Abstract
Purpose
The aim of our study was to investigate the relationship between albumin-bilirubin (ALBI) grade and recurrence in patients who underwent TACE sequential ablation. We developed and validated a nomogram to predict low levels of ALBI patients’ recurrence.
Patients and Methods
A total of 880 patients undergoing TACE combined ablation at Beijing Youan Hospital from January 2014 to December 2021 were retrospectively enrolled, including 415 patients with L-ALBI (≤-2.6) and 465 patients with high levels (>-2.6) of ALBI (H-ALBI). L-ALBI patients were randomized in a 7:3 ratio into the training cohort (N=289) and validation cohort (N=126). Multivariate Cox regression followed by random survival forest was carried out to identify independent risk factors for prediction nomogram construction. An examination of nomogram accuracy was performed using the C-index, receiver operating characteristic (ROC), calibration curves, and decision curve analysis (DCA) curves. According to the nomogram, the patients were divided into low-risk, intermediate-risk, and high-risk groups. Kaplan-Meier (KM) curves were applied to compare the difference in recurrence-free survival (RFS) among the three groups.
Results
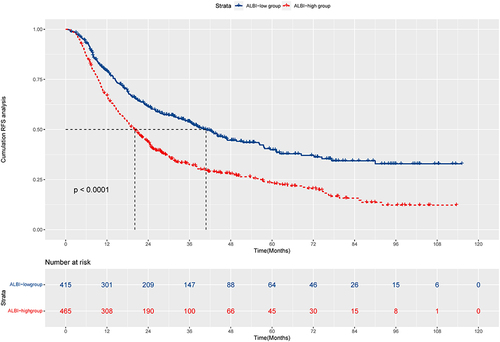
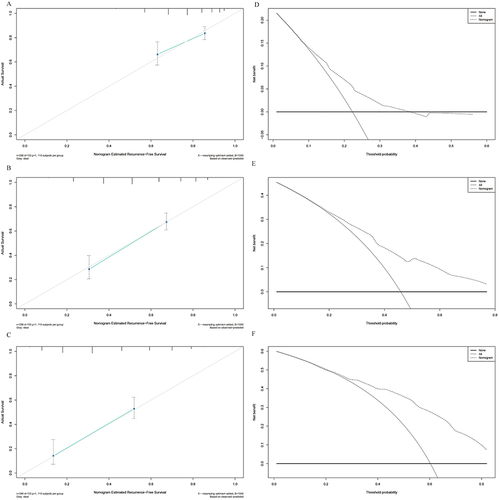
The median RFS in L-ALBI patients was significantly longer than the H-ALBI patients (40.8m vs 20.1m, HR:1.71, 95% CI:1.44–2.04, P<0.0001). The nomogram was composed of five variables, such as age, Barcelona Clinic Liver Cancer (BCLC) stage, globulin, gamma-glutamyl transferase to lymphocyte ratio (GLR), and international normalized ratio (INR). The C-index (0.722 and 0.731) and 1-, 3-, and 5-year AUCs (0.725, 0.803, 0.870, and 0.764, 0.816, 0.798) of the training and validation cohorts proved the good predictive performance of the nomogram. Calibration curves and DCA curves demonstrated good consistency and good clinical utility. There were significant differences in RFS between the low-risk, intermediate-risk, and high-risk groups (P<0.0001).
Conclusion
L-ALBI Patients who underwent TACE combined ablation had better recurrence-free survival than patients with H-ALBI. The nomogram developed and validated in our study had good predictive ability in recurrence for L-ALBI patients.
Introduction
Hepatocellular carcinoma (HCC) accounts for approximately 90% of liver cancer and is the sixth leading cause of cancer and the fourth leading cause of cancer-related mortality worldwide, which has caused an enormous burden on global health.Citation1,Citation2 Despite the decline in incidence, HCC remains the second most common cancer, with 36000 new cases reported annually.Citation3,Citation4 For HCC patients in the early-stage, curative treatments such as resection, ablation, and liver transplantation are recommended. Several studies have reported that the efficacy and safety of ablation therapy are comparable with surgery.Citation5 Still, disease recurrence after curative therapy remains an issue, and the 5-year recurrence rate of HCC patients after ablation is as high as 50–70%.Citation6 Transcatheter arterial chemoembolization (TACE) has become the first-choice therapy in patients with intermediate-stage HCC, but the median progression-free survival (PFS) is only five months.Citation7 Hence prognosis of HCC is still a problem of worldwide major concern.
