Abstract
Purpose
Liver resection and ablation remain the most common therapeutic options for Barcelona Clinic Liver Cancer (BCLC) stage 0-A hepatocellular carcinoma (HCC), but there is a lack of evidence to show which is the most suitable therapy. This study aimed to make concurrent multi-arm comparisons of the short-term and long-term outcomes of percutaneous ablation (PA), open (OLR) or laparoscopic liver resection (LLR) for these patients.
Patients and Methods
This was a retrospective observational cohort study. A series of generalized propensity score methods for multiple treatment groups were performed to concurrently compare the clinical outcomes of these three treatment options to balance potential confounders. Regression standardization was used to account for hazard of all-cause mortality and recurrence of intergroup differences.
Results
Of the 1778 patients included, 1237, 307 and 234 underwent OLR, LLR and PA, respectively. After overlap weighting, which was the optimal adjustment strategy, patients in the minimally invasive group (LLR and PA groups) had few postoperative complications and short postoperative hospital stays (both P < 0.001). The 5-year recurrence-free survival (RFS) rate and 5-year overall survival (OS) rate were significantly higher in the LLR group when compared with the OLR and PA groups (RFS: 55.6% vs 48.0% vs 30.2%, P < 0.001; OS: 89.1% vs 79.7% vs 84.0%, P = 0.020). Multivariable Cox analysis and regression standardization showed that LLR was an independent factor for better RFS when compared with OLR and PA. In subgroup analysis, the long-term outcomes of patients with BCLC stage A HCC were consistent with the whole population.
Conclusion
In the observational study using various covariate adjustment analysis with excellent balance, LLR is not only minimally invasive, but also provides better RFS and equivalent OS for patients with BCLC stage 0-A HCC when compared with OLR and PA.
Introduction
Primary liver cancer ranks as the seventh most common cancers and the second-leading cause of cancer-related mortality all over the world.Citation1 Hepatocellular carcinoma (HCC) accounts for more than 80% of primary liver cancers.Citation2 Due to advances in surveillance technology and popularization of screening programs in patients with high risks of HCC, an increasing number of patients with HCC are diagnosed in the early stage.Citation3 According to the Barcelona Clinic Liver Cancer (BCLC) strategy for treatment recommendation, liver resection, ablation and transplantation are the recommended treatment modalities for HCC in the very early stage (BCLC stage 0) and early stage (BCLC stage A).Citation4 Of note, liver transplantation is not commonly used because of organ shortage.Citation5 Therefore, liver resection and ablation remain the most common options for BCLC stage 0-A HCC. Currently, laparoscopic liver resection (LLR) has become more and more popular with many surgeons owing to its safety and minimal invasiveness, although open liver resection (OLR) is still considered as the gold standard operation to treat HCC.Citation6 The efficacy of OLR, LLR and percutaneous ablation (PA) for HCC patients is worthy of further discussion.
Currently, a growing number of randomized controlled trials (RCTs) have investigated clinical outcomes of OLR, LLR or PA for HCC patients. PA has shown comparable long-term outcomes to liver resection in early-stage or small HCC patients,Citation7–10 although one trial favored OLR for better overall survival (OS) and recurrence-free survival (RFS) for HCC meeting Milan criteria.Citation11 LLR has demonstrated similar long-term outcomes to OLR, with superior short-term results for solitary HCC less than 5 cm in cirrhotic patients,Citation12 although direct RCT comparisons with PA are currently lacking.
Given the accumulation of substantial observational data over the years, an increasing number of cohort studies have investigated the efficacy of OLR, LLR or PA for HCC patients. Observational cohort studies generally indicate that surgical resection provides longer RFS and comparable OS to PA in early-stage HCC patients,Citation13,Citation14 although some show varied results for HCC ≤ 2 cm.Citation15–18 LLR and OLR have shown similar RFS and OS in multiple studies,Citation19–22 while LLR showed superior RFS and comparable OS compared to PA.Citation23–25 However, due to variations. In study populations among cohort studies, conclusive evidence favoring one treatment over the other remains lacking.
While conducting a large-sample, multi-arm RCT would be ideal for comprehensive evaluation of these treatments,Citation26 sample size requirements complicate this approach.Citation27 Besides, current two-group RCTs lack consistency in patient populations, thereby impeding direct comparisons among OLR, LLR and PA. This study aims to address these gaps by simultaneously analyzing short-term and long-term outcomes of OLR, LLR and PA in BCLC stage 0-A HCC patients using advanced generalized propensity score analysis (GPSA) methods which are developed for concurrent multi-arm treatment comparisons, providing a higher level of evidence for clinical outcomes of these three curative therapies.
