diameter exceeds 5 cm, the incidence of intrahepatic metastasis and portal vein invasion significantly increases.Citation59
In this study, we established a nomogram model that included the HALP score and evaluated its ability to predict early recurrence in patients with early-stage HCC after undergoing radical liver resection. The model underwent multi-center external validation. The probability of early recurrence can be estimated for each patient based on the risk score calculated using our nomogram. The model demonstrated excellent discrimination in both the training and external validation cohorts, with 2-year AUCs of 0.756 and 0.764, respectively. Kaplan-Meier survival curves indicated that this model could effectively identify patients at high risk of recurrence, and it also had good applicability in the external cohort of this study. Currently, the efficacies of transarterial chemoembolization (TACE), hepatic arterial infusion chemotherapy (HAIC), radiotherapy, and molecular targeted therapy in the adjuvant treatment of HCC are being studied.Citation60 Therefore, our nomogram can help to identify patients at high risk of recurrence after radical liver resection and enable these patients to benefit from adjuvant therapy.
Our study has several limitations. Firstly, this is a retrospective study; therefore, the conclusions require further validation. Secondly, the nomogram was derived from BCLC stage 0/A HCC patients who underwent liver resection and may not be applicable to HCC patients with other BCLC stages or those who received other treatments. Finally, the mechanism by which the HALP score predicts the prognosis of HCC patients needs to be further studied.
Conclusion
In Conclusion, we developed and externally validated a novel nomogram based on the HALP score that can predict the DFS after hepatectomy in BCLC stage 0/A HCC patients. This model exhibited a good predictive ability and can assist doctors in making personalized treatment decisions for BCLC stage 0/A HCC patients.
Abbreviations
ER, early recurrence; HCC, hepatocellular carcinoma; HALP, hemoglobin, albumin, lymphocyte and platelet score; BCLC, Barcelona Clinic of Liver Cancer; DFS, disease-free survival; ROC, receiver operating characteristic; C-index, concordance index; DCA, decision curve analysis; HBV DNA, hepatitis B virus DNA; MVI, microvascular invasion; AUC, area under the receiver operating characteristic curve; OS, overall survival; LR, liver resection; ICC, intrahepatic cholangiocarcinoma; HBsAg, hepatitis B surface antigen; AFP, alpha-fetoprotein; TBIL, total bilirubin; ALB, albumin; EBL, estimated blood loss; OT, operation time; E-S, Edmondson-Steiner grade; CSPH, clinically significant portal hypertension; ALBI, albumin-bilirubin grade; CT, computed tomography; MRI, magnetic resonance imaging; IQR, interquartile ranges; HR, hazard ratio; CI, confidence interval; OLR, open liver resection; MILR, minimally invasive liver resection; NAR, non-anatomical anatomical resection; AR, anatomical resection.
Ethics Statement
All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The authors are grateful to all patients who provided data for this study.
Data Sharing Statement
The datasets used and analyzed in this study can be obtained from the corresponding author upon reasonable request.
Additional information
Funding
References
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. doi:10.3322/caac.21834
- Chen ZH, Zhang XP, Feng JK, et al. Actual long-term survival in hepatocellular carcinoma patients with microvascular invasion: a multicenter study from China. Hepatol Int. 2021;15(3):642–650. doi:10.1007/s12072-021-10174-x
- European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
- Sugawara Y, Hibi T. Surgical treatment of hepatocellular carcinoma. Biosci Trends. 2021;15(3):138–141. doi:10.5582/bst.2021.01094
- Bruix J, Sherman M. Practice Guidelines Committee AAftSoLD. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–1236. doi:10.1002/hep.20933