In comparison with other solid cancers, the treatment and prognosis of HCC depend not only on the tumor biology but also on underlying liver function reserve.Citation8,Citation9 For decades, the Child-Pugh (CTP) classification system has been widely used to assess patient hepatic function. There have been studies that incorporate Child-Pugh into the nomogram achieved strong survival predictive accuracy.Citation10 However, clinical assessment of hepatic encephalopathy and ascites is limited by subjectivity.Citation11 Therefore, a scoring system for serum albumin and bilirubin was developed based on laboratory data from a large international patient cohort, which was simpler and more objective than the CTP classification system.Citation12,Citation13 The albumin-bilirubin (ALBI) grade was proven to be a reliable model for assessing liver function. Several studies have shown that ALBI grade is superior to CTP classification both in predicting the prognosis of hepatectomy, TACE, and Yi-90 radioembolization.Citation14–16 Even in patients with early-stage HCC, ALBI grade is associated with recurrence and long-term survival and is sensitive enough to determine outcome. In a recent meta-analysis that described 95 studies exploring the relationship between ALBI and HCC, Bannaga et al reported that ALBI grade was higher than CTP classification and AFP in predicting HCC survival.Citation17 Patients with the most favorable group (ALBI grade 1) had the highest 5-year OS rate of 77.9–88.5%. The OS rates of ALBI 2 and 3 HCC patients were 38.6%-73.8%.Citation18 Nevertheless, the predictive role of ALBI grade for HCC patients’ recurrence undergoing TACE combined with ablation needs to be further demonstrated.
Although the good predictive performance of ALBI, previous research suggested that the five-year recurrence rate remained high in patients with low levels of ALBI (L-ALBI).Citation19,Citation20 And postoperative monitoring of L-ALBI patients is easy to ignore because L-ALBI patients have a better prognosis than patients with high levels of ALBI (H-ALBI). Consequently, the aim of our study was to investigate the relationship between ALBI grade and recurrence in patients who underwent TACE sequential ablation. Finally, we developed and validated a nomogram for clinicians to predict L-ALBI patients’ recurrence and improve their treatment planning.
Methods and Materials
Patients Selection
This retrospective cohort study reviewed 880 HCC patients who underwent TACE combined with ablation at Beijing Youan Hospital from January 2014 to December 2021. HCC diagnosis was based on the guideline of the American Association for the Study of Liver Diseases (ASSLD).Citation2,Citation21 The HCC patients were comprised of 415 individuals with low levels (≤-2.6) of ALBI (L-ALBI) and 465 individuals with high levels (>-2.6) of individuals (H-ALBI). L-ALBI patients were randomized in a 7:3 ratio into the training cohort (N=289) and the validation cohort (N=126). The inclusion criteria were like following: (1) Aged 18–75 years. (2) Received TACE combined with ablation and achieved complete ablation. (3) Child-Pugh classification A or B. (4) did not have extrahepatic metastasis or vascular invasion. The exclusion criteria were as follows: (1) received other anti-tumor treatments before ablation. (2) with second primary malignant tumors. (3) clinical follow-up data incomplete.
Our research was approved by the Medical Ethics Committee of Beijing Youan Hospital and conducted in accordance with the Helsinki Declaration. The requirement for informed consent was waived by the Ethics Commission because the study was based on deidentified data.
Clinicopathologic Characteristics
The demographic and clinicopathological characteristics were collected retrospectively and analyzed. Demographics included age, gender, hypertension, and diabetes. Clinicopathological data was composed of tumor size, tumor number, hemoglobin (Hb), alanine transaminase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), direct bilirubin (DBIL), γ-glutamyl transpeptidase (GGT), albumin (ALB), globulin, des-gamma-carboxyprothrombin (DCP), neutrophil-to-lymphocyte ratio (NLR), prealbumin (Palb), platelet-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR), and gamma-glutamyl transferase to lymphocyte ratio (GLR). The formula for the ALBI score was as follows: (log10 TBIL [µmol/L] × 0.66) − (albumin [g/L] × 0.085).Citation12
Therapeutic Procedure
All patients were treated with TACE by two interventional radiologists with more than 5 years of experience. Detailed treatment procedures have been described in previous studies and will not be described in this study. Ablation was performed after TACE within 2 weeks by radiofrequency (RFA) and microwave (MWA). The ablation range completely covered the tumor to the edge of 0.5–1.0cm to prevent marginal residue and recurrence. The detailed protocol of ablation was described in our previous study. Contrast-enhanced CT/MRI was provided to evaluate the tumor response after one month.
