Abstract
Purpose
To observe and assess the efficacy and safety of donafenib combined with transarterial chemoembolization (TACE) to treat unresectable hepatocellular carcinoma (HCC).
Patients and Methods
This prospective, single-arm, single-center, phase II clinical study enrolled 36 patients with initial unresectable HCC who had not undergone any systemic treatment. The patients received donafenib plus TACE (n = 26) or donafenib plus TACE plus programmed death receptor 1 inhibitors (n = 10). The primary endpoint was short-term efficacy, with secondary endpoints including progression-free survival (PFS), time to response (TTR), disease control rate (DCR), and adverse events. The tumor feeding artery diameter was also measured.
Results
Efficacy evaluation of all 36 patients revealed 6 cases of complete response, 19 of partial response, 8 of stable disease, and 3 of progressive disease. Six (16.7%) patients successfully underwent conversion surgery, all achieving R0 resection, and 2 (5.6%) achieved a complete pathological response. The objective response rate (ORR) was 69.4% and the DCR was 91.7%. The median PFS was 10.7 months, the median overall survival was not reached, and the median TTR was 1.4 months. The median survival rates at 6, 12, and 18 months were 85.0%, 77.6%, and 71.3%, respectively. The median PFS rates at 6, 12, and 18 months were 65.3%, 45.6%, and 34.2%, respectively. Treatment-related adverse events (TRAEs) occurred in all 25 subjects, including 4 (11.3%) grade 3 TRAEs. No grade 4 or 5 TRAEs occurred. The tumor feeding artery diameter was significantly decreased following treatment (P = 0.036). Multivariable analysis revealed the sum of baseline target lesion diameters, best tumor response, and combined immunotherapy as independent predictors of PFS.
Conclusion
TACE plus donafenib reduced the tumor feeding artery diameter in patients with unresectable HCC. The safety profile was good, and a high ORR was achieved.
Introduction
Hepatocellular carcinoma (HCC) accounts for 75–85% of primary liver cancer cases and is a major cause of cancer-related deaths worldwide, seriously threatening human health.Citation1,Citation2 Great advancements in the treatment modes for HCC have been made in recent years, and factors such as the patient body strength, degree of cirrhosis, tumor load, and cancer stage require full consideration. Surgical resection, liver transplantation, local ablation, and other treatments are generally used for early HCC, with local and systemic treatments mainly reserved for advanced HCC. Comprehensive treatment further improves the curative effect and prolongs patient survival.Citation3,Citation4 However, for patients with advanced HCC who are unsuitable or refuse treatment, the main local treatment method is transarterial chemoembolization (TACE), which significantly improves the outcomes of patients with Barcelona Clinic Liver Cancer (BCLC) stage B and some with stage C.Citation5–7
In recent years, targeted therapy, immunotherapy, and other innovative methods have provided new treatment options for patients with advanced liver cancer, and these have become the standard first-line treatment for patients with advanced HCC.Citation8–11 Donafenib, an oral small-molecule multitarget tyrosine kinase inhibitor (TKI), is an improved form of sorafenib, with significantly enhanced molecular stability and improved pharmacokinetics.Citation12 It is also the only single-agent targeted drug superior to sorafenib in first-line head-to-head studies on advanced HCC. The positive results of the ZGDH3 trial, which showed improved overall survival (OS) compared with sorafenib, led to the approval of donafenib for treating unresectable or metastatic HCC.Citation3 Like sorafenib, lenvatinib, and atilizumab plus bevacizumab, donafenib is recommended as first-line systemic therapy for patients with unresectable HCC.Citation13
With the advent of the immune era, the use of systemic therapies, such as TKIsCitation9 and immune checkpoint inhibitors (ICIs),Citation14,Citation15 have shown good effects in HCC treatment. Compared with a single-agent treatment, combination therapy shows a better trend in tumor response rate, survival outcome, and transformation resection in patients with advanced liver cancer due to the combination of the different antitumor mechanisms of TACE, TKIs, and immune checkpoint inhibitors, which may exert synergistic and toxic effects.Citation16–18 To the best of our knowledge, there are few clinical studies on TACE combined with donafenib with or without immune checkpoint inhibitors in the treatment of advanced liver cancer. Thus, this real-world study aimed to evaluate the efficacy and safety of donafenib combined with TACE with or without immune checkpoint inhibitors in the treatment of advanced HCC with a view to providing novel therapeutic approaches for clinical diagnosis and treatment.