Materials and Methods
Study Design and Patients
This study was a retrospective observational cohort study and following the STROBE guidelines.Citation28 It was reviewed and approved by the Institutional Review Board of the First Affiliated Hospital of Sun Yat-sen University, and the requirement for informed consent was waived due to the nature of the retrospective cohort study. The confidentiality of patient data was ensured, and the study was performed according to the Declaration of Helsinki. From January 1, 2012 to December 31, 2021, data from consecutive patients who received curative OLR, LLR or ablation at the First Affiliated Hospital of Sun Yat-sen University were collected. All patients were followed up at the end of 2023. All patients were diagnosed according to the guidelines of the European Association for the Study of the Liver (EASL).Citation29
The inclusion criteria were as follows: 1) primary HCC with pathological confirmation, 2) no previous cancer-related therapies, 3) with very-early-stage or early-stage HCC (BCLC stage 0-A), 4) receiving curative therapies and 5) Eastern Cooperative Oncology Group performance status of 0–1. The following exclusion criteria were considered: 1) with history of other malignancies, 2) with visible tumor thrombus or identified extrahepatic metastasis and 3) with insufficient clinical or follow-up information. According to the center’s policies, each patient was discussed in a multidisciplinary team (MDT) meeting which included liver surgeons, radiologists and interventional oncologist.
Exposures
Open Liver Resection
OLR was performed by experienced surgeons with more than 10 years of liver surgery experience in the study center. Patients were placed in a supine position and intraoperative ultrasound was routinely performed. Pringle’s maneuver was routinely used with a clamp/unclamp time of 10/5 min. An ultrasonic scalpel was used for liver parenchymal transection.
Laparoscopic Liver Resection
For LLR, the patient was placed in a supine position and the camera port was placed above the umbilicus. Carbon dioxide pneumoperitoneum pressure was maintained at 12–14 mmHg.
The choice of the type of liver resection was mainly determined by MDT according to the liver function, tumor location and tumor size.
Percutaneous Ablation
As previously described,Citation30 PA was performed by two experienced doctors with more than 10 years of tumor ablation experience. Ablation was performed using real-time ultrasound or computed tomography (CT) guidance. The choice of imaging-guided percutaneous ablation, including radiofrequency ablation, microwave ablation, ethanol injection and combination ablation, mainly depends on the tumor size and location.
Follow-Up
Treatment response was evaluated by contrast-enhanced CT or magnetic resonance imaging (MRI) examination 1 month after liver resection or ablation. Thereafter, all HCC patients were followed up by conventional ultrasound, contrast-enhanced ultrasound, CT or MRI, and serum alpha-fetoprotein (AFP) level every 3 months for the first 2 years, every 6 months from 2 to 5 years, and annually after 5 years. The diagnosis of recurrence was based on the guidelines of EASL.Citation29
Potential Confounders
Preoperative variables, including sex, age, HBV infection, cirrhosis, Child-Pugh class, tumor number, tumor size, tumor site, AFP, aspartate aminotransferase (AST) and alanine aminotransferase (ALT), were considered as potential confounders due to these factors were essential for clinical decision and treatment strategy making.
Outcomes
The primary outcome of this study was OS. OS was defined as the time interval from surgery or ablation to death or censoring at last follow-up (31 December 2023). Another long-term outcome was RFS as a secondary outcome. RFS was defined as the time interval from surgery to recurrence or censoring at the end of follow-up. Other secondary outcomes included postoperative hospital stay and postoperative complications. Surgical complications were classified according to the Clavien-Dindo classification.Citation31
Generalized Propensity Score Analysis
Since there were three treatment groups to compare concurrently in this study, to reduce the bias of potential confounders, various GPSA methods for multiple treatment comparisons were performed.Citation32 In GPSA, generalized propensity scores (GPS) are usually estimated using the multinomial logistic regression or generalized boosted model with multiple treatment groups as the outcome variable and potential confounding variables as covariates.Citation33 The GPS matching and the generalized boosted model-based inverse probability weighting are two common methods with target inference of average treatment effect for data with multiple treatment groups.Citation33,Citation34 However, GPS matching would reduce more than half of the sample size after matching. Results after inverse probability weighting would be violated by extreme propensity scores.Citation35 Therefore, more weighting methods were recently introduced to avoid extreme propensity scores. The overlap weighting and matching weights were introduced by providing average treatment effects in the overlap population and subset, respectively.Citation36,Citation37 These weights were also computed from GPS with different formulas other than inverse probability weighting. We then compared their performance on balancing confounding factors by using kernel density plots and absolute standardized mean differences (SMDs). The average SMD of each covariate <0.1 was considered to be well balanced.Citation38 Finally, we performed Three-way matching, TriMatch, TriMatch with exact matching, inverse probability weighting, inverse probability weighting with trimming, overlap weighting and matching weights analysis by including the potential confounders mentioned above, and selected the most appropriate method with better performance of balance for further statistical analyses.