Follow-Up
All patients were followed up every three months for the first year, and then every six months thereafter in the outpatient clinic. The contents of follow-ups included liver function, blood tests, and imaging examinations to monitor recurrence. Recurrence was defined as the appearance of a new enhanced lesion with radiographic features. Recurrence-free survival (RFS) was defined as the time from initial treatment to the diagnosis to relapse.
Statistical Analysis
Categorical variables were expressed as number (percentage) and continuous variables were presented as mean±standard deviations. Differences between groups were compared by chi-square tests, t-test, and Mann–Whitney test. Survival was estimated by the Kaplan-Meier (KM) curve with the Log rank test. The significance of variables for RFS which were used to construct the nomogram was analyzed by multivariate Cox regression analysis followed by the random survival forest. The prediction ability of the nomogram was assessed by the area under the receiver operating characteristic (ROC) curves (AUCs). The calibration curve and decision curve analysis (DCA) were evaluated to test the calibration performance and clinical utility. Based on the nomogram risk score, patients were categorized into low-risk, medium-risk, and high-risk groups, and the KM curves were generated.
All analyses were performed by SPSS (version 26.0, IBM, Armonk, NY, USA) and R software (version 4.1.3). P-values less than 0.05 were considered to be statistically significant (two-tailed tests).
Result
Baseline Characteristics
A total of 880 HCC patients submitted to TACE combined with ablation in Beijing Youan Hospital were enrolled in our study, including 706 (80.2%) males and 174 (19.8%) females. At the time of diagnosis, 221 (25.1%) patients had hypertension, and 184 (20.9%) patients had diabetes. There were 271 (30.8%) patients with multiple tumor numbers and 312 (35.5%) patients with large tumors (≥30mm). The 880 patients included 415 (46.1%) L-ALBI patients and 465 (53.9%) H-ALBI patients ().
Table 1 Demographics and Clinical Characteristics for HCC Patients
We randomly allocated L-ALBI patients into a training cohort (N=289) and a validation cohort (N=126), in a 7:3 radio. The clinicopathological features were similar between the training cohort and the validation cohort (). In the two cohorts, the majority of the patients were male (84.4% vs.80.2%, P=0.355) and the average was over 50 years (55.5±9.53 vs.55.9±9.10, P=0.504). BCLC A had the highest percentage of patients (51.6% vs.55.6%, P=0.746). For characteristics of the tumor, most tumors were solitary (72.0% vs.73.8%, P=0.907) and tumor size was less than 30mm (67.8% vs.66.7%, P=0.790).
Table 2 Demographics and Clinical Characteristics for Training and Validation Sets
The Efficacy of ALBI in HCC Patients Undergoing TACE Combined with Ablation
At July 30, 2023, median follow-up times was 44.1 months in our study. The KM curves showed the mRFS in L-ALBI patients was significantly longer than in H-ALBI patients (40.8m vs.20.1m, P<0.001, HR:1.71, 95% CI:1.44–2.04, ). The 1-, 3-, and 5-year RFS rates of L-ALBI patients were 79.2%, 54.0%, and 39.8%, which were higher than H-ALBI patients (1-year: 67.1%; 3-year: 32.4%; 5-year: 23.4%). Although the L-ALBI patients had a better prognosis, the recurrence remained high and this group of patients was more likely to be overlooked. Therefore, it is necessary to establish a nomogram for L-ALBI patients to predict the recurrence after ablation.
Significant Variables of RFS of HCC
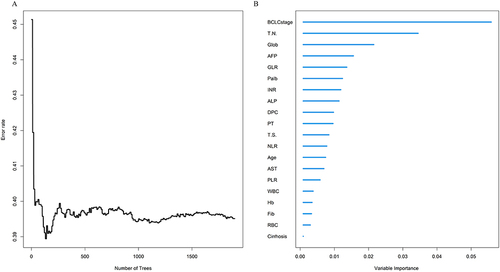
The variables were used to build an RFS model in the training cohort. With the increase in the number of random survival forest, the prediction error rate decreased significantly; The error rates tended to be stable when the number of tree was more than 600 (). According to the VIMP method, the importance of variables was ranked, in the order of age, cirrhosis, DCP, BCLC stage, tumor number, tumor size, WBC, NLR, RBC, Hb, PLR, AST, Palb, globulin, GLR, ALP, PT, INR, AFP and Fibrinogen (Fib) (). Based on the Results of the random survival forest, multivariate survival analysis was performed to reveal the recurrence-related factors (). The final results obtained were age (HR: 1.03, 95% CI: 1.01–1.04), BCLC stage (HR: 1.67, 95% CI: 1.33–2.09), globulin (HR: 1.04, 95% CI: 1.01–1.08), INR (HR: 11.23, 95% CI: 2.12–59.11) and GLR (HR: 1.00, 95% CI: 1.00–1.01).