Materials and Methods
Patient Selection
This prospective, single-arm, single-center phase II clinical trial was initiated by the researcher. The study subjects were selected from patients at the Affiliated Tumor Hospital of Shandong First Medical University.This study was conducted in accordance with the Declaration of Helsinki. The study received approval from the Ethics Committee of Affiliated Tumor Hospital of Shandong First Medical University, and all patients provided informed consent.After discussion by the multidisciplinary team (MDT), it was deemed unresectable (unresectable was defined as fulfilling any of the following conditions: large tumor; multiple tumors involving both the left and right lobes of the liver; invasion of major blood vessels such as the main portal vein and inferior vena cava; the patient was unable to tolerate surgical treatment; there were extrahepatic metastatic lesions).Citation19
The inclusion criteria were as follows: (1) patients with liver cancer who strictly met the clinical diagnostic criteria for primary liver cancer as per the Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition)Citation20 or were diagnosed by histopathology or cytology, (2) patients who received TACE plus donafenib or TACE plus donafenib with immune checkpoint inhibitors as the first-line treatment, (3) patients aged 18–75 years with a survival time >3 months, (4) patients with an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–1 before TACE and Child–Pugh grade A or B, and (5) patients with least one measurable lesion.
The exclusion criteria were as follows: (1) previous systemic antitumor therapy, (2) diffuse liver cancer, (3) intractable hepatic encephalopathy, refractory ascites, or hepatorenal syndrome, (4) previous tumor history, (5) and (5) incomplete data.
Treatment Protocol
According to the judgment of the investigator, TACE was administered conventionally or using drug-eluting beads. The administration of multiple TACE treatments depended on evidence of viable tumors or intrapathological recurrence as observed by contrast-enhanced computed tomography or magnetic resonance imaging.
Donafenib was administered orally 3 days after TACE at an initial dose of 200 mg twice daily. A immune checkpoint inhibitors (carrelizumab or tislelizumab: 200 mg, once every 3 weeks, 1 cycle) was administered within 1–2 weeks after TACE treatment. If patients experienced side effects during the use of immune checkpoint inhibitors and donafenib, the dose was reduced or drug administration was suspended or stopped. If donafenib-related adverse reactions could be tolerated, the dose was adjusted to 200 mg/day.
Evaluation of Treatment Response and Follow-Up
All patients were regularly followed up at intervals of 4–6 weeks after the first treatment until reaching the OS endpoint or the last follow-up date (December 31, 2023).
All patients underwent a comprehensive examination before and after treatment, including tests for serum tumor markers, an electrocardiogram, and an examination of the chest, abdomen, and pelvis. Routine blood analysis, blood biochemistry, urine, stool, and liver and kidney function tests were performed before each treatment cycle. Tumor responses were evaluated by two radiologists, both with >10 years’ experience, using the modified Response Evaluation Criteria in Solid Tumors (mRECIST)Citation21 to compare tumor response between the two groups, including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The objective response rate (ORR) and disease control rate (DCR) were also assessed. Progression-free survival (PFS) was defined as the time between the first TACE treatment and the date of disease progression or death.Citation21 OS was defined as the time from the first TACE treatment to death from any cause or the date of the last follow-up.Citation21 Treatment-related adverse events (TRAEs) were assessed using Common Terminology Criteria for Adverse Events (version 5.0).Citation22 The occurrence of common adverse events (AEs), such as hand–foot syndrome, rash, fatigue, diarrhea, hypertension, alopecia, and gastrointestinal bleeding, as well as any other AEs, were recorded.