Statistical Analysis
As the retrospective nature of the study, the sample size was determined by the study period and not conducted using statistical estimation. The baseline characteristics and outcomes were described and compared among the three study groups in both the unmatched and matched/weighed cohorts. Continuous variables with normal distribution were described using mean and standard deviation (SD) or median and interquartile range (IQR), if data are not normally distributed. ANOVA or Kruskal–Wallis tests were used to compare the differences among the three study groups if appropriate. Categorical variables were described using frequencies and proportions and compared among three study groups using the chi-square test or Fisher's exact test. Missing covariates were imputed by its median value as a very small portion of missing were found (<1%).
Survival analysis for the three groups were estimated using the Kaplan–Meier curve, and compared by the Log rank test. Univariable and multivariable Cox proportional hazard models were performed to evaluate the treatment effect of the three study groups with OS and RFS both before and after GPSA. The multivariable Cox model included all the potential confounders mentioned above. Hazard ratio (HR) and its 95% confidence interval (95% CI) was estimated for each Cox model. Proportional hazard assumption was tested by weighted residuals using cox.zph function in R and no violation was found. Subgroup analysis was performed according to BCLC stage (0 and A). The P value was corrected by Bonferroni method for multiple treatment comparisons. Flexible parametric survival regression standardization was used to estimate standardized survival.Citation39,Citation40 The hazard which was defined as the slope of the survival curve and the hazard difference between OS and RFS of intergroups before and after GPSA were reported by graphs over time.
One of the authors QZ a medical statistician performed the statistical analysis. A two-tailed p value less than 0.05 was considered statistically significant. All statistical analyses were performed using R version 3.6.0 software (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Characteristics
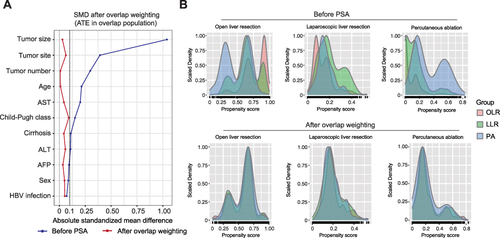
A total of 1778 patients with BCLC stage 0-A HCC met the study inclusion criteria, 1237 of which were treated with OLR, 307 underwent LLR and 234 underwent PA (). The baseline characteristics of the 1778 patients were summarized in . Most characteristics of the three groups were unbalanced before adjustment. Of note, compared with the LLR and PA groups, the tumor size in the OLR group was significantly larger, and the proportion of tumors in the bilobar liver was significantly higher. While compared with the OLR and LLR groups, the proportion of multifocal tumors was significantly higher in the PA group. Therefore, seven GPSA methods were conducted to balance the covariates among the OLR, LLR and PA groups. SMD ( and S1) and density plot of GPS () were used to assess the effects of balancing. As represented by the overlap weighting, all the SMDs with baseline characteristics were <0.1 ( and ) and the overlap of density plots of GPS for each group () was reasonable, suggesting that the potential confounders were well balanced with no significant differences in preoperative variables among the three groups.
Table 1 Baseline Characteristics of HCC Patients Included Before and After Generalized Propensity Score Analysis
Figure 1 Flow diagram of patient collection. A total of 1778 patients with BCLC stage 0-A HCC which met the study inclusion criteria (OLR: 1237; LLR: 307; PA: 234) were included for analysis.
Figure 2 Assessment of balance of overlap weighting analysis. (A) The absolute SMDs of potential confounders before and after overlap weighting analysis. (B) Density plot of generalized propensity score in OLR, LLR and PA groups before and after overlap weighting analysis.
Intraoperative and Postoperative Outcomes
As Table S1 shown, the operation time was significantly longer in the LLR group compared with the OLR group (4.25 hours vs 3.20 hours, P < 0.001). In addition, more intraoperative blood loss was observed in the OLR group compared with the LLR group (P = 0.001). Conversion to open surgery occurred in 32 patients in the LLR group due to: uncontrollable bleeding (n = 7), difficulty in dissecting tumor from major vessels (n = 11), and poor exposure or no progression after a long time (n = 14). In the PA group, most of the patients (n = 182) underwent radiofrequency ablation, 17.1% (n = 40) underwent microwave ablation, 4.3% (n = 10) underwent ethanol injection, and the remaining underwent combination ablation. There was no intraoperative death in the three groups.