- Calderaro J, Petitprez F, Becht E, et al. Intra-tumoral tertiary lymphoid structures are associated with a low risk of early recurrence of hepatocellular carcinoma. J Hepatol. 2019;70(1):58–65. doi:10.1016/j.jhep.2018.09.003
- Jung SM, Kim JM, Choi GS, et al. Characteristics of early recurrence after curative liver resection for solitary hepatocellular carcinoma. J Gastrointest Surg. 2019;23(2):304–311. doi:10.1007/s11605-018-3927-2
- Zheng Z, Guan R, Zou Y, et al. Nomogram based on inflammatory biomarkers to predict the recurrence of hepatocellular carcinoma-a multicentre experience. J Inflamm Res. 2022;15:5089–5102. doi:10.2147/JIR.S378099
- Shi Y, Wang Y, Niu K, Zhang W, Lv Q, Zhang Y. How CLSPN could demystify its prognostic value and potential molecular mechanism for hepatocellular carcinoma: a crosstalk study. Comput Biol Med. 2024;172:108260. doi:10.1016/j.compbiomed.2024.108260
- Shi Y, Wang Y, Zhang W, et al. N6-methyladenosine with immune infiltration and PD-L1 in hepatocellular carcinoma: novel perspective to personalized diagnosis and treatment. Front Endocrinol. 2023;14:1153802. doi:10.3389/fendo.2023.1153802
- Yang C, Wang H, Liu J, et al. Pre- to postoperative alpha-fetoprotein ratio-based nomogram to predict tumor recurrence in patients with hepatocellular carcinoma. Front Oncol. 2023;13:1134933. doi:10.3389/fonc.2023.1134933
- Chen XL, Xue L, Wang W, et al. Prognostic significance of the combination of preoperative hemoglobin, albumin, lymphocyte and platelet in patients with gastric carcinoma: a retrospective cohort study. Oncotarget. 2015;6(38):41370–41382. doi:10.18632/oncotarget.5629
- Jiang H, Li H, Li A, et al. Preoperative combined hemoglobin, albumin, lymphocyte and platelet levels predict survival in patients with locally advanced colorectal cancer. Oncotarget. 2016;7(44):72076–72083. doi:10.18632/oncotarget.12271
- Peng D, Zhang CJ, Gong YQ, et al. Prognostic significance of HALP (hemoglobin, albumin, lymphocyte and platelet) in patients with bladder cancer after radical cystectomy. Sci Rep. 2018;8(1):794. doi:10.1038/s41598-018-19146-y
- Zhao Z, Yin XN, Wang J, Chen X, Cai ZL, Zhang B. Prognostic significance of hemoglobin, albumin, lymphocyte, platelet in gastrointestinal stromal tumors: a propensity matched retrospective cohort study. World J Gastroenterol. 2022;28(27):3476–3487. doi:10.3748/wjg.v28.i27.3476
- Xu H, Zheng X, Ai J, Yang L. Hemoglobin, albumin, lymphocyte, and platelet (HALP) score and cancer prognosis: a systematic review and meta-analysis of 13,110 patients. Int Immunopharmacol. 2023;114:109496. doi:10.1016/j.intimp.2022.109496
- Zhou J, Yang D. Prognostic significance of Hemoglobin, Albumin, Lymphocyte and Platelet (HALP) Score in hepatocellular carcinoma. J Hepatocell Carcinoma. 2023;10:821–831. doi:10.2147/JHC.S411521
- Xia F, Huang Z, Zhang Q, et al. Clinically significant portal hypertension (CSPH) on early-stage HCC following hepatectomy: what’s the impact? Eur J Surg Oncol. 2023;49(4):771–779. doi:10.1016/j.ejso.2022.11.005
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi:10.1200/JCO.2014.57.9151
- Wu W, Xu W, Sun W, et al. Forced vital capacity predicts the survival of interstitial lung disease in anti-MDA5 positive dermatomyositis: a multi-centre cohort study. Rheumatology. 2021;61(1):230–239. doi:10.1093/rheumatology/keab305
- Bluthner E, Bednarsch J, Malinowski M, et al. Dynamic liver function is an independent predictor of recurrence-free survival after curative liver resection for HCC - A retrospective cohort study. Int J Surg. 2019;71:56–65. doi:10.1016/j.ijsu.2019.08.033
- Wei T, Zhang XF, Xue F, et al. Multi-institutional development and external validation of a nomogram for prediction of extrahepatic recurrence after curative-intent resection for hepatocellular carcinoma. Ann Surg Oncol. 2021;28(12):7624–7633. doi:10.1245/s10434-021-10142-7