Statistical Methods
Data were analyzed using SPSS version 25.0 statistical software (IBM Corp., Armonk, NY, USA). Continuous data were presented as the mean ± standard deviation, and comparisons between the two groups were performed by t-test. Categorical data were compared using chi-square test or Fisher exact test. The Kaplan–Meier method was used to draw survival curves, and log rank tests were used to compare the differences in survival between the two groups. The prognostic risk factors of OS and PFS were analyzed by Cox regression. P-values <0.05 were considered statistically significant.
Results
Patients
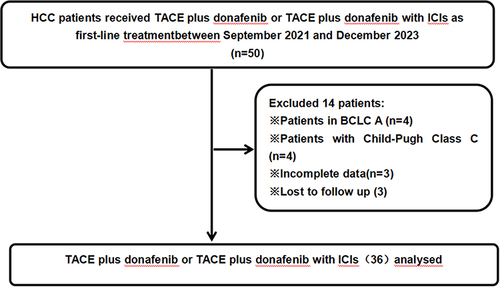
presents a flowchart showing the participant selection process. From September 2021 to December 2023, 50 patients received TACE combined with donafenib with or without immune checkpoint inhibitor. Screening according to the inclusion criteria resulted in the exclusion of 14 patients, including 3 due to incomplete data and 3 due to lack of follow-up data. The remaining 36 patients participated in the study (TACE + donafenib, n = 26; TACE + donafenib + immune checkpoint inhibitors, n = 10). Appropriate PD-1 drugs were selected according to the patient’s medical insurance and condition. Among the 36 patients, 33 were male (91.7%), and the median age was 62.0 years. There were 17 (47.2%) cases with an ECOG PS of 1. Complications included 33 (91.7%) cases with hepatitis B virus infection and 11 (30.6%) with portal vein tumor thrombus. Baseline alpha-fetoprotein (AFP) levels ≥400 ng/mL were present in 18 (50.0%) cases. The median maximum tumor diameter value was 9.1 cm (interquartile range: 5.1–12.4). The institutional distribution is presented in .
Table 1 Baseline Characteristics of Patients
Effectiveness Analysis
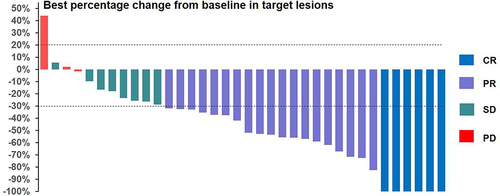
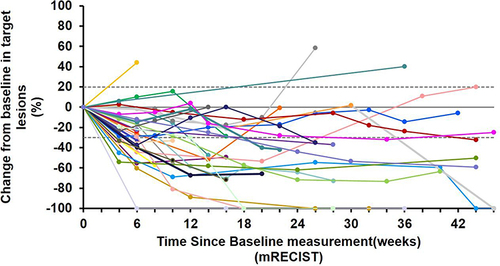
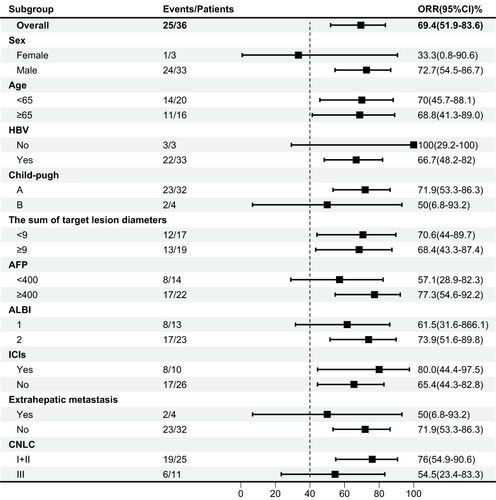
As of February 11, 2024, the median follow-up time was 17.6 months. All 36 subjects were eligible for efficacy evaluation, and the median number of TACE treatments was 2.3 (range: 1–5). According to mRECIST, the ORR was 69.4% (25/36) and the DCR was 91.7% (33/36) (). Compared with the baseline, the target lesion burden decreased in 32 (88.9%) patients (). CR was achieved in 6 (16.7%) patients, PR in 19 (52.8%), SD in 8 (22.2%), and PD in 3 (8.8%) (). Six (16.7%) patients successfully underwent conversion surgery and achieved R0 resection. Complete pathological response was achieved in two patients. presents the time change from baseline in target lesions (mRECIST). The tumor feeding artery diameter decreased from 3.9 ± 1.2 mm to 3.1 ± 1.1 mm at 4 weeks after TACE treatment, and the difference was statistically significant (P = 0.004).