Table 3 Multivariate Cox Proportional Hazards Regression to Predict Recurrence Based on Random Survival Forest and Multivariate Cox Regression
Figure 2 Screening of variables based on random survival forest. (A) Error rate of random survival rate forest; (B) out-of-bag variable importance ranking.
Nomogram Construction
The nomogram was constructed based on the prognostic factors identified by the multivariate Cox regression (). The C-index was 0.722 (95% CI: 0.68–0.76) and the 1-, 3-, and 5-year AUCs were 0.725, 0.803, and 0.870 in the training cohort (). The calibration curves depicted that the predicted outcome was broadly consistent with the actual outcome (). Moreover, the decision curve analysis (DCA) testified that the nomogram had a high net benefit ().
Figure 3 Nomogram, including Age, globulin, GLR, BCLC stage, and INR for 1-, 3-, and 5-year RFS in HCC patients with low levels of ALBI. The nomogram is valued to obtain the probability of 1-, 3-, and 5- years recurrence by adding up the points identified on the points scale for each variable.
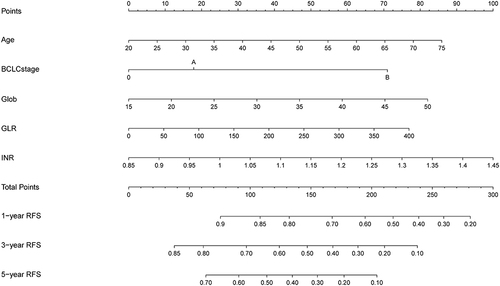
Figure 4 1-, 3-, and 5-year ROC curves of the nomogram in the training cohort.
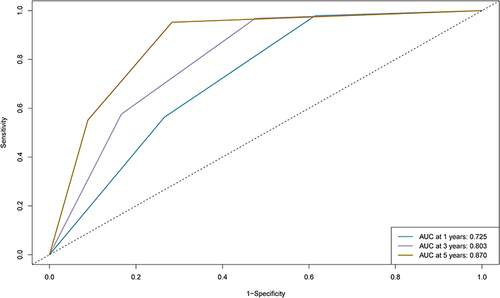
Figure 5 Calibration curves and DCA curves of the nomogram in the training cohort. (A) 1-year calibration curves of the nomogram in the training cohort. (B) 3-year calibration curves of the nomogram in the training cohort. (C) 5-year calibration curves of the nomogram in the training cohort. (D) 1-year DCA curves of the nomogram in the training cohort. (E) 3-year DCA curves of the nomogram in the training cohort. (F) 5-year DCA curves of the nomogram in the training cohort.
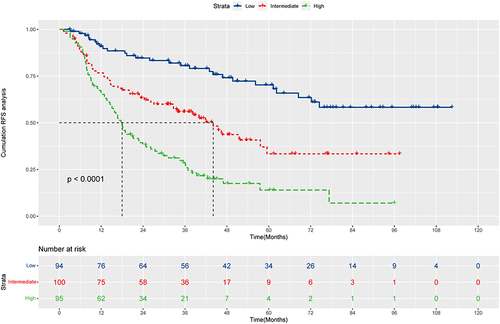
We calculated the risk scores of HCC patients and divided them into low-risk (N=94), intermediate-risk (N=100), and high-risk (N=95) groups. And there were apparent variances in RFS between the low-risk, intermediate-risk, and high-risk groups (P<0.0001, ). The median RFS was not reached for the low-risk group with 1-, 3-, and 5-year RFS rates of 90.9%, 80.6%, and 70.3%, respectively. The intermediate-risk group had a median RFS of 44.1 months (95% CI: 32.6–74.5m) with 1-, 3-, and 5-year RFS rates of 76.7%, 55.9%, and 33.4%, respectively. The high-risk group had a median RFS of 18.0 months (95% CI: 14.9–23.9m) with 1-, 3-, and 5-year RFS rates of 66.3%, 28.6%, and 14.0%, respectively.