After overlap weighting in , patients in the LLR and PA groups recovered significantly faster than those in the OLR group (7 days vs 3 days vs 9 days, P < 0.001). The incidence of postoperative complications was significantly higher in the LLR group compared with the OLR and PA groups (P < 0.001). However, the postoperative complications were more severe in the OLR group compared with LLR and PA groups (Clavien-Dindo grade II: P = 0.022; Clavien-Dindo grade IIIa: P = 0.007) while most of postoperative complications in LLR and PA groups were Clavien-Dindo grade I. Notably, five patients occurred liver failure and one patient occurred multiple organ dysfunctional syndromes in the OLR group, and one patient occurred liver failure in the LLR group. No early postoperative death occurred in any of the three groups, suggesting the safety of these three treatments.
Table 2 Postoperative Data of HCC Patients Before and After Generalized Propensity Score Analysis
Long-Term Oncological Outcomes
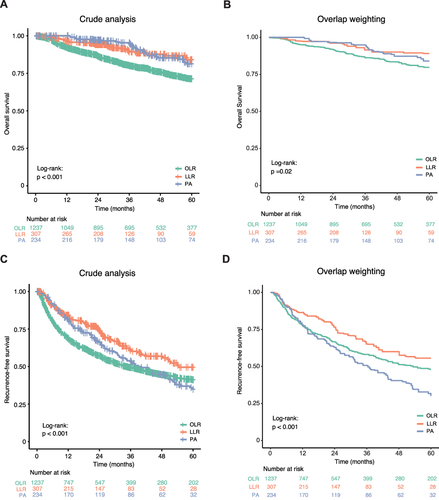
The median follow-up time for all patients was 39.5 (22.2, 63.5) months. Before GPSA, the 5-year OS rates of OLR, LLR and PA groups were 71.3%, 84.0% and 81.3%, respectively (P < 0.001, ). After the overlap weighting, the 5-year OS rates of the OLR, LLR and PA groups were 79.7% versus 89.1% versus 84.0%, respectively (P = 0.020, ). Compared with patients in the LLR and PA groups, patients in the OLR group had a significantly shorter OS. Before GPSA, the 5-year RFS rates of OLR, LLR and PA groups were 41.2%, 49.5% and 34.8%, respectively (P < 0.001, ). After the overlap weighting, the 5-year RFS rates of OLR, LLR and PA groups were 48.0% versus 55.6% versus 30.3%, respectively (P < 0.001, ). Patients in the LLR group had a significantly longer RFS than those in the OLR and PA groups. As shown in Figure S2, consistent with the results obtained by overlap weighting analysis, the results obtained by other GPSA methods except Three-way matching analysis also showed superior long-term outcomes in the LLR group.
Figure 3 Kaplan–Meier curves for long-term outcomes of BCLC stage 0-A HCC patients who underwent OLR, LLR and PA before and after overlap weighting analysis. (A and B) Overall survival before (A) and after (B) overlap weighting analysis. (C and D) Recurrence-free survival before (C) and after (D) overlap weighting analysis.
Univariable and multivariable Cox proportional hazard analyses were performed before and after GPSA to evaluate the relationships between the three treatment options and OS or RFS (). LLR was independently associated with better RFS compared with OLR (LLR vs OLR: HR=0.74, P = 0.006) and PA (PA vs LLR: HR = 1.69, P < 0.001) in crude multivariable Cox analysis and was also significantly better than OLR (LLR vs OLR: HR = 0.70, P = 0.016) and PA (PA vs LLR: HR = 1.83, P < 0.001) in overlap-weighted multivariable Cox analysis. In terms of OS, LLR was independently associated with better OS compared with OLR in crude (LLR vs OLR: HR = 0.61, P = 0.014) and overlap-weighted multivariable Cox analysis (LLR vs OLR: HR = 0.49, P = 0.015). No significant difference was observed between LLR and PA groups or PA and OLR groups in crude or overlap-weighted multivariable Cox analysis (both P > 0.05), suggesting LLR or OLR was not independently associated with better OS compared to PA.