Table 2 Tumor Response According to mRECIST
Survival Analysis
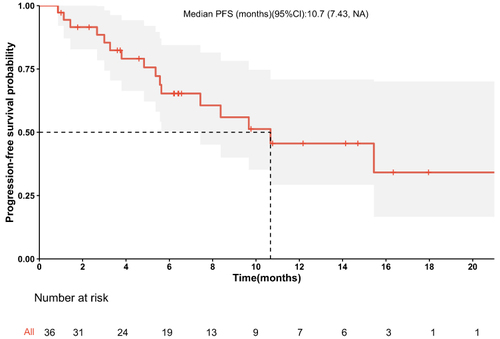
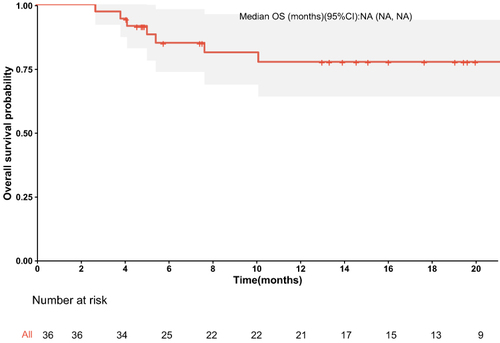
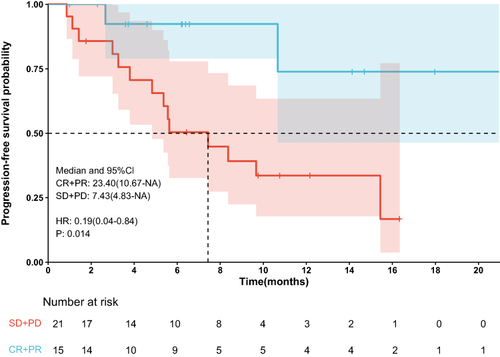
The median PFS was 10.7 months (95% confidence interval [CI]: 8.37–NA months) (), the median duration of response was 11.4 months, the median OS time was not reached (), the median time to response (TTR) was 1.4 months (95% CI: 0.8–6.9 months), and the onset time was relatively fast. Furthermore, patients in the early tumor response group (CR+PR) had longer median PFS (23.4 vs 7.43 months, P < 0.014) (95% CI: 10.67–NA months vs 95% CI: 4.83-NA months) compared with those in the late tumor group (SD+PD) (). Patients in the BCLC B group had longer median PFS (10.67 vs 5.63 months, P =0.048) (95% CI: 7.43–NA months vs 95% CI: 2.67-NA months) compared with those in the BCLC C group, the median OS time was not reached.The median survival rates at 6, 12, and 18 months were 85.0%, 77.6%, and 71.3%, respectively. The median PFS rates at 6, 12, and 18 months were 65.3%, 45.6%, and 34.2%, respectively. The ORR was consistent among all subgroups, including those with a tumor size ≥9 cm or extrahepatic metastasis ().
Analysis of Prognostic Factors
presents the results of univariate and multivariate analyses of the matched cohorts. Cox proportional hazards model showed that the sum of baseline target lesion diameters (<9 vs ≥9 cm) (hazard ratio [HR] = 0.13 [95% CI: 0.03–0.68]; P = 0.016), immune checkpoint inhibitor (yes vs no) (HR = 0.327 [95% CI: 0.116–0.922]; P = 0.034), and best tumor response (CR+PR vs SD+PD) (HR = 0.09 [95% CI: 0.02–0.48]; P = 0.032) were independent prognostic factors for PFS.