Validate of Nomogram
To validate the performance of the resulting nomogram, we performed internal validation by using an independent validation cohort. In the validation cohort, the C-index was 0.731 (95% CI: 0.67–0.78), with the 1-, 3-, and 5-year AUCs of 0.764, 0.816, and 0.798, and these indicated the validity of the nomogram in predicting RFS (Figure S1). Besides, the calibration curves revealed optimal agreement between the nomogram and the observation and the DCA curves also showed good clinical utility (Figure S2). Patients in the validation cohort were similarly categorized into low-risk (N=48), medium-risk (N=37), and high-risk (N=41) groups, and the KM curves of the three groups were significantly different (P<0.0001, Figure S3). In the validation cohort, the median RFS was not reached for the low-risk group with 1-, 3-, and 5-year RFS rates of 92.6%, 82.2%, and 62.7%, respectively. The intermediate-risk group had a median RFS of 47.8 months (95% CI: 29.2m-NA) with 1-, 3-, and 5-year RFS rates of 86.3%, 49.1%, and 35.8%, respectively. The high-risk group had a median RFS of 23.1 months (95% CI: 15.6–38.7m) with 1-, 3-, and 5-year RFS rates of 68.8%, 26.5%, and 21.2%, respectively.
Discussion
Morbidity and mortality caused by HCC continue to be substantial worldwide.Citation4,Citation22 Originally developed to evaluate liver function in patients with HCC, the ALBI grade was developed.Citation19 In our study, we found that L-ALBI patients had a better prognosis than H-ALBI patients treated with TACE sequential ablation (P<0.0001). A nomogram was created and validated to predict recurrence for L-ALBI patients.
The L-ALBI group had a median RFS of up to 40.8 months. But the recurrence rates at 1-, 3-, and 5-year were 79.2%, 53.9%, and 39.8%, respectively, so the recurrence rate remains high. Besides, L-ALBI patients are more likely to be ignored because of the better prognosis. It is necessary to create a nomogram to accurately predict recurrence in this group of patients. In a ratio of 7:3, we divide the L-ALBI patients into the training cohort and the validation cohort in the context of the advantage of eight years of follow-up. The random survival forest and multivariate Cox proportional hazards regression model were performed to screen prognostic variables for RFS in the training cohort. Random survival forest, a machine learning, takes into account the interactions between variables compared to the univariate Cox regression. According to the accurate screening, a nomogram was established to predict the relapse in L-ALBI patients. The nomogram is used by converting the corresponding predictor value into the corresponding nomogram score and then adding the score value, and the 1-, 3-, and 5-year RFS rates are obtained from the values shown at the intersection points. The C-index, ROC curve, calibration curve, and DCA curve revealed the good predictive performance of the nomogram. Based on the nomogram, patients can be accurately stratified into low-risk, medium-risk, and high-risk groups. In addition, we used an independent validation cohort to internally validate the nomogram, and the results further proved the reliability. With the help of this well-established nomogram, clinicians would be able to make more individualized treatments, control modifiable risk factors, and frequent follow-ups. Therefore, the nomogram can help reduce the recurrence rates and improve the survival rates of patients.
The nomogram includes age, BCLC stage, globulin, GLR, and INR. Several studies have shown that liver weight and portal blood flow velocity are reduced in older patients, which can lead to the liver that is less repairable than in younger patients.Citation23 Due to the low immunity in the elderly, tumor progression after treatment is faster than in younger patients, leading to a high rate of recurrence and poor prognosis.Citation24 The BCLC stage, which integrates factors such as liver function, tumor load, and physical condition, is the most widely used staging system for HCC. There is a great deal of heterogeneity among tumors at different stages, and both OS and RFS are related to the stage of BCLC.Citation25,Citation26 The lymphocytes of the immune system play a critical role in the immune response of the body and contain potent antitumor properties.Citation27 GGT, a cell surface enzyme, has been shown to be a marker for several cancers. In the course of the destruction of hepatocytes, GGT in cells is released into the blood, resulting in an increased concentration of GGT.Citation28 The increased GLR was independently associated with the poor prognosis of HCC patients and can be a potential indicator of early recurrence.Citation29–31 INR is an important index of liver synthetic function. As the severity of liver disease worsens, the production of VII factors is also decreased. INR is also recommended for assessing survival in patients with severe liver disease and is included in the MELD score. A study suggested that the high INR to albumin ratio is an independent risk factor for worse DFS and OS in patients with surgically treated HCC.Citation32 Globulins consist of a variety of pro-inflammatory proteins, including C-reactive protein, α2-macroglobulin, prothrombin, fibrinogen, and serum amyloid A.Citation33 Primary metabolism of human immunoglobulins is carried out by the liver, and individuals with severe hepatic insufficiency experience decreased immunoglobulin clearance, leading to hyperglobulinemia. A malignancy patient’s poor clinical outcome can be attributed to inflammation, which alters tumor cell biological characteristics and destroys immune function.Citation34,Citation35