- Qin S, Chen M, Cheng AL, et al. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023;402(10415):1835–1847. doi:10.1016/S0140-6736(23)01796-8
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
- Beumer BR, Buettner S, Galjart B, et al. Systematic review and meta-analysis of validated prognostic models for resected hepatocellular carcinoma patients. Eur J Surg Oncol. 2022;48(3):492–499. doi:10.1016/j.ejso.2021.09.012
- Chun YS, Pawlik TM, Vauthey JN. 8th edition of the AJCC cancer staging manual: pancreas and hepatobiliary cancers. Ann Surg Oncol. 2018;25(4):845–847. doi:10.1245/s10434-017-6025-x
- Pang S, Shi Y, Xu D, et al. Screening of hepatocellular carcinoma patients with high risk of early recurrence after radical hepatectomy using a nomogram model based on the gamma-glutamyl transpeptidase-to-albumin ratio. J Gastrointest Surg. 2022;26(8):1–9. doi:10.1007/s11605-022-05326-9
- Zhang XP, Chen ZH, Zhou TF, et al. A nomogram to predict early postoperative recurrence of hepatocellular carcinoma with portal vein tumour thrombus after R0 liver resection: a large-scale, multicenter study. Eur J Surg Oncol. 2019;45(9):1644–1651. doi:10.1016/j.ejso.2019.03.043
- Shi S, Zhao YX, Fan JL, Chang LY, Yu DX. Development and external validation of a nomogram including body composition parameters for predicting early recurrence of hepatocellular carcinoma after hepatectomy. Acad Radiol. 2023;30(12):2940–2953. doi:10.1016/j.acra.2023.05.022
- Toshida K, Itoh S, Kayashima H, et al. The hemoglobin, albumin, lymphocyte, and platelet score is a prognostic factor for Child-Pugh A patients undergoing curative hepatic resection for single and small hepatocellular carcinoma. Hepatol Res. 2023;53(6):522–530. doi:10.1111/hepr.13885
- Sargin ZG, Dusunceli I. The effect of HALP Score on the prognosis of gastric adenocarcinoma. J Coll Physicians Surg Pak. 2022;32(9):1154–1159.
- Feng JF, Wang L, Yang X. The preoperative hemoglobin, albumin, lymphocyte and platelet (HALP) score is a useful predictor in patients with resectable esophageal squamous cell carcinoma. Bosn J Basic Med Sci. 2021;21(6):773–781. doi:10.17305/bjbms.2021.5666
- Hu SJ, Zhao XK, Song X, et al. Preoperative maximal voluntary ventilation, hemoglobin, albumin, lymphocytes and platelets predict postoperative survival in esophageal squamous cell carcinoma. World J Gastroenterol. 2021;27(4):321–335. doi:10.3748/wjg.v27.i4.321
- Dagmura H, Daldal E, Okan I. The efficacy of hemoglobin, albumin, lymphocytes, and platelets as a prognostic marker for survival in octogenarians and nonagenarians undergoing colorectal cancer surgery. Cancer Biother Radiopharm. 2022;37(10):955–962. doi:10.1089/cbr.2020.4725
- Yalav O, Topal U, Unal AG, Eray IC. Prognostic significance of preoperative hemoglobin and albumin levels and lymphocyte and platelet counts (HALP) in patients undergoing curative resection for colorectal cancer. Ann Ital Chir. 2021;92:283–292.
- Zhang D, Zeng H, Pan Y, et al. Liver tumor markers, HALP score, and NLR: simple, cost-effective, easily accessible indexes for predicting prognosis in ICC patients after surgery. J Pers Med. 2022;12(12):2041. doi:10.3390/jpm12122041
- Toshida K, Itoh S, Nakayama Y, et al. Preoperative HALP score is a prognostic factor for intrahepatic cholangiocarcinoma patients undergoing curative hepatic resection: association with sarcopenia and immune microenvironment. Int J Clin Oncol. 2023;28(8):1082–1091. doi:10.1007/s10147-023-02358-2
- Marasco G, Poggioli F, Colecchia A, et al. A nomogram-based prognostic model for advanced hepatocellular carcinoma patients treated with sorafenib: a multicenter study. Cancers. 2021;13(11):2677. doi:10.3390/cancers13112677
- Unwith S, Zhao H, Hennah L, Ma D. The potential role of HIF on tumour progression and dissemination. Int J Cancer. 2015;136(11):2491–2503. doi:10.1002/ijc.28889
- Fu X, Yang Y, Zhang D. Molecular mechanism of albumin in suppressing invasion and metastasis of hepatocellular carcinoma. Liver Int. 2022;42(3):696–709. doi:10.1111/liv.15115