Table 3 Univariate and Multivariate Predictors of PFS
Safety
All 25 patients experienced TRAEs (), including 5 (14.7%) with TRAEs ≥ grade 3, which were mainly manifested by elevated alanine aminotransferase (2.9%) and aspartate aminotransaminase (5.9%) serum levels, hypertension (2.9%), and rash (2.9%). Hypertension related to donafenib (mainly grade 1 and 2) developed in 11 (32.3%) patients. A reduction in platelet count induced by hepatic arterial infusion chemotherapy combined with target immunity was observed in 15 patients, reaching grade 3 in 2 patients, which was relieved after treatment with drugs such as avatripopa. Two patients (5.9%) developed immune-related serious AEs, including one case of immune-related pneumonia (grade 2) and one case of hypothyroidism (grade 2). All patients recovered after drug withdrawal and steroid treatment. There were no treatment-related deaths and no TRAEs >grade 4. All TRAEs were relieved following symptomatic treatment or discontinuation of drug administration.
Table 4 Treatment-Related Adverse Events
Discussion
Due to the high malignancy and complexity of liver cancer, its poor prognosis, and its serious impact on human health, there is an urgent need for effective treatment. TACE-based comprehensive treatment is gradually evolving,Citation23 particularly for patients with BCLC stage B, where the ORR of TACE alone is as high as 50%.Citation8 However, as the number of TACE treatments increases, the effective rate decreases and liver damage occurs, with tumor recurrence and distant metastasis developing in some patients. The application of targeted drugs and immunotherapy has led to significant progress in treatment prospects for HCC. The current treatment trend for advanced HCC is combination therapy. Thus, our study evaluated the efficacy and safety of TACE combined with donafenib with or without immune checkpoint inhibitors for the treatment of advanced liver cancer. The treatment regimens achieved satisfactory tumor remission and good efficacy, with some patients achieving transformation resection, providing further evidence for the treatment of initial unresectable HCC.
At present, there are many different molecular targeted drugs and ICIs available to treat HCC. Many studies have focused on whether different drug combinations can achieve good therapeutic effects and which combination regimen provides greater survival benefits for HCC patients. There are many limitations to using TACE treatment alone. However, the ORR and DCR of TACE combined with systemic therapy for unresectable HCC ranges from 35–70% and 75%–96%, respectively, which fully shows the synergistic effect between local therapy and systemic therapy, and that combination therapy achieves a higher tumor control rate compared with TACE alone.Citation24,Citation25 The IMbrave150 study showed that atezolizumab plus bevacizumab achieved an ORR of 27.3% and a median PFS of 6.8 months for HCC.Citation16 Likewise, the CARES-310 study showed that camrelizumab plus apatinib achieved an ORR of 25.4% and a median PFS of 5.6 months.Citation26 In our study, TACE combined with donafenib with or without immune checkpoint inhibitors achieved a high ORR (69.4%) and DCR (91.7%). Six patients successfully underwent conversion surgery and achieved R0 resection, and two patients achieved complete pathological remission. Moreover, the therapeutic onset time was short, with a median TTR of 1.4 months. The results also showed a long-term survival benefit, with a median PFS of 10.7 months. The median survival rates at 6, 12, and 18 months were 85.0%, 77.6%, and 71.3%, respectively, exceeding those of most monotherapy and targeted combined immunotherapy. Compared with different combinations of systemic therapies, local therapy combined with systemic therapy appears to further improve efficacy. The basic principle is clear: TACE reduces the tumor burden and activates immunogenic cell death.Citation27 Similar to sorafenib and other TKIs, donafenib inhibits platelet-derived growth factor receptors and vascular endothelial growth factor receptors and also blocks the RAF/MEK/ERK signaling pathway, leading to vascular normalization.Citation28 The normalization of tumor vasculature promotes immune cell infiltration of the tumor area and enhances immune killing, further controlling tumor recurrence and metastasis.Citation29
Although multimodal treatment may improve the curative effect, the side effects and serious adverse reactions cannot be ignored because they affect patients’ quality of life. The quality of life of cancer patients is crucial, particularly because it has been shown to affect long-term prognosis.Citation30 The REFLECT study reported that clinical responders had a lower risk of exacerbation and higher quality of life scores compared with clinical nonresponders.Citation31 In our study, TACE combined with donafenib with or without PD-1 did not show new safety signals and was well-tolerated by patients. The combination of multiple treatment modes did not appear to cause any unusual overlapping toxicity, and no grade 4 or 5 TRAEs occurred. The above results were comparable to those previously reported,Citation32,Citation33 indicating the safety and tolerability of combined TACE treatment.