Table 3 Univariable and Multivariable Cox Proportional Hazards Modeling for RFS and OS in HCC Patients Before and After Generalized Propensity Score Analysis
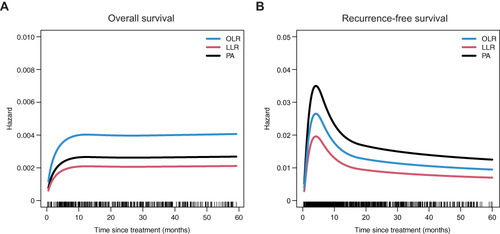
The hazards of all-cause mortality and recurrence over time among the three groups after overlap weighting were shown in . The hazard of all-cause mortality reached the highest level within a year and remained unchanged thereafter (). LLR showed the lowest hazard of all-cause mortality and followed by PA, but there was no significant difference between the two groups. However, the hazards of mortality in both LLR and PA were significantly lower than that of OLR. The hazard differences with 95% CI were depicted in Figure S3A. The hazard of recurrence rose to the highest level within half a year, then fell back in 1 year, and decreased slightly over time after 1 year, suggesting the highest risk of tumor recurrence a half-year after OLR, LLR or PA (). PA showed the lowest hazard of recurrence compared to LLR, and LLR showed the lower hazard of recurrence compared with OLR. The hazard differences of recurrence with 95% CI were depicted in Figure S3B.
Subgroup Analysis
Subgroup analysis was performed according to the BCLC stage (0 and A) in both the original and overlap weighted cohort. The results obtained by overlap weighting analysis were depicted as below. In the BCLC stage 0 HCC patients, although no statistically significant differences in the OS and RFS were observed among the three groups, the Kaplan–Meier curves and multivariable Cox proportional hazard analyses showed that patients in the LLR group tended to have better RFS compared with the OLR and PA groups (Table S2, Figure S4A and B). In the BCLC stage A cohort, consistent with the whole population, patients in the LLR group had significantly longer RFS than those in the OLR and PA groups, and patients in the LLR and PA groups tended to have better OS compared with the OLR group (Table S3, Figure S4C and D).
Discussion
Minimally invasive techniques, including laparoscopic hepatectomy and percutaneous ablation, have been well developed in the treatment of HCC. According to the Balliol IDEAL classification, long-term oncological outcomes are recommended to evaluate the efficacy of laparoscopic hepatectomy in treating HCC.Citation41 However, high-level studies on long-term outcomes in BCLC stage 0-A HCC patients after OLR, LLR or PA are lacking. Due to the challenges of conducting multi-arm randomized controlled trials in clinical practice, we employed multiple GPSA methods to balance baseline characteristics of the study cohorts, aiming for more reliable comparisons in real-world settings. Our study found that patients in the minimally invasive group (LLR and PA groups) had significantly fewer postoperative complications and faster recovery compared with patients who underwent OLR. Moreover, patients in the LLR group had significantly longer RFS compared with patients in the OLR and PA groups and significantly longer OS compared with patients in the OLR group. These findings demonstrated LLR as an effective and safe approach in the treatment of BCLC stage 0-A HCC.
The debate over whether resection or ablation is the superior treatment for BCLC stage 0-A HCC has been extensively discussed previously. Accumulating evidence, including high-level systematic reviews and meta-analysis, consistently indicates that liver resection yields better RFS than ablation while provides similar OS for HCC patients.Citation13,Citation14,Citation42,Citation43 In line with these findings, our study also revealed that patients in the either LLR or OLR group had significant longer RFS than those in the PA group. However, upon further stratification of patients receiving liver resection into OLR and LLR groups, we found that patients in the PA group had similar OS to those in the LLR group and longer OS than those in the OLR group. The superior RFS associated with surgical resection is likely attributed to the complete removal of tumors, thereby eliminating micro metastases. Conversely, the high recurrence rates observed after ablation might be attributed to repeated puncture to the tumor, the change of biological behaviors of tumor cells and the remodeling of tumor microenvironment.Citation44 Despite the shorter RFS, percutaneous ablation could provide comparable OS mainly due to the repeatability of ablation for tumor recurrence.Citation45 In terms of short-term outcomes, patients undergoing ablation showed less postoperative complication rate and faster recovery compared with the surgical group (LLR and OLR groups), suggesting that PA is a more minimally invasive approach and might be associated with better quality of life.