Different treatment modalities of HCC have demonstrated prognostic value of ALBI grade, including liver resection, TACE, and systemic treatment.Citation36–39 Our study confirmed that ALBI grade could also predict the prognosis of patients with HCC patients underwent TACE combined with ablation. The ALBI score is calculated from objective factors of serum albumin and bilirubin and is based on statistical evidence rather than clinical observation. It was previously illustrated that ALBI grade was more effective than Milan criteria in predicting recurrence after ablation in HCC patients.Citation40 The incidence of HCC markedly increased with low serum albumin levels.Citation41 In a basic study, albumin inhibited the growth and invasion of HCC cells.Citation42 Despite these strengths, the ALBI grade still has limitations. Recurrence rates remained higher in the L-ALBI group, and ALBI alone was not sufficient to predict relapse in HCC patients. The efficacy of surveillance will be decreased because of the better prognosis in the L-ALBI group. Thereby, models for risk assessment in individual patients are still needed.
The BCLC guidelines for the treatment of HCC recommend TACE for patients with intermediate-stage HCC. For early-stage HCC, TACE is able to mark tumors that are not clearly visible on imaging and reduce tumor size by embolizing tumor vessels, shortening ablation time, and increasing the success rate of ablation.Citation27 The results of several studies indicated improved OS and RFS when TACE and ablation were used in combination for the treatment of HCC, compared with TACE alone.Citation43–45 On that account, we chose TACE sequential ablation, and further prospective multicenter studies are needed to confirm the efficacy.
However, there were some limitations in our research. Firstly, it was a single-center study, which should be followed up by multicenter studies in the future. Furthermore, as the current study was a retrospective study, there may have been unavoidable selection bias. Nevertheless, we used a follow-up period of up to eight years to explore the impact of ALBI levels in the prognosis of HCC patients after sequential ablation with TACE and created an accurate and reliable nomogram to better guide clinical practice for L-ALBI patients with HCC.
Conclusion
In summary, Patients with low levels of ALBI who underwent TACE combined with ablation had better recurrence-free survival than patients with high levels of ALBI. However, the rates of 1-, 3-, and 5-year RFS rates in L-ALBI patients were 79.2%, 54.0%, and 39.8%, which remained high. Therefore, we created an accurate and reliable nomogram to predict recurrence for L-ALBI patients based on random survival forest and multivariate Cox regression. The nomogram, including age, BCLC stage, GLR, globulin, and INR, demonstrated adequate discrimination ability, which could better guide clinical decisions.
Ethics Approval and Consent to Participate
The study protocol was approved by the Ethics Committee of Beijing You ‘an Hospital and complied with the requirements of the Declaration of Helsinki. As a retrospective study, the requirement for patient written informed consent was waived.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The authors highly appreciate all patients who participated in the study.
Data Sharing Statement
Data to support the study findings are available on request from the corresponding author.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
- Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345–1362. doi:10.1016/s0140-6736(22)01200-4
- Xia C, Dong X, Li H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J. 2022;135(5):584–590. doi:10.1097/cm9.0000000000002108
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
- Bai XM, Cui M, Yang W, et al. The 10-year survival analysis of radiofrequency ablation for solitary hepatocellular carcinoma 5 cm or smaller: primary versus recurrent HCC. Radiology. 2021;300(2):458–469. doi:10.1148/radiol.2021200153
- Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. doi:10.1038/s41572-020-00240-3
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
- Lin PT, Teng W, Jeng WJ, et al. Dynamic change of albumin-bilirubin score is good predictive parameter for prognosis in chronic hepatitis C-hepatocellular carcinoma patients receiving transarterial chemoembolization. Diagnostics. 2022;12(3):1.
- Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(5 Suppl 1):S35–50. doi:10.1053/j.gastro.2004.09.014
- Su K, Shen Q, Tong J, et al. Construction and validation of a nomogram for HBV-related hepatocellular carcinoma: a large, multicenter study. Ann Hepatol. 2023;28(4):101109. doi:10.1016/j.aohep.2023.101109
- Durand F, Valla D. Assessment of prognosis of cirrhosis. Semin Liver Dis. 2008;28(1):110–122. doi:10.1055/s-2008-1040325
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi:10.1200/jco.2014.57.9151
- Kuo YH, Wang JH, Hung CH, et al. Albumin-Bilirubin grade predicts prognosis of HCC patients with sorafenib use. J Gastroenterol Hepatol. 2017;32(12):1975–1981. doi:10.1111/jgh.13783
- Wang YY, Zhong JH, Su ZY, et al. Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br J Surg. 2016;103(6):725–734. doi:10.1002/bjs.10095
- Gui B, Weiner AA, Nosher J, et al. Assessment of the Albumin-Bilirubin (ALBI) grade as a prognostic indicator for hepatocellular carcinoma patients treated with radioembolization. Am J Clin Oncol. 2018;41(9):861–866. doi:10.1097/coc.0000000000000384
- Mishra G, Majeed A, Dev A, et al. Clinical utility of albumin bilirubin grade as a prognostic marker in patients with hepatocellular carcinoma undergoing transarterial chemoembolization: a systematic review and meta-analysis. J Gastrointest Cancer. 2023;54(2):420–432. doi:10.1007/s12029-022-00832-0
- Bannaga A, Arasaradnam RP. Neutrophil to lymphocyte ratio and albumin bilirubin grade in hepatocellular carcinoma: a systematic review. World J Gastroenterol. 2020;26(33):5022–5049. doi:10.3748/wjg.v26.i33.5022
- Demirtas CO, D’Alessio A, Rimassa L, Sharma R, Pinato DJ. ALBI grade: evidence for an improved model for liver functional estimation in patients with hepatocellular carcinoma. JHEP Rep. 2021;3(5):100347. doi:10.1016/j.jhepr.2021.100347
- Toyoda H, Johnson PJ. The ALBI score: from liver function in patients with HCC to a general measure of liver function. JHEP Rep. 2022;4(10):100557. doi:10.1016/j.jhepr.2022.100557
- Long H, Xie X, Huang G, Huang T, Xie X, Liu B. Prognostic role of albumin-bilirubin grade in hepatocellular carcinoma after ultrasound-guided percutaneous radiofrequency ablation: a single-center experience over a decade. Surg Laparosc Endosc Percutan Tech. 2022;32(3):350–356. doi:10.1097/sle.0000000000001049
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
- Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun. 2021;41(10):1037–1048. doi:10.1002/cac2.12197
- Schmucker DL. Aging and the liver: an update. J Gerontol a Biol Sci Med Sci. 1998;53(5):B315–20. doi:10.1093/gerona/53a.5.b315
- Wang Q, Qiao W, Zhang H, et al. Nomogram established on account of Lasso-Cox regression for predicting recurrence in patients with early-stage hepatocellular carcinoma. Front Immunol. 2022;13:1019638. doi:10.3389/fimmu.2022.1019638
- Ho MC, Hasegawa K, Chen XP, et al. Surgery for intermediate and advanced hepatocellular carcinoma: a consensus report from the 5th Asia-pacific primary liver cancer expert meeting (APPLE 2014). Liver Cancer. 2016;5(4):245–256. doi:10.1159/000449336
- Qiao W, Fan Z, Wang Q, Jin R, Hu C. Development and Validation of a Nomogram to Predict the Recurrence of HCC Patients Undergoing CECT After Ablation. J Hepatocell Carcinoma. 2024;11:65–79. doi:10.2147/jhc.S441540
- Wang Q, Qiao W, Liu B, et al. The monocyte to lymphocyte ratio not only at baseline but also at relapse predicts poor outcomes in patients with hepatocellular carcinoma receiving locoregional therapy. BMC Gastroenterol. 2022;22(1):98. doi:10.1186/s12876-022-02180-6
- Zhao Z, Zhu Y, Ni X, et al. Serum GGT/ALT ratio predicts vascular invasion in HBV-related HCC. Cancer Cell Int. 2021;21(1):517. doi:10.1186/s12935-021-02214-1
- Li S, Xu W, Liao M, et al. The significance of gamma-glutamyl transpeptidase to lymphocyte count ratio in the early postoperative recurrence monitoring and prognosis prediction of AFP-negative hepatocellular carcinoma. J Hepatocell Carcinoma. 2021;8:23–33. doi:10.2147/jhc.S286213
- Zhao H, Liu X, Xu R, Guo X, Shen A. Predictive value of and relationship between the gamma-glutamyl transpeptidase to lymphocyte ratio and CT features in hepatocellular carcinoma patients with postoperative adjuvant TACE. J buon. 2021;26(4):1346–1354.