Our clinical study has certain limitations. First, the sample size is small, so the possibility of bias in the results cannot be ruled out. Second, the follow-up time is relatively short. Third, because this is a single-arm study, there is no control group, and the level of evidence is somewhat poor. Thus, large, multicenter prospective clinical studies are necessary to further verify the efficacy and safety of TACE combined with donafenib with or without immune checkpoint inhibitors in patients with unresectable HCC.
Conclusion
To summarize, TACE combined with donafenib with or without immune checkpoint inhibitors had a short therapeutic onset time for patients with unresectable HCC, showing high ORR, PFS, and DCR, as well as controllable toxicity. Additionally, treatment resulted in significant narrowing of the tumor feeding artery. Thus, TACE combined with donafenib with or without immune checkpoint inhibitors is a potentially safe and effective first-line treatment for HCC and is worthy of further promotion in clinical practice.
Abbreviations
AE, Adverse events; BCLC, Barcelona Clinic Liver Cancer; CI, Confidence interval; CR, Complete response; DCR, Disease control rate; ICI, Immune checkpoint inhibitors; ORR, Objective response rate; OS, Overall survival; PD, Progressive disease; PFS, Progression-free survival; PR, Partial response; SD, Stable disease; TKI, Tyrosine kinase inhibitor; TRAE, Treatment-related adverse events; TTR, Time to response.
Ethics Approval and Informed Consent
This study was approved by the Medical Ethics Committee of our hospital and registered in the Chinese Clinical Trial Registry (ChiCTR2100054041). All subjects voluntarily enrolled and signed written informed consent.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
Thank all the staff authors for their contributions to this study.
Data Sharing Statement
The data that support the findings of this study are available via a data access agreement. Please contact the corresponding author for this request.
Additional information
Funding
References
- European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
- Zhu XD, Sun HC. Emerging agents and regimens for hepatocellular carcinoma[J]. J Hematol Oncol. 2019;12(1):110. doi:10.1186/s13045-019-0794-6
- Zhu XD, Tang ZY, Sun HC. Targeting angiogenesis for liver cancer: past, present, and future[J]. Genes Dis. 2020;7(3):328–335. doi:10.1016/j.gendis.2020.03.010
- Chang Y, Jeong SW, Young Jang J, Jae Kim Y. Recent updates of transarterial chemoembolilzation in hepatocellular carcinoma. Int J Mol Sci. 2020;21(21):8165. PMID: 33142892; PMCID: PMC7662786. doi:10.3390/ijms21218165.
- Han K, Kim JH. Transarterial chemoembolization in hepatocellular carcinoma treatment: Barcelona clinic Liver cancer staging system. World J Gastroenterol. 2015;21(36):10327–10335. PMID: 26420959; PMCID: PMC4579879. doi:10.3748/wjg.v21.i36.10327.
- Miyayama S, Arai Y, Matsui O. Transarterial chemoembolization for hepatocellular carcinoma with vascular invasion. Br J Radiol. 2022;95(1138):20211316. PMID: 35143258; PMCID: PMC9815726. doi:10.1259/bjr.20211316.