- Feng F, Zheng G, Wang Q, et al. Low lymphocyte count and high monocyte count predicts poor prognosis of gastric cancer. BMC Gastroenterol. 2018;18(1):148. doi:10.1186/s12876-018-0877-9
- Itoh S, Yugawa K, Shimokawa M, et al. Prognostic significance of inflammatory biomarkers in hepatocellular carcinoma following hepatic resection. BJS Open. 2019;3(4):500–508. doi:10.1002/bjs5.50170
- Wen S, Chen Y, Hu C, et al. Combination of tertiary lymphoid structure and neutrophil-to-lymphocyte ratio predicts survival in patients with hepatocellular carcinoma. Front Immunol. 2021;12:788640. doi:10.3389/fimmu.2021.788640
- Tada T, Kumada T, Hiraoka A, et al. Neutrophil-to-lymphocyte ratio is associated with survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib. Liver Int. 2020;40(4):968–976. doi:10.1111/liv.14405
- Honn KV, Tang DG, Crissman JD. Platelets and cancer metastasis: a causal relationship? Cancer Metastasis Rev. 1992;11(3–4):325–351. doi:10.1007/BF01307186
- Midorikawa Y, Takayama T, Higaki T, et al. High platelet count as a poor prognostic factor for liver cancer patients without cirrhosis. Biosci Trends. 2020;14(5):368–375. doi:10.5582/bst.2020.03230
- Zhang H, Wu J, Liu Y, et al. Identification reproducible microbiota biomarkers for the diagnosis of cirrhosis and hepatocellular carcinoma. AMB Express. 2023;13(1):35. doi:10.1186/s13568-023-01539-6
- Iwakiri Y, Trebicka J. Portal hypertension in cirrhosis: pathophysiological mechanisms and therapy. JHEP Rep. 2021;3(4):100316. doi:10.1016/j.jhepr.2021.100316
- Jothimani D, Rela M, Kamath PS. Liver cirrhosis and portal hypertension: how to deal with esophageal varices? Med Clin North Am. 2023;107(3):491–504. doi:10.1016/j.mcna.2023.01.002
- Berzigotti A, Reig M, Abraldes JG, Bosch J, Bruix J. Portal hypertension and the outcome of surgery for hepatocellular carcinoma in compensated cirrhosis: a systematic review and meta-analysis. Hepatology. 2015;61(2):526–536. doi:10.1002/hep.27431
- Cortese S, Tellado JM. Impact and outcomes of liver resection for hepatocellular carcinoma in patients with clinically significant portal hypertension. Cir Cir. 2022;90(5):579–587. doi:10.24875/CIRU.22000041
- Lopez-Lopez V, Brusadin R, Lopez-Conesa A, et al. Preoperative transarterial chemoembolization for laparoscopic liver resection in Child A cirrhotic patients with hepatocellular carcinoma. Langenbecks Arch Surg. 2021;406(3):763–771. doi:10.1007/s00423-020-02056-x
- Liu J, Zhang H, Xia Y, et al. Impact of clinically significant portal hypertension on outcomes after partial hepatectomy for hepatocellular carcinoma: a systematic review and meta-analysis. HPB. 2019;21(1):1–13. doi:10.1016/j.hpb.2018.07.005
- Qin L, Li C, Xie F, Wang Z, Wen T. Combination of albumin-bilirubin grade and clinically significant portal hypertension predicts the prognosis of patients with hepatocellular carcinoma after liver resection. Biosci Trends. 2021;15(1):41–49. doi:10.5582/bst.2021.01064
- Wang Q, Qiao W, Zhang H, et al. Nomogram established on account of Lasso-Cox regression for predicting recurrence in patients with early-stage hepatocellular carcinoma. Front Immunol. 2022;13:1019638. doi:10.3389/fimmu.2022.1019638
- Erstad DJ, Tanabe KK. Prognostic and therapeutic implications of microvascular invasion in hepatocellular carcinoma. Ann Surg Oncol. 2019;26(5):1474–1493. doi:10.1245/s10434-019-07227-9
- Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7(3):462–503. doi:10.1002/1097-0142(195405)7:3<462::AID-CNCR2820070308>3.0.CO;2-E
- Cong WM, Bu H, Chen J, et al. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol. 2016;22(42):9279–9287. doi:10.3748/wjg.v22.i42.9279
- Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232(1):10–24. doi:10.1097/00000658-200007000-00003
- Guo B, Chen Q, Liu Z, Chen X, Zhu P. Adjuvant therapy following curative treatments for hepatocellular carcinoma: current dilemmas and prospects. Front Oncol. 2023;13:1098958. doi:10.3389/fonc.2023.1098958