- Zhang W, Bi Y, Yang K, et al. A new model based on gamma-glutamyl transpeptidase to lymphocyte ratio and systemic immune-inflammation index can effectively predict the recurrence of hepatocellular carcinoma after liver transplantation. Front Oncol. 2023;13:1178123. doi:10.3389/fonc.2023.1178123
- Haruki K, Shiba H, Saito N, et al. Risk stratification using a novel liver functional reserve score of combination prothrombin time-international normalized ratio to albumin ratio and albumin in patients with hepatocellular carcinoma. Surgery. 2018;164(3):404–410. doi:10.1016/j.surg.2018.02.022
- Deng Y, Pang Q, Miao RC, et al. Prognostic significance of pretreatment albumin/globulin ratio in patients with hepatocellular carcinoma. Onco Targets Ther. 2016;9:5317–5328. doi:10.2147/ott.S109736
- Wang S, Deng Y, Yu X, et al. Prognostic significance of preoperative systemic inflammatory biomarkers in patients with hepatocellular carcinoma after microwave ablation and establishment of a nomogram. Sci Rep. 2021;11(1):13814. doi:10.1038/s41598-021-93289-3
- Li J, Li Z, Hao S, et al. Inversed albumin-to-globulin ratio and underlying liver disease severity as a prognostic factor for survival in hepatocellular carcinoma patients undergoing transarterial chemoembolization. Diagn Interv Radiol. 2023;29(3):520–528. doi:10.5152/dir.2022.211166
- Zhao S, Wang M, Yang Z, et al. Comparison between child-Pugh score and albumin-bilirubin grade in the prognosis of patients with HCC after liver resection using time-dependent ROC. Ann Transl Med. 2020;8(8):539. doi:10.21037/atm.2020.02.85
- Xu W, Li R, Liu F. Novel prognostic nomograms for predicting early and late recurrence of hepatocellular carcinoma after curative hepatectomy. Cancer Manag Res. 2020;12:1693–1712. doi:10.2147/cmar.S241959
- Chen PC, Chiu NC, Su CW, et al. Albumin-bilirubin grade may determine the outcomes of patients with very early stage hepatocellular carcinoma after radiofrequency ablation therapy. J Chin Med Assoc. 2019;82(1):2–10. doi:10.1097/jcma.0000000000000001
- Ni JY, Fang ZT, Sun HL, et al. A nomogram to predict survival of patients with intermediate-stage hepatocellular carcinoma after transarterial chemoembolization combined with microwave ablation. Eur Radiol. 2020;30(4):2377–2390. doi:10.1007/s00330-019-06438-8
- Zhang J, Zhao L, Zhou Y, Ding J, Zhang Q, Jing X. The comparison between albumin-bilirubin grade and Child-Pugh grade for assessing the prognosis of hepatocellular carcinoma after thermal ablation: a propensity score-matched analysis. Transl Cancer Res. 2022;11(8):2523–2535. doi:10.21037/tcr-22-244
- Carr BI, Guerra V. Serum albumin levels in relation to tumor parameters in hepatocellular carcinoma patients. Int J Biol Markers. 2017;32(4):e391–e396. doi:10.5301/ijbm.5000300
- Bağırsakçı E, Şahin E, Atabey N, Erdal E, Guerra V, Carr BI. Role of albumin in growth inhibition in hepatocellular carcinoma. Oncology. 2017;93(2):136–142. doi:10.1159/000471807
- Peng ZW, Zhang YJ, Liang HH, Lin XJ, Guo RP, Chen MS. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: a prospective randomized trial. Radiology. 2012;262(2):689–700. doi:10.1148/radiol.11110637
- Zhang YJ, Chen MS, Chen Y, Lau WY, Peng Z. Long-term outcomes of transcatheter arterial chemoembolization combined with radiofrequency ablation as an initial treatment for early-stage hepatocellular carcinoma. JAMA Network Open. 2021;4(9):e2126992. doi:10.1001/jamanetworkopen.2021.26992
- Endo K, Kuroda H, Oikawa T, et al. Efficacy of combination therapy with transcatheter arterial chemoembolization and radiofrequency ablation for intermediate-stage hepatocellular carcinoma. Scand J Gastroenterol. 2018;53(12):1575–1583. doi:10.1080/00365521.2018.1548645