Moreover, in our study, the LLR group showed significantly better OS and RFS than OLR group in patients receiving a hepatectomy. A recent prospective observational study using propensity score matching with 56 patients for each group compared long-term outcomes of laparoscopic and open liver resection for patients with BCLC stage 0-A HCC, and found that there was no significant difference between the laparoscopic and open hepatectomy groups in the 5-year RFS and OS.Citation19 Similarly, other studies also showed that LLR and OLR provided comparable RFS and OS.Citation20–22 However, of note, Zhu P et alCitation19 study found that when they combined LLR and robotic-assisted liver resection into a group (minimally invasive hepatectomy group), the 5-year RFS rate was lower in the minimally invasive hepatectomy group compared to OLR, consistent with our findings. The lower RFS rates observed after minimally invasive hepatectomy may be attributed to the potential reduced immunosuppressive effects associated with minimally invasive treatments.Citation46,Citation47 Additionally, the type of hepatectomy might account for these differences in our study. In our study, the proportions of patients receiving anatomical hepatectomy was significantly higher in the LLR group than in the OLR group, also suggesting the safety of the laparoscopic hepatectomy. It is suggested that LLR may facilitate the complete removal of tumor burden and potential micro metastases, such as microvascular invasion, which could explain its superior RFS and OS outcomes. In addition, the LLR group showed lower complication rates and shorter postoperative stays than the OLR group, yielding better short-term outcomes. Given short-term and long-term outcomes, LLR might be a superior treatment option for BCLC stage 0-A HCC if the preserve of liver function is allowed. Overall, our results demonstrate the safety and efficacy of LLR for patients with BCLC stage 0-A HCC.
Due to the minimally invasiveness and therapeutic efficacy of laparoscopic hepatectomy for HCC patients, the adoption of laparoscopic hepatectomy has increased rapidly in the recent years. The indications for LLR are uncertain, and several factors, including tumor size, location, type/extent of liver resection and presence of liver cirrhosis, affect the complexity of LLR.Citation48 Therefore, it is cautious for surgeons to select patients available for LLR. Currently, according to the EASL guideline, LLR is appropriate for very early and early HCC mainly located in superficial or antero-lateral liver positions.Citation29 Nevertheless, the role of LLR in some situations remains controversial, such as for difficultly located HCC and for multiple or giant lesions. Owing to the rapidly developed techniques, laparoscopic hepatectomy for liver segments, which were difficult to resect, such as S1, S7 and S8, have been conducted at some specialized centers, suggesting that more and more HCC patients could benefit from LLR.Citation49 As a minimally invasive approach, LLR provides better RFS and OS compared to OLR, while another minimally invasive approach, percutaneous ablation, is restricted by tumor location and inappropriate for lesions located near large vessels, such as a primary or secondary branch of the portal vein, subphrenic lesions and lesions near extrahepatic organs.Citation50 In recent years, a growing number of evidence has found that laparoscopic ablation could overcome the technique difficulty of percutaneous ablation and provided better therapeutic outcomes than percutaneous ablation for subphrenic lesions, suggesting the superiority of the laparoscopic approach.Citation51,Citation52 Therefore, despite the complexity and difficulty of LLR, it is worthy of promoting to surgeons.
There were a few limitations in this study. First, this study was still a non-randomized study which might lead to some unavoidable bias. Thus, we performed various GPSA methods to overcome potential bias and several methods achieved excellent balance. And most of the results from those methods were consistent. Second, this study was conducted at a single institution, and whether our findings could be generalized to other centers was unknown. Third, most patients included in this study had hepatitis B. It is unknown whether our conclusions could be obtained in HCC patients with other etiological factors. In the future, multicenter randomized controlled trials with large sample sizes are required for further validation.
Conclusion
In conclusion, laparoscopic hepatectomy was safe and effective for the treatment of patients with BCLC stage 0-A HCC in this large observational cohort study after using various covariates adjustment analysis methods suitable for concurrent multiple treatment comparisons. Laparoscopic hepatectomy is not only minimally invasive but also provides superior long-term outcomes compared with open liver resection and percutaneous ablation.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
- Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi:10.1056/NEJMra1713263
- Singal AG, Zhang E, Narasimman M, et al. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: a meta-analysis. J Hepatol. 2022;77(1):128–139. doi:10.1016/j.jhep.2022.01.023
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
- Wong RJ, Devaki P, Nguyen L, Cheung R, Nguyen MH. Ethnic disparities and liver transplantation rates in hepatocellular carcinoma patients in the recent era: results from the Surveillance, Epidemiology, and End Results registry. Liver Transpl. 2014;20(5):528–535. doi:10.1002/lt.23820
- Schmelzle M, Krenzien F, Schöning W, Pratschke J. Laparoscopic liver resection: indications, limitations, and economic aspects. Langenbecks Arch Surg. 2020;405(6):725–735. doi:10.1007/s00423-020-01918-8
- Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243(3):321–328. doi:10.1097/01.sla.0000201480.65519.b8
- Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57(4):794–802. doi:10.1016/j.jhep.2012.05.007
- Fang Y, Chen W, Liang X, et al. Comparison of long-term effectiveness and complications of radiofrequency ablation with hepatectomy for small hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29(1):193–200. doi:10.1111/jgh.12441
- Takayama T, Hasegawa K, Izumi N, et al. Surgery versus radiofrequency ablation for small hepatocellular carcinoma: a randomized controlled trial (SURF Trial). Liver Cancer. 2021;11(3):209–218. doi:10.1159/000521665
- Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252(6):903–912. doi:10.1097/SLA.0b013e3181efc656
- El-Gendi A, El-Shafei M, El-Gendi S, Shawky A. Laparoscopic versus open hepatic resection for solitary hepatocellular carcinoma less than 5 cm in cirrhotic patients: a randomized controlled study. J Laparoendosc Adv Surg Tech A. 2018;28(3):302–310. doi:10.1089/lap.2017.0518
- Wang JH, Wang CC, Hung CH, Chen CL, Lu SN. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol. 2012;56(2):412–418. doi:10.1016/j.jhep.2011.05.020
- Hung HH, Chiou YY, Hsia CY, et al. Survival rates are comparable after radiofrequency ablation or surgery in patients with small hepatocellular carcinomas. Clin Gastroenterol Hepatol. 2011;9(1):79–86. doi:10.1016/j.cgh.2010.08.018
- Peng ZW, Lin XJ, Zhang YJ, et al. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: a retrospective comparative study. Radiology. 2012;262(3):1022–1033. doi:10.1148/radiol.11110817
- Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2008;47(1):82–89. doi:10.1002/hep.21933
- Chu HH, Kim JH, Kim PN, et al. Surgical resection versus radiofrequency ablation very early-stage HCC (</=2 cm Single HCC): a propensity score analysis. Liver Int. 2019;39(12):2397–2407. doi:10.1111/liv.14258
- Liu PH, Hsu CY, Hsia CY, et al. Surgical Resection Versus Radiofrequency Ablation for Single Hepatocellular Carcinoma </= 2 cm in a Propensity Score Model. Ann Surg. 2016;263(3):538–545. doi:10.1097/SLA.0000000000001178
- Zhu P, Liao W, Zhang WG, et al. A prospective study using propensity score matching to compare long-term survival outcomes after robotic-assisted, laparoscopic or open liver resection for patients with BCLC stage 0-A hepatocellular carcinoma. Ann Surg. 2023;277(1):e103–e111. doi:10.1097/SLA.0000000000005380
- Takahara T, Wakabayashi G, Beppu T, et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: a multi-institutional Japanese study. J Hepatobiliary Pancreat Sci. 2015;22(10):721–727. doi:10.1002/jhbp.276
- Han HS, Shehta A, Ahn S, Yoon YS, Cho JY, Choi Y. Laparoscopic versus open liver resection for hepatocellular carcinoma: case-matched study with propensity score matching. J Hepatol. 2015;63(3):643–650. doi:10.1016/j.jhep.2015.04.005
- Troisi RI, Berardi G, Morise Z, et al. Laparoscopic and open liver resection for hepatocellular carcinoma with Child-Pugh B cirrhosis: multicentre propensity score-matched study. Br J Surg. 2021;108(2):196–204. doi:10.1093/bjs/znaa041
- Ogiso S, Seo S, Eso Y, et al. Laparoscopic liver resection versus percutaneous radiofrequency ablation for small hepatocellular carcinoma. HPB. 2021;23(4):533–537. doi:10.1016/j.hpb.2020.08.009
- Song J, Wang Y, Ma K, et al. Laparoscopic hepatectomy versus radiofrequency ablation for minimally invasive treatment of single, small hepatocellular carcinomas. Surg Endosc. 2016;30(10):4249–4257. doi:10.1007/s00464-015-4737-1
- Lee DH, Kim JW, Lee JM, et al. Laparoscopic liver resection versus percutaneous radiofrequency ablation for small single nodular hepatocellular carcinoma: comparison of treatment outcomes. Liver Cancer. 2021;10(1):25–37. doi:10.1159/000510909
- Piantadosi S. Clinical Trials: A Methodologic Perspective. 2nd ed. Wiley; 2006.