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/jjhep.2021.11.018
- Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 noninferiority trial[J]. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
- Kambhampati S, Bauer KE, Bracci PM, et al. Nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh class B cirrhosis: safety and clinical outcomes in a retrospective case series[J]. Cancer. 2019;125(18):3234–3241. doi:10.1002/cncr.32206
- Lim H, Ramjeesingh R, Liu D, et al. Optimizing survival by changing the landscape of targeted therapy for intermediate and advanced hepatocellular carcinoma: a systematic review[J]. J Natl Cancer Inst. 2020;113(2):123–136. doi:10.1093/jnci/djaa119
- Li X, Qiu M, Wang S, Zhu H, Feng B, Zheng L. A Phase I dose-escalation, pharmacokinetics and food-effect study of oral donafenib in patients with advanced solid tumours. Cancer Chemother Pharmacol. 2020;85(3):593–604. doi:10.1007/s00280-020-04031-1
- Bureau of Medical Administration and National Health Commission of the People’s Republic of China. Standardization for diagnosis and treatment of hepatocellular carcinoma (2022 edition). Zhonghua Gan Zang Bing Za Zhi. 2022;30(4):367–388. doi:10.3760/cma.j.cn501113-20220413-00193.
- Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193–202. doi:10.1200/JCO.19.01307
- El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an openlabel, non-comparative, Phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi:10.1016/S0140-6736(17)31046-2
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
- Meyer T, Fox R, Ma YT, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebocontrolled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2(8):565–575. doi:10.1016/S2468-1253(17)30156-5
- Yau T, Kang YK, Kim TY, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the checkMate 040 randomized clinical trial. JAMA Oncol. 2020;6(11):e204564. doi:10.1001/jamaoncol.2020.4564
- Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29(3):339–364. doi:10.1159/000327577
- Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer. 2020;9(6):682–720. doi:10.1159/000509424
- Llovet JM, Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol. 2020;72(2):288–306. doi:10.1016/j.jhep.2019.09.026
- Common Terminology Criteria for Adverse Events (CTCAE). Protocol development cancer therapy evaluation program (CTEP). Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference5x7.pdf. Accessed August 12, 2018.
- Kobayashi S, Tajiri K, Murayama A, et al. Drug-eluting bead-transcatheter arterial chemoembolization for advanced hepatocellular carcinoma refractory to conventional lipiodol-based transcatheter arterial chemoembolization[J]. J Hepatocell Carcinoma. 2020;7:181–189. doi:10.2147/JHC.S273929
- Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69(8):1492–1501. doi:10.1136/gutjnl-2019-318934
- Peng Z, Fan W, Zhu B, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: a Phase III, randomized clinical trial (LAUNCH). J Clin Oncol. 2023;41(1):117–127. doi:10.1200/JCO.22.00392
- Qin S, Chan SL, Gu S, et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study. Lancet. 2023;402(10408):1133–1146. doi:10.1016/S0140-6736(23)00961-3
- Zhong B-Y, Jin Z-C, Chen -J-J, Zhu HD, Zhu XL. Role of transarterial chemoembolization in the treatment of hepatocellular carcinoma. J Clin Transl Hepatol. 2023;11(2):480–489. doi:10.14218/JCTH.2022.00293
- Keam SJ, Duggan S. Donafenib: first approval. Drugs. 2021;81(16):1915–1920. doi:10.1007/s40265-021-01603-0
- Llovet JM, Castet F, Heikenwalder M, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19(3):151–172. doi:10.1038/s41571-021-00573-2
- Quinten C, Maringwa J, Gotay CC, et al. Patient self-reports of symptoms and clinician ratings as predictors of overall cancer survival. J Natl Cancer Inst. 2011;103(24):1851–1858. doi:10.1093/jnci/djr485
- Vogel A, Qin S, Kudo M, et al. Lenvatinib versus sorafenib for first-line treatment of unresectable hepatocellular carcinoma: patient-reported outcomes from a randomised, open-label, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol. 2021;6(8):649–658. doi:10.1016/S2468-1253(21)00110-2
- Cao F, Yang Y, Si T, et al. The efficacy of TACE combined with lenvatinib plus sintilimab in unresectable hepatocellular carcinoma: a multicenter retrospective study. Front Oncol. 2021;11:783480. doi:10.3389/fonc.2021.783480
- Yang XG, Sun YY, Li DS, Xu GH, Huang XQ. Efficacy and safety of drug-eluting beads transarterial chemoembolization combining immune checkpoint inhibitors in unresectable intrahepatic cholangiocarcinoma: a propensity score matching analysis. Front Immunol. 2022;13:940009. doi:10.3389/fimmu.2022.940009