- Juszczak E, Altman DG, Hopewell S, Schulz K. Reporting of multi-arm parallel-group randomized trials: extension of the CONSORT 2010 statement. JAMA. 2019;321(16):1610–1620. doi:10.1001/jama.2019.3087
- von EE, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi:10.1016/S0140-6736(07)61602-X
- European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
- Xie W, Tan J, Li B, et al. Comparison of hepatic resection with percutaneous ablation for hepatocellular carcinoma in the caudate lobe within Milan criteria. J Gastrointest Surg. 2022;26(2):323–332. doi:10.1007/s11605-021-05111-0
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi:10.1097/01.sla.0000133083.54934.ae
- Feng P, Zhou XH, Zou QM, Fan MY, Li XS. Generalized propensity score for estimating the average treatment effect of multiple treatments. Stat Med. 2012;31(7):681–697. doi:10.1002/sim.4168
- McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013;32(19):3388–3414. doi:10.1002/sim.5753
- Rassen JA, Shelat AA, Franklin JM, Glynn RJ, Solomon DH, Schneeweiss S. Matching by propensity score in cohort studies with three treatment groups. Epidemiology. 2013;24(3):401–409. doi:10.1097/EDE.0b013e318289dedf
- Yu Y, Zhang M, Shi X, Caram MEV, Little RJA, Mukherjee B. A comparison of parametric propensity score-based methods for causal inference with multiple treatments and a binary outcome. Stat Med. 2021;40(7):1653–1677. doi:10.1002/sim.8862
- Yoshida K, Hernandez-Diaz S, Solomon DH, et al. Matching weights to simultaneously compare three treatment groups: comparison to three-way matching. Epidemiology. 2017;28(3):387–395. doi:10.1097/EDE.0000000000000627
- Thomas L, Li F, Pencina M. Overlap weighting: a propensity score method that mimics attributes of a randomized clinical trial. JAMA. 2020;323(23):2417–2418. doi:10.1001/jama.2020.7819
- Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. doi:10.1002/sim.3697
- Syriopoulou E, Wästerlid T, Lambert PC, Andersson TM. Standardised survival probabilities: a useful and informative tool for reporting regression models for survival data. Br J Cancer. 2022;127(10):1808–1815. doi:10.1038/s41416-022-01949-6
- Liu XR, Pawitan Y, Clements M. Parametric and penalized generalized survival models. Stat Methods Med Res. 2018;27(5):1531–1546. doi:10.1177/0962280216664760
- Hirst A, Philippou Y, Blazeby J, et al. No surgical innovation without evaluation: evolution and further development of the IDEAL framework and recommendations. Ann Surg. 2019;269(2):211–220. doi:10.1097/SLA.0000000000002794
- Xu XL, Liu XD, Liang M, Luo BM. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: systematic review of randomized controlled trials with meta-analysis and trial sequential analysis. Radiology. 2018;287(2):461–472. doi:10.1148/radiol.2017162756
- Shin SW, Ahn KS, Kim SW, Kim TS, Kim YH, Kang KJ. Liver Resection Versus Local Ablation Therapies for Hepatocellular Carcinoma Within the Milan Criteria: a Systematic Review and Meta-analysis. Ann Surg. 2021;273(4):656–666. doi:10.1097/SLA.0000000000004350
- Yang J, Guo W, Lu M. Recent Perspectives on the mechanism of recurrence after ablation of hepatocellular carcinoma: a mini-review. Front Oncol. 2022;12:895678. doi:10.3389/fonc.2022.895678
- Pompili M, Saviano A, de Matthaeis N, et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma </=3 cm. Results of a multicenter Italian survey. J Hepatol. 2013;59(1):89–97. doi:10.1016/j.jhep.2013.03.009
- Kuhry E, Jeekel J, Bonjer HJ. Effect of laparoscopy on the immune system. Semin Laparosc Surg. 2004;11(1):37–44. doi:10.1177/107155170401100107
- Tang F, Tie Y, Tu C, Wei X. Surgical trauma-induced immunosuppression in cancer: recent advances and the potential therapies. Clin Transl Med. 2020;10(1):199–223. doi:10.1002/ctm2.24
- Abu Hilal M, Aldrighetti L, Dagher I, et al. The Southampton consensus guidelines for laparoscopic liver surgery: from indication to implementation. Ann Surg. 2018;268(1):11–18. doi:10.1097/SLA.0000000000002524
- Ibuki S, Hibi T, Tanabe M, et al. Short-term outcomes of “Difficult” laparoscopic liver resection at specialized centers: report from INSTALL (International Survey on Technical Aspects of Laparoscopic Liver Resection)-2 on 4478 patients. Ann Surg. 2022;275(5):940–946. doi:10.1097/SLA.0000000000004434
- Teratani T, Yoshida H, Shiina S, et al. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology. 2006;43(5):1101–1108. doi:10.1002/hep.21164
- Kwak MH, Lee MW, Ko SE, et al. Laparoscopic radiofrequency ablation versus percutaneous radiofrequency ablation for subphrenic hepatocellular carcinoma. Ultrasonography. 2022;41(3):543–552. doi:10.14366/usg.21241
- Eun HS, Lee BS, Kwon IS, et al. Advantages of laparoscopic radiofrequency ablation over percutaneous radiofrequency ablation in hepatocellular carcinoma. Dig Dis Sci. 2017;62(9):2586–2600. doi:10.1007/s10620-017-4